Abstract
[11C]MRB is one of the most promising radioligands used to measure brain norepinephrine transporters (NET) with positron emission tomography (PET). The objective of this study was to evaluate the suitability of [11C]MRB for drug occupancy studies of NET using atomoxetine (ATX), a NET uptake inhibitor used in the treatment of depression and attention-deficit hyperactivity disorder (ADHD). A second goal of the study was identification of a suitable reference region. Ten PET studies were performed in three anesthetized rhesus monkeys following an infusion of ATX or placebo. [11C]MRB arterial input functions and ATX plasma levels were also measured. A dose-dependent reduction of [11C]MRB volume of distribution was observed after correction for [11C]MRB plasma free fraction. ATX IC50 was estimated to be 31±10 ng/mL plasma. This corresponds to an effective dose (ED50) of 0.13 mg/kg, which is much lower than the therapeutic dose of ATX in ADHD (1.0–1.5 mg/kg). [11C]MRB binding potential BPND in the thalamus was estimated to be 1.8±0.3. Defining a reference region for a NET radiotracer is challenging due to the widespread and relatively uniform distribution of NET in the brain. Three regions were evaluated for use as reference region: caudate, putamen and occipital cortex. Caudate was found to be the most suitable for preclinical drug occupancy studies in rhesus monkeys. The IC50 estimate obtained using MRTM2 BPND without arterial blood sampling was 21±3 ng/mL (using caudate as the reference region). This study demonstrated that [11C]MRB is suitable for drug occupancy studies of NET.
Introduction
The brain norepinephrine transporter (NET) is responsible for norepinephrine reuptake, as well as dopamine reuptake in some regions such as the frontal cortex. (Morón et al., 2002). The ability to quantify the density of these transporters in vivo with positron emission tomography (PET) would provide an important means to study the role of NET in various psychiatric and behavioral disorders in which they are implicated, such as attention deficit hyperactivity disorder (ADHD) (Spencer et al., 2002), substance abuse and depression (Barker and Blakely, 1995; Ding et al., 2006, review and references within). However, PET imaging of norepinephrine transporters has long been hampered by the lack of suitable radioligand (Ding et al., 2005, 2006). Drawbacks of early candidate radiotracers included very low specific binding and the lack of a suitable reference region. The challenges for NET imaging are in part fundamentally due to the widespread distribution of NET in the brain, and the lower contrast in transporter density between NET-poor and NET-rich regions (Smith et al., 2006) than for the other monoamine transporters like DAT (Kaufman et al., 1991) or SERT (Zeng et al., 2006). Furthermore, the locus coeruleus, the highest NET density region, is difficult to localize and quantify with PET due to its small volume. Currently, the best radioligands available are derivatives of reboxetine: (S,S)-[11C]methylreboxetine ([11C]MRB, also known as [11C]MeNER) (Ding et al., 2003; Schou et al., 2003; Wilson et al., 2003) and its [18F]fluoromethyl derivatives ([18F]F-MeNER-D2 (Schou et al., 2004) and [18F]FRB (Ding et al., 2005, Lin et al., 2005). They have higher specific binding and lower variability in non-specific binding than previous radioligands (Ding et al., 2006).
When [11C]MRB was initially tested in a drug occupancy study in humans using Atomoxetine (ATX), a reduction in [11C]MRB binding was reported, but there was no obvious dose-dependent effect (Logan et al., 2007). ATX is a potent norepinephrine transporter reuptake inhibitor (Ki ~ 5 nM) currently used in the treatment of depression and ADHD (Spencer et al., 2002). One hypothesis was that the doses of ATX examined in the human PET study, though clinically relevant, were too high. That is, the three tested doses all reached saturation, the plateau portion of the corresponding dose-occupancy curve, and, as a result, there was no obvious dose-dependent effect. Another occupancy study of ATX using [18F]F-MeNER-D2 in rhesus monkeys showed a reasonable dose-dependent occupancy though only in one of the two monkeys studied and the IC50 (or ED50) was not determined (Seneca et al., 2006). In a second occupancy study of ATX using [18F]F-MeNER-D2 in rhesus monkeys, the ATX IC50 was quantified as 16 ng/mL plasma (Takano et al., 2009a), which is about 7 times lower than the lowest plasma levels achieved in the aforementioned [11C]MRB human study.
The goal of the study was to re-evaluate the suitability of [11C]MRB for occupancy studies in non-human primates using lower doses of ATX than those used in the previous PET study with [11C]MRB (Logan et al., 2007), and with a continuous ATX infusion paradigm, a protocol similar to the aforementioned [18F]F-MeNER-D2 studies. A second goal of this study is to determine if there is a suitable reference region for [11C]MRB.
Materials and methods
Study design
In order to maintain a constant concentration of ATX in the plasma during the PET acquisition time, a continuous infusion protocol was used. This protocol was similar to the one used in the study with [18F]F-MeNER-D2 (Seneca et al., 2006). Two infusion rates were used (Fig. 1): 2 h before the beginning of each PET scan, the first ATX infusion at the “loading” rate was begun; then, 1 h before the injection of [11C]MRB and the beginning of the PET scan, the infusion rate was lowered by 37.5% (i.e., “maintenance” rate). This infusion rate (MIRATX) was maintained until the end of PET scan. Initially the maintenance rates were 0.01 mg/kg/h (low dose), 0.05 mg/kg/h (medium dose) and 0.012 mg/kg/h (high dose). After analysis of the data from the first two monkeys (6 scans), the medium and high-dose rates were lowered to 0.03 mg/kg/h and 0.09 mg/kg/h, respectively.
Fig. 1.
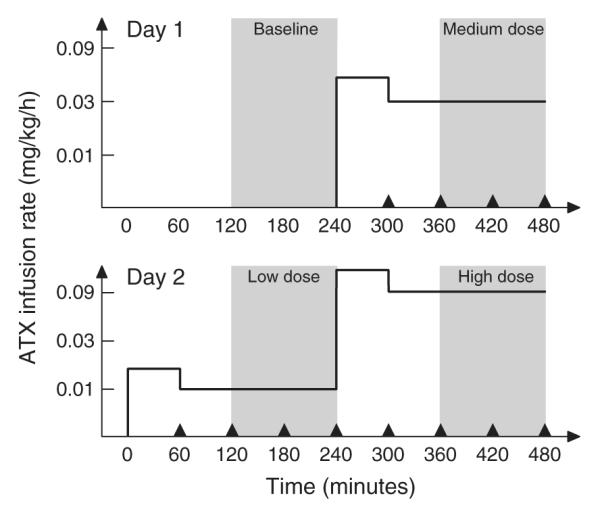
Study timelines for the two-day, four-dose studies (Day 1: baseline and medium-dose studies; Day 2: low- and high-dose studies). The solid line represents the ATX infusion rates (i.e., loading rate followed by maintenance rate for each study), as used for monkey #3 (i.e., the maintenance rates were 0.01 mg/kg/h (low dose), 0.03 mg/kg/h (medium dose) and 0.09 mg/kg/h (high dose)). The grey areas highlight the PET scanning times. The black triangles represent the PK sampling times.
The design was to have each monkey have four PET scans on 2 study days. These 2 study days were scheduled 2 weeks apart to allow recovery from the effects of the anesthesia and blood drawing. On each study day, two PET scans were acquired, beginning 4 h apart. The first study day consisted of the baseline and medium-dose scans, the second study day consisted of the low- and high-dose scans.
Study population
Three non-human primates (Rhesus macaque, 5.5±1.4 years old and weighing 6.7±1.4 kg) were scanned. Only two monkeys were scanned four times. The second monkey could not be scanned on the second study day, due to the unavailability of arterial samples. Thus, only baseline and medium-dose scans were acquired for that animal. All study procedures were performed under a protocol approved by the Yale University Institutional Animal Care and Use Committee.
Radiochemistry
Both the MRB standard and precursor were prepared previously (Lin and Ding, 2004). [11C]MRB was synthesized with [11C]methyliodide ([11C]MeI) as previously reported (Lin and Ding, 2004) except, radiolabeling was carried out using either the TRACERLab FXC automated synthesis module (GE Medical Systems), or using the AutoLoop (Bioscan, Washington, DC, USA) by modifying previously reported method (Wilson et al., 2003) (see supplementary information for details). C-11 methyl iodide ([11C]CH3I) was produced from [11C] CO2, as previously described (Nabulsi et al., 2010). The total radio-synthesis time was ~40 min with an average specific activity of 264±107 MBq/nmol (7±3 mCi/nmol) at the end of synthesis (n=10). The final formulated product was >98% radiochemically pure. The average injected dose was 247±20 MBq with an average specific radioactivity of 129±46 MBq/nmol at the time of injection. The average injected mass was 0.57±0.23 μg.
PET imaging
Animals were initially anesthetized with an intramuscular injection of ketamine hydrochloride, intubated and transported to the PET facility. They were maintained on oxygen and isoflurane (1.5–3%) throughout the study. Scans were performed with the High Resolution Research Tomograph (HRRT) (Siemens/CTI, Knoxville, TN, USA) PET scanner which acquires 207 simultaneous slices with 1.2 mm inter-slice distance and resolution of ~3 mm. Following a 6-min transmission scan, [11C]MRB was injected over 2 min by an infusion pump (Harvard PHD 22/2000, Harvard Apparatus, Holliston, Massachusetts, United States). List-mode data were acquired for 120 min. Dynamic scan data were reconstructed with all corrections (attenuation, normalization, scatter, randoms, and deadtime) using the MOLAR algorithm (Carson et al., 2003; Johnson et al., 2004) with 2 iterations and 30 subsets. A sequence of 33 frames was reconstructed with the following timing: 6×30 s; 3×1 min; 2×2 min; 22×5 min.
Arterial input function measurement
In order to measure the time–concentration curve of [11C]MRB, 21 blood samples were drawn at 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 15, 20, 25, 30, 40, 50, 60, 75, 90, 105, 120 min after the injection of [11C]MRB. Sample volumes were approximately 0.5 mL. Samples drawn at 10-, 20-, 40-, 60- and 90-min postinjection had a volume of 1.5 mL in order to measure the fraction of unchanged [11C]MRB in plasma by HPLC. Plasma was separated from blood cells by centrifugation (3900g for 5 min at 4 °C; Allegra X-22R Centrifuge, Beckman Coulter, Fullerton, CA, USA). Whole blood and plasma samples were counted in a cross-calibrated well counter (Wizard 1480, Perkin Elmer, Waltham, MA, USA).
For the metabolite samples, plasma proteins were precipitated using acetonitrile (1 mL/mL plasma) and centrifugation (14,000g for 5 min; Minispin plus centrifuge, Eppendorf, Westbury, NY, USA). The supernatant was counted and 0.2 mL was analyzed by reverse-phase HPLC. The HPLC system consisted of an isocratic pump (LC-20A1, Shimadzu, Kyoto, Japan), an injector (7725i, Rheodyne, Rohnert Park, CA, USA) equipped with a 2 mL sample loop, a Luna C18(2) column (semi-prep, 4.6×250 mm, 10 μm, Phenomenex, Torrance, CA, USA). The column was eluted with a mixture of acetonitrile and aqueous 0.1 M ammonium formate (pH 4.2) in a ratio of 32:68 at a flow rate of ~4.2 mL/min. The output of the HPLC column was connected first to an UV detector (SPD-20A, Shimadzu, Kyoto, Japan), then to an inline gamma counter (Flow-Count Radio-HPLC Detector, Bioscan, Washington, DC, USA) and finally to a fraction collector (CF-1 Fraction Collector, Spectrum Chromatography, Houston, TX, USA). Collection tubes were manually shifted based on the detection of the parent compound peak with the inline gamma counter. Fractions were then counted in a well counter.
The ratios of the radioactivity concentrations in supernatant and plasma were obtained and fitted to a quadratic curve (fS). The unchanged (i.e., unmetabolized) fraction of the tracer in the supernatant was fitted to an integrated gamma function:
| (1) |
The unchanged tracer fraction in plasma was then computed as the product of the functions fS and fH. Both functions were extrapolated from 90 min (time of the last sample analyzed by HPLC) to 120 min.
[11C]MRB plasma free fraction
For measurement of tracer plasma free fraction (fp), ~740 kBq of [11C] MRB was added (<25 μl/mL blood from the dose vial) to a blood sample withdrawn before injection to produce a blood standard. After mixing and centrifugation of red blood cells, plasma filtrate was separated from plasma proteins using ultrafiltration tubes (Centrifree UF device number 4104, Millipore, Billerica, MA, USA) and centrifugation (1100g for 20 min; IEC Medilite centrifuge, Thermo Fisher Scientific, Waltham, MA, USA). Plasma and filtrate samples were counted in triplicate and free fraction (fP) was estimated as the ratio of the mean concentrations in filtrate and plasma.
ATX plasma levels
In addition to the samples used to determine [11C]MRB radioactive concentration in plasma, four 3-mL blood samples were drawn 60, 120, 180, and 240 min post-ATX infusion (or 60 min before and 0, 60 and 120 min after tracer injection) to determine the ATX concentration in plasma. Red blood cells were precipitated; the plasma was frozen and then shipped for analysis. The plasma level of ATX was quantified with capillary gas chromatography-mass spectrometry (Analytical Psycho-pharmacology Laboratory, Nathan Kline Institute, Orangeburg, NY). The rate of variation of ATX concentration in plasma during the PET scan was estimated by fitting the 0-, 60- and 120-min sample values (post tracer injection) with a mono-exponential function.
Magnetic resonance imaging
MR images were acquired for each rhesus monkey on a Siemens 3.0T Trio scanner, using an extremity coil. T1-weighted images were acquired in the coronal plane with a spin echo sequence (TE=3.34, TR =2530, flip angle=7°, section thickness=0.50 mm, field-of-view = 140 mm, image matrix = 256 × 256 × 176 pixels, matrix size=0.5469×0.5469×0.5000 mm). Non-brain was removed and cropped to 176×176×176 pixels using MEDx software (Medical Numerics Inc, Germantown, MD, USA) before co-registration with PET Images.
Regional TAC computation
In order to compute regional time activity curves (TAC), selected regions of interest (ROIs) where drawn on a template brain (a representative MR image of a monkey brain, from a monkey not included in the study) for the caudate nucleus (0.43 cm3), putamen (0.38 cm3), occipital cortex (5.2 cm3), anterior cingulate cortex (0.077 cm3), thalamus (0.25 cm3), midbrain (0.61 cm3) and brainstem (1.85 cm3). To transfer this template of regions to the PET dynamic images, two transformations were estimated: first, a nonlinear transform between the template MR image and each monkey MR image was computed using the bioimagesuite software (www.bioimagesuite.org); then, a rigid coregistration matrix was estimated between each monkey MR and an early PET sum image from each study (0–10 or 5–10 min postinjection), using a normalized mutual information algorithm (FLIRT, www.fmrib.ox.ac.uk/fsl/flirt/index.html).
Quantification of [11C]MRB distribution volume
Based on previous modeling studies of [11C]MRB kinetics (Logan et al., 2005; Gallezot et al., 2007), [11C]MRB volume of distribution (VT) was estimated using the multilinear analysis MA1 (Ichise et al., 2002) and the following equation:
| (2) |
where the parameter b is equivalent to the y-intercept in the Logan plot (Logan et al., 1990). When MA1 was applied to the regional time activity curves, t* was set to 30 min. Parametric images of the VT of [11C]MRB were also computed using MA1. In these pixel-by-pixel analyses, t* was set to 10 min.
Quantification of [11C]MRB binding potential without arterial blood sampling
In order to avoid using arterial blood sampling, the ratio of the VT of [11C]MRB in a target region (or voxel) to that in a reference region, VT/VRef, was estimated using MRTM2 (Ichise et al., 2003). MRTM2 is based on the multilinear reference tissue model (MRTM) (Ichise et al., 1996, 2003) and the following equation:
| (3) |
where the parameter b’ is the y-intercept in the Logan plot of the reference region. The parameter b’ is also equal to the value of the parameter b, in Eq. (2), for the reference region. Therefore, in theory, the parameter b’ should be identical in all voxels or regions for any given scan. However, in MRTM, one value of b’ is estimated per ROI or voxel. With MRTM2, only one value of b’ is estimated per scan in order to reduce the variability of the other parameter estimates.
MRTM2 is a two-step procedure. In the first step, MRTM was applied to all brain voxels to produce parametric images of BPND, b and b’, as well as a parametric image of the standard error σb’ of parameter b’ determined from the linear fit. Then a global estimate of b’ was computed using the following weighted mean formula over all brain voxels:
| (4) |
Then, in the second step, MRTM was reapplied to all brain voxels using the aforementioned b’ value as a fixed parameter, to produce improved parametric images of BPND and b. To compute these MRTM2 parametric images, only the caudate nucleus was used as reference region.
Estimation of ATX IC50 and ED50
Three indexes of [11C]MRB binding were compared to highlight the dose-dependent effect of ATX and quantify its IC50: the normalized distribution volume VT/fP, and the binding potentials BPF and BPND (Innis et al., 2007). VT/fP and BPF values were obtained from the MA1 VT estimates. BPND values were obtained either from the MA1 VT estimates or directly from the MRTM2 analyses. The effect of ATX on these three indexes was quantified using the following equations:
| (5) |
| (6) |
| (7) |
where CATX is the average concentration of ATX measured in the plasma during the PET scan (i.e., the average of the three samples taken 120, 180 and 240 min after the beginning of the ATX infusion), Bmax is the NET density, Kd is [11 C]MRB equilibrium dissociation constant, VND and VRef are the non-displaceable distribution volumes in the target tissue and in the reference region, respectively, and fND and fP are the free fractions of [11 C] MRB in the target tissue (excluding specific binding) and in plasma, respectively. Eq. (5) is a 3-parameter equation (VND/fP, Bmax/Kd and the IC50). Eqs. (6) and (7) are 2-parameter equations (Bmax/Kd and the IC50 for Eq. (6); fNDBmax/Kd and the IC50 for Eq. (7)), and are based on the assumption that VND in the target tissue is equal to VT in the reference region (i.e., VND=VRef). Three candidate reference regions were evaluated: putamen, caudate nucleus and occipital cortex. IC50 estimates were obtained using each target region by pooling data from all monkeys. For each region, data from all monkeys was pooled, and fitted with one set of parameters, ignoring the effects of the population variability of VND/fP, Bmax/Kd, fNDBmax/Kd and IC50 in this small sample.
Alternatively, the percentage of occupancy was estimated for each scan using the Lassen plot (Lassen et al., 1995; Cunningham et al., 2010). This method assumes that all the regions included in the analysis have identical non-specific binding (i.e. VND/fP) and ATX occupancy level. Therefore, regions which were estimated to have different VND/fP values were excluded (see results). In the Lassen plot, the reduction of VT/fP between a baseline scan and a post-drug scan is plotted against the corresponding VT/fP estimates at baseline. Then, ATX occupancy was estimated for each scan as the slope of the regression line. Then, ATX IC50 was estimated by fitting the curve representing ATX occupancy versus the ATX plasma concentration CATX using the following equation:
| (8) |
Finally, the IC50 estimates (ng/mL plasma) were converted to ED50 estimates (mg/kg/h) using the linear correlation between the measured ATX plasma levels and the infusion rates.
Data weights, parameter estimation and statistical analyses
Parameters VT (for MA1) and BPND (for MRTM2) were estimated using multilinear weighted least squares, with weights based on the noise-equivalent counts in each frame. For the estimation of ATX IC50, nonlinear parameter estimation was performed using a Marquardt–Levenberg algorithm (Marquardt, 1963) and custom software for IDL 6.2 (ITT Visual Information Solutions, Boulder, CO, USA). Standard errors on the fitted parameters were estimated using the theoretical covariance matrix (Beck and Arnold, 1977, chapter 7.7.1).
Standard errors on the plasma free fraction (σfP) were estimated using the standard deviation of the triplicate measurements of plasma (σC***plasma) and ultrafiltrate (σC***ultrafiltrate) samples, and the error propagation equation. Similarly, the standard error on the normalized distribution volumes VT/fP were also computed using the error propagation equation.
F tests were used to determine whether the parameters IC50 and VND/fP could be shared across NET-rich regions. Similarly, F tests were used to determine whether the parameter Bmax/Kd could be set to zero in the candidate reference regions, or if the value of VND/fP estimated from a reference region was compatible with data from the high-binding regions.
Results
ATX plasma levels
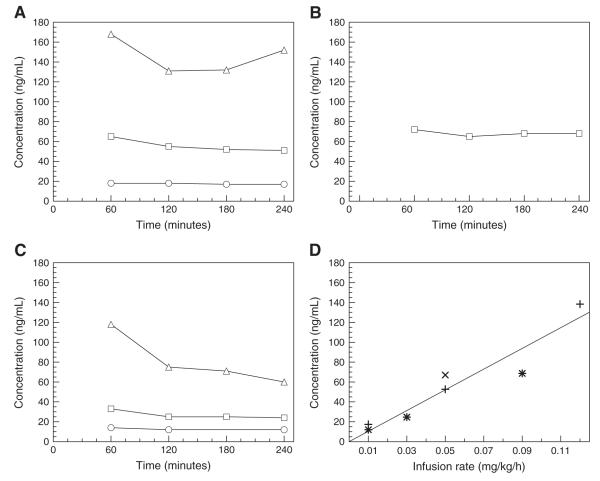
For all monkeys and all ATX doses, ATX plasma levels could be considered stable 120 min after the beginning of the first infusion (i.e. throughout the PET scan), and ranged between 12 ng/mL and 132 ng/mL (Fig. 2A–C). The percent change per hour of ATX plasma level during the PET scan was −1±6%/h. Moreover, ATX plasma levels and the infusion rate were well correlated (Fig. 2D). The slope of the regression line was 1.04 kg×h/L (body weight×time/plasma volume) (r2=0.902, n=7, intercept set to zero).
Fig. 2.
(A, B, C) ATX plasma concentration measured for monkeys 1, 2, and 3, respectively, for the low (circle), medium (square) and high (triangle) dose scans. (D) Correlation between the ATX plasma levels and the final infusion rates (infusion II) for monkey 1 (+), 2 (×) and 3 (*), respectively. The regression line was obtained by pooling data from all monkeys (slope=1.04 kg×h/L, r2=0.902, n=7).
[11C]MRB input function
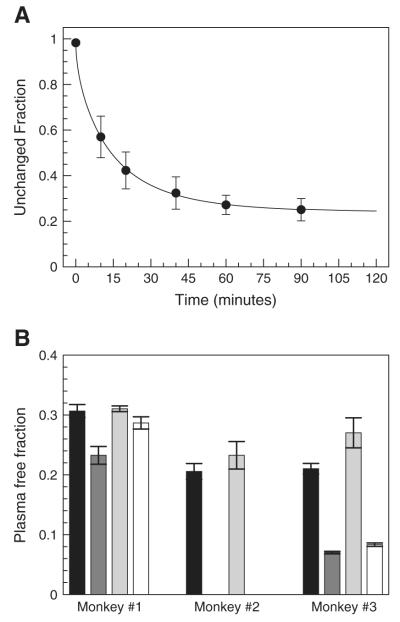
The radioactivity concentration in whole blood was always slightly lower than that in plasma. The whole blood to plasma concentration ratio was 0.94±0.13 throughout the scans. The fraction of unmetabolized [11C]MRB in plasma quickly decreased initially and then stabilized (Fig. 3A): it was 42±8% and 25±4% at 20 and 90 min post-injection, respectively. Since the last parent fraction measurement was only done at 90 min, the metabolite correction from 90 to 120 min was based on the extrapolation of the fits obtained with Eq. (1) and shown in Fig. 3A. The relative difference between the distribution volumes estimated using 120 min worth of PET data (with extrapolation of the unchanged fraction curve) versus 90 min was +5±6% in the high binding regions and +3±2% in the NET-poor regions.
Fig. 3.
(A) Unchanged fraction of [11C]MRB in plasma (average±SD, n=10). The solid line represents the average of the fitted fH curves used for metabolite correction. (B) [11C]MRB plasma free fraction measured by ultrafiltration for the baseline (black), low (dark grey), medium (light grey) and high (white) ATX dose scans. Errors bars represent the standard errors estimated from the triplicate measurements of plasma and ultrafiltrate activity.
[11C]MRB plasma free fraction fP was highly variable from scan to scan, with two main trends (Fig. 3B). First, there was a moderate increase from the first to the second scan of the day (+17%±10%). Also, there was a higher day-to-day variability (−16% and −68% for monkey #1 and #3, respectively). The relative standard errors on the free fraction fP measurement were less than 10% for all studies (5% in average) suggesting that these differences were not due to measurement error.
[11C]MRB distribution volumes and normalized distribution volumes
Typical regional time–activity curves at baseline and the corresponding fitted curves obtained with MA1 in one monkey are shown in Fig. 4. [11C]MRB distribution volumes (VT) estimated using MA1 (t*=30 min) are listed in Table 1. The relative standard errors on VT estimates were less than 9% for all studies and ROIs (3% in average). Although the distribution volumes were variable from day to day, especially for monkey #3, VT values estimated in the candidate reference regions were highly correlated with the plasma free fraction fP (e.g., in the caudate nucleus VT=25.2 fP+0.3, r2=0.920, n=10). The normalized volumes of distribution values (VT/fP) decreased in a dosedependent manner with increased ATX doses for all monkeys in all the NET-rich regions (Fig. 5). This relationship was not clearly detectible when VT values were used without fP normalization.
Fig. 4.
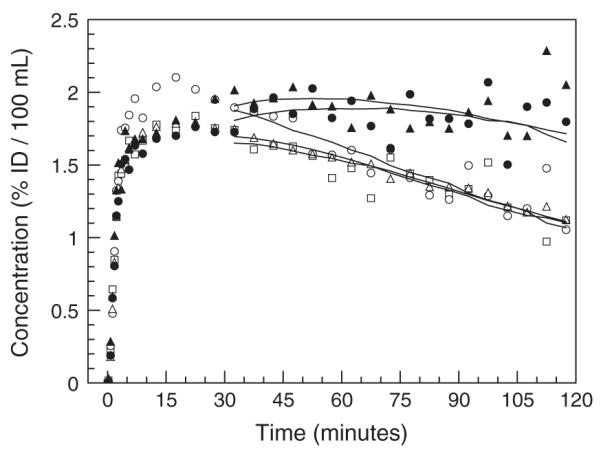
Example of regional time activity curves obtained in one monkey (#2) at baseline in the caudate nucleus (open squares), putamen (open circles), occipital cortex (open triangles), anterior cingulate cortex (solid triangles) and thalamus (solid circles). Solid lines correspond to the MA1 fits.
Table 1.
[11C]MRB distribution volumes (VT).
| Distribution Volume VT (mL/cm3) |
|||||
|---|---|---|---|---|---|
| Region | Monkey | Baseline |
ATX low dose |
ATX medium dose |
ATX high dose |
| Day 1 | Day 2 | Day 1 | Day 2 | ||
| Caudate | #1 | 7.7 | 6.2 | 8.7 | 6.6 |
| #2 | 6.5 | 6.5 | |||
| #3 | 5.9 | 2.1 | 6.3 | 1.7 | |
| Putamen | #1 | 8.9 | 6.8 | 9.9 | 7.6 |
| #2 | 6.6 | 8.7 | |||
| #3 | 6.3 | 2.4 | 7.2 | 2.2 | |
| Occipital | #1 | 8.4 | 6.2 | 8.2 | 6.2 |
| #2 | 6.6 | 6.4 | |||
| #3 | 6.4 | 2.0 | 5.6 | 1.5 | |
| Anterior | #1 | 13.3 | 8.5 | 11.0 | 7.4 |
| Cingulate | #2 | 10.1 | 8.7 | ||
| #3 | 10.9 | 2.8 | 8.3 | 1.8 | |
| Thalamus | #1 | 15.5 | 10.4 | 11.6 | 8.0 |
| #2 | 10.7 | 8.2 | |||
| #3 | 12.7 | 3.4 | 8.5 | 2.0 | |
| Midbrain | #1 | 13.3 | 9.0 | 10.1 | 6.3 |
| #2 | 10.5 | 7.5 | |||
| #3 | 9.4 | 3.0 | 7.8 | 1.9 | |
| Brainstem | #1 | 16.3 | 9.9 | 11.5 | 6.6 |
| #2 | 10.2 | 7.3 | |||
| #3 | 11.1 | 3.1 | 8.2 | 2.0 | |
Fig. 5.
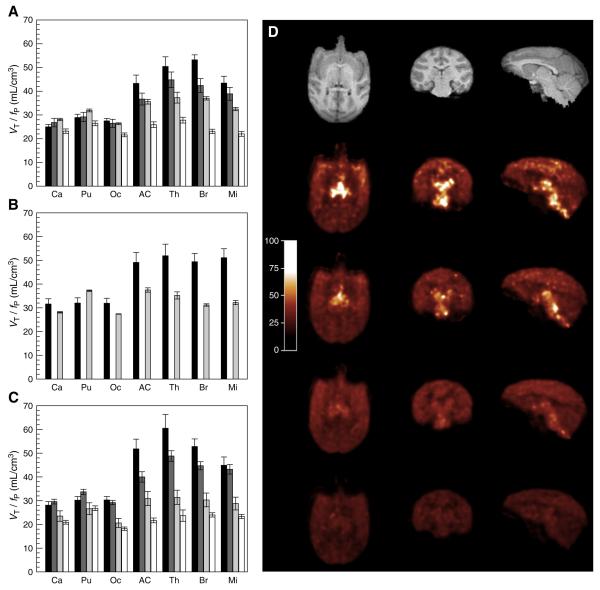
Dose-dependent occupancy of ATX in NET-rich regions in three monkeys. (A to C) Normalized volume of distribution (VT/fP) computed using MA1 at baseline (black bars) and at the low (dark grey bars), medium (light grey bars) and high (white bars) doses of ATX in the monkeys 1 to 3. The brain regions are: Ca = caudate, Pu = putamen, Oc = occipital cortex, AC = anterior cingulate cortex, Th = thalamus, Br = brainstem, Mi = midbrain. Error bars represent the estimated standard errors. (D) From top to bottom: Anatomic MR image, and parametric images of the normalized volume of distribution (VT/fP) at baseline and after blockade with 0.03 mg/kg/h, 0.06 mg/kg/h and 0.12 mg/kg/h of ATX. Images are from monkey #1 and were computed using MA1.
Estimation of ATX IC50 in high binding regions
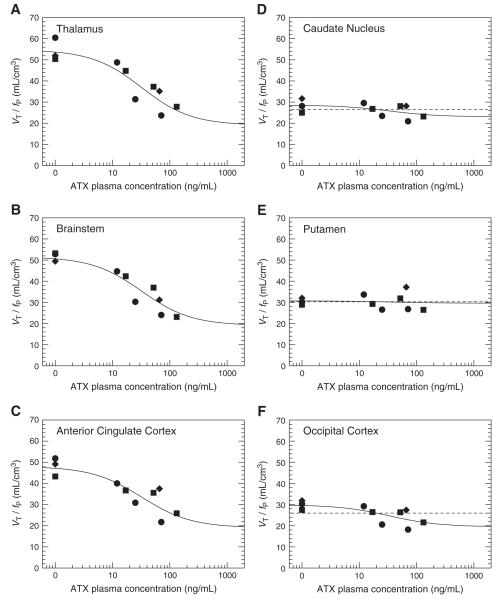
Plots of VT/fP values versus the plasma concentration of ATX are displayed in Fig. 6A to C for three NET-rich regions. Using only the normalized distribution values VT/fP, ATX IC50 values were estimated in four NET-rich regions, without using a reference region. It is possible to fit the data from these four regions with a global IC50 value and a global VND/fP value without significantly increasing the residual sum of squares (F6,28=0.17, p=0.98). The global IC50 estimate was 32±13 ng/mL (rSE =39%) and the global VND/fP estimate was 19±4 mL/cm3 (rSE=22%). The corresponding fitted curves are displayed in Fig. 6A to C. This corresponds to a global effective dose (ED50) of 0.031 mg/kg/h using the relationship between plasma levels and the maintenance infusion shown in Fig. 2D (i.e., CATX=MIRATX×1.042 kg×h/L). Based on this global IC50 estimate and the measured ATX plasma concentration, the percentage of occupancy of NET by ATX achieved in this study was estimated to range from 27% (at 12 ng/mL) to 80% (at 132 ng/mL).
Fig. 6.
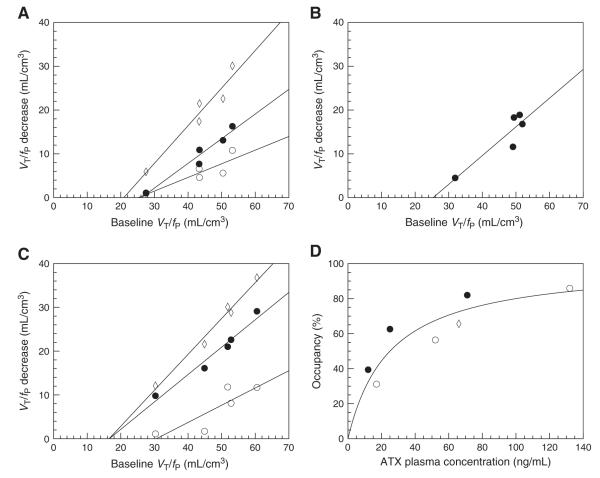
The relationship between the normalized distribution volumes (VT/fP) and the plasma levels of ATX three NET-rich regions: thalamus (A), brainstem (B) and anterior cingulate cortex (C); and in three candidate reference regions: caudate (D), putamen (E) and occipital cortex (F). Data obtained from monkey 1, 2 and 3 and represented with circles, diamonds and squares, respectively. In subfigures A to C, the solid line represents the fit obtained using Eq. (5) to estimate the ATX IC50. The parameters IC50 and VND/fP were shared across all NET-rich regions. In subfigures D to F, the two lines represent the fits obtained using Eq. (5), with the IC50 set to the value estimated in NET-rich regions (32 ng/mL), and Bmax/Kd set to zero (dashed line) or fitted (solid line). According to the F test, Bmax/Kd was significantly different from zero in the occipital only.
Using the global VND/fP estimate, BPND was computed as VT/fP at baseline divided by VND/fP, minus one, and was estimated to range from 1.4±0.2 in the midbrain to 1.8±0.3 in the thalamus (n=3). These BPND values being very sensitive to the estimation of VND/fP, a more conservative approach was also used to estimate a lower bound for BPND: BPND was computed was VT/fP at baseline divided by VT/fP at the highest doses of ATX, minus one. That is, a 100% occupancy was assumed at the highest doses of ATX in this second approach. With this method BPND was estimated to range from 1.0±0.04 in the midbrain to 1.2±0.5 in the thalamus (n=2, since one monkey did not have a high-dose scan).
Evaluation of candidate reference regions
The relative standard error on the aforementioned IC50 estimate was large (39%) due to the need to estimate the extra parameter VND/fP. To try to reduce that uncertainty of the IC50 estimate, and also to potentially avoid arterial blood sampling, the use of a reference region was investigated. The normalized distribution volume values VT/fP at baseline (n=3) were very similar for all three candidate reference regions, i.e., 30±2 mL/cm3, 30±2 mL/cm3 and 28±3 mL/cm3 for putamen, occipital cortex, and caudate nucleus, respectively. These baseline VT/fP values were higher than the VT/fP values after blockade by the highest dose of ATX in NET-rich regions: VT/fP was estimated to be 24±3, 26±3, 23±1 and 24±1 in the anterior cingulate cortex, thalamus, midbrain and brainstem, respectively, after blockade by the highest dose of ATX (n=2). These baseline VT/fP values were also higher than the previously estimated value of VND/fP in the four high-binding regions (i.e., 19±4 mL/cm3). These differences could be due to differences in VND between the candidate reference regions and the high-binding regions, or due to the presence of specific binding in the candidate reference regions.
To test for the presence of specific binding, the curves of VT/fP values versus the plasma concentration of ATX in the three reference regions were fitted using Eq. (5), with the IC50 parameter fixed to the global value estimated in high-binding regions (i.e., 32 ng/mL). The data was fitted twice, with or without the assumption that Bmax/Kd can be fixed to zero (Fig. 6D to F), and the two fits were compared using the F test. In the occipital cortex, assuming that Bmax/Kd is equal to zero produced a significantly worse fit (F1,8=8.4, p<0.02). Bmax/Kd was estimated to be 10±4 mL/cm3 and VND/fP was estimated to be 20±3 mL/cm3. Thus, a significant specific binding was detected in occipital cortex; this region was thus excluded from the list of candidate reference regions. In the putamen, assuming that Bmax/Kd is equal to zero did not produce a significantly worse fit (F1,8=0.07, p=0.8). VND/fP was then estimated to be 30±1 mL/cm3. In the caudate, there was a trend-level, but not significant, difference between the two fits (F1,8=3.0, p=0.12). With the two-parameter fit, Bmax/Kd was estimated to be 6±4 mL/cm3 and VND/fP was estimated to be 23±2 mL/cm3. With the one-parameter fit, VND/fP was estimated to be 26±1 mL/cm3.
To test whether the estimate of VND/fP from the putamen (or caudate) is significantly different from the estimate of VND/fP from the high-binding regions, the high binding region curves were fitted twice, with or without fixing VND/fP to the value estimated in the putamen (or caudate), and the two fits were compared using the F test. Using the VND/fP value estimated in the putamen (i.e., 30 mL/cm3) as a fixed parameter to fit the high-binding region data lead to significantly worse fits (F1,34=17.6, p<0.001). Similarly, using the VND/fP value estimated in the caudate (i.e., 26 mL/cm3) as a fixed parameter to fit the high-binding region data lead to significantly worse fits (F1,34=6.0, p<0.02). Thus, putamen and caudate are also not perfectly valid reference regions.
Estimation of the bias on IC50 estimates when using caudate or putamen as the reference region
Using the caudate as the reference region and the binding potential BPND (deduced from MA1 VT estimates), ATX IC50 was estimated to be 21±4 ng/mL (rSE=18%), which is 36% lower than the IC50 estimate obtained without using a reference region. Similarly, ATX IC50 was estimated to be 19±3 ng/mL (rSE=16%) when using BPF (bias=−39%). Using the putamen as the reference region and either BPND or BPF, ATX IC50 was estimated to be 9±2 ng/mL (rSE=28%), which is 72% lower than the estimate without using a reference region. Thus, the IC50 estimate was both more biased and less precise when using the putamen rather than the caudate nucleus. Additionally, the IC50 estimates obtained using caudate-based BPND or BPF values are not significantly lower than the IC50 estimate obtained using VT/fP (F1,34<2.3, p>0.14), but the IC50 estimates obtained using putamen-based BPND or BPF values are significantly lower (F1,34=12.6, p<0.002).
In order to avoid arterial blood sampling, [11C]MRB binding potentials were also estimated directly using MRTM2 and the caudate nucleus as a reference region. The estimates of BPND obtained with MRTM2 and MA1 were highly correlated (y =0.993 x+ 0.021, r2=0.949, n=70, where x and y represent MA1 and MRTM2 regional BPND estimates, respectively). MRTM2 BPND parametric images are visually almost identical to MA1 BPND images, except for a few artifacts in the images from scans following administration of the highest doses of ATX (data not shown). Consequently, the IC50 estimate obtained using MRTM2 BPND estimates (21±3 ng/mL) was very similar to the one obtained using MA1 BPND estimates (21±4 ng/mL).
Improving IC50 estimates by using occipital as a low binding region
Another approach was tested to reduce the standard error on the IC50 estimate: fitting the four high binding regions together with the occipital cortex since both VND/fP and the IC50 seemed similar in these five regions, using Eq. (5). The relative standard error on VND/fP and IC50 estimates could be slightly reduced to 15% and 31% (from 22% and 39%, respectively, without including the occipital in the analysis) with negligible changes in the estimates themselves: VND/fP and IC50 estimates are then 19± 3 mL/cm3 and 31±10 ng/mL (from 19±4 mL/cm3 and 32±13 ng/mL, respectively). Bmax/Kd was estimated to 11±4 in the occipital cortex.
Estimating ATX IC50 using the Lassen plot
Lassen plots obtained for the three animals are shown in Fig. 7A–C. The four high binding regions and the occipital were included in the analysis, since the previous analyses showed that VND/fP and the IC50 were not significantly different in these regions. ATX occupancy estimates were 31±10%, 56±7% and 86±13% for the low-, medium- and high-dose scans for monkey 1, 65±18% for monkey 2, and 39±14%, 63±7% and 82±6% for the low-, medium- and high-dose scans for monkey 3. The relationship between these ATX occupancy estimates and the measured concentration of ATX in plasma is shown in Fig. 7D. ATX IC50 was estimated to be 28±7 ng/mL with this method. This IC50 value is in excellent agreement with the value obtained above using the nonlinear fit.
Fig. 7.
Lassen plots obtained for the low (open circles), medium (solid circles) and high (open diamonds) doses for monkeys 1 to 3 are displayed in subfigures A to C. The solid lines represent the regression lines for each set of data. In subfigure D, occupancies estimated as the slope of these regression lines are plotted versus the ATX plasma concentration (monkey 1: open circles; monkey 2: open diamonds, monkey 3: solid circles). Solid line represent the line of best fit obtained with Eq. (8) (pooling data from all monkeys), and corresponding to an IC50 estimate of 28±7 ng/mL.
Discussion
In this study, we demonstrated that [11C]MRB is a suitable ligand for determination of NET occupancy. We found that [11C]MRB binding can be inhibited in a dose-dependent manner by infusion of ATX. An important methodological consideration was the day-to-day variability of [11C]MRB VT estimates in anesthetized non-human primate, which can be explained by variations of [11C]MRB plasma free fraction. Thus VT/fP is a better index to quantify [11C]MRB in vivo binding than VT. Finally, the suitability of three putative reference regions was investigated. None of them was found to be an ideal reference region, the caudate being the region introducing the least bias in IC50 estimates based on BPND calculations.
Variability of VT estimates and plasma free fraction fP
The substantial variability in the VT estimates for [11C]MRB from scan to scan in anesthetized baboons was previously reported (Logan et al., 2005). Consequently, the normalization of the volumes of distribution estimates by some reference region values was found to be critical, even though no ideal reference region could be identified. In this study, high variations of the VT estimates were also observed. As a result, without correction by fP, no dose-dependent reduction of VT estimates could be consistently observed. However, the VT estimates in NET-poor regions were highly correlated with the estimates of the plasma free fraction fP. This result is consistent with the interpretation that a higher plasma free fraction will lead to higher brain uptake and higher values of VT as well as the non-specific distribution volume VND, specifically VND=fP/fND (Innis et al., 2007; Eq. (19)). Moreover, the normalized distribution volumes (VT/fP) decreased in a dose-dependent manner with increased ATX doses in the NET-rich regions, and were almost stable or slightly decreasing in the NET-poor regions. Thus, the normalized volume of distribution VT/fP appears to be a suitable index of [11C]MRB binding for occupancy studies. It is also worth noting that the range of the plasma free fraction fP values observed in this study (8% to 31%), is favorable as it can be accurately measured for each study.
Using the estimate of VND/fP in NET-rich regions (19 mL/cm3), [11C] MRB tissue free fraction fND is on the order of 5% (since fND=fP/VND). In theory, BPND should be equal to fND Bmax/Kd. Therefore, using in vitro estimates of MRB dissociation constant Kd (2.5 nM) (Melloni et al., 1984) and of the density of the norepinephrine transporters in the thalamus of rhesus monkeys (23–72 nM) (Smith et al., 2006), and the present estimate of fND, then an expected value of the binding potential BPND in the thalamus can be computed as 0.46–1.44, in good agreement with the present measured values (1.8±0.3 based on the VND/fP estimates from the fits with Eq. (5); 1.2±0.5 based on VT/fP estimates from the high-dose scans).
The cause of the variations of the plasma free fraction fP across experiments in these anesthetized animals could not be explained. No correlations were found between the estimates of the plasma free fraction fP and physiological recordings (pCO2, heart rate, respiration rate, level of isoflurane) measured during the anesthesia (data not shown). Interestingly, this fP variation was not observed in humans (Hannestad et al., 2010).
Estimation of ATX IC50 for NET
Based on our PET studies, the preferred method to estimate the IC50 of ATX with [11C]MRB was to fit the VT/fP values obtained for each scan with Eq. (5), using the four high binding regions and the occipital cortex. Since ATX IC50 and [11C]MRB VND/fP were not found to be statistically different across these regions, this method is very similar to the Lassen plot method (Lassen et al. 1995; Cunningham et al., 2010), which assumes that these two variables have identical values in all regions. Consequently, the estimates were very similar (31 vs. 28 ng/mL plasma). The differences between the method used in this study and the Lassen plot method lies in the nature of the estimators (nonlinear versus linear fit; uncorrelated versus correlated variables used for the x- and y-axes), and some hypotheses used when estimating VND/fP (one VND/fP value estimated for all scans and monkeys using Eq. (5), versus seven VND/fP values estimated from the Lassen plots).
The IC50 of ATX for norepinephrine transporters estimated in this study, i.e. 31 ng/mL plasma or 122 nM (ATX molecular weight=255 g/ mol), is compatible with results from previous in vitro studies. Using similar hypotheses as for a radiotracer, the IC50 of a drug in vivo can be linked to its in vitro affinity (Kd) and its plasma free fraction (fP), by the equation IC50=Kd/fP. ATX in vitro Kd was estimated to be 3.4 nM in rats (Wong et al., 1982) and 2–5 nM in humans (Tatsumi et al., 1997; Bymaster et al., 2002). Plasma free fraction of ATX was estimated to be 11–13% in rats (Mattiuz et al., 2003; Kielbasa et al., 2009), 3% in dogs (Mattiuz et al., 2003) and 1.3% in humans (Sauer et al., 2003). The IC50 predicted from these Kd and fP values would be between 15 nM and 385 nM (i.e., 4–98 ng/mL). Despite the approximations in these calculated values, especially due to the species differences, the order of magnitude matches with the present IC50 estimate.
The present IC50 estimate is also compatible with a previous [18F] F-MeNER-D2 study in which ATX IC50 was estimated to 16 ng/mL (Takano et al., 2009a). Moreover, the IC50 estimated using MRTM2 and caudate as a reference region, as in that [18F]F-MeNER-D2 study, is even closer (21 ng/mL).
The present estimate of the IC50 of ATX, 31 ng/mL plasma, is much lower than the plasma levels which were measured during the previous study of ATX and [11C]MRB in humans (Logan et al., 2007): there, the plasma levels of ATX were 116, 206, and 421 ng/mL for the three clinically relevant doses of 25, 50 and 100 mg oral ATX, respectively. Based on the present IC50 estimate, the lack of an obvious dose-dependent effect in that study was indeed due to the fact that those three clinically relevant doses all reached saturation with over 78–93% occupancy; i.e., the plateau portion of the corresponding dose-occupancy curve. This extrapolation assumes comparable free fraction of ATX in humans and non-human primates.
Use of a reference region with [11C]MRB in occupancy studies
Norepinephrine transporters are distributed throughout the brain, with relatively low contrast between transporter-poor and transporter-rich regions (locus coeruleus excluded). In rhesus monkeys, the lowest densities measured by autoradiography (Smith et al., 2006) were detected in putamen and caudate: in these two regions, the density of NET is about 5 fmol/mg of wet brain tissue, which represents between 7% and 22% of the densities measured in the various sub-nuclei of the thalamus in the same study. In the present study, no significant level of specific binding was detected in the caudate (p=0.12) and putamen (p=0.8). However, the non-displaceable binding appeared higher in the caudate (VND/fP=26 mL/cm3) and putamen (VND/fP=30 mL/cm3) than in the NET-rich region (VND/fP=19 mL/cm3). This lead to an underestimation of BPND or BPF values, and subsequently to an underestimation of the IC50 of ATX (by 36–39% with caudate and 72% with putamen). This non-uniform level of non-specific binding throughout the brain has been reported for [11C]MRB in baboons (Logan et al., 2005) and humans (Logan et al., 2007). This issue is also not specific to [11C]MRB: a higher level of non-specific binding in the putamen was also reported for previous radiotracers developed to target norepinephrine transporters (such as [11C]oxaprotiline and [11C] lortalamine) (Ding et al., 2005). It is also worth noting that such small between-region difference in non-specific binding may exist for many PET tracers. However, the effect of such variation is more pronounced, and therefore more easily detectible, for tracers with relatively low BPND values. An alternative possibility is that the apparent higher non-specific binding in the basal ganglia is in fact due to a small amount of specific binding, but that the variability of the VT/fP estimates was too high, or the number of experiment too low, to detect a statistically significant blockade in these NET-poor regions. Finally, one limitation of the present study is that VND/fP was estimated based on fits of the occupancy data. Studies at higher occupancy levels than in this study (i.e., >90%) would be useful to confirm these findings.
The occipital cortex was investigated since in one in vitro autoradiography study in humans using [18F]F-MeNER-D2, the lowest concentration of NET was found in the occipital cortex (Schou et al., 2005). However, a significant reduction of [11C]MRB VT/fP after ATX infusion was observed in the present study. Conversely, the level of non-specific binding in the occipital cortex seems to be similar to the one in the main regions of interest. The difference between this study and the aforementioned in vitro autoradiography study could be due to difference of species, as well as differences in the delineation of the region of interest. In fact, the suitability of the occipital cortex as a reference region in humans for [11C]MRB has been assessed by blocking studies in humans: [11C]MRB average VT in the occipital cortex was reduced by 10% after the administration of 100 mg of ATX (Logan et al., 2007), and a reduction of 9±17% for VT and 5±15% for VT/fP was observed after the administration of 40 mg of methylphenidate (n=9;Hannestad et al., 2010). Therefore, the fraction of displaceable binding in the occipital cortex for [11C]MRB may be higher in rhesus monkeys than in humans.
Since the normalized distribution volume (VT/fP) can be measured accurately and shown to reduce in a dose-dependent manner, it is not necessary to use a reference region for occupancy studies. However, only relying on VT/fP to quantify [11C]MRB binding in an occupancy study would lead to a potentially larger uncertainty in the estimated IC50, since there is one additional parameter to be estimated (i.e., the normalized non-displaceable volume of distribution VND/fP). To improve the estimation of this extra parameter, an additional scan using a very high dose of the blocking drug (targeting >90% occupancy) would be required. However, using such a high blocking dose of the drug may not be feasible due to the potential toxicity of the drug. Alternatively, using a “reference” region to avoid estimating VND/fP could lead to some bias in the estimated IC50, when the so called “reference” region contains some specific binding, or when that region’s non-specific binding is not representative of other regions of interest. This presents a classical trade-off between maximizing precision (i.e., reducing the standard error of the IC50 estimate) and accuracy (i.e., reducing the bias in that IC50 estimate). In this study, the caudate nucleus was the “reference” region, which leads to the least bias (36–39%). This bias is not significant as compared to the “gold standard” IC50 estimate obtained using only VT/fP values. Moreover, similar results were obtained while using the binding potentials BPF and BPND. The latter could be estimated without arterial blood sampling by using MRTM2 and provided equivalent results to those obtained with the arterial data, i.e., without introducing further bias.
Interestingly, this issue of selecting a reference region seems to be less troublesome in humans since all three NET-poor regions (caudate, putamen and occipital) were considered as reasonable reference regions based on the results of our occupancy study of methylphenidate (Hannestad et al., 2010). Thus, using MRTM2 with a reference region to quantify [11C]MRB binding without arterial sampling for occupancy studies is feasible in non-human primates and adequate in humans.
Visualization of the locus coeruleus
The region with the highest density of NET, the locus coeruleus, is very small (~10 mm3 in humans) (Hoogendijk et al., 1995), and thus challenging to quantify accurately with PET, even with the HRRT and the MOLAR algorithm. [11C]MRB binding in the locus coeruleus might still produce a visible signal in rhesus monkeys, but unambiguously identifying the locus coeruleus is not simple: it is not visible on standard T1-weighted MR images, and there are other NET-rich nuclei in the brainstem where the signal may be higher (due to lower partial volume effect) or whose signal may overlap the locus coeruleus signal (due to limited resolution). Noise in the parametric images may also be an issue. Therefore, we limited our quantification to a more global brainstem region.
Comparison of [11C]MRB and [18F]MeNER-D2 for preclinical occupancy studies in rhesus monkeys
In rhesus monkeys, [11C]MRB binding potentials are higher than those of [18F]MeNER-D2: for example in the thalamus, [11C]MRB BPND was 1.8±0.3, while [18F]MeNER-D2 BPND was 0.26±0.06 (Seneca et al., 2006) or 0.7±0.1 (Takano et al., 2009a,b). Interestingly, BPND values for [11C]MRB and [18F]MeNER-D2 were found to be similar in humans (Logan et al., 2007; Ding et al., 2010; Arakawa et al., 2008).
The biggest drawback for [18F]MeNER-D2 is its in vivo defluorination which leads to a high uptake of radioactivity in the skull. Though some regions of interest are away from the skull (thalamus, midbrain and brainstem), some part of the cortex, such as the supplemental motor area (Logan et al., 2007), have high concentration of NET and may be potentially useful drug targets. The continuously increasing radioactivity in the skull resulting from in vivo defluorination of [18F]MeNER-D2 during the entire scanning time produces great challenges to accurate quantification of NET binding in the brain cortical areas.
Conversely, the brain time–activity curves are noisy in the late frames with [11C]MRB, as noted in a previous report (Schou et al., 2003). However, we found that the relative standard errors on the VT estimates were only 3±2% (max=9%). Another potential drawback of [11C]MRB is that the results from this study suggest that there is up to ~30% specific binding, or a higher non-specific binding, in the NET-poor regions (caudate, putamen and occipital), which makes it difficult to choose an ideal reference region. This issue seems to be less troublesome in humans since occipital cortex was considered as a reasonable reference region (Hannestad et al., 2010). It is not clear whether an ideal reference region is available for [18F]F-MeNER-D2 in rhesus monkeys, since a detailed comparison of binding potentials estimated using arterial blood sampling versus using a reference region was not performed in the two published occupancy studies in rhesus monkey (Seneca et al., 2006; Takano et al., 2009a).
In general, F-18 tracers are considered advantageous due to their longer half-live which usually provides better count levels at the end of the scan, especially for tracers with slow kinetics. However, drug occupancy studies may be executed more efficiently with C-11 tracers. Occupancy studies in humans typically include a measurement of baseline receptor availability and at least two measures of occupancy per subject, measured at appropriate times post-dose. In anesthetized animals, it is common to test a range of doses within the same animal. The short half-life of C-11 permits two of these scans to be performed in the same day on the same subject. This reduces the number of visits needed, and scans on one day also tend to have less variability, allowing the study to be accomplished with a smaller sample size of subjects. Furthermore, when proper quantification with arterial input function is needed in humans, thus, the C-11 design is clearly more practical since it can be accomplished in a 2-day study design, with the use of arterial lines in one arm per day.
Conclusions
This study demonstrates that [11C]MRB binding in vivo can be inhibited in a dose-dependent manner by ATX, and the variability of [11C]MRB distribution volumes in anesthetized monkeys could be explained and corrected by the underlying variability of the plasma free fraction of [11C]MRB. Therefore [11C]MRB is suitable for drug occupancy studies of norepinephrine transporters. Since no ideal reference region could be identified, the gold standard method to estimate the IC50 of drugs with [11C]MRB would be the Lassen plot method or the nonlinear fits described in this study. However, it is feasible to use MRTM2 with a reference region to quantify [11C]MRB binding without arterial sampling for occupancy studies. Among the candidate reference regions evaluated, the caudate nucleus has the most desirable properties in rhesus monkeys.
The ED50 of ATX was estimated to be 0.028 mg/kg/h (or 0.13 mg/kg), which is much lower than the therapeutic dose of ATX in ADHD (1.0–1.5 mg/kg). This explains why no obvious dose-dependent effect could be seen in the previous PET occupancy study of ATX in humans (Logan et al., 2007).
Acknowledgments
The authors thank the staff of the Yale University PET Center for their technical expertise and support. Funding for this study was provided by the Yale Pfizer Bioimaging Alliance. This publication was also made possible by CTSA Grant Number UL1RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
References
- Arakawa R, Okumura M, Ito H, Seki C, Takahashi H, Takano H, Nakao R, Suzuki K, Okubo Y, Halldin C, Suhara T. Quantitative analysis of norepinephrine transporter in the human brain using PET with (S, S)-18F-FMeNER-D2. J. Nucl. Med. 2008;49:1270–1276. doi: 10.2967/jnumed.108.051292. [DOI] [PubMed] [Google Scholar]
- Barker EL, Blakely RD. Norepinephrine and serotonin transporters: molecular targets of antidepressant drugs. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. Raven Press; New York, NY: 1995. pp. 321–334. [Google Scholar]
- Beck J, Arnold K. Parameter estimation in engineering and science. Wiley; New York, NY: 1977. [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Carson RE, Barker WC, Liow J, Johnson CA. Design of a motion-compensation OSEM list-mode algorithm for resolution-recovery reconstruction for the HRRT. IEEE Nucl. Sci. Symp. Conf. Rec. 2003;5:3281–3285. [Google Scholar]
- Cunningham VJ, Rabiner E, Slifstein M, Laruelle M, Gunn RN. Measuring drug occupancy in the absence of a reference region: the Lassen plot re-visited. J. Cereb. Blood Flow Metab. 2010;30:46–50. doi: 10.1038/jcbfm.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YS, Lin KS, Garza V, Carter P, Alexoff D, Logan J, Shea C, Xu Y, King P. Evaluation of a new norepinephrine transporter PET ligand in baboons, both in brain and peripheral organs. Synapse. 2003;50:345–352. doi: 10.1002/syn.10281. [DOI] [PubMed] [Google Scholar]
- Ding YS, Lin KS, Logan J, Benveniste H, Carter P. Comparative evaluation of positron emission tomography radiotracers for imaging the norepinephrine transporter: (S, S) and (R, R) enantiomers of reboxetine analogs ([11C]methylreboxetine, 3-Cl-[11C]methylreboxetine and [18F]fluororeboxetine), (R)-[11C]nisoxetine, [11C] oxaprotiline and [11C]lortalamine. J. Neurochem. 2005;94:337–351. doi: 10.1111/j.1471-4159.2005.03202.x. [DOI] [PubMed] [Google Scholar]
- Ding YS, Lin KS, Logan J. PET imaging of norepinephrine transporters. Curr. Pharm. Des. 2006;12:3831–3845. doi: 10.2174/138161206778559687. [DOI] [PubMed] [Google Scholar]
- Ding YS, Singhal T, Planeta-Wilson B, Gallezot JD, Nabulsi N, Labaree D, Ropchan J, Henry S, Williams W, Carson RE, Neumeister A, Malison RT. PET imaging of the effects of age and cocaine on the norepinephrine transporter in the human brain using (S,S)-[(11)C]O-methylreboxetine and HRRT. Synapse. 2010;64:30–38. doi: 10.1002/syn.20696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallezot JD, Planeta-Wilson B, Wang GJ, Carson RE, Ding YS. Kinetic modeling of the net radioligand [C-11]MRB in humans: a test-retest study. J. Cereb. Blood Flow Metab. 2007;27(Suppl 1):23. [Google Scholar]
- Hannestad J, Gallezot J-D, Planeta-Wilson B, Lin S-F, Williams WA, van Dyck CH, Malison RT, Carson RE, Ding YS. Clinically relevant doses of dethylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol Psychiatry. 2010;68:854–860. doi: 10.1016/j.biopsych.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendijk WJ, Pool CW, Troost D, van Zwieten E, Swaab DF. Image analyser-assisted morphometry of the locus coeruleus in Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis. Brain. 1995;118:131–143. doi: 10.1093/brain/118.1.131. [DOI] [PubMed] [Google Scholar]
- Ichise M, Ballinger JR, Golan H, Vines D, Luong A, Tsai S, Kung HF. Noninvasive quantification of dopamine D2 receptors with iodine-123-IBF SPECT. J. Nucl. Med. 1996;37:513–520. [PubMed] [Google Scholar]
- Ichise M, Toyama H, Innis RB, Carson RE. Strategies to improve neuroreceptor parameter estimation by linear regression analysis. J. Cereb. Blood Flow Metab. 2002;22:1271–1281. doi: 10.1097/01.WCB.0000038000.34930.4E. [DOI] [PubMed] [Google Scholar]
- Ichise M, Liow JS, Lu JQ, Takano A, Model K, Toyama H, Suhara T, Suzuki K, Innis RB, Carson RE. Linearized reference tissue parametric imaging methods: Application to [11C]DASB positron emission tomography studies of the serotonin transporter in human brain. J. Cereb. Blood Flow Metab. 2003;23:1096–1112. doi: 10.1097/01.WCB.0000085441.37552.CA. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J. Cereb. Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Johnson CA, Thada S, Rodriguez M, Zhao Y, Iano-Fletcher AR, Liow J, Barker WC, Martino RL, Carson RE. Software architecture of the MOLAR-HRRT reconstruction engine. IEEE Nucl. Sci. Symp. Conf. Rec. 2004;6:3956–3960. [Google Scholar]
- Kaufman MJ, Spealman RD, Madras BK. Distribution of cocaine recognition sites in monkey brain: I. in vitro autoradiography with [3H]CFT. Synapse. 1991;9:177–187. doi: 10.1002/syn.890090304. [DOI] [PubMed] [Google Scholar]
- Kielbasa W, Kalvass JC, Stratford R. Microdialysis evaluation of atomoxetine brain penetration and central nervous system pharmacokinetics in rats. Drug Metab. Dispos. 2009;37:137–142. doi: 10.1124/dmd.108.023119. [DOI] [PubMed] [Google Scholar]
- Lin KS, Ding YS. Synthesis, enantiomeric resolution, and selective C-11 methylation of a highly selective radioligand for imaging the norepinephrine transporter with positron emission tomography. Chirality. 2004;16:475–481. doi: 10.1002/chir.20055. [DOI] [PubMed] [Google Scholar]
- Lin KS, Ding YS, Kim SW, Kil KE. Synthesis, enantiomeric resolution, F-18 labeling and biodistribution of reboxetine analogs: promising radioligands for imaging the norepinephrine transporter with Positron Emission Tomography. Nucl. Med. Biol. 2005;32:415–422. doi: 10.1016/j.nucmedbio.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Lassen NA, Bartenstein PA, Lammertsma AA, Prevett MC, Turton DR, Luthra SK, Osman S, Bloomfield PM, Jones T, Patsalos PN. Benzodiazepine receptor quantification in vivo in humans using [11C]flumazenil and PET: application of the steady-state principle. J. Cereb. Blood Flow Metab. 1995;15:152–165. doi: 10.1038/jcbfm.1995.17. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wolf A, Dewey SL, Schlyer D, Macgregor R, Hitzemann, Bendriem, Gatley S, Christman D. Graphical analysis of reversible radioligand binding from time–activity measurements applied to [N-11C -methyl]-(-)-cocaine PET studies in human subjects. J. Cereb. Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- Logan J, Ding YS, Lin KS, Pareto D, Fowler JS, Biegon A. Modeling and analysis of PET studies with norepinephrine transporter ligands: The search for a reference region. Nucl. Med. Biol. 2005;32:531–542. doi: 10.1016/j.nucmedbio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Logan J, Wang GJ, Telang F, Fowler JS, Alexoff D, Zabroski J, Jayne M, Hubbard B, King P, Carter P, Shea C, Xu Y, Muench L, Schlyer D, Learned-Coughlin S, Cosson V, Volkow ND, Ding YS. Imaging the norepinephrine transporter in humans with (S, S)-[ 11C]O-methyl reboxetine and PET: Problems and progress. Nucl. Med. Biol. 2007;34:667–679. doi: 10.1016/j.nucmedbio.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Marquardt D. An algorithm for least square estimation of nonlinear parameters. SIAM J. Appl. Math. 1963;11:431–441. [Google Scholar]
- Mattiuz EL, Ponsler GD, Barbuch RJ, Wood PG, Mullen JH, Shugert RL, Li Q, Wheeler WJ, Kuo F, Conrad PC, Sauer JM. Disposition and metabolic fate of atomoxetine hydrochloride: Pharmacokinetics, metabolism, and excretion in the Fischer 344 rat and beagle dog. Drug Metab. Dispos. 2003;31:88–97. doi: 10.1124/dmd.31.1.88. [DOI] [PubMed] [Google Scholar]
- Melloni PCG, Della Torre A, Bonsignori A, Buonamici M, Pozzi ORS, Rossi AC. Potential antidepressant agents. A-aryloxy-benzyl derivatives of ethanol-amine and morpholine. Eur. J. Med. Chem. 1984;19:235–242. [Google Scholar]
- Morón JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: Evidence from knock-out mouse lines. J. Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabulsi N, Huang Y, Weinzimmer D, Ropchan J, Frost JJ, McCarthy T, Carson RE, Ding YS. High-resolution imaging of brain 5-HT1B receptors in the rhesus monkey using [11C]P943. Nucl. Med. Biol. 2010;37:205–214. doi: 10.1016/j.nucmedbio.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer J, Ponsler GD, Mattiuz EL, Long AJ, Witcher JW, Thomasson HR, Desante KA. Disposition and metabolic fate of atomoxetine hydrochloride: The role of CYP2D6 in human disposition and metabolism. Drug Metab. Dispos. 2003;31:98–107. doi: 10.1124/dmd.31.1.98. [DOI] [PubMed] [Google Scholar]
- Schou M, Halldin C, Sóvágó J, Pike VW, Gulyás B, Mozley PD, Johnson DP, Hall H, Innis RB, Farde L. Specific in vivo binding to the norepinephrine transporter demonstrated with the PET radioligand, (S, S)-[ 11C]MeNER. Nucl. Med. Biol. 2003;30:707–714. doi: 10.1016/s0969-8051(03)00079-9. [DOI] [PubMed] [Google Scholar]
- Schou M, Halldin C, Sóvágó J, Pike VW, Hall H, Gulyás B, Mozley PD, Dobson D, Shchukin E, Innis RB, Farde L. PET evaluation of novel radiofluorinated reboxetine analogs as norepinephrine transporter probes in the monkey brain. Synapse. 2004;53:57–67. doi: 10.1002/syn.20031. [DOI] [PubMed] [Google Scholar]
- Schou M, Halldin C, Pike VW, Mozley PD, Dobson D, Innis RB, Farde L, Hall H. Post-mortem human brain autoradiography of the norepinephrine transporter using (S, S)-[18F]FMeNER-D2. Eur. Neuropsychopharmacol. 2005;15:517–520. doi: 10.1016/j.euroneuro.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Seneca N, Gulyás B, Varrone A, Schou M, Airaksinen A, Tauscher J, Vandenhende F, Kielbasa W, Farde L, Innis RB, Halldin C. Atomoxetine occupies the norepinephrine transporter in a dose-dependent fashion: A PET study in nonhuman primate brain using (S, S)-[18F]FMeNER-D2. Psychopharmacology (Berl) 2006;188:119–127. doi: 10.1007/s00213-006-0483-3. [DOI] [PubMed] [Google Scholar]
- Smith HR, Beveridge TJ, Porrino LJ. Distribution of norepinephrine transporters in the non-human primate brain. Neuroscience. 2006;138:703–714. doi: 10.1016/j.neuroscience.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Wilens TE, Faraone SV. Novel treatments for attention-deficit/hyperactivity disorder in children. J. Clin. Psychiatry. 2002;63(Suppl 12):16–22. [PubMed] [Google Scholar]
- Takano A, Gulyás B, Varrone A, Maguire RP, Halldin C. Saturated norepinephrine transporter occupancy by atomoxetine relevant to clinical doses: a rhesus monkey study with (S, S)-[(18)F]FMeNER-D(2) Eur. J. Nucl. Med. 2009a;36:1308–1314. doi: 10.1007/s00259-009-1118-9. [DOI] [PubMed] [Google Scholar]
- Takano A, Gulyás B, Varrone A, Halldin C. Comparative evaluations of norepinephrine transporter radioligands with reference tissue models in rhesus monkeys: (S, S)-[(18)F]FMeNER-D(2) and (S, S)-[(11)C]MeNER. Eur. J. Nucl. Med. 2009b;36:1892–1895. doi: 10.1007/s00259-009-1194-x. [DOI] [PubMed] [Google Scholar]
- Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur. J. Pharmacol. 1997;340:249–258. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- Wilson AA, Johnson DP, Mozley D, Hussey D, Ginovart N, Nobrega J, Garcia A, Meyer J, Houle S. Synthesis and in vivo evaluation of novel radiotracers for the in vivo imaging of the norepinephrine transporter. Nucl. Med. Biol. 2003;30:85–92. doi: 10.1016/s0969-8051(02)00420-1. [DOI] [PubMed] [Google Scholar]
- Wong DT, Threlkeld PG, Best KL, Bymaster FP. A new inhibitor of norepinephrine uptake devoid of affinity for receptors in rat brain. J. Pharmacol. Exp. Ther. 1982;222:61–65. [PubMed] [Google Scholar]
- Zeng Z, Chen TB, Miller PJ, Dean D, Tang YS, Sur C, Williams DL. The serotonin transporter in rhesus monkey brain: Comparison of DASB and citalopram binding sites. Nucl. Med. Biol. 2006;33:555–563. doi: 10.1016/j.nucmedbio.2006.02.007. [DOI] [PubMed] [Google Scholar]