Abstract
A wide variety of phytochemicals, mostly flavonoids or polyphenolics, have been shown to possess anti-carcinogenic activities. Among these are the grape seed proanthocyanidins (GSPs), which are the active ingredients of grape-seed extract (GSE). Substantial in vitro and preclinical in vivo studies have demonstrated the chemopreventive efficacy of GSPs against various forms of cancers in different tumor models. In this issue of the journal, Derry and colleagues show that administration of GSE in the diet reduces azoxymethane-induced colon carcinogenesis in an A/J mouse model. The results of this innovative and comprehensive study indicate that inhibition of azoxymethane-induced colon cancer by dietary GSE is mediated through the induction of apoptosis that is associated with alterations in microRNA (miRNA) and cytokine expression profiles as well as β-catenin signaling. Notably, the demonstration that microRNA expression is affected by dietary GSE suggests a novel underlying mechanism for the chemopreventive action of GSE in colon cancer and, potentially, other cancers.
Natural products including dietary components, herbs and spices have been used for the prevention of many diseases, including cancer, for centuries. Today, there is renewed interest in natural phytochemicals as promising options for the development of more effective chemopreventive or chemotherapeutic strategies for various types of cancer. Full realization of this potential, however, requires an improved knowledge of the effects of these natural products. Two major lines of enquiry are being pursued: first, identification of the components of the plant, or the dietary agent derived from the plants that are responsible for the anti-cancer effects and second, elucidation of the mechanisms of action of these components and identification of the specific mechanisms that play a role in reducing the risk of cancer.
Grape seed extract (GSE), which is readily prepared from grape (Vitis vinifera) seeds, is a by-product of the grape juice and wine industries. Indeed, it is available in the USA and sold as an over-the-counter health dietary supplement in the form of capsule or tablet formulations containing 100–500 mg. It has been shown that GSE is a mixture of several polyphenolic components. Proanthocyanidins, which include dimers, trimers, tetramers, and oligomers/polymers of monomeric catechins and/or (−)-epicatechins, are considered to be a major fraction of GSE (1, 2). The proanthocyanidins are naturally occurring compounds that are found in numerous different plants. They are distributed variously among the fruits, vegetables, nut, seeds, flowers and bark. The proanthocyanidins represent the major type of polyphenols in red wine; however, the seeds of grapes are a particularly rich source. The current interest in the biology of GSE originated with reports describing epidemiological data related to the population of France that showed that despite consumption of a diet high in saturated fat, this population manifests a low incidence of coronary heart disease. To describe these observations, the term “French Paradox” was coined. It was suggested that moderate red wine consumption may be associated with a lower incidence of coronary heart disease in this population. Since red wine is a rich source of GSPs, this prompted interest in testing the cellular effects of GSE and GSPs, including assessment of their chemopreventive or chemotherapeutic efficacy.
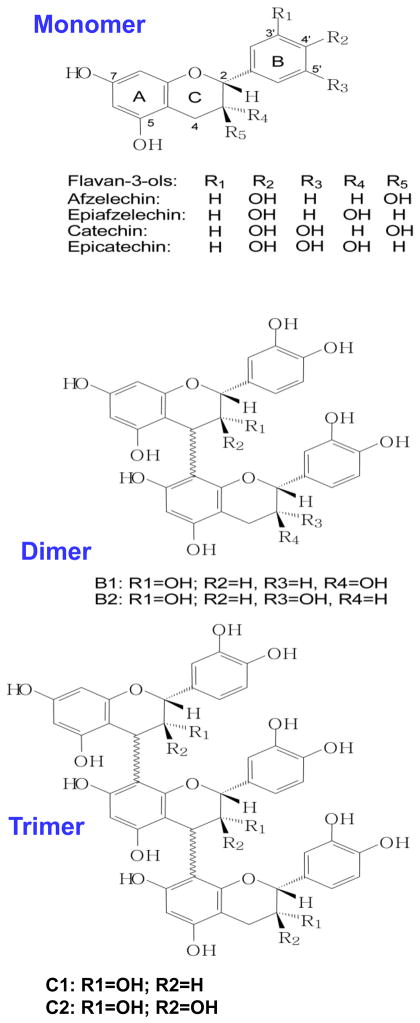
Proanthocyanidins are synonymous with condensed tannins and also known as oligomeric proanthocyanidins, pycno-genols or leukocyanidins, and oligomers or polymers of flavan-3-ols. The monomeric units in these oligomers are linked primarily through C4→C8 bond, but the C4→C6 linkages also are present (Fig. 1). These linkages are called B-type linkages (1, 2). The presence of high amounts of oligomeric procyanidins and polymeric compounds (such as tannins) as a result of condensation of flavan-3-ols is responsible for various aesthetic and taste-related qualities of red wine. These agents may also contribute significantly to the pharmacological properties of grape seeds. The most common types of ingredients and linkages are shown in Figure 1. The most abundant types of proanthocyanidins in plants are the procyanidins, which consist exclusively of epicatechin units. Various factors can affect the content of the phenolic constituents in grapes. The content is known to be affected by agro-ecological factors such as the cultivar; the year of production, which is of interest for the most part because of the climatic condition during that year; the geographic location, which affects soil chemistry, the use of fertilizers, etc.; and the degree of fruit maturation. The GSPs have been subjected to toxicity testing, including analysis of the acute and sub-chronic toxicity in rats and genotoxicity testing. The results of this testing indicate that these compounds are of low toxicity and have no genotoxic potential (2, 3).
Figure 1.
Chemical structures of the monomers (flavan-3-ols), dimers (B1, B2) and trimers (C1, C2) present in grape seed extract.
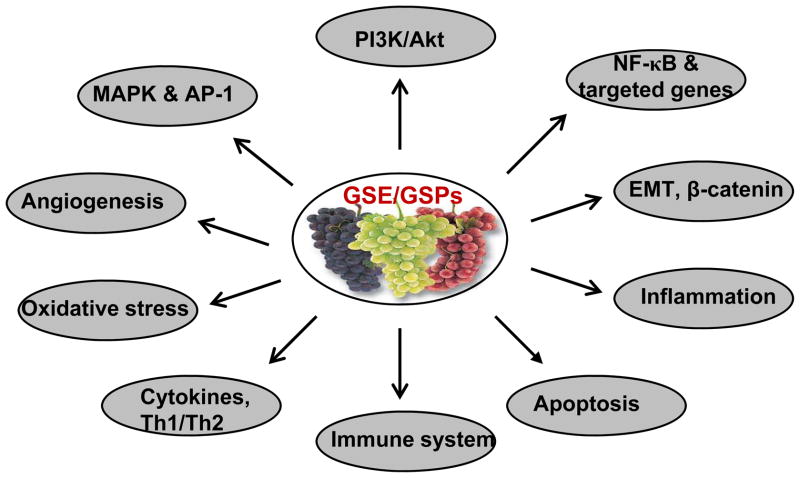
During the last decade, multiple preclinical studies have been carried out using in vitro approaches and in vivo analyses in animal models that show the protective effects of GSE and its active constituents grape seed proanthocyanidins (GSPs) against skin (4, 5), breast (6), prostate (7, 8), head and neck (9, 10) and lung (11, 12) cancers. The grape seed constituents possess anti-oxidant and anti-inflammatory properties. A number of studies, including those from our laboratories, have identified multiple molecular targets that are affected by treatment with GSPs. Specific molecules that have been shown to be affected include mitogen-activated protein kinases (MAPKs), nuclear factor-kappaB (NF-κB), phosphatidylinositol-3 kinase (PI3K), 5-lipooxygenase, cytokines, vascular endothelial growth factor (VEGF), angiogenic factors, immunomodulatory factors, and the epidermal growth factor receptor (EGFR)-Shc-ERK1/2-ELK1-AP-1 pathway, etc (2). Collectively, these studies suggest that GSPs can affect tumor cell proliferation, apoptosis, invasion, angiogenesis, cell cycle regulators and the generation of inflammatory mediators (Fig. 2).
Figure 2.
Molecular targets of GSE or GSPs in prevention of cancers of different organs.
Both topical treatment of GSE or dietary administration of GSPs have been shown to prevent 7,12-dimethylbenz(a)anthracene (DMBA)-initiated and 12-O-tetradecanoyl phorbol-13-acetate (TPA)-promoted two-stage chemical carcinogenesis in mice in terms of tumor incidence, tumor multiplicity and tumor size (4,13). Extensive work has demonstrated the chemopreventive effect of GSPs against ultraviolet (UV) radiation-induced skin carcinogenesis and its related molecular mechanisms. In these studies, the diet of the test animals was supplemented with GSPs and the effect on UV-induced skin tumors determined. Dietary GSPs inhibit UV-induced skin tumor development in SKH-1 hairless mice in terms of a reduction in percentage of mice with tumors, tumor multiplicity per animal and the size of the tumors (5), which was associated with the inhibition of UV-induced oxidative stress and inflammatory responses, such as suppression of inducible cyclooxygenase-2 (COX-2) and its prostaglandin (PG) metabolites (14). Further, the molecular targets involved in prevention of photocarcinogenesis by dietary GSPs were shown to involve the enhancement of DNA repair and xeroderma pigmentosum group A-dependent mechanisms (15). As UV-induced suppression of the immune system has been implicated as a risk factor for nonmelanoma and melanoma skin cancers (16), prevention of UV-induced immunosuppression represents a potential strategy for the prevention of skin cancers. Dietary administration of GSPs significantly inhibits UV-induced suppression of the contact hypersensitivity response to contact sensitizer, 2, 4-dinitrofluorobenzene, and this effect has been shown to be associated with the enhanced production of the immunostimulatory cytokine, interleukin (IL)-12, and reduced expression of the immunosuppressive cytokine, IL-10, in mouse skin and draining lymph nodes of UV-exposed mice (17). Protection of the immune system by GSPs in this model also was mediated through IL-12-dependent stimulation of CD8+ effector T cells and inactivation of CD4+ T cells (18), and functional activation of dendritic cells in mice and these effects of GSPs have been associated with the enhanced repair of UVB-induced DNA damage in mouse skin compared to those mice that were not given a diet supplemented with GSPs (19). Of particular interest, chemoprevention by GSPs also involved reactivation of silenced tumor suppressor genes in skin cancer cells as a consequence of epigenetic reprogramming (20).
Both in vitro studies and in vivo studies in animals have generated additional insights into the mechanisms underlying the anti-cancer effects of GSPs/GSE. Recently, we provided compelling evidence that GSPs have the ability to reverse the epithelial-mesenchymal transition (EMT) in cancer cells by affecting the expression of various proteins that orchestrate the migration of invasive cancer cells during metastasis. Using in vitro cell culture models, we have shown that treatment of melanoma (21), non-small cell lung cancer (NSCLC) cells (22), pancreatic cancer cells (23) and head and neck squamous cell carcinoma (HNSCC) cells (9) with GSPs significantly inhibited the migration ability or invasive potential of these cells. GSPs inhibit melanoma cell invasiveness by reduction of COX-2 expression, PGE2 production and reversal of EMT in these cells (21). This characteristic of GSPs is important because melanoma is the leading cause of death from skin disease due, in large part, to its propensity to metastasize (24). Moreover, its incidence in children is increasing rapidly (25). HNSCC is responsible for approximately 20,000 deaths and affects more than 40,000 people in the United States annually (26). EGFR is overexpressed in 90% of HNSCCs and is considered as a critical target in its treatment. Using a cell invasion assay, we found that treatment of HNSCC cells with GSPs resulted in inhibition of cell invasion, which was associated with the reduced levels of EGFR and its down-stream target NF-κB (9). Overexpression of EGFR and high NFκB activity play key roles in the EMT, which is of critical importance in the processes underlying metastasis, and it was found that treatment of cancer cells with GSPs reversed the EMT process and promoted the mesenchymal-epithelial transition instead. Bioactive components of GSPs also inhibit the growth of HNSCC cells in vitro as well as in vivo in an athymic nude mouse model by targeting multiple signaling molecules/pathways (10). These targets include: (i) inhibition of cell proliferation, (ii) induction of apoptosis, (iii) regulation of dysregulated cell cycle and its checkpoints, and (iv) reactivation of tumor suppressor proteins. Similar effects of GSPs also were observed when pancreatic cancer cells were treated with GSPs (23). Pancreatic cancer is an aggressive malignancy that is frequently diagnosed at an advanced stage with poor prognosis (27). Using in vitro studies and an in vivo athymic nude mouse model, Prasad et al. (28) have shown that GSPs inhibit pancreatic cancer cell growth through induction of apoptosis and by targeting the PI3K/Akt pathway. The PI3K/Akt pathway is a fundamental signaling pathway that mediates several cellular processes, including cell proliferation, growth, cell survival and motility (29). Lung cancer remains the leading cause of cancer related deaths in the United States and worldwide. NSCLC represents approximately 80% of all types of lung cancer. Although a combination of chemotherapy and radiation therapy can improve survival of the patients, most patients die of disease progression, often resulting from acquired or intrinsic resistance to chemotherapeutic drugs (30). Thus, it is of interest that the results of a recent study revealed that treatment of human NSCLC cells with GSPs induces apoptosis by loss of mitochondrial membrane potential in both in vitro and in vivo models (31). This preclinical study also indicated that dietary GSPs inhibit the growth of human NSCLC xenografts by targeting insulin-like growth factor binding protein-3 and inhibition of tumor angiogenesis (11). The previously mentioned GSPs-induced inhibitory effects on PGE2 and PGE2 receptors also have been associated with the inhibition of human NSCLC cells growth (12). As the metastasis of lung cancer cells is considered as a major cause of death, the effect of GSPs on lung cancer cell migration/invasion was also investigated. Punathil and Katiyar have reported that treatment of human NSCLC cells with GSPs resulted in inhibition of migration of these cells, and the inhibition of cancer cell migration by GSPs was associated with the sequential inhibition of nitric oxide, guanylase cyclase and MAPK pathways (22). Similarly, administration of dietary GSE inhibited the growth and progression of prostate cancer in the TRAMP mouse model with these effects being associated with its inhibition of prostate cancer cell proliferation and angiogenesis (32, 33). These studies indicate that GSPs may be highly efficacious if used as an adjuvant during the therapeutic intervention of highly aggressive metastatic cancers in humans. Collectively, the information derived from these studies provide important leads in terms of potential chemopreventive strategies and the identification of specific molecular markers that could be used to evaluate the chemopreventive efficacy of the GSPs against various form of cancers.
With respect to chemoprevention of colon cancer by GSE, in vivo studies using Fischer 344 rats have shown that dietary GSE significantly inhibits azoxymethane (AOM)-induced formation of colonic aberrant crypt foci (ACF) in terms of crypt multiplicity (34). This study also showed that feeding of GSE inhibited AOM-induced cell proliferation and enhanced apoptosis in colon including ACF, which was associated with the reduction in the expression of COX-2, iNOS, cyclin D1 and survivin. The reduction in colonic ACF by GSE also was correlated with a reduction in AOM-induced increase in β-catenin and NF-κB as compared to the group of rats that were not given GSE in diet. In another study, the same group demonstrated that treatment of colon carcinoma HT29 cells with GSE inhibited cell growth and induced apoptotic cell death, which was associated with the induction of Cip1/p21 protein and phosphorylation of ERK1/2 (35).
In this issue, Agarwal and colleagues (36) report the results of an extension of their previous studies and demonstrate a mechanism-based chemopreventive action of GSE in models of colorectal carcinogenesis. Employing the A/J mouse model, these authors showed that dietary GSE prevents AOM-induced colon carcinogenesis and that this was associated with the anti-proliferative and pro-apoptotic activities of GSE. This study provides elegant mechanistic evidence to support the concept that GSE targets cytokine/inflammation signaling pathways by modulating the expression of microRNAs (miRNAs). Notably, the authors employed a highly relevant model, i.e., AOM-induced colon tumorigenesis in A/J mice that resembles inflammation-associated human colorectal carcinogenesis in terms of the progression of disease from aberrant crypt foci to polyps, adenoma and carcinomas (36). Treatment of dietary GSE resulted in a significant reduction in both colon and small intestine tumor multiplicity and size with a concomitant reduction in proliferation-related biomarkers and increase in apoptosis-related proteins. A unique aspect of this study is the application of anatomical gadolinium-enhanced T1-weighted magnetic resonance imaging non-invasive technology to track the progression of the disease. This demonstrated the slow progression of the disease in real time with respect to GSE feeding. A second important aspect of this study is the comprehensive evaluation of potential targets. The authors verified that NF-κB, β-catenin and MAPKs signaling pathways are targeted by GSE as well as a significant reduction in the expression of cytokines/chemokines and other inflammation predicting biomarkers. Notably, they also extended the analyses to include analysis of miRNAs. The importance of miRNA in cancer biology is becoming increasingly apparent with numerous reports now demonstrating a correlation between their expression and disease prognosis in a variety of cancers. It is therefore of considerable importance that these authors found that GSE modulated the expression profile of a broad spectrum of miRNAs (miR-19a, miR-20a miR-let7a, miR-205, miR-135b, miR-196a, miR-21, miR-148a and miR-103), as well as the miRNA processing machinery. Further in-depth investigations, which were not a part of this current study, are required to understand the mechanism by which phytochemicals like GSE may affect miRNA homeostasis. Undoubtedly, this study provides a direction for further translational investigations with a view to develop chemopreventive agents that can specifically modulate the expression of miRNA and abrogate colon cancer development. Additionally, the angiogenesis regulatory pathway, another important target pathway of these bioactive GSE, needs to be further investigated.
The chemopreventive studies employing various dietary polyphenols conducted in various cell culture and murine models of multiple cancer-types are of great interest and suggest that these complex mixtures that are present as secondary metabolites in plants are pharmacologically active and important. It should be noted, however, that the results of clinical trials of these phytochemicals are often not very encouraging (37). Major questions have been raised about their bioavailability and degradation profiles in human tissues and must be resolved. This issue is complicated by the difficulties inherent in obtaining pharmacokinetic values for complex mixtures. Safety issues, particularly the possibility of heavy metal contamination of the agents and the estrogenic activities associated with many of the phytochemicals, are other impediments in the development of these agents. From the in vitro and in vivo animal data and molecular target identification studies, it appears that the use of GSE/GSPs alone or in an adjuvant setting with other chemotherapeutic agents or drugs in cancer patients may be highly promising. Thus, the current challenge is to accelerate laboratory studies that will advance the knowledge of the mechanisms of action of GSE in a manner that facilitates clinical applications.
Acknowledgments
Grant support: The work reported from the author’s laboratory is supported from the Veterans Administration Merit Review Award (SKK) and National Institutes of Health (CA166883, SKK).
Footnotes
Disclosure of Potential Conflicts of Interest
There is no conflict of interest to declare.
Authors’ Contributions:
Writing, review and/or revision of the manuscript: S.K. Katiyar, M. Athar
References
- 1.Prieur C, Rigaud J, Cheynier V, Moutounet M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry. 1994;36:781–9. [Google Scholar]
- 2.Nandakumar V, Singh T, Katiyar SK. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 2008;375:162–7. doi: 10.1016/j.canlet.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamakoshi J, Saito M, Kataoka S, Kikuchi M. Safety evaluation of proanthocyanidins-rich extract from grape seeds. Food Chemical Toxicol. 2002;40:599–607. doi: 10.1016/s0278-6915(02)00006-6. [DOI] [PubMed] [Google Scholar]
- 4.Zhao J, Wang J, Chen Y, Agarwal R. Anti-tumor-promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation-promotion protocol and identification of procyanidin B5-3′-gallate as the most effective antioxidant constituent. Carcinogenesis. 1999;20:1737–45. doi: 10.1093/carcin/20.9.1737. [DOI] [PubMed] [Google Scholar]
- 5.Mittal A, Elmets CA, Katiyar SK. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: Relationship to decreased fat and lipid peroxidation. Carcinogenesis. 2003;24:1379–88. doi: 10.1093/carcin/bgg095. [DOI] [PubMed] [Google Scholar]
- 6.Mantena SK, Baliga MS, Katiyar SK. Grape seed proanthocyanidins induce apoptosis and inhibit metastasis of highly metastatic breast carcinoma cells. Carcinogenesis. 2006;27:1682–91. doi: 10.1093/carcin/bgl030. [DOI] [PubMed] [Google Scholar]
- 7.Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int J Cancer. 2004;108:733–40. doi: 10.1002/ijc.11620. [DOI] [PubMed] [Google Scholar]
- 8.Raina K, Singh RP, Agarwal R, Agarwal C. Oral grape seed extract inhibits prostate tumor growth and progression in TRAMP mice. Cancer Res. 2007;67:5976–82. doi: 10.1158/0008-5472.CAN-07-0295. [DOI] [PubMed] [Google Scholar]
- 9.Sun Q, Prasad R, Rosenthal E, Katiyar SK. Grape seed proanthocyanidins inhibit the invasive potential of human HNSCC cells by targeting EGFR and the epithelial-to-mesenchymal transition. PLoS ONE. 2012;7:e31093. doi: 10.1371/journal.pone.0031093. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Prasad R, Katiyar SK. Bioactive phytochemical proanthocyanidins inhibit growth of head and neck squamous cell carcinoma cells by targeting multiple signaling molecules. PLoS ONE. 2012;7:e46404. doi: 10.1371/journal.pone.0046404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akhtar S, Meeran SM, Katiyar N, Katiyar SK. Grape seed proanthocyanidins inhibit the growth of human non-small cell lung cancer xenografts by targeting IGFBP-3, tumor cell proliferation and angiogenic factors. Clin Cancer Res. 2009;15:821–31. doi: 10.1158/1078-0432.CCR-08-1901. [DOI] [PubMed] [Google Scholar]
- 12.Sharma SD, Meeran SM, Katiyar SK. Proanthocyanidins inhibit in vitro and in vivo growth of human non-small cell lung cancer cells by inhibiting the prostaglandin E2 and prostaglandin E2 receptors. Mol Cancer Ther. 2010;9:569–80. doi: 10.1158/1535-7163.MCT-09-0638. [DOI] [PubMed] [Google Scholar]
- 13.Meeran SM, Vaid M, Punathil T, Katiyar SK. Dietary grape seed proanthocyanidins inhibit 12-O-tetradecanoyl phorbol-13-acetate-caused skin tumor promotion in 7, 12-dimethylbenz(a)anthracene-initiated mouse skin, which is associated with the inhibition of inflammatory responses. Carcinogenesis. 2009;30:520–8. doi: 10.1093/carcin/bgp019. [DOI] [PubMed] [Google Scholar]
- 14.Sharma SD, Meeran SM, Katiyar SK. Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative stress and activation of mitogen-activated protein kinases and nuclear factor-κB signaling in in vivo SKH-1 hairless mice. Mol Cancer Ther. 2007;6:995–1005. doi: 10.1158/1535-7163.MCT-06-0661. [DOI] [PubMed] [Google Scholar]
- 15.Vaid M, Sharma SD, Katiyar SK. Proanthocyanidins inhibit photocarcinogenesis through enhancement of DNA repair and xeroderma pigmentosum Group A-dependent mechanism. Cancer Prev Res. 2010;3:1621–9. doi: 10.1158/1940-6207.CAPR-10-0137. [DOI] [PubMed] [Google Scholar]
- 16.Meunier L, Raison-Peyron N, Meynadier J. UV-induced immunosuppression and skin cancers. Rev Med Interne. 1998;19:247–54. doi: 10.1016/S0248-8663(97)89326-5. [DOI] [PubMed] [Google Scholar]
- 17.Sharma SD, Katiyar SK. Dietary grape-seed proanthocyanidin inhibition of ultraviolet B-induced immune suppression is associated with induction of IL-12. Carcinogenesis. 2006;27:95–102. doi: 10.1093/carcin/bgi169. [DOI] [PubMed] [Google Scholar]
- 18.Vaid M, Singh T, Li A, Katiyar N, Sharma S, Elmets CA, et al. Proanthocyanidins inhibit UV-induced immunosuppression through IL-12-dependent stimulation of CD8+ effector T cells and inactivation of CD4+ T cells. Cancer Prev Res. 2011;4:238–47. doi: 10.1158/1940-6207.CAPR-10-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaid M, Singh T, Prasad R, Elmets CA, Xu H, Katiyar SK. Bioactive grape proanthocyanidins enhance immune reactivity in UV-irradiated skin through functional activation of dendritic cells in mice. Cancer Prev Res. 2013;6:242–52. doi: 10.1158/1940-6207.CAPR-12-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaid M, Prasad R, Singh T, Jones V, Katiyar SK. Grape seed proanthocyanidins reactivate silenced tumor suppressor genes in human skin cancer cells by targeting epigenetic regulators. Toxicol Appl Pharmacol. 2012;263:122–30. doi: 10.1016/j.taap.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaid M, Singh T, Katiyar SK. Grape seed proanthocyanidins inhibit melanoma cell invasiveness by reduction of PGE2 synthesis and reversal of epithelial-to-mesenchymal transition. PLoS ONE. 2011;6:e21539. doi: 10.1371/journal.pone.0021539. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Punathil T, Katiyar SK. Inhibition of non-small cell lung cancer cell migration by grape seed proanthocyanidins is mediated through the inhibition of nitric oxide, guanylate cyclase, and ERK1/2. Mol Carcinogen. 2009;48:232–42. doi: 10.1002/mc.20473. [DOI] [PubMed] [Google Scholar]
- 23.Prasad R, Katiyar SK. Grape seed proanthocyanidins inhibit migration potential of pancreatic cancer cells by promoting mesenchymal-to-epithelial transition and targeting NF-κB. Cancer Lett. 2013 doi: 10.1016/j.canlet.2012.08.003. in press. [DOI] [PubMed] [Google Scholar]
- 24.American Cancer Society. [Accessed 2011];Cancer facts and figures. Available: http://www.cancer.org/
- 25.Strouse JJ, Fears TR, Tucker MA, Wayne AS. Pediatric melanoma: risk factor and survival analysis of the surveillance, epidemiology and end results database. J Clin Oncol. 2005;23:4735–41. doi: 10.1200/JCO.2005.02.899. [DOI] [PubMed] [Google Scholar]
- 26.Arbes SJ, Jr, Olshan AF, Caplan DJ, Schoenbach VJ, Slade GD, Symons MJ. Factors contributing to the poorer survival of black Americans diagnosed with oral cancer (United States) Cancer Cause Control. 1999;10:513–23. doi: 10.1023/a:1008911300100. [DOI] [PubMed] [Google Scholar]
- 27.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–57. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 28.Prasad R, Vaid M, Katiyar SK. Grape proanthocyanidins inhibit pancreatic cancer cell growth in vitro and in vivo through induction of apoptosis and by targeting the PI3K/Akt pathway. PLoS ONE. 2012;7:e43064. doi: 10.1371/journal.pone.0043064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–62. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 30.Ferrigno D, Buccheri G. Second-line chemotherapy for recurrent non-small cell lung cancer: do new agents make a difference? Lung Cancer. 2000;29:91–104. doi: 10.1016/s0169-5002(00)00112-4. [DOI] [PubMed] [Google Scholar]
- 31.Singh T, Sharma SD, Katiyar SK. Grape seed proanthocyanidins induce apoptosis by loss of mitochondrial membrane potential of human non-small cell lung cancer cells in vitro and in vivo. PLoS ONE. 2011;6:e27444. doi: 10.1371/journal.pone.0027444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raina K, Singh RP, Agarwal R, Agarwal C. Oral grape seed extract inhibits prostate tumor growth and progression in TRAMP mice. Cancer Res. 2007;67:5976–82. doi: 10.1158/0008-5472.CAN-07-0295. [DOI] [PubMed] [Google Scholar]
- 33.Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int J Cancer. 2004;108:733–40. doi: 10.1002/ijc.11620. [DOI] [PubMed] [Google Scholar]
- 34.Velmurugan B, Singh RP, Agarwal R, Agarwal C. Dietary feeding of grape seed extract prevents azoxymethane-induced colonic aberrant crypt foci formation in fischer 344 rats. Mol Carcinog. 2010;49:641–52. doi: 10.1002/mc.20643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaur M, Tyagi A, Singh RP, Sclafani RA, Agarwal R, Agarwal C. Grape seed extract upregulates p21 (Cip1) through redox-mediated activation of ERK1/2 and posttranscriptional regulation leading to cell cycle arrest in colon carcinoma HT29 cells. Mol Carcinogen. 2011;50:553–62. doi: 10.1002/mc.20739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Derry M, Raina K, Balaiya V, Jain AK, Shrotriya S, Huber KM, et al. Grape seed extract efficacy against azoxymethane-induced colon tumorigenesis in A/J mice: interlinking miRNA with cytokine signaling and inflammation. Cancer Prev Res. 2013;6:XXX–XXX. doi: 10.1158/1940-6207.CAPR-13-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vigna GB, Costantini F, Aldini G, Carini M, Catapano A, Schena F, et al. Effect of a standardized grape seed extract on low-density lipoprotein susceptibility to oxidation in heavy smokers. Metabolism. 2003;52:1250–7. doi: 10.1016/s0026-0495(03)00192-6. [DOI] [PubMed] [Google Scholar]