Abstract
In this study, we tested the hypothesis that the Angiopoietin 1 (Ang1)/Tie2 pathway mediates simvastatin-induced vascular integrity and migration of neuroblasts after stroke. Rats were subjected to 2 hrs of middle cerebral artery occlusion (MCAo) and treated, starting 1 day after stroke with or without simvastatin (1 mg/kg, daily) for 7 days. Simvastatin treatment significantly decreased blood–brain barrier (BBB) leakage and concomitantly, increased Ang1, Tie2 and Occludin expression in the ischaemic border (IBZ) compared to the MCAo control group. Simvastatin also significantly increased doublecortin (DCX, a marker of migrating neuroblasts) expression in the IBZ compared to control MCAo rats. DCX was highly expressed around vessels. To further investigate the signalling pathway of simvastatin-induced vascular stabilization and angiogenesis, rat brain microvascular endothelial cell (RBMEC) culture was employed. The data show that simvastatin treatment of RBMEC increased Ang1 and Tie2 gene and protein expression and promoted phosphorylated-Tie2 activity. Simvastatin significantly increased endothelial capillary tube formation, an index of angiogenesis, compared to non-treated control. Inhibition of Ang1 or knockdown of Tie2 gene expression in endothelial cells significantly attenuated simvastatin-induced capillary tube formation. In addition, simvastatin significantly increased subventricular zone (SVZ) explant cell migration compared to non-treatment control. Inhibition of Ang1 significantly attenuated simvastatin-induced SVZ cell migration. Simvastatin treatment of stroke increases Ang1/Tie2 expression and thereby reduces BBB leakage and promotes vascular stabilization. Ang1/Tie2 expression induced by simvastatin treatment promotes neuroblast micro-vascular coupling after stroke.
Keywords: stroke, angiopoietin 1, Tie2, simvastatin, vascular stabilization
Introduction
Vascular remodelling promotes neurorestoration and is an important goal for neurorestorative therapy of ischaemic neural tissues [1]. Blood vessel/nerve interactions, ultimately, play essential roles for the neurovascular network and brain function. Neuroblasts migrate to blood vessels in an area exhibiting early vascular remodelling and persistently increase vessel density, and bursts of angiogenesis are concurrent with neurogenesis [2, 3]. New neurons in the adult brain preferentially migrate to the immediate vicinity of existing vasculature, an area that has been referred to as a ‘vascular niche’. Endothelial cells release soluble factors that stimulate the self-renewal of neural progenitor cells and enhance neurogenesis [4]. Neurogenesis and angiogenesis are causally linked through vascular production of angiogenic factors [2, 5]. Angiopoietin 1 (Ang1) is an endothelial growth factor that functions as a ligand for the endothelial-specific receptor tyrosine kinase, Tie2. Ang1/Tie2 plays an important role in vascular integrity and neovascularization. Ang1 also promotes after-stroke neuroblast migration [2]. In vivo blockage of angiogenesis with intraventricular infusion of a neutralized Tie2 antibody substantially attenuates migration of neuroblasts newly born in the subventricular zone (SVZ) to the ischaemic region [2]. Neuronal recruitment and angiogenesis are mechanistically linked [6].
Vascular remodelling stimulates neurogenesis and enhances functional recovery after stroke [7, 8]. Vascular maturation and stabilization are required for functional angiogenesis [9]. The stabilization of endothelial cell barrier function within newly formed capillaries is a critical feature of angiogenesis. Vascular stabilization, which is defined as the investment of mural cells to the endothelial cell layer of nascent vessels, is critical for vascular development, angiogenesis and the maintenance of the established vasculature [9, 10]. The Ang1/Tie2 system controls pericyte recruitment, endothelial cells survival, and is implicated in blood vessel formation and vascular stabilization [11]. Ang1/Tie2 not only promotes angiogenesis and vascular maturation, Ang-1 is also expressed in the motor neurons in the ventral neural tube, and provides a cue for the sprouting vessels [12].
The use of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors, or statins, constitutes a well-established and potent strategy for reducing cholesterol levels in human beings. Beyond lowering cholesterol, statins also exert many major beneficial effects, including enhancement of endothelial function, angiogenesis and reductions of inflammatory responses [13, 14]. Statin treatment of stroke animals induces angiogenesis and neurogenesis as well as improves functional outcome after stroke [15, 16]. Whether simvastatin regulates vascular stabilization and neuroblast migration to cerebral vasculature has not been investigated. In this study, we test the hypothesis that simvastatin increases Ang1/Tie2 expression, which mediates simvastatin-induced vascular stabilization and neuroblast microvasculature coupling after stroke.
Methods
MCAo model and simvastatin administration
Young adult male Wistar rats weighing 270–300 g (n= 30, age 2–3 month) were employed in all our experiments. All experimental procedures have been approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital. Rats were anaesthetized with isoflurane. Transient right middle cerebral artery occlusion (MCAo) was induced for 2 hrs by advancing a 4-0 surgical nylon suture (18.5–19.5 mm) with an expanded (heated) tip from the external carotid artery into the lumen of the internal carotid artery to block the origin of the MCA [17]. After MCA, rats were randomly divided into two groups (n= 15/group): Group 1: MCAo alone for control. Group 2: simvastatin (1 mg/kg) was gavaged starting at 24 hrs after MCAo and daily for 7 days. Our previous studies have shown that 1 mg/kg simvastatin improved neurological functional outcome after stroke [15], therefore, the dose of 1 mg/kg simvastatin was employed in this study. Rats (n= 4/group) were sacrificed at 5 days after MCAo for BBB leakage measurement. Rats (n= 3/group) were sacrificed at 8 days after MCAo and brain tissue was isolated from the ischaemic core (core), ischaemic border zone (IBZ), which is adjacent to the ischaemic core, and the contra-lateral hemisphere (Contra.) (see Fig. 1A) for Western blot and real-time PCR assay. The ischaemic SVZ was isolated for SVZ explant cell migration assay. Rats (n= 8/group) were sacrificed at 14 days after MCAo for immunostaining.
Figure 1.
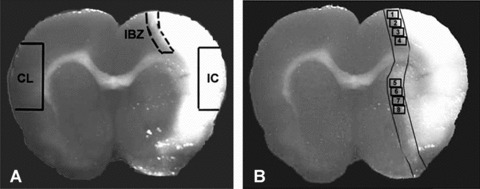
Panel A shows the regions where brain tissue samples were isolated from ischemic brain core (IC), ischemic border zone (IBZ) and homologous contralateral tissue (CL) for Western blot and real time PCR assay. Panel B shows the immunostaining measurement eight areas in the ischemic border (IBZ).
Quantitative evaluation of Evans blue dye extravasation
A total of 2% Evans blue dye in saline was injected intravenously as a BBB permeability tracer at 4 hrs before sacrifice at 5 days after MCAo. Coronal brain sections from bregma −1 to 1 mm were divided into the right and left hemispheres. Evans blue dye fluorescence intensity was determined by a microplate fluorescence reader (excitation 620 nm and emission 680 nm). Calculations were based on the external standards dissolved in the same solvent. The amount of extravasated Evans blue dye was quantified as micrograms per ischaemic hemisphere [18, 19].
Immunohistochemical staining
The brains were fixed by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde before being embedded in paraffin. A standard paraffin block was obtained from the centre of the lesion (bregma –1 mm to +1 mm). A series of 6 μm thick sections were cut from the block. Every 10th coronal section for a total five sections was used for immunohistochemical staining. Antibody against Ang1 (rabbit polyclonal lgG, 1:2000, Abecam, Cambridge, MA, USA), Tie2 (rabbit poly-clonal IgG antibody, 1:80 dilution, Santa Cruz, Santa Cruz, CA, USA), Occludin (Mouse monoclonal lgG antibody, 1:200 dilution, Zymed, San Francisco, CA, USA) and doublecortin (DCX), a protein expressed in migrating neurons (C-18, goat polyclonal IgG antibody, 1:200 dilution, Santa Cruz), were employed, respectively. Control experiments consisted of staining brain coronal tissue sections as outlined above, but the primary antibodies were omitted, as previously described [20].
Double immunofluorescence staining
To specifically identify Ang1 or Tie2-reactive cells localized with DCX, double immunofluorescence staining was employed. Fluorescein isothio-cyanate (FITC) (Calbiochem, Gibbstown, NJ, USA) and cyanine-5.18 (CY5, Jackson Immunoresearch, West Grove, PA, USA) were used for double-label immunoreactivity. Each coronal section was first treated with the primary anti-Ang1, anti-Tie2 antibody with Cy3, and was then followed by anti-DCX with FITC staining. Control experiments consisted of staining brain coronal tissue sections as outlined above, but omitted the primary antibodies, as previously described [20].
Quantitation
For quantitative measurements of Ang1, Tie2, Occludin and DCX, five slides from each brain, with each slide containing eight fields from the IBZ, (Fig. 1B) were digitized under a 20× objective (Olympus BX40) using a 3-CCD colour video camera (Sony DXC-970MD) interfaced with an MCID image analysis system (Imaging Research, St. Catharines, Canada) [21]. The data are presented as a percentage of positive area in each field.
Real-time PCR
Brain tissue from the IBZ was harvested and total RNA was isolated with TRIzol (Invitrogen, Carlsbad, CA, USA), following a standard protocol [22]. Quantitative PCR was performed with the SYBR Green real-time PCR method. Quantitative PCR was performed on an ABI 7000 PCR instrument (Applied Biosystems, Foster City, CA, USA) using 3-stage program parameters provided by the manufacturer, as follows: 2 min. at 50°C, 10 min. at 95°C, and then 40 cycles of 15 sec. at 95°C and 1 min. at 60°C. Each sample was tested in triplicate, and analysis of relative gene expression data was performed with the 2 −ΔΔCT method. The following primers for realtime PCR were designed using Primer Express software (ABI). Ang1: FWD: TAT TTT GTG ATT CTG GTG ATT; REV: GTT TCG CTT TAT TTT TGT AATG. Tie2: FWD: CGG CCA GGT ACA TAG GAG GAA; REV: TCA CAT CTC CGA ACA ATC AGC. GAPDH: FWD: AGA ACA TCA TCC CTG CAT CC; REV: CAC ATT GGG GGT AGG AAC AC.
Western blot
Protein was isolated from brain tissue and cultured cells with TRIzol (Invitrogen) following standard protocol. Protein concentrations were determined by a DC protein assay kit (Bio-Rad, Hercules, CA, USA). Membrane lysate was used. Protein samples were electrophoresed on gradient sodium dodecyl sulfate–polyacrylamide gel (Bio-Rad) and subsequently electrotransferred to nitrocellulose membranes. Membranes were treated with blocking buffer for 1 hr at room temperature, followed by incubation with primary antibodies for anti-β-actin (1:2000; Sigma, St. Louis, MO, USA), anti-Ang1 (1 μg/mL; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and anti-Tie2 (1 μg/mL; Santa Cruz Biotechnology) for 16 hrs at 4°C. The membranes were washed with blocking buffer without milk, and then incubated with horseradish per-oxidase-conjugated secondary antibody in blocking buffer. The bands were visualized with an enhanced chemiluminescence kit (SuperSignal; Pierce, Rockford, IL, USA).
Analysis of tyrosine phosphorylation of Tie2
Cells were collected and suspended in lysis buffer for 30 min on ice, followed by centrifugation at 15,000 g for 15 min. The Tie2 protein was immunoprecipitated from the cell lysate using protein Glutathione Sepharose 4B gel beads (Amersham Biosciences, Picataway, NJ, USA) coated with anti-Tie2 antibody. After electrophoresis under reducing conditions performed with a gradient gel polyacrylamide (Bio-Rad), the immunoprecipitated Tie2 proteins were transblotted onto a nitrocellulose membrane. The membrane was incubated with either 1 μg/mL anti-Tie2 antibody or anti-phosphotyrosine (PTyr) antibody in a blocking buffer for 1 hr. The membrane was washed and incubated with horseradish peroxidase-conjugated IgG, then developed, as described above.
Rat brain microvascular endothelial cell (RBMEC) culture
Rats were subjected to 2 hrs of MCAo and sacrificed at 7 days after MCAo. Rat brains were collected. The IBZ area tissue was isolated and digested in collagenase/dispase, and the microvessels separated by centrifugation in a Percoll (Sigma) gradient. Microvessels were seeded in flasks coated with rat-tail collagen and the medium was changed every 2 or 3 days. RBMECs were treated with or without simvastatin (0.1 μM) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with sodium pyruvate with 10% foetal bovine serum (FBS), 1% antibiotic/antimyotic (n= 3/group). Cells were left overnight before harvesting for real-time PCR and Western blot analysis.
Tie2 siRNA for mouse brain endothelial cells (MBECs)
Tie2 siRNA (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was transfected using Lipofectamine 2000 (Invitrogen) following standard protocol. Briefly, MBECs were plated in 10 cm plates and allowed to culture until they were 80% confluent. They were then transfected with 8 μg Tie2 siRNA in serum-free media for 6 hrs. Afterwards, DMEM +20% FBS was added and cells incubated overnight. The following day, media was changed and 48 hrs later cells were passaged for the following experiments. Our previous studies have shown that Tie2 knockdown MBECs by siRNA significantly decreased (70–80%) Tie2 gene and protein expression [23]. The knockdown of Tie2 gene expression in MBECs was used in the following capillary tube formation assay.
Capillary-like tube formation assay
Briefly, 0.1 ml growth factor-reduced Matrigel (Becton Dickinson, San Jose, CA, USA) was added per well, and MBECs (2 × 104 cells) were added and incubated in (1) regular cell culture medium (DMEM) for control; (2) simvastatin (0.1 μM); (3) simvastatin (0.1 μM) with anti-Ang1 antibody (1 μg/ml, Chemicon, Temecula, CA, USA); (4) Tie2 knockdown MBECs and (5) Tie2 knockdown MBECs treated with simvastatin (0.1 μM) for 5 hrs. All assays were performed in n= 6/group. For quantitative measurements of capillary tube formation, matrigel wells were digitized under a 4 × objective (Olympus BX40) for measurement of total tube length of capillary tube formation using a video camera (Sony DXC-970MD) interfaced with the multiple channel image display (MCID) image analysis system (Imaging Research, St. Catharines, Canada) at 5 hrs [24]. Tracks of endothelial cells organized into networks of cellular cords (tubes) were counted and averaged in randomly selected three microscopic fields [25].
SVZ explant cultures and cell migration measurement in vitro
To further test whether simvastatin affects neuroblast migration, we use an in vitro SVZ explant culture model. Rats were subjected 2 hrs MCAo and treated with or without simvastatin (1 mg/kg) daily for 7 days. Rats were sacrificed at 8 days after MCAo and SVZ explants were isolated from the ipsilateral of MCAo and simvastatin-treated rats. SVZ tissue was minced with a scalpel into pieces of ∼0.1 mm in each dimension. Explants were cultured within Matrigel in wells with 500 μl of Neuralbasal-A Medium containing 2% B27 supplement (Invitrogen). The cultured SVZ explants were treated in the absence or presence of anti-Ang1 inhibitor (1 μg/ml) for 7 days. Cell migration from the SVZ explant was measured using a phase contrast microscope and photographed at 4 × magnification with a digital camera. The average linear distance of cell migration from the edge of the SVZ explant were captured and measured at day 7 using the MCID software [26]. This average distance was assessed in each explant culture.
Statistical analysis
Independent Samples t-test was used to test for Ang1, Tie2, DCX and Occludin gene and protein expression between two groups with and without simvastatin treatment in vitro and in vivo. One-way ANOVA and least significant difference (LSD) analysis after post hoc test was used for testing the data of capillary tube formation and SVZ cell migration in vitro. The data are presented as mean ±SE; P < 0.05 is considered significant.
Results
Simvastatin treatment decreases BBB leakage and increases Ang1, Tie2 and Occludin expression in the ischaemic brain
To test whether simvastatin treatment of stroke rats decreases BBB leakage, vascular permeability was quantitatively evaluated by fluorescent detection of extravasated Evans blue dye [18, 19]. Figure 2A shows that simvastatin treatment of stroke significantly decreases BBB leakage compared to control MCAo rats.
Figure 2.
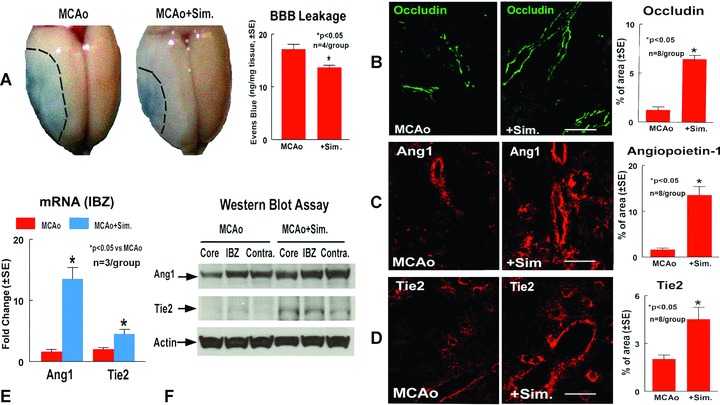
Simvastatin treatment decreases BBB leakage and increase vascular stabilization and Ang1, Tie2 expression in the ischemic brain. Panel A shows BBB leakage measured by Evans blue in MCAo and simvastatin treated rats, and the quantitative data. Panels B–D show Occludin (B), Ang1 (C) and Tie2 (D) expression in the IBZ in MCAo rats and simvastatin treated rats, and the quantitative data, respectively. Scale bar B, C and D= 50 μm Panel E shows Ang1 and Tie2 gene expression in the IBZ measured by real time PCR. Panel F shows Ang1 and Tie2 protein expression in the ischemic core, IBZ and contralateral hemisphere measured by Western blot assay.
The Ang1/Tie2 axis plays a crucial role in mediating vascular stabilization and maturation [11]. The pericyte-derived multimeric Ang-1/Tie-2 pathway induces the expression of tight junction protein Occludin [27]. Occludin, is a tight junction protein, and an index of microvascular integrity [28]. To identify the mechanism by which simvastatin treatment decreased BBB leakage, Ang1/Tie2 and Occludin expression were measured in the ischaemic brain. Figure 2B–D show that simvastatin treatment significantly increases Occludin (B), Ang1 (C), and Tie2 (D) expression in the ischaemic border compared to control MCAo animals.
To confirm the immunostaining data and to measure Ang1 and Tie2 gene and protein expression, real-time PCR and Western blot assay were performed. Rats were subjected to MCAo and treated with or without simvastatin 1 mg/kg daily for 7 days and sacrificed at 8 days after MCAo. Brain tissues were isolated from ischaemic core, IBZ and contra-lateral hemisphere. Real-time PCR and Western blot assay was performed. Figure 2E shows that simvastatin treatment after stroke increases Ang1 and Tie2 gene (E) expression in the ischaemic border area compared to control MCAo rats. Figure 2F shows that simvastatin treatment also increases Ang1 and Tie2 protein (F) expression in the ischaemic core and IBZ. These data suggest that simvastatin decreases BBB leakage, increases vascular integrity and up-regulates Ang1/Tie2 and tight junction protein expression in the ischaemic brain after stroke.
Simvastatin treatment promotes neuroblast migration
Ang1 not only regulates angiogenesis and vascular stabilization, but also affects neuroblast migration after stroke [5]. DCX, is a marker of migrating developmental neuronal cells. To test whether simvastatin promotes neuronal migration, DCX immunostaining was performed in the coronal section. To test whether simvastatin-mediated increase of Ang1/Tie2 expression is related to neuroblast migration, DCX/Ang1 and DCX/Tie2 double immunostaining was performed. Figure 3 show that simvastatin treatment (B and C) significantly increases DCX expression in the ischaemic border compared to control MCAo rats (A and C). The DCX positive cells were primarily located around vessels (D). The double immunostaining show that DCX positive cells also close to Tie2 (E) and Ang1 (F) reactive cells. These data suggest that simvas-tatin increases neuroblast migration, which may be mediated by increased Ang1/Tie2 expression and vascular remodelling.
Figure 3.
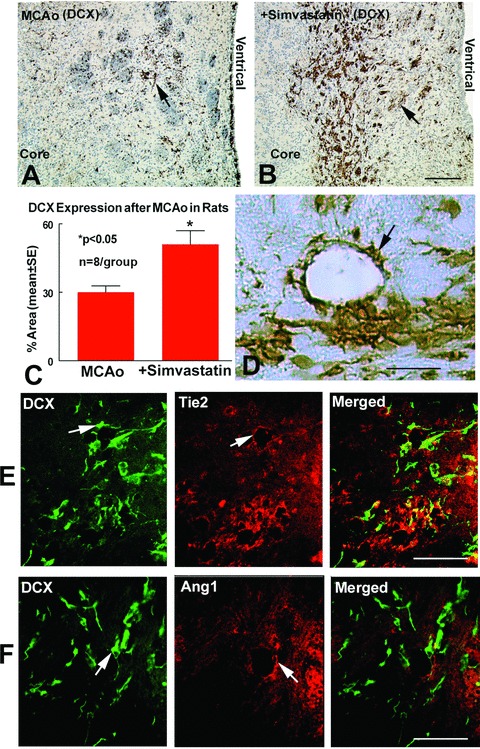
Simvastatin treatment increases DCX expression in the ischemic brain. Panels A–C show DCX expression in the IBZ in MCAo rats (A), simvastatin-treated rats (B), and the quantitative data (C). Panel D shows that the DCX positive cells were primarily located around vessels. Panels E and F show double immu-nostaining DCX/Tie2 (E) and DCX/Ang1 (F). DCX positive cells expression were close to Ang1 and Tie2 positive cells. Scale bar B= 100 μm, D 25 μm, E and F= 50 μm
Simvastatin increases brain endothelial cell Ang1, Tie2 gene and protein expression and Tie2 activity
To gain insight into the mechanism by which simvastatin promotes vascular stabilization and neuroblast-vascular coupling, RBMECs culture were employed. Figure 4 shows that simvastatin treatment significantly increases RBMEC Ang1 gene (A), Ang1/Tie2 protein expression, and also promotes phospho-Tie2 activity (B) compared to non-treatment RBMEC control.
Figure 4.
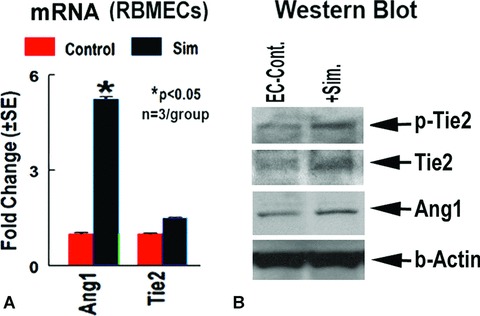
Simvastatin regulates Ang1, Tie2 gene and protein expression and phospho-Tie2 activity in cultured RBMECs in vitro. Panel A shows Ang1 and Tie2 gene expression. Panel B shows Western blot and immunoprecipitation assay.
Simvastatin increases capillary tube formation; inhibition of Ang1/Tie2 pathway attenuates simvastatin-induced capillary tube-like formation
To test the mechanism of simvastatin regulation of angiogenesis, capillary tube-like formation assays were performed in vitro. Figure 5 shows capillary tube formation after 5 hrs in culture.
Figure 5.
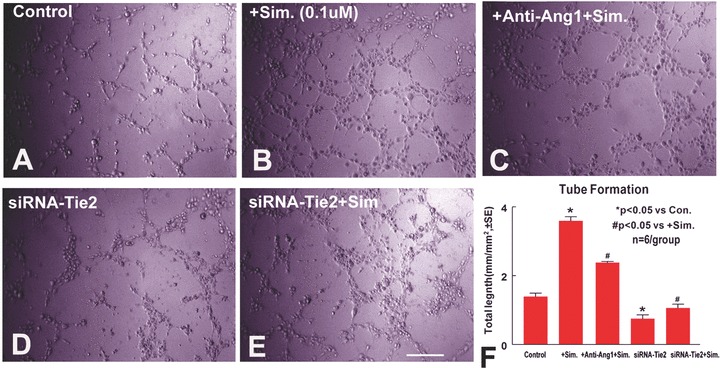
Simvastatin promotes capillary tube formation. Inhibition of Ang1 or knockdown Tie2 expression in MBECs attenuates simvastatin-induced capillary tube formation: Panels A–E show tube formation in DMEM (A), simvastatin treatment (B), simvastatin with anti-Ang1 antibody (C), Tie2 knockdown MBECs (D) Tie2 knockdown MBECs treated with simvastatin (E). Panel F shows quantitative data of capillary tube formation at 5 hrs after culture (n= 6/group). Scale bar E= 250 μm
Simvastatin (B and F) significantly increases capillary tube formation compared to non-treatment control at 5 hrs after culture (A and F, P < 0.05). Inhibition of Ang1 (C and F) significantly attenuates, but not completely inhibits simvastatin-induced tube formation (P < 0.05). Knockdown Tie2 expression in endothelial cell (E and F) significantly inhibits simvastatin-induced tube formation (P < 0.05). These data indicate that simvastatin induces angiogenesis. Ang1 partially mediates simvastatin-induced tube formation. In addition, Tie2 expression in endothelial cells is essential for simvastatin-induced tube formation.
Simvastatin induces SVZ explant cell migration. Inhibition of Ang1 attenuates simvastatin-induced SVZ cell migration
To further test whether simvastatin induces neuroblast migration, we use an in vitro SVZ explant culture model. SVZ explants were isolated from MCAo and simvastatintreated rats. SVZ explants were cultured in matrigel and treated with Anti-Ang1 antibody for 7 days. SVZ explant migration length was measured at 7 days after culture. Our data (Fig. 6) show that simvastatin treatment (B and D) significantly increases SVZ explant cell migration compared to MCAo control SVZ explant (A and D). Inhibition of Ang1 using an anti-Ang1 antibody (C and D) significantly attenuates simvastatin-induced SVZ cell migration. These data indicate that simvastatin increases Ang1 expression, which promotes SVZ neuroblast migration.
Figure 6.
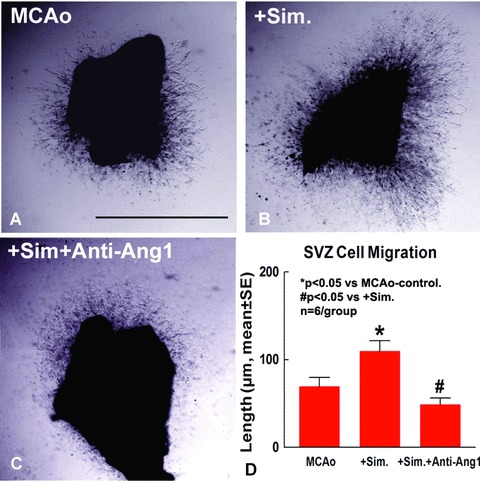
Simavstatin treatment increases SVZ cell migration, inhibition of Ang1 attenuates simvas-tatin-induced SVZ cell migration. Panels A–C show SVZ explant cell migration in MCAo control (A), simvastatin treatment (B), simvastatin with anti-Ang1 antibody (C). Panel D shows quantitative data of SVZ explant cell migration (n= 6/group). Scale bar A 500 μm.
Discussion
The present study demonstrated that treating stroke rats with simvastatin induces Ang1/Tie2 expression and increases phospho-Tie2 activity, as well as promotes angiogenesis, vascular stabilization and decreases BBB leakage. Inhibition of Ang-1 or knockdown Tie2 expression in endothelial cells by siRNA attenuates simvastatin-induced angiogenesis. In addition, simvastatin-induced Ang1/Tie2 expression increases SVZ cell migration. Inhibition of Ang1 attenuates simvastatin-induced SVZ cell migration.
During angiogenic vascular remodelling, supporting cells such as pericytes and smooth muscle cells are recruited to the vessels to provide structural support and stability for the vascular walls. Vascular remodelling also requires the mobilization of endothelial progenitor cells (EPCs) from the bone marrow and homing of progenitor cells to ischaemic tissue. The Ang1/Tie2 system controls pericyte recruitment, endothelial cell survival, and is implicated in blood vessel formation and vascular stabilization [11]. Ang1 acts directly on mural cells or their precursors to facilitate their recruitment to new blood vessels [29, 30]. Ang1-induced Tie2 phosphorylation is an essential process for vasculogenesis and maintaining vascular endothelial integrity [31]. Previous studies have found that the nitric oxide donor, (Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl) aminio] diazen-1-ium-1,2-diolate (DETA-NONOate), increases expression of Ang1/Tie2 and plays an important role in regulating angiogene-sis and vascular integrity after stroke [32]. Nagaraja et al. found that simvastatin administered 30 min after stroke promotes acute neuroprotective effects and decreases BBB leakage [33]. Our data indicate that simvastatin promotes Ang1 and Tie2 gene and protein expression, significantly increases Tie2 phosphorylation and decreases BBB leakage as well as increases the tight junction protein, Occludin expression, in the ischaemic brain. In addition, statins facilitate the EPC mobilization and proliferation, and enhance endothelial differentiation of peripheral blood mononuclear cells in patients with hypercholesterolaemia [34–36]. Our previous studies have found that statins promote angiogenesis after stroke [15]. Inhibiting the Ang1/Tie2 pathway using anti-Ang1 antibody or Tie2, siRNA subsequently attenuates simvastatin-induced angiogenesis. Therefore, Ang1/Tie2 pathway may play a role in simvastatin-induced angiogenesis and vascular stabilization.
The ‘vascular niche’ may reflect coordinated development and maintenance of both the nervous and vascular systems [37]. Vascular signalling via angiogenesis influences neural progenitor cell migration and survival [38]. Immature neurons localize in the peri-infarct cortex in a neurovascular niche, where neurogenesis is causally linked to angiogenesis through the vascular factors [2, 6, 16, 38–40]. Ang1 has a unique role in the nervous system in addition to its angiogenic role [41]; it is a chemoattractant for neuronal migration and supports neurite outgrowth from dorsal root ganglion cells positive for Tie2 receptor [2, 5, 42]. The overlap in molecular signalling between after-stroke angiogenesis and neurogenesis suggests a continuum of vascular and neural reorganization in the tissue adjacent to stroke. Our data show that simvastatin increases Ang1 and Tie2 expression in the ischaemic brain as well as promotes neuroblast migration to Ang1 reactive cells. Inhibition of the Ang1/Tie2 pathway significantly attenuates simvastatin-induced SVZ cell migration. Therefore, the Ang1/Tie2 pathway may regulate the simvastatin-induced SVZ neuroblast migration to the cerebral microvasculature after stroke.
In conclusion, our data indicate that simvastatin up-regulates the Ang1/Tie2 expression, which partially mediates simvastatin-induced angiogenesis and vascular stabilization after stroke. Up-regulation of Ang1/Tie2 by simvastatin treatment also promotes neuroblast migration after stroke.
Acknowledgments
The authors wish to thank Cynthia Roberts, Yuping Yang and Qinge Lu for technical assistance. This work was supported by NINDS RO1 NS047682 (J.C), P50 NS23393 (M.C) and AHA 0750048Z (J.C).
References
- 1.Hurtado O, Pradillo JM, Alonso-Escolano D, et al. Neurorepair versus neuroprotection in stroke. Cerebrovasc Dis. 2006;21:54–63. doi: 10.1159/000091704. [DOI] [PubMed] [Google Scholar]
- 2.Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–16. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thored P, Wood J, Arvidsson A, et al. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–9. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- 4.Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–40. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 5.Ohab JJ, Carmichael ST. Poststroke neurogenesis: emerging principles of migration and localization of Immature neurons. Neuroscientist. 2007 doi: 10.1177/1073858407309545. [DOI] [PubMed] [Google Scholar]
- 6.Li Q, Ford MC, Lavik EB, Madri JA. Modeling the neurovascular niche: VEGF-and BDNF-mediated cross-talk between neural stem cells and endothelial cells: an in vitro study. J Neurosci Res. 2006;84:1656–68. doi: 10.1002/jnr.21087. [DOI] [PubMed] [Google Scholar]
- 7.Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–8. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwai M, Cao G, Yin W, et al. Erythropoietin promotes neuronal replacement through revascularization and neurogenesis after neonatal hypoxia/ischemia in rats. Stroke. 2007;38:2795–803. doi: 10.1161/STROKEAHA.107.483008. [DOI] [PubMed] [Google Scholar]
- 9.Hirschi KK, Rohovsky SA, D’Amore PA. Cell-cell interactions in vessel assembly: a model for the fundamentals of vascular remodelling. Transpl Immunol. 1997;5:177–8. doi: 10.1016/s0966-3274(97)80034-2. [DOI] [PubMed] [Google Scholar]
- 10.Hirschi KK, D’Amore PA. Control of angiogenesis by the pericyte: molecular mechanisms and significance. Exs. 1997;79:419–28. doi: 10.1007/978-3-0348-9006-9_18. [DOI] [PubMed] [Google Scholar]
- 11.Iurlaro M, Scatena M, Zhu WH, et al. Rat aorta-derived mural precursor cells express the Tie2 receptor and respond directly to stimulation by angiopoietins. J Cell Sci. 2003;116:3635–43. doi: 10.1242/jcs.00629. [DOI] [PubMed] [Google Scholar]
- 12.Nagase T, Nagase M, Yoshimura K, et al. Angiogenesis within the developing mouse neural tube is dependent on sonic hedgehog signaling: possible roles of motor neurons. Genes Cells. 2005;10:595–604. doi: 10.1111/j.1365-2443.2005.00861.x. [DOI] [PubMed] [Google Scholar]
- 13.Aviram M, Rosenblat M, Bisgaier CL, Newton RS. Atorvastatin and gemfibrozil metabolites, but not the parent drugs, are potent antioxidants against lipoprotein oxidation. Atherosclerosis. 1998;138:271–80. doi: 10.1016/s0021-9150(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 14.Diomede L, Albani D, Sottocorno M, et al. In vivo anti-inflammatory effect of statins is mediated by nonsterol meval-onate products. Arterioscler Thromb Vasc Biol. 2001;21:1327–32. doi: 10.1161/hq0801.094222. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Zhang ZG, Li Y, et al. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–51. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Zhang C, Jiang H, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–90. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Sanberg PR, Li Y, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–8. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 18.Asahi M, Wang X, Mori T, et al. Effects of matrix metalloproteinase-9 gene knockout on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–32. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang ZG, Zhang L, Croll SD, Chopp M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience. 2002;113:683–7. doi: 10.1016/s0306-4522(02)00175-6. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Jiang N, Powers C, Chopp M. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke. 1998;29:1972–80. doi: 10.1161/01.str.29.9.1972. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Zhang ZG, Li Y, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–9. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Zacharek A, Chen J, Cui X, et al. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007;27:1684–91. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haralabopoulos GC, Grant DS, Kleinman HK, et al. Inhibitors of basement membrane collagen synthesis prevent endothe-lial cell alignment in matrigel in vitro and angiogenesis in vivo. Lab Invest. 1994;71:575–82. [PubMed] [Google Scholar]
- 25.Rikitake Y, Hirata K, Kawashima S, et al. Involvement of endothelial nitric oxide in sphingosine-1-phosphate-induced angio-genesis. Arterioscler Thromb Vasc Biol. 2002;22:108–14. doi: 10.1161/hq0102.101843. [DOI] [PubMed] [Google Scholar]
- 26.Dutton R, Bartlett PF. Precursor cells in the subventricular zone of the adult mouse are actively inhibited from differentiating into neurons. Dev Neurosci. 2000;22:96–105. doi: 10.1159/000017431. [DOI] [PubMed] [Google Scholar]
- 27.Hori S, Ohtsuki S, Hosoya K, et al. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem. 2004;89:503–13. doi: 10.1111/j.1471-4159.2004.02343.x. [DOI] [PubMed] [Google Scholar]
- 28.Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282:H1485–94. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metheny-Barlow LJ, Tian S, Hayes AJ, Li LY. Direct chemotactic action of angiopoietin-1 on mesenchymal cells in the presence of VEGF. Microvasc Res. 2004;68:221–30. doi: 10.1016/j.mvr.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Tammela T, Saaristo A, Lohela M, et al. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642–8. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- 31.Koh GY, Kim I, Kwak HJ, et al. Biomedical significance of endothelial cell specific growth factor, angiopoietin. Exp Mol Med. 2002;34:1–11. doi: 10.1038/emm.2002.1. [DOI] [PubMed] [Google Scholar]
- 32.Zacharek A, Chen J, Zhang C, et al. Nitric oxide regulates Angiopoietin1/Tie2 expression after stroke. Neurosci Lett. 2006;404:28–32. doi: 10.1016/j.neulet.2006.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagaraja TN, Knight RA, Croxen RL, et al. Acute neurovascular unit protection by simvastatin in transient cerebral ischemia. Neurol Res. 2006;28:826–30. doi: 10.1179/174313206X153914. [DOI] [PubMed] [Google Scholar]
- 34.Shao H, Tan Y, Eton D, et al. Statin and stromal cell derived factor-1 additively promote angiogenesis by enhancement of progenitor cells incorporation into new vessels. Stem Cells. 2008;26:1376–84. doi: 10.1634/stemcells.2007-0785. [DOI] [PubMed] [Google Scholar]
- 35.Gomez-Cerezo JF, Pagan-Munoz B, Lopez-Rodriguez M, et al. The role of endothelial progenitor cells and statins in endothelial function: a review. Cardiovasc Hematol Agents Med Chem. 2007;5:265–72. doi: 10.2174/187152507782109836. [DOI] [PubMed] [Google Scholar]
- 36.Park KW, Hwang KK, Cho HJ, et al. Simvastatin enhances endothelial differentiation of peripheral blood mononuclear cells in hypercholesterolemic patients and induces pro-angiogenic cytokine IL-8 secretion from monocytes. Clin Chim Acta. 2008;388:156–66. doi: 10.1016/j.cca.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 37.Ward NL, Lamanna JC. The neurovascular unit and its growth factors: coordinated response in the vascular and nervous systems. Neurol Res. 2004;26:870–83. doi: 10.1179/016164104X3798. [DOI] [PubMed] [Google Scholar]
- 38.Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–60. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 39.Leventhal C, Rafii S, Rafii D, et al. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–64. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- 40.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 41.Ward NL, Putoczki T, Mearow K, et al. Vascular-specific growth factor angiopoietin 1 is involved in the organization of neuronal processes. J Comp Neurol. 2005;482:244–56. doi: 10.1002/cne.20422. [DOI] [PubMed] [Google Scholar]
- 42.Kosacka J, Figiel M, Engele J, et al. Angiopoietin-1 promotes neurite outgrowth from dorsal root ganglion cells positive for Tie-2 receptor. Cell Tissue Res. 2005;320:11–9. doi: 10.1007/s00441-004-1068-2. [DOI] [PubMed] [Google Scholar]


