Abstract
Parietal–frontal networks in primate brains are central to mediating actions. Physiological and anatomical investigations have shown that the parietal–frontal network is consistently organized across several branches of primate evolution that include prosimian galagos, New World owl and squirrel monkeys, and Old World macaque monkeys. Electrical stimulation with 0.5-sec trains of pulses delivered via microelectrodes evoked ethologically relevant actions from both posterior parietal cortex (PPC) and frontal motor cortex (FMC). Reaching, grasping, defensive, and other complex movement patterns were evoked from domains that had a characteristic organization in both FMC and PPC. Although a PPC domain (e.g. reaching) may be connected with other PPC domains (e.g. grasping and defensive), its connections with FMC are preferential for a matching domain (reaching). Similarly, electrical stimulation of a PPC domain and concurrent optical imaging of FMC, showed activation patterns consistent with the preferential connectivity of PPC and FMC domains. The evidence for similar arrangements of interconnected functional domains in PPC and FMC of members of three major branches of the primate radiation suggests that the parietal–frontal networks emerged early in the evolution of primates. The small size of PPC in the close relatives of primates including lagomorphs, rodents, and tree shrews, suggests a limited involvement of PPC in motor behavior before archaic primates emerged. However, functional domains may have evolved in motor cortex before the emergence of archaic primates.
Keywords: motor cortex, posterior parietal cortex, grasping, prosimians
INTRODUCTION
This review addresses the general question of how motor behaviors are generated. As in other mammals, primates are characterized by many behaviors that are conducted in the same way across individuals within the species, and even across species in larger taxonomic groups [Platt & Ghazanfar, 2010]. Many of these behaviors seem to require little or no experience to be performed, although this can be difficult to evaluate when nervous systems take a long time to mature, as is the case in humans. This long development time may be one of the reasons that researchers in psychology have long emphasized the role of learning in human behavior [Elsner & Hommel, 2004], and have discounted the importance of neural networks for adaptive behaviors that emerge in brain development without considerable relevant experience [Pinker, 2002]. In addition, there are a number of specific motor sequences and skills that humans and other primates acquire only with great difficulty and practice. Thus, it is common to characterize cortical motor systems in primates and other mammals as general-purpose systems that are organized to produce simple movements that can be combined in any number of ways to produce a great range of motor behaviors. This impression is reinforced by the depictions of the organization of motor cortex as highly somatotopically organized so that neurons capable of moving specific body parts or muscles, the twitch of a finger for example, are arranged in an orderly pattern. Thus, inputs could activate various parts of the motor map in any pattern [Leyton & Sherrington, 1917; Penfield & Boldrey, 1937; Woolsey et al., 1952], much as any number of tunes can be played on the orderly arrangement of keys on a piano [Schieber, 2001].
However, in order to reconsider this view, it is important to recognize that the most detailed motor maps in primary motor cortex (M1) and elsewhere are based on electrical stimulation of cortical neurons with microelectrodes with low levels of current for very brief periods of time. These detailed maps are produced with penetrating microelectrodes, with the tip in or near cortical layer 5 so that only a few neurons are activated and only for a small fraction of a second [Schieber, 2001]. In earlier studies, motor cortex was often stimulated with higher levels of current, for longer periods, and with surface electrodes that activated more neurons. Under these conditions, more complex movement sequences were produced that were difficult to interpret, and were not fully explored. The summary map of the organization of motor cortex in the chimpanzee by Leyton and Sherrington [1917] was a map of the first movement evoked at each stimulation site, and not the full sequence of movements. These early motor maps, as for subsequent sensory maps of somatosensory cortex, emphasized the orderly arrangement of body parts, as the hindlimb, trunk, forelimb, and face were consistently represented in a mediolateral sequence in primary motor cortex. However, when the subsequent results of more detailed microstimulation maps of motor cortex were considered, even when the stimulation of neurons was brief and at threshold levels, so that only “first movements” were observed, the motor maps were only globally somatotopic. Locally, they were disordered, quite unlike the sensory maps. Thus, in the forelimb portion of primary motor cortex of various primates, stimulation sites evoking movements of fingers were mixed with sites evoking wrist, elbow, or shoulder movements, and sites for different finger movements were scattered and mixed [Donoghue et al., 1992; Gould et al., 1986; Qi et al., 2010, 2000; Wu et al., 2000]. Consistent with such microstimulation results, corticospinal neurons that control a single muscle of the hand are broadly distributed in M1, and form multiple “hot spots,” and are mixed with neurons for controlling other muscles such as those for shoulder, wrist, and elbow movements [Rathelot & Strick, 2006]. What could account for this puzzling difference in sensory and motor maps?
Recently, Graziano and co-workers challenged the traditional view of the organization of motor cortex in primates by stimulating motor cortex in awake macaque monkeys for longer times with slightly higher levels of current. This change in stimulation parameters produced sequences of motor movements that seemed ethologically relevant, and the sequences differed according to where in motor cortex neurons were stimulated. Using a behaviorally relevant stimulation time of 0.5 sec, Graziano and co-workers divided motor and premotor cortex into a patchwork of zones, with each zone devoted to a different sequence of movements [Graziano & Aflalo, 2007; Graziano et al., 2005; 2002b]. Microstimulation sites within each functional zone evoked similar movements, with slight variations depending on location. More medially in motor cortex, coordinated movements of the forelimbs and hindlimbs were evoked as if the monkey were climbing or leaping (which was not possible as the stimulated monkeys were in monkey chairs with the head fixed). More laterally in motor cortex, sites evoked defensive movements of the face and forearm as if to protect the head from a blow. Nearby sites evoked chewing and licking, while sites in zones between these more lateral and more medial zones evoked behaviors such as hand-to-mouth, reach-to-grasp, and grasping to manipulate. This evidence that different parts of motor cortex were involved in specific, useful behaviors, and that these zones were similarly located in different monkeys, provided strong evidence that motor cortex is not a general-purpose organ. Elements of movements are not represented in a series of distributed columns of neurons to be played like a piano by a distant player to form any complex sequence called upon. Instead, motor cortex has functionally distinct territories that intrinsically organize movements into adaptive sequences. Because the functional zones are similarly distributed across individuals, they likely form early in development, possibly before the behaviors are first needed, but at least with minimal experience.
Our own research was motivated by these findings of Graziano and co-workers. We had two main questions. First, is frontal motor cortex (FMC) similarly organized into functional zones (domains) in other primates? Using similar stimulation procedures, but in anesthetized primates, we found functionally distinct domains in motor and premotor cortex of two species of New World monkeys, owl monkeys (Aotus nancymaae) and squirrel monkeys (Saimiri sciureus), and in prosimian galagos (Otolemur garnetti). Wealso replicated some of the findings of Graziano and co-workers in FMC of macaque monkeys (Macaca fascicularis, mulatta, and radiata).
Second, we wondered whether similar or even matching functional domains exist in posterior parietal cortex (PPC), and if so, if they are arranged in a similar manner in all primates. There was already considerable evidence that such domains might exist in the PPC of macaque monkeys, as much of PPC of macaques is thought to be involved in the planning and initiation of specific behaviors. Thus, neurons in an anterior region of PPC were found to be activated during grasping [Sakata et al., 1995], and neurons in a posterior parietal reach region (PPC) are active during reaching [Snyder et al., 1997]. Also, electrical stimulation of a lateral region, the lateral intraparietal area (LIP) evoked eye movements [Constantin et al., 2007; Their & Andersen, 1998], as did electrical stimulation of the frontal eye field (FEF) [Bruce & Goldberg, 1985; Huerta et al., 1987], a subdivision of motor cortex. As LIP and the FEF are interconnected, they appear to work together to direct eyes toward objects of potential interest. Most importantly, when part of PPC, the ventral intraparietal area (VIP), was stimulated with 0.5-sec trains of electrical pulses in awake monkeys, defensive hand and head movements were produced [Cooke et al., 2003], much like those produced by stimulating motor cortex. Thus, we included PPC in our stimulation studies of anesthetized owl monkeys, squirrel monkeys, and galagos.
We were fortunate to find that domains for specific behaviors could be revealed in PPC, as well as in FMC, in anesthetized primates where neurons are generally less responsive. However, the use of anesthetized primates allowed us more control within our experiments. Given our success in activating functional domains in motor cortex and PPC, we determined cortical and thalamic connections of these domains, used optical imaging to see how domains interact during electrical stimulation, and investigated the function of the roles of behaviorally matched domains by stimulating one while another was inactivated. Collectively, these experiments provide the beginning of an understanding of the properties of the cortical networks involving domains in PPC and FMC.
Functional Domains in Galagos
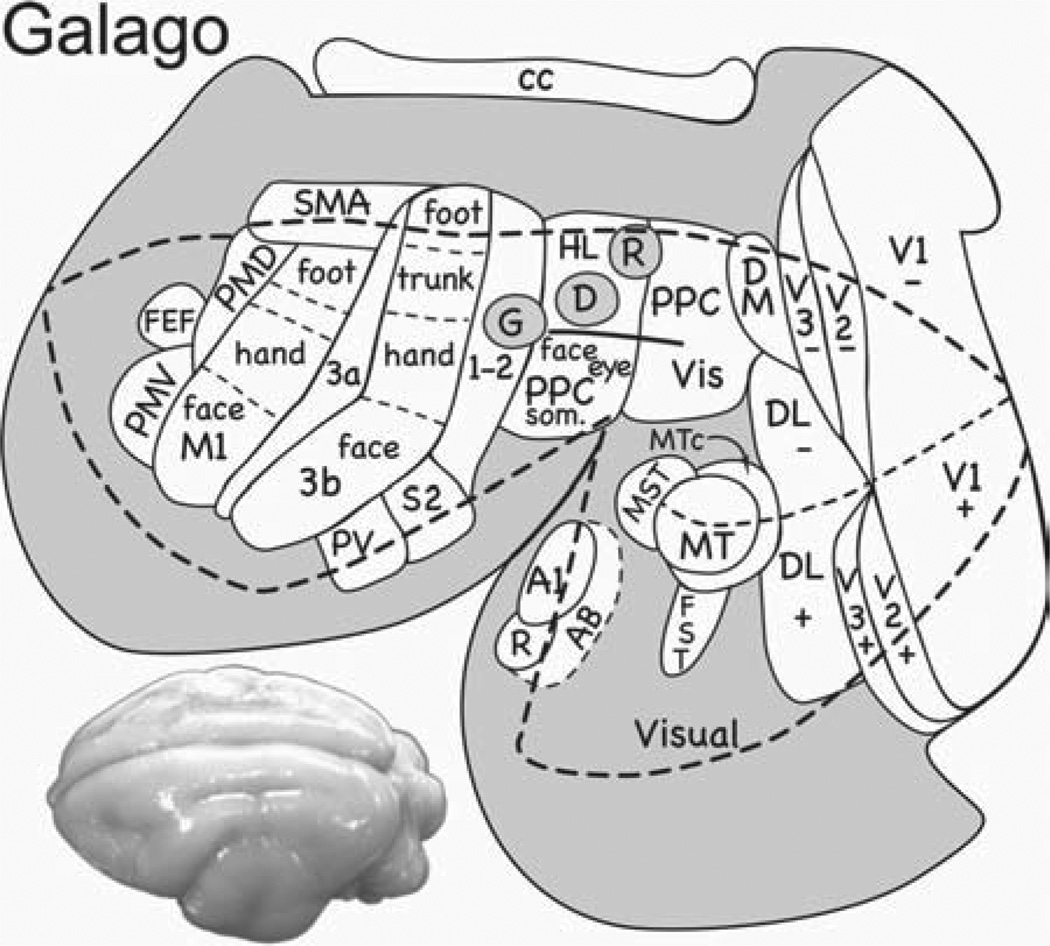
We started our experiments in galagos (O. garnetti), a primate in the prosimian radiation that has been separate for over 70 million years from the anthropoid radiation that includes monkeys [Purvis, 1995; Steiper & Seiffert, 2012]. Earlier studies established that the major frontal motor areas of monkeys are also present in galagos [Wu et al., 2000], but not much was known about PPC. Microstimulation results from galagos are summarized in Figure 1. PPC was most fully explored, and a surprisingly large number of domains were revealed. Most medially in PPC a domain was found where stimulation produced coordinated movements of both hindlimbs, usually with both forelimbs, as if attempting to climb or run. More laterally and somewhat more posterior, a domain produced reaching movements. A domain for forelimb defensive movements was slightly more anterior. More laterally, we found cortical domains for hand-to-body movements, and for grasping. Face-defensive, face-aggressive, and eye-movement domains were located most laterally in PPC. In motor and premotor cortex, these domains were arranged in a similar mediolateral manner, relating somewhat to the overall somatotopy of motor cortex as revealed by movements evoked with short trains of pulses and threshold levels of current (hindlimb medial and face lateral). The evoked movements appeared within 50 msec of when the train of electrical pulses started, and were completed by the end of the stimulation or soon thereafter [Stepniewska et al., 2009b]. When tracers were injected into PPC domains identified by microstimulation, connections were with other PPC domains, and functionally matched domains in FMC [Stepniewska et al., 2009a]. Functional domains in frontal cortex sometimes overlapped premotor and primary motor cortex, thereby involving both fields, and sometimes they were clearly separate in premotor and motor cortex.
Fig. 1.
Functional domains for ethologically relevant behaviors in prosimian galagos. Sensory and motor areas are outlined on a flattened view of the cortical surface of the brain. The mediolateral array of movement domains in the anterior half of posterior parietal cortex (PPC) is interconnected with matching domains in motor and premotor cortex (not shown). The domain for grasping (G) is partly or largely in area 2 of anterior parietal cortex, suggesting that part of this region is closely aligned with PPC. Domains for defensive movements of the face and arm (D) and reaching (R) are also shown. Motor areas include primary motor cortex (M1), dorsal and ventral premotor areas (PMD and PMV), the supplementary motor area (SMA), and the frontal eye field (FEF). Somatosensory areas include the primary area (S1 or area 3b), area 3a, area 1–2 (as separate areas 1 and 2 have not been distinguished), the second somatosensory area (S2), and the ventral parietal area (PV). Visual areas include V1, V2 and V3, dorsomedial (DM), dorsolateral (DL), middle temporal (MT), middle temporal crescent (MTc), middle superior temporal (MST), and inferior temporal (IT) areas. Representations of upper (+) and lower (−) visual quadrants are indicated for some visual areas. The auditory region includes primary auditory cortex (A1), the rostral area (R), and the auditory belt (AB). A dorsolateral view of a galago brain is on the lower left.
The visual inputs to the functional domains in the anterior half of PPC were largely those relayed from the posterior half of PPC that has dense visual inputs, but where no movement domains were observed. However, some inputs to PPC domains were directly from visual areas. The somatosensory inputs to PPC domains were largely from secondary somatosensory areas located in the upper bank and fundus of the lateral fissure. Domains in FMC received inputs from other frontal motor areas, somatosensory areas, and PPC [Fang et al., 2005; Stepniewska et al., 2009a].
When we used optical imaging to reveal activation sites in motor and premotor cortex while electrically stimulating sites within PPC domains, only functionally matched domains in frontal cortex were activated at levels above thresholds for imaging [Stepniewska et al., 2011]. When the stimulus trains were reduced in length so that no movements were evoked, the same frontal domains were activated, but less intensively and for less time. Thus, we have evidence that electrical stimulation of PPC domains produces highly specific activation patterns in FMC, and that the movement patterns depend on the duration and intensity of electrical stimulation in PPC. In addition, we know from related experiments, that movements cannot be evoked from PPC domains when the activity of matching domains in premotor and especially in primary motor cortex is chemically blocked. Thus, the functions of PPC domains depend on their activating connections with frontal motor and premotor cortex.
Functional Domains in New World Monkeys
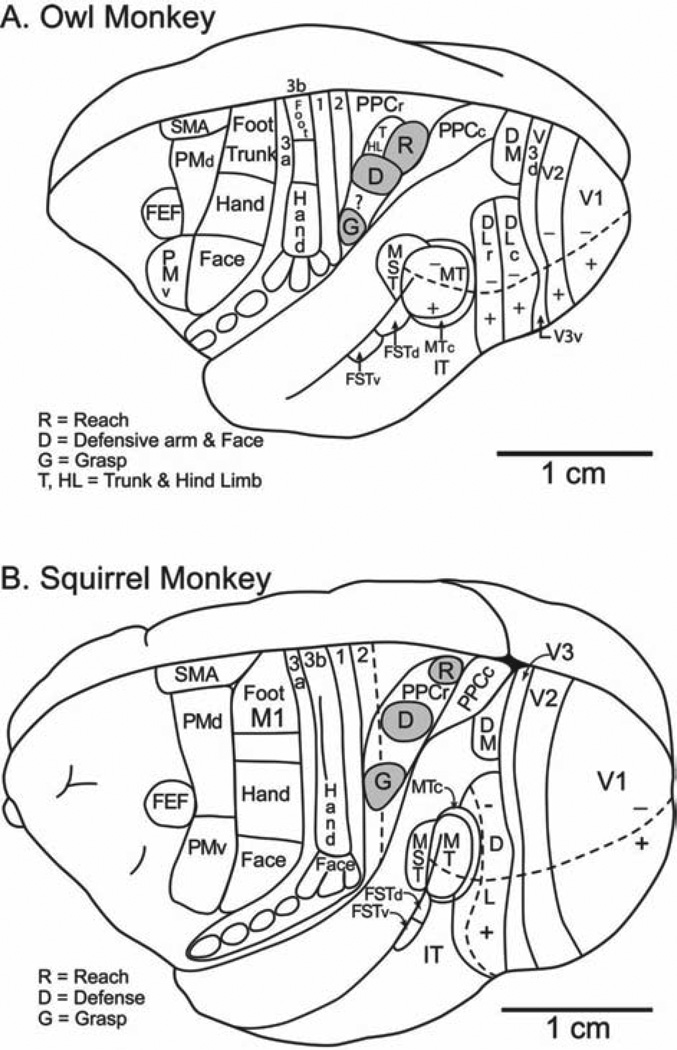
Most of the functional domains found in PPC and FMC of galagos were also found with the same stimulation methods in both New World owl monkeys and squirrel monkeys. These monkeys were included in our study as much was already known about cortical organization in these primates from previous investigations. Most importantly, motor and sensory areas had been identified. Our studies of functional domains in New World monkeys are ongoing, and many questions remain. Initially, we focused on determining the locations and connections of the domains for grasping, forearm defensive movements, and reaching. The locations of these domains in PPC and FMC are shown for owl monkeys and squirrel monkeys in Figure 2. Note that in both these New World monkeys, the arrangement of these three domains in PPC is the same with the grasping domain most lateral, the forearm defense more medial, and the reaching domain most medial [Gharbawie et al., 2011a]. Also, the arrangement of domains in PPC has a rostrocaudal slant, with the grasp domain largely in area 2, and the reach domain most caudal in PPC. A similar arrangement of domains was found in prosimian galagos (Fig. 1). Thus, the cortical organization of PPC domains is remarkably similar in nocturnal (crepuscular) prosimian galagos, nocturnal owl monkeys, and diurnal squirrel monkeys. The connections of these domains are also similar across these primates, with matching PPC and FMC domains preferentially interconnected, and with PPC domains having visual and somatosensory inputs, while FMC domains having connections with other frontal motor areas, prefrontal cortex, and somatosensory areas [Gharbawie et al., 2011a]. The thalamic connections are also similar, with PPC having more connections with the somatosensory thalamus and FMC having more connections with the motor thalamus [Gharbawie et al., 2010].
Fig. 2.
Functional domains in (A) owl monkeys and (B) squirrel monkeys. Domains for reaching defense, and grasping have been identified in posterior parietal and frontal motor cortex. Other domains are under investigation. Conventions as in Figure 1.
Functional Domains in Old World Macaque Monkeys
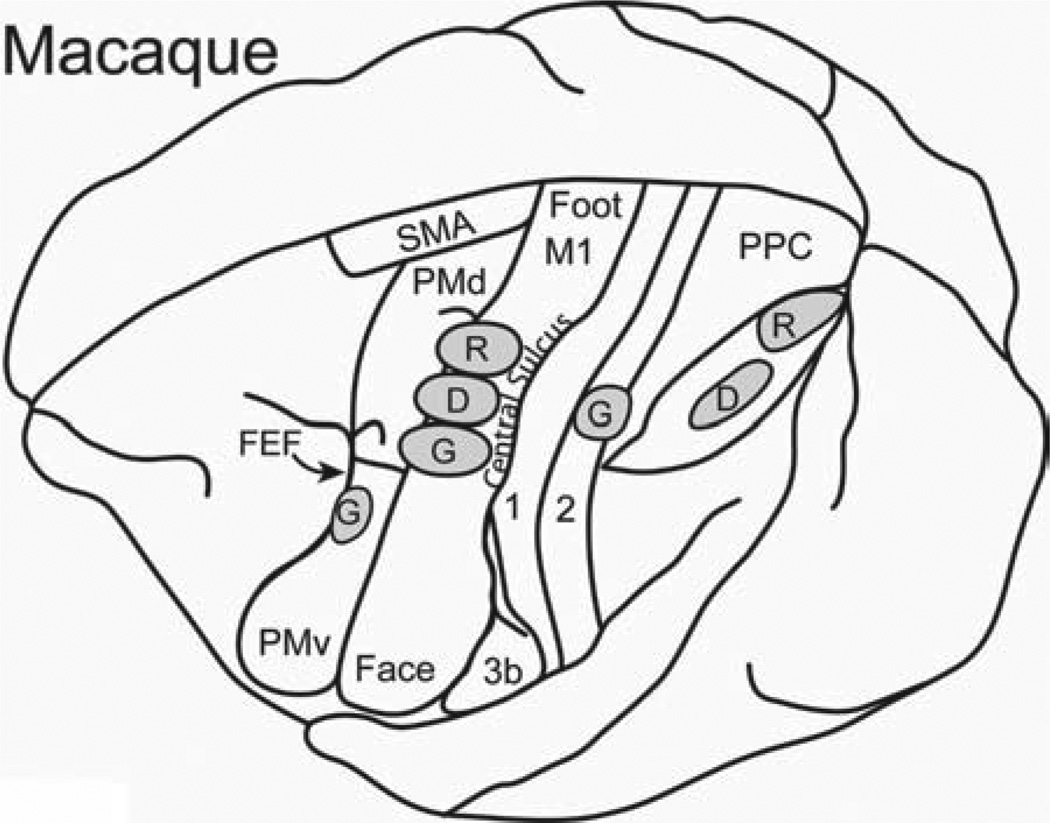
While the motor cortex of Old World macaques is harder to explore with microelectrodes as much of M1 is located in the anterior bank of the central sulcus [Qi et al., 2000], a number of domains have been identified by Graziano and co-workers in the parts of motor and premotor cortex that is exposed on the dorsal surface of the frontal lobe [Graziano et al., 2005, 2002a]. We have obtained comparable, but less extensive results from frontal cortex of macaque monkeys [Gharbawie et al., 2011b]. This cortex includes the rostral part of M1, and the dorsal and ventral premotor areas, PMD and PMV. We transposed some of the domains onto a dorsolateral view of the brain of a macaque monkey, where we also illustrate the locations of a grasping domain, a defensive domain, and a reaching domain in PPC (Fig. 3). The posterior grasping domain, which is largely or completely located along the caudal margin of area 2, was identified by long trains (0.5 sec) of electrical pulses in our recent microstimulation experiments [Gharbawie et al., 2011a, 2011b]. While the stimulation of other nearby regions of cortex in area 2 and PPC did not produce grasping movements, neurons along the lateral bank of the anterior tip of the intraparietal sulcus, the anterior intraparietal area, AIP, and adjoining cortex in the 7b region are also involved in grasping behavior, as the area 2 grasping domain has connections with these regions, and neurons in these regions are activated during grasping [Sakata et al., 1995]. Thus, in macaques at least, we proposed that the grasping network includes several parallel pathways from PPC to FMC in addition to the pathway from the area 2 grasping domain to matching domains in premotor and motor cortex [Gharbawie et al., 2011b].
Fig. 3.
Functional grasp (G), defense (D), and reach (R) domains in macaque monkeys. Other domains have been demonstrated for motor and premotor cortex, and the reach domain in PPC is based on microelectrode recordings, as microstimulation results have not been reported. Based on Gharbawie et al. [2011b]; Graziano et al. [2002a, 2002b]; Cooke et al. [2003]. Conventions as in Figure 1.
The existence of other functional domains within PPC is suggested by the results of other studies. Previously, Cooke et al. [2003] found that 0.5-sec trains of pulses in the VIP, of PPC of macaque monkeys evoked defensive movements of the forearm and face. This defensive domain is located just caudal and slightly medial to our area 2 grasping domain. While microstimulation has not yet been used to identify a domain for reaching in PPC, this domain would be expected from the results in galagos and New World monkeys to be caudal and medial to the defense domain in VIP. Cortex in this region was called the posterior parietal reach region (PPR) by Anderson and Buneo [2002] because neurons are active in this cortex just before and during a reach. While the evidence is incomplete, it appears that grasping, defensive, and reaching domains exist in PPC of macaque monkeys in the same spatial arrangement as in galagos and New World monkeys. Matching domains, as well as other domains, exist in FMC. The LIP, where electrical stimulation evokes saccadic eye movements [Constantin et al., 2007; Keating et al., 1983; Kurylo & Skavenski, 1991; Shibutani et al., 1984; Thier & Andersen, 1998] can be considered another domain of PPC of macaques that is shared with other primates, and projects to a matching domain in the FMC, the FEF.
We conclude from these results that most or all primates have cortical networks that involve subregions of PPC and FMC in producing common useful behaviors. It seems likely that these networks emerge early in brain development and require little or no experience to be functional, although they may be modified by experience. The functional domains in M1 and premotor cortex are not quite cortical areas, as they are embedded in larger cortical areas. PPC and FMC domains are specialized for specific movement sequences that are important for survival, and would be costly to be learned by trial and error.
Similar arrays of functional domains in PPC and FMC likely exist in humans. A wealth of data, from functional imaging studies in humans, supports the conclusion that PPC has separate regions for reaching, grasping, defensive, and eye movements [e.g. Davare et al., 2007; Serano & Huang, 2006; Vesia et al., 2010]. But there is also considerable evidence that PPC in humans has expanded and further differentiated so that regions with new functions, such as promoting tool use, have evolved [Durand et al., 2009; Frey, 2007; Orban et al., 2006; Peeters et al., 2009]. Thus, the constellation of functional domains has expanded in humans, and perhaps even in macaques, but this premise awaits further study.
The Evolution of Functional Domains in PPC and FMC
It seems likely that functional domains in FMC emerged sometime in the early eutherian ancestors of primates, while PPC domains emerged more recently in the immediate ancestors of modern primates. M1, PMD, and PMV in primates have a mosaic or fractured type of functional organization where vertical arrays of neurons next to each other, produce different small movements, or no movements when stimulated briefly with threshold levels of current [Gould et al., 1986; Qi et al., 2002; Schieber, 2001; Wu et al., 2000]. The region of M1 that represents the hand, for example, has rerepresentations of finger movements in different parts of the region, along with rerepresentations of wrist, elbow, and shoulder movements. This type of organization would seem necessary if several different functional zones were to coexist but be spatially separate in M1. There is no evidence yet of functional domains in motor cortex of nonprimate mammals, but in tree shrews [Remple et al., 2006] and squirrels [Cooke et al., 2011], M1 has a fractured mosaic representation of movements in threshold maps, much like those in primates. Thus, specialized domains for different movement sequences could have evolved in M1 of early eutherian mammals.
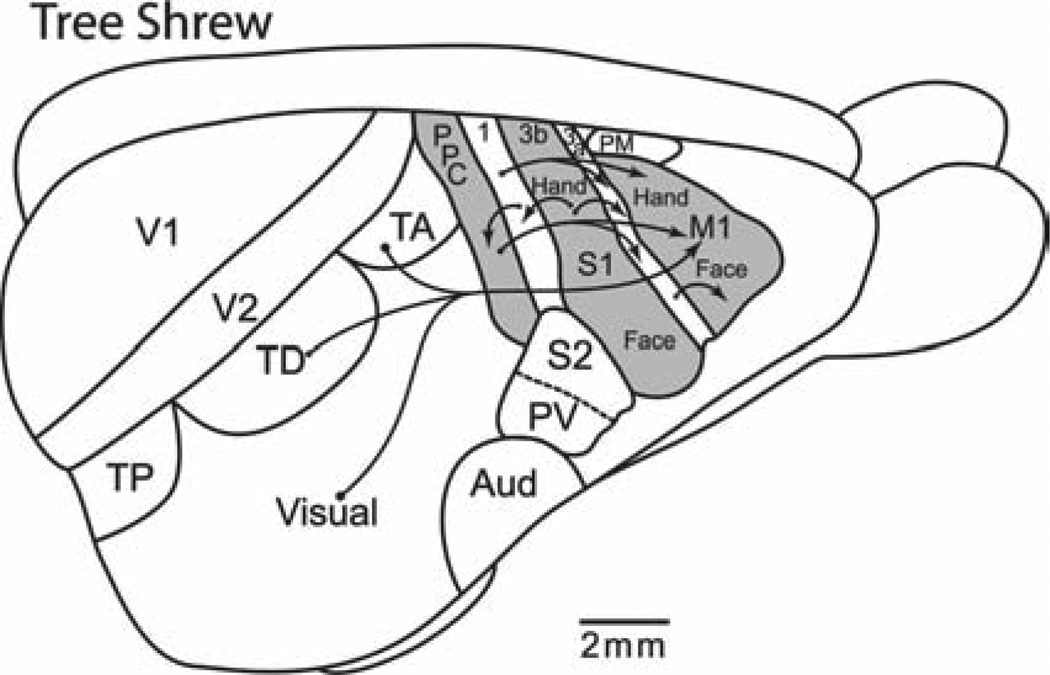
Present-day monotremes and marsupials do not appear to have a separate motor region of frontal cortex, but instead motor functions of cortex are mediated by somatosensory cortex [Kaas, 2007]. Thus, we can infer that early mammals also lacked a region of motor cortex that was separate from somatosensory cortex. However, the high degree of somatotopy in somatosensory cortex would seem to constrain the development of functionally independent motor domains within somatosensory cortex. Thus, the structures promoting ethologically relevant behaviors in early mammals could have been subcortical. Although, the evolution of cortical domains for complex behaviors could have evolved in mammalian evolution with the emergence of motor cortex in eutherian (placental) mammals, similar domains probably did not exist in PPC. Rodents, lagomorphs, and tree shrews, the closest living relatives of primates that have been studied, all have very little PPC (Fig. 4), and this cortex may do little more than provide sensory information to motor cortex. In rodents and tree shrews, more direct sensory projections to FMC come from visual and somatosensory areas [Cooke et al., 2011; Remple et al., 2007].
Fig. 4.
Posterior parietal cortex and frontal motor cortex in tree shrews. Although tree shrews are closely related to primates, they have little posterior parietal cortex. Thus, functional domains may not exist in PPC, but they may in M1. TP, TD, and TA are posterior, dorsal, and anterior divisions of temporal visual cortex. Most of the rest of temporal cortex is visual. A small premotor area (PM) is medial to M1. FST is the fundal visual area of the superior temporal sulcus. Arrows indicate connections from visual areas to M1, from posterior parietal cortex to M1, and somatosensory areas to PPC and M1.
The Significance of Cortical Networks for Ethologically Relevant Behaviors
The finding of cortical domains and networks for adaptive, innate movement sequences is in many ways quite surprising. While there is a long tradition in neuroethology of describing neural “centers” where the interactions of groups of neurons produce a stereotyped sequence of movements, these behaviors have often been described in animals with little or no neocortex [Ewert, 1980], or otherwise have been attributed to or identified with neuron groups or centers of the brainstem [Bignall & Schramm, 1974; Randall, 1965]. The microstimulation results not only demonstrate that neocortex is intimately involved in many such behaviors in primates, but in initiating and maintaining such behaviors, rather than inhibiting them, a role that has been often attributed to cortex. Moreover, the behaviors have been evoked from three divisions of neocortex that are relatively new additions to mammalian neocortex. The primary motor area, M1, appeared with the emergence of placental mammals more than 100 mya, but long after the first mammals appeared [Kaas, 2007]. Areas of premotor cortex have been identified in members of two of the major branches of placental mammals, Laurasiatheria and Euarchontoglires, but not in two others, Afrotheria and Xenarthra. This may reflect limited efforts to demonstrate the existence of such areas, but this lack of information allows for the possibility that premotor cortex evolved well after the emergence of M1 in placental mammals. Finally, PPC is greatly enlarged in primates, but not in the close relatives of primates. The occupation of much of PPC by sensorimotor domains suggests that these domains are one of the main reasons for the expansion of PPC in primates.
Another surprise is that the domains are not centers in the traditional sense, as matching domains are interconnected to form in three hierarchical stages in PPC, premotor cortex, and primary motor cortex. While the three stages are functionally related, stimulation of domains in M1 can evoke the behaviors without contributions from the other two stages. Of course, motor cortex projections bring other groups of neurons into the network, including brainstem and spinal cord motor neurons, as well as neurons in the basal ganglia, and the motor thalamus. As the actions and connection patterns of PPC are not completely necessary, it is important to determine what they add. As postulated for prefrontal cortex, PPC at least adds complexity to the movement planning process.
Our results raise several important research questions. We can evoke behaviors with electrical stimulation of domains, but what is the nature of the afferent inputs that select and trigger the behaviors? Domains at each of the three stages of cortical processing receive different feedforward activating inputs, so we can now start to formulate hypotheses of how these networks are activated, and test these hypotheses. For example, both visual and somatosensory inputs dominate the domains in PPC, whereas premotor cortex receives major inputs from prefrontal cortex. Primary motor cortex receives major inputs from the motor thalamus, which is activated by projections from the cerebellum and basal ganglia, and movements can be evoked by electrical stimulation of the motor thalamus. A related question is how do different domains in the same cortical region interact? Do they mutually inhibit each other so that one behavior dominates? A third set of questions concerns the neural interactions within a domain. Are they critical to the production of the movement sequence? Do the domains have the capacity to produce variations in behavior? Does experience modify their activity or enlarge their territory? In addition, how does the feedforward output of each domain affect the domains and neuron groups that they activate? Are the outputs of domains modified by sensory feedback? How do the functions of the domains and their networks vary across primate taxa? Finally, the most difficult research question concerns what the parietal–frontal components of each network add to the behavioral sequences that are similar in mammals without the same cortical components? We hope to answer at least some of these questions.
ACKNOWLEDGMENTS
Research reported here was conducted in compliance with the American Society of Primatologists’ Principles for the Ethical Treatment of Primates.
REFERENCES
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Ann Rev Neurosci. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- Bignall EE, Schramm L. Behavior of chronically decerebrated kittens. Exp Neurol. 1974;42:519–531. doi: 10.1016/0014-4886(74)90075-2. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Constantin AG, Wang H, Martinez-Trujillo JC, Crawford JD. Frames of reference for gaze saccades evoked during stimulation of lateral intraparietal cortex. J Neurophysiol. 2007;98:696–709. doi: 10.1152/jn.00206.2007. [DOI] [PubMed] [Google Scholar]
- Cooke DF, Padberg J, Zahner T, Krubitzer L. The functional organization and cortical connections of motor cortex in squirrels. Cereb Cortex. 2011;22:1959–1978. doi: 10.1093/cercor/bhr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke DF, Taylor CR, Moore T, Graziano MSA. Complex movements evoked by microstimulation of the ventral intraparietal area. Proc Natl Acad Sci USA. 2003;100:6163–6168. doi: 10.1073/pnas.1031751100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Andres M, Clerget E, Thonnard J-L, Olivier E. Temporal dissociation between hand shaping and grip force scaling in the anterior intraparietal areas. J Neurosci. 2007;27:3974–3980. doi: 10.1523/JNEUROSCI.0426-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP, Leibovic S, Sanes JN. Organization of the forelimb area in squirrel monkey motor cortex: representation of digit, wrist, and elbow muscles. Exp Brain Res. 1992;89:1–19. doi: 10.1007/BF00228996. [DOI] [PubMed] [Google Scholar]
- Durand J-B, Peeters R, Norman JF, Todd JT, Orban GA. Parietal regions processing visual 3D shape extracted from disparity. NeuroImage. 2009;46:1114–1126. doi: 10.1016/j.neuroimage.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Elsner B, Hommel B. Contiguity and contingency in action-effect learning. Psych Res. 2004;68:138–154. doi: 10.1007/s00426-003-0151-8. [DOI] [PubMed] [Google Scholar]
- Ewert JP. Neuroethology. Berlin: Springer-Verlag; 1980. [Google Scholar]
- Fang PC, Stepniewska I, Kaas JH. Ipsilateral cortical connections of motor, premotor, frontal eye, and posterior parietal fields in a prosimian primate, Otolemur garnetti. J Comp Neurol. 2005;490:305–333. doi: 10.1002/cne.20665. [DOI] [PubMed] [Google Scholar]
- Frey SH. Neurological specializations for processing faces and objects. In: Kaas JH, Preuss TM, editors. Evolution of nervous systems. Vol. 4. Oxford: Elsevier; 2007. pp. 395–406. Primates. [Google Scholar]
- Gharbawie OA, Stepniewska I, Burish MJ, Kaas JH. Thalamocortical connections of functional zones in posterior parietal cortex and frontal cortex motor regions in New World monkeys. Cereb Cortex. 2010;20:2391–2410. doi: 10.1093/cercor/bhp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharbawie OA, Stepniewska I, Kaas JH. Cortical connections of functional zones in posterior parietal cortex and frontal cortex motor regions in New World monkeys. Cereb Cortex. 2011a;21:1981–2002. doi: 10.1093/cercor/bhq260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharbawie OA, Stepniewska I, Qi H-X, Kaas JH. Multiple parietal-frontal pathways mediate grasping in macaque monkeys. J Neurosci. 2011b;31:11660–11677. doi: 10.1523/JNEUROSCI.1777-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould HJ, Cusick CG, Pons TP, Kaas JH. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. J Comp Neurol. 1986;247:297–325. doi: 10.1002/cne.902470303. [DOI] [PubMed] [Google Scholar]
- Graziano M, Aflalo T. Rethinking cortical organization: moving away from discrete areas arranged in hierarchies. Neuroscientist. 2007;13:138–147. doi: 10.1177/1073858406295918. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Atlalo TNS, Cooke PF. Arm movements evoked by electrical stimulation in the motor cortex of monkeys. J Neurophysiol. 2005;94:4209–4223. doi: 10.1152/jn.01303.2004. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Taylor CSR, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002a;34:841–851. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Taylor CSR, Moore T, Cooke DF. The cortical control of movement revisited. Neuron. 2002b;36:349–362. doi: 10.1016/s0896-6273(02)01003-6. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. II. Cortical connections. J Comp Neurol. 1987;265:332–361. doi: 10.1002/cne.902650304. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Reconstructing the organization of the neocortex of the first mammals and subsequent modifications. In: Kaas JH, Krubitzer LA, editors. Evolution of nervous systems. London: Elsevier; 2007. pp. 27–48. [Google Scholar]
- Keating EG, Gooley SG, Pratt SE, Kelsey JE. Removing the superior colliculus silences eye movements normally evoked from stimulation of the parietal and occipital eye fields. Brain Res. 1983;269:145–148. doi: 10.1016/0006-8993(83)90971-x. [DOI] [PubMed] [Google Scholar]
- Kurylo DD, Skavenski AA. Eye movements elicited by electrical stimulation of area PG in the monkey. J Neurophysiol. 1991;65:1243–1253. doi: 10.1152/jn.1991.65.6.1243. [DOI] [PubMed] [Google Scholar]
- Leyton ASF, Sherrington CS. Observations on the excitable cortex of the chimpanzee, orang-utan, and gorilla. Quart J Exp Physiol. 1917;11:137–222. [Google Scholar]
- Orban GA, Claeys K, Nelissen K, Smans R, Sunaert S, Todd JT, Wardak C, Durand J-B, Vanduffel W. Mapping the parietal cortex of human and non-human primates. Neuropsychologia. 2006;44:2647–2667. doi: 10.1016/j.neuropsychologia.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Peeters R, Simone L, Nelissen K, Fabbri-Destro M, Vanduffel W, Rizzolatti G, Orban GA. The representation of tool use in humans and monkeys: common and uniquely human features. J Neurosci. 2009;29:11523–11539. doi: 10.1523/JNEUROSCI.2040-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Boldrey E. Somatic motor and sensory representations in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;37:389–443. [Google Scholar]
- Pinker S. The blank slate. New York: Viking; 2002. [Google Scholar]
- Platt ML, Ghazanfar A. Primate neuroethology. New York: Oxford University Press, Inc; 2010. [Google Scholar]
- Purvis A. A composite estimate of primate phylogeny. Phil Trans R Soc Lond B. 1995;348:405–421. doi: 10.1098/rstb.1995.0078. [DOI] [PubMed] [Google Scholar]
- Qi HX, Jain N, Collins CE, Lyon DC, Kaas JH. Functional organization of motor cortex of adult macaque monkeys is altered by sensory loss in infancy. Proc Natl Acad Sci USA. 2010;107:3192–3197. doi: 10.1073/pnas.0914962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi HX, Lyon DC, Kaas JH. Cortical and thalamic connections of the parietal ventral somatosensory area in marmoset monkeys (Callithrix jacchus) J Comp Neurol. 2002;443:168–182. doi: 10.1002/cne.10113. [DOI] [PubMed] [Google Scholar]
- Qi HX, Stepniewska I, Kaas JH. Reorganization of primary motor cortex in adult macaque monkeys with longstanding amputations. J Neurophysiol. 2000;84:2133–2147. doi: 10.1152/jn.2000.84.4.2133. [DOI] [PubMed] [Google Scholar]
- Randall WL. The behavior of cats (Felis catus) with lesions in the caudal midbrain region. Behavior. 1965;23:107–139. [Google Scholar]
- Rathelot J-A, Strick PL. Muscle representation in the macaque motor cortex: an anatomical perspective. Proc Natl Acad Sci USA. 2006;103:8257–8262. doi: 10.1073/pnas.0602933103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remple MS, Reed JL, Stepniewska I, Kaas JH. The organization of frontoparietal cortex in the tree shrew (Tupaia blangeri): I. Architecture, microelectrode maps and corticospinal connections. J Comp Neurol. 2006;497:133–154. doi: 10.1002/cne.20975. [DOI] [PubMed] [Google Scholar]
- Remple MS, Reed JL, Stepniewska I, Lyon DC, Kaas JH. The organization of frontoparietal cortex in the tree shrew (Tupaia belangeri): II. Connectional evidence for a frontal-posterior parietal network. J Comp Neurol. 2007;501:121–149. doi: 10.1002/cne.21226. [DOI] [PubMed] [Google Scholar]
- Sakata H, Taira M, Murata A, Mine S. Neural mechanisms of visual guidance of hand action in the parietal cortex of the monkey. Cereb Cortex. 1995;5:429–438. doi: 10.1093/cercor/5.5.429. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Constraints on somatotopic organization in the primary motor cortex. J Neurophysiol. 2001;86:2125–2143. doi: 10.1152/jn.2001.86.5.2125. [DOI] [PubMed] [Google Scholar]
- Serano MI, Huang R-S. A human parietal face area contains aligned head-centered visual and tactile maps. Nat Neurosci. 2006;9:1337–1343. doi: 10.1038/nn1777. [DOI] [PubMed] [Google Scholar]
- Shibutani H, Sakata H, Hyvärinen J. Saccade and blinking evoked by microstimulation of the posterior parietal association cortex of the monkey. Exp Brain Res. 1984;55:1–8. doi: 10.1007/BF00240493. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386:167–170. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- Steiper ME, Seiffert ER. Evidence for a convergent slowdown in primate molecular rates and its implications for the timing of early primate evolution. Proc Natl Acad Sci USA. 2012;109:6008–6011. doi: 10.1073/pnas.1119506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Cerkevich CM, Fang PC, Kaas JH. Organization of posterior parietal cortex in galagos: II. Ipsilateral cortical connections of physiologically identified zones within anterior sensorimotor region. J Comp Neurol. 2009a;517:783–807. doi: 10.1002/cne.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Fang PC, Kaas JH. Organization of posterior parietal cortex in galagos: I. Functional zones identified by microstimulation. J Comp Neurol. 2009b;517:765–782. doi: 10.1002/cne.22181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Friedman RM, Gharbawie OA, Cerkevich CM, Roe AW, Kaas JH. Optical imaging in galagos reveals parietal-frontal circuits underlying motor behavior. Proc Natl Acad Sci USA. 2011;108:15033–15034. doi: 10.1073/pnas.1109925108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thier P, Andersen RA. Electrical microstimulation distinguishes distinct saccade-related areas in the posterior parietal cortex. J Neurophysiol. 1998;80:1713–1735. doi: 10.1152/jn.1998.80.4.1713. [DOI] [PubMed] [Google Scholar]
- Vesia M, Prime SL, Yan X, Sergio LE, Crawford JD. Specificity of human parietal saccade and reach regions during transcranial magnetic stimulation. J Neurosci. 2010;30:13053–13065. doi: 10.1523/JNEUROSCI.1644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey CN, Settlage PH, Meyer DR, Sencer W, Hamuy TP, Travis AM. Pattern of localization in precentral and “supplementary” motor areas and their relation to the concept of a premotor area. Assn Res Nerv Ment Dis. 1952;30:238–264. [PubMed] [Google Scholar]
- Wu CW, Bichot NP, Kaas JH. Converging evidence from microstimulation, architecture, and connections for multiple motor areas in the frontal and cingulate cortex of prosimian primates. J Comp Neurol. 2000;423:140–177. doi: 10.1002/1096-9861(20000717)423:1<140::aid-cne12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]