Abstract
Cutaneous leishmaniasis is a major world health problem. Diagnosis is suspected on evocative clinical presentation in patients living in or coming from endemic areas. Several methods have been used. The smear is a simple investigation used in endemic regions. The culture enables to identify the specimen. PCR has a high sensitivity. Montenegro’s reaction is used in the epidemiological study.
Pentavalent antimony derivatives remain the mainstay of systemic treatment. Their efficiency is well established. Their toxicity should be researched. Other treatments can be utilized, such as miltefosine. Local therapy is used in uncomplicated lesions. Injections of the pentavalent antimony derivate, cryotherapy and paromomycin ointmentsis are important options and should be used more frequently in Old World leishmaniasis.
Keywords: antimony, cutaneous leishmaniasis, diagnosis, Glucantime, leishmania, mucosal leishmaniasis, PCR, treatment
Introduction
Leishmaniasis is a disease caused by a heterogeneous group of protozoan parasites that belong to the genus Leishmania and is transmitted by the bite of certain species of sand fly (subfamily Phlebotominae). Two genres transmit Leishmania to humans: Phlebotomus and Lutzomyia.[1] Most species of leishmania cause diseases predominantly for animals and humans are incidentally infected.
Most forms of the disease are transmissible only from animals (zoonotic leishmaniasis) but some can be spread between humans (anthroponotic leishmaniasis). Human infection is caused by more than 20 different species that infect mammals. A single species can produce more than one clinical form of the disease, and each form can be caused by multiple species. The different clinical presentations of the disease depend on which of these species causes the infection and on host-related factors. The skin, mucosa, and mononuclear phagocytic system may be affected giving three forms of leishmaniasis: cutaneous leishmaniasis, mucocutaneous leishmaniasis and visceral leishmaniasis.
Cutaneous leishmaniasis (CL) is endemic in 88 countries, particularly localized in areas of the tropics and subtropics of Africa, in settings ranging from rain forests in America to deserts in western Asia.[2,3] The increase in ecological tourism has extended this problem to developed countries. We distinguish old world and new world CL.[4]
Cutaneous leishmaniasis of the Old World is wide-spread in the Middle East, Mediterranean littoral, Arabian Peninsula, Africa, Near Asia, Indian Subcontinent and other areas.
In the Old World, cutaneous leishmaniasis is due to L. major (zoonotic cutaneous leishmaniasis), L. tropica (anthroponotic cutaneous leishmaniasis), L. aethiopica and some zymodemes of L. infantum.
The aim of this article is to summarize diagnosis methods and recent treatments of Old World cutaneous leishmaniasis.
Clinical manifestations
Initially, an erythematous papule is seen at the site of inoculation, usually uncovered sites: face [Fig. 1], upper limbs, lower extremities. The papule enlarges and breaks, forming a painless ulcer with a well-demarcated raised border, making 0,5 to 10 cm diameter. A depressed scar is the final result after healing, which constitutes the main problem of this disease.
Figure 1.
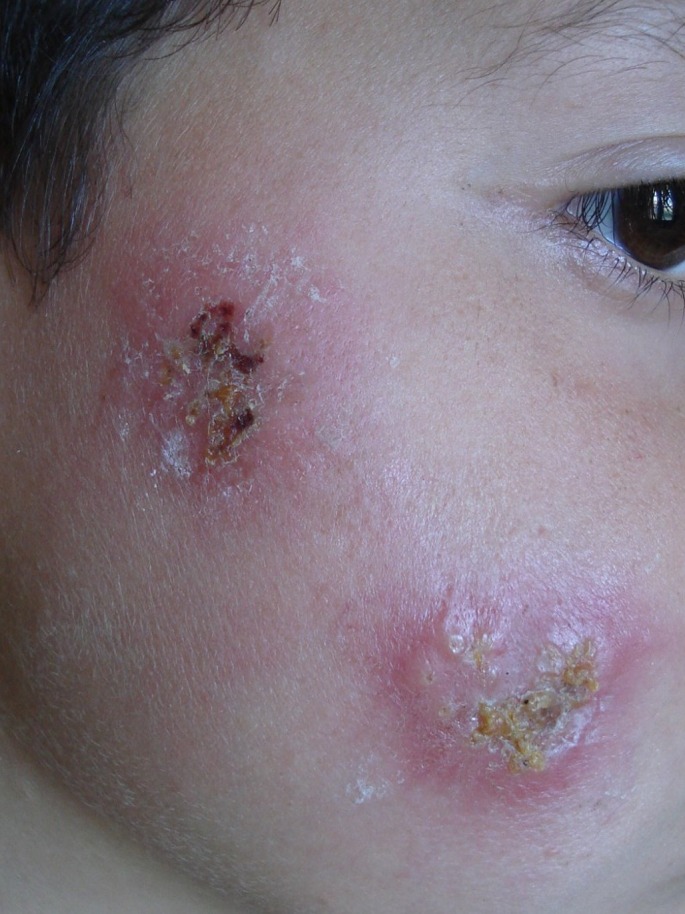
Cutaneous leishmaniasis of the face in children.
The incubation period is from several months to over a year. Lesions are few (fewer than three in major cases). The lesions tend to resolve within 2-4months inmajority of cases.
The rural form of leishmaniasis is due to L. major infection. The incubation period is usually 1-4 weeks and rarely surpasses 2 months. Multiple ulcero-crusted nodules [Fig. 2] may be found and lesions resolve after a period ranging from 3 months to 2 years.
Figure 2.
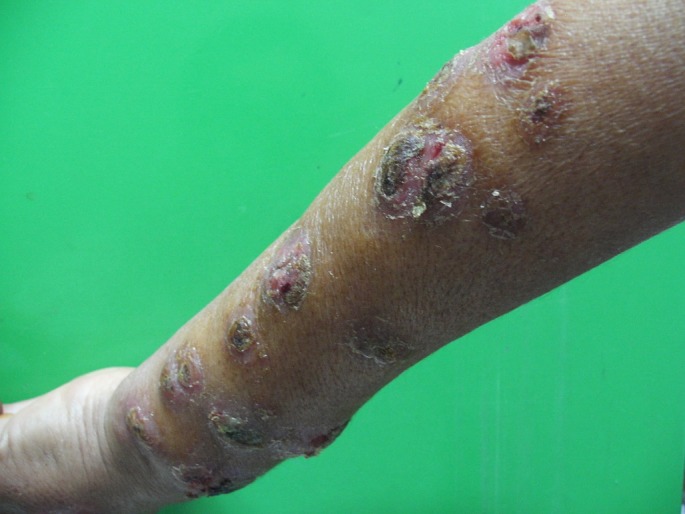
Multiple lesion of cutaneous leishmaniasis.
CL due to L. aethiopica is characterized by a slow development, late ulceration, and delayed healing requiring 1-3 years. It may result in a global cutaneous involvement leading to diffuse cutaneous leishmaniasis. L. infantum causes papules and nodules with little ulceration that recover slowly.
In some cases, L. tropica infection, a particular form can be observed such as a leishmaniasis recidiva cutis or leishmaniasis recidivans.
In HIV-infected patients, leishmania coinfection may cause post kala azar dermal leishmaniasis.
A clinical polymorphism is observed. In fact, beside ulcero- crusted typical form, unusual clinical forms were reported: lupoid leishmaniasis [Fig. 3] miming lupus vulgaris lesions, erysipeloid form with an atypical inflammatory presentation, verrucous or xanthomatous nodular lesions particularly found in diffuse CL, sporotrichoid lymphatic spread lesions, psoriasiform, zosteriform, linear, mycetomatous, squamous cell carcinoma-like and eczematous morphologies. This clinical polymorphism shows that the clinical diagnosis is not usually evident and other pathologies should be distinguished.
Figure 3.
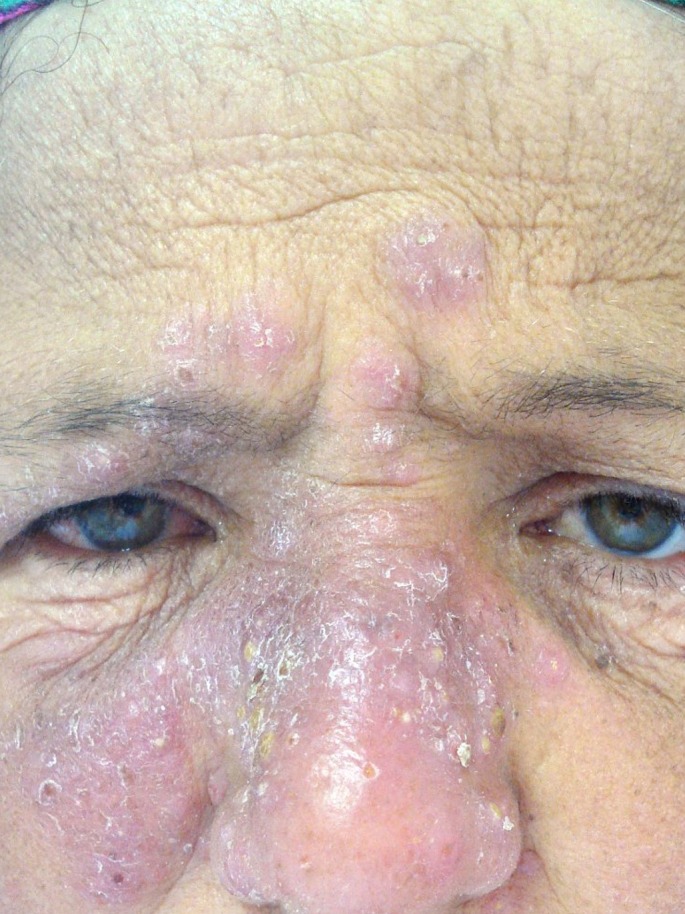
Lupoid cutaneous leishmaniasis.
Diffuse cutaneous leishmaniasis is characterized by the presence of nodular lesions that do not ulcerate. It is observed in South America, Central America and Ethiopia.
Atypical manifestations of cutaneous leishmaniasis occur in AIDS patients. The recurrence of skin lesions at the site of old scars has been reported years after the resolution of the primary infection in patients which have immunodeficiency. The clinical presentation is often severe and the current treatment is rarely effective.[5]
Diagnostic methods
CL diagnosis is suspected on evocative clinical presentations in patients living in or coming from endemic areas. Those manifestations are not specific; many other diseases such as Virchow’s hanseniasis, paracoccidioidomycosis, tropical ulcer, syphilis, cutaneous tuberculosis, atypical mycobacteriosis, cutaneous sporotrichosis must be distinguished from ulcerated or non ulcerated CL.
Therefore, the diagnosis is achieved through an association of clinical, epidemiological and laboratory characteristics. Several methods have been used for the diagnosis of leishmaniasis including direct investigation and serological tests.[6,7,8]
Smear
Parasite isolation is performed on material obtained from scratches from the lesion margins, using a sterile surgical blade (vaccinostyle, scalpel). In case of non ulcerated lesions (nodular, sporotrichoid, lupoid), aspirated punctures are performed using disposable syringes containing 0.3 to 0.5 ml of sterile saline solution.
Smears are bleached using May-Grünwald-Giemsa stain to identify amastigotes forms by means of optical microscopy with sensitivity rate ranging from 64%[9] to 80%[10] depending on technique quality. The specificity of the dermal smear is excellent (100%).
Culture
The parasite can be isolated in NNN/Schneider medium (incubation at 28°C) from a tissue fragment removed from the border of an active lesion. Culture usually requires 3 to 10 days to grow and sometimes more with some leishmania New World species. Specimen should not be discarded unless they are negative for 4 weeks. Positivity of the culture varies depending on presence of amastigotes in the smear. Specificity is about 100%, but sensitivity rate is in challenge (40% in our experience, 84% according to Faber et al (2003).[9]
PCR
The characterization of the leishmania species is based on biochemical criteria (electrophoresis of isoenzymes) or genetic criteria using various molecular methods including PCR and the monoclonal antibody technique with specific panel. Those techniques are only used in sophisticated centers because of their high cost. Besides diagnostic and prognosis purposes, the identification of parasite species allows a better understanding of leishmaniasis epidemiology.
PCR is only used in sophisticated centers because of their technique and their high cost. It does not require previous isolation procedures which are especially difficult under field conditions or in laboratories in disease-endemic areas where technical resources are poor.[11,12] Based on distinct loci of kinetoplast kDNA (mitochondrion genome) or nuclear DNA such as rDNA, mini-exon, β-tubulin, gp63, G6PD, cytochrome b[12], it shows species identification and parasites quantification. In an Iranian study, PCR-RFLP (restriction fragment length polymorphism) assay with serosity materials punctured from CL patients using Hae III enzyme is useful for the rapid identification of Leishmania species.[11] This technique offers the best sensitivity and specificity rates (98.8% nd 100% respectively in a prospective Iranian study 2008),[13] more likely with the ITS1 PCR (using the internal transcribed spacer region, located between the 18S and 5.8S rRNA genes) in comparison with kDNA PCR according to Bensoussan et al (Israel, 2006).[14]
The combination of parasitological techniques is slightly more sensitive (100%) than the PCR assay (98.8%) in detection of Old World CL.[13]
Histology
Skin biopsies should be taken from the margin of the lesion. Although a 4 mm punch may be used, the elliptical biopsy taken with a scalpel is preferred. Histopathologic analysis of infected tissues stained with hematoxylin-eosin allows a diagnostic confirmation of the disease in most cases. The histopathological presentation of CL shows a great variability, but a predominant pattern characterized by the presence of unorganized granuloma without necrosis. The leishmania organisms are typically intensely blue with Giemsa stain. The Leishman-Donovan bodies, that are 2 to 4 μm in diameter and round or oval are usually seen in macrophages, but mat be also present in the extracellular areas.[15]
Different histological features may be found. According to a prospective study conducted in Saudi Arabia in 2005,[16] four distinct groups may be individualized. Type A, where macrophages are heavily parasitized and vacuolated with few lymphocytes, correlates with an early immune response or an anergic diffuse CL. Type B and C consist in a mixed inflammatory response with or without necrosis and present the most common types in Old World CL. Finally, type D, characterized by a tuberculoid granuloma with absent or low parasite load, correlates with chronic forms such as lupoid leishmaniasis or the end stage of spontaneous healing.
Immunological tests
Montenegro’s reaction
The main classical serological test is Montenegro’s reaction. This test reveals leishmania infection and therefore it is used in epidemiological studies to determine infection. This test consists in intradermic inoculation of 0.1 ml of the antigen (leishmania) into the anterior face of the forearm. The skin test is read after 48 or 72 hours, and induration equal to or more than 5 mm is considered positive. Habitually, the positivity is detected after 4 months of the appearance of the lesions. Although this test has an excellent sensitivity rate of around 90% in cases of New World leishmaniasis,[8] it is quietly useful in detection of Old World leishmania infections. False negativity may occur with diffuse anergic CL and with CL of less than 1 month duration. Moreover, skin test may show false positive results among individuals from endemic areas because of the occurrence of subclinical infections, or in case of past infection.
This test can be useful for the diagnosis of travelers living in nonendemic areas.
Serological diagnosis
More commonly serodiagnosis is indirect immunofluorescence (IIF) and ELISA. Those serological tests were not a routine procedure for the diagnosis of CL in old world due to the lower sensitivity of the tests and across reactivity with other infections. In fact, theses tests may present limitations such as undetectable or low titer of antibody, absence of correlation between circulating antibody levels and the disease stage, and the existence of cross-reactions with other species: false-positive results are found in patients with trypanosomosis, toxoplasmosis or paracoccidioidomycosis and even healthy individuals.[17] Those limitations lead to find out immunological approaches which allow the detection of antiLeishmania antibodies, like flow cytometry, western blot test, Enzyme immunoassay using Leishmania antigens.[8,17] Actually, IIF does not belong to diagnostic arsenal of CL.
Table I summarizes the sensibility and specificity of different investigations for cutaneous leishmaniasis and mucocutaneous leishmaniasis.
Table 1. Diagnostic tests in cutaneous leishmaniasis.
| Technique | Sensitivity | Specificity |
|---|---|---|
| Smear | 64% to 80% | 100% |
| Culture | 40% to 84% | 100% |
| PCR | 98.8% in CL 47.4% to 83.3% in ML |
100% |
| Histopathology | 68% | - |
| Montenegro’s reaction | 90% | - |
Treatment
CL is not a severe disease; however it may be hardly tolerated by patients because of three reasons: first, its particular localization in uncovered areas which makes an esthetic bother for the patient especially when it lies on the face. Second, its spontaneous evolution is long (some lesions may require years to heal). Finally, the anesthetic scar that may be caused particularly if it was not well treated. For those reasons, therapeutic abstention is rarely admitted, despite the high rate of spontaneous recovery specially noted with Old World CL.
The treatment depends on the causative leishmania; however, specie identification is fastidious, time-consuming and not always available. Then, the choice of the drug should be inferred from the geographic setting of the patient, epidemiological data of leishmania distribution as well as the clinical courses.
Several means have been used and some of them for more than 50 years. Recently, the development of new therapeutic procedures has been one of the most interesting sectors of human and veterinarian medicine. The main target of new drugs is to fight the parasite in its different aspects: physiologic and biochemical, and to promote the immune response of the host.
Until now, there has been no medication that seems to be effective, little expensive, easy to manipulate and without any side effects at the same time.
The main particularity that interferes with the evaluation of the efficiency of one or another treatment is the spontaneous healing tendency of CL: this leads to a perpetual question: whether the recovery was spontaneous or due to the drug used for treated patients? The response requires more controlled studies, comparing tested treatment to placebo or comparing drugs in order to obtain valid results. In fact, in literature, there are not many controlled studies.
Systemic treatments
Pentavalent antimony derivatives (PAD)
Currently there is meglumine antimoniate (Glucantime®), used preferentially by the francophone and stibogluconate (Pentostam®), which is preferred by the anglophone.
Meglumine antimoniate is prescribed in one injection per day through intramuscular shut however stibogluconate requires a slow intravenous injection. WHO recommendations are 20 mg pentavalent antimony/kg/day (10 mg/kg/day in enfant) with an unlimited daily dose for 20 days and for 30 days for patients with mucous involvement.[18] The effective dose should be gradually reached: ¼ the first day, ½ the second day, ¾ the third day and the full dose since the fourth day. In our experience, we perceive ½ the dose on the first day and a full dose on the second day.
The action of this molecule is not clearly identified: it may interfere with parasite enzymes implicated in DNA synthesis.
Several adverse effects have been reported. Stibogluconate-intolerance symptoms including fever, arthralgias, myalgias, rush, digestive troubles, which occur at the onset of the medication, are dose-independent and do not induce to stop the treatment. In contrast, stibio-toxicity manifestations (cardio- toxicity, pancreatitis, hepatic troubles, pancytopenia, and rarely, renal toxicity) which are often serious, depend on the dose and occur by the second week (the fact that casts doubt the interest of gradual doses in the beginning of the treatment).[19] Thus, contra-indications such as pregnancy, hepatic, cardiac, renal failure must be considered and close supervision must be conducted according to the diagram proposed by Morizot et al [Table II].[20]
Table 2. Criteria to discontinue treatment with pentavalent antimony.
| 20 mg of pentavalent antimony /kg/day x 10-20 days biologic surveillance | ||||
| Day | day 3 | day 7 | day 14 | day 20 |
| ECG NFS Transaminases Amylase Lipase |
Amylase Lipase |
ECG NFS Transaminases Amylase Lipase |
ECG NFS Transaminases Amylase Lipase |
ECG NFS Transaminases Amylase Lipase |
|
Criteria to stop (arbitrary and purely indicative criteria): ECG: prolongation of the QT interval, T wave inversion, NFS: thrombopenia < 35000/mm3, leucopenia < 1500/mm3 Transaminases: increasing > 5N Amylase: >3N after 72h, >5N later Lipase: >10N after 72h, >15N later | ||||
PAD have variable success rates in the treatment of Old World CL while Tunisian retrospective study showed a 75% recovery (1999-2006).[19] An Algerian controlled study (1986) found no significant difference in response rate between Placebo (48%) and intramuscular meglumine antimoniate (55%).[21]
Drug resistance has been reported with PAD: it may be a primary resistance, or may be due to sub therapeutic doses of the medication or treatment shorter than recommended.[4]
Pentamidine
Pentamidine has been used in VL treatment for years. Pentamidine isothionate (Pentacarinat®) is actually used in different protocols to heal CL. The most common treatment is the intramuscular injection of 2-4 mg/kg Pentamidine-base (< 240 mg) each 2 or 3 days for weeks until recovery.
Pentamidine interferes with the DNA synthesis of the parasite leading to its death. This drug seems to be more acceptable than PAD (short time treatment, shorter hospitalization, less expensive). However, several side effects have been noted: hypertension, tachycardia, digestive troubles, skin problems, elevated muscles enzymes, renal failure, and pancreatic toxicity limiting the use of the drug as a first choice in CL.
Pentamidine has proved its efficiency in the treatment of CL in the Old World; it seems to be more interesting with American leishmaniasis.
Medication failure depends mainly on the timing of the treatment and the withdrawal of patients before achieving the therapy.[18]
Metronidazol
Metronidazol or Flagyl® has been used for years for the treatment of CL with a dose of 1,5 g/day for adult and 25 mg/kg/day for children during 15-30 days. This drug is not known as a leishmanicide, however, many studies have shown that it may be considered as a therapeutic alternative against Old World CL leading to 66% recovery in Tunisian retrospective series.[23,24] Considering the self-limiting aspect of L. tropica and L. major, such low rate may not be significant and there is a need for more controlled studies to confirm the real contribution of metronidazol in CL healing.
Amphotericin B
Since it was tested in 1959, amphotericin B has been used for the treatment of CL. Two variants are available: Fungisone® prescribed with the dose of 1 mg/kg/day each 2 days in IV perfusion of glucose serum (< 50 mg/day for adult; < 25 mg/day for children) and liposomal amphotericin B (Ambiosome®, Amphocil®) used with a total dose of 18 mg/kg in IV injections during 10 days. The latter variant is more widely applied due to its presumed higher safety profile.
Amphotericin B is active on OWL species in vitro; no controlled study confirms the efficiency of the drug in the treatment of the disease.[1] The high cost and the nephrotoxicity limit the prescription of Amphotericin B in endemic regions. It should be utilized in the treatment of resistant CL.
Azoles
As for amphotericin B, some azole antifungal drugs may be successfully used in the treatment of old world cutaneous leishmaniasis. Many molecules are tested: ketoconzole (200-600 mg/day for 28 days); fluconazole (200 mg/day for 6 weeks); itraconazole (100-400 mg/day for 6-8 weeks). These drugs interfere with the parasite cell membrane biosynthesis. Their efficiency varies between studies. Its use is limited by the hepatic toxicity that it may cause. Itraconazole has a cure rate of 60-70% when a lesion is due to L. tropica (Inde, 1990)[27] or L. major (Kuwait, 1991),[28] especially for the child (where it is more tolerated than PAD);[29] In a recent study, a case of cutaneous sporotrichoid leishmaniasis unresponsive to intralesional pentavalent antimonial therapy, is completely resolved after treatment with oral itraconazole.[30]
Fluconazole (200 mg/day for 6 weeks) was tested in a double blind essay in Iran (2002)[31] to treat CL caused by L. major: 79% healing were reported versus 34% in the placebo group. Its use is limited because of its high cost.
Miltefosine
Miltefosine (hexadecylphosphocholine) is an oral antitumor agent discovered in 1980 and it was rapidly abandoned because of its hematologic toxicity. In 1992, it was tried as an antileishmanial drug with good results. Since then, miltefosine (50-100 mg/kg/day for 28 days in oral use) has been prescribed in CL. It is well tolerated despite the few adverse effects that it may generate (digestive troubles, increasing concenteration of transaminases and creatinine, dizzy spell, abortion in pregnant women). Miltefosine was used in an Iranian randomized trial to treat CL due to L. major, the cure rate reached 90%.[31]
Other systemic drugs
Allopurinol, a xanthine oxydase inhibitor has been demonstrated to be an effective treatment for CL in Asia (74% recovery). For Iranian patients infected with L. major, the addition of allopurinol reduced the antimoine dosage to the half to achieve the same efficiency.
Doxycycline seems beneficial in the Old World CL. Used with a dose of 200 mg/day during 15-30 days; it allowed healing of 71% of patients in a non controlled Tunisian study. The main side effect here is phototoxicity.[32]
Azithromycin seems to be interesting for children in endemic areas thanks to its excellent tolerance.[33] However, in Iranian prospective study, the efficiency of azithromycin was compared with Glucantime in treatment of Old World leishmaniasis. Among 49 patients, 22 received 500 mg/day azithromycin for 5 days/month. Treatment cycles were repeated monthly to a maximum of 4 months; 27 patients received 60 mg/kg intramuscular meglumine antimoniate for 20 days. Azithromycin was determined to be not as effective as Glucantime in treatment of Old World CL.[34]
Rifampicine has shown a variable success rate on old world CL ranging from 0% to 80%.[35]
Oral zinc sulfate seems to be efficient in old world cutaneous leishmaniasis particularly in Iraqi patients.[18]
Other molecules have been tested with controversial results: antimalaric substances, avlosulfon, sulfamethoxazole-trimethoprim, bleomycine, terbinafine, and systemic paromomycin.
Local treatment
Intralesionnel injection of pentavalent antimony derivates
Local infiltration with PAD has been used in the old world localized CL after considering contra indications such as lymphatic invasion, peri-cartilaginous (ears, nose), peri-articular or peri-orificial sites. WHO recommends an injection of 1-3 ml under the edges of the lesion. The infiltration could be given every 5-7 days, for a total of 2-5 times.[36] The injection is useful for the early non inflamed lesions.
Some side effects were reported: infectious complications and stibio-intolerance particularly described in case of cephalic lesion. A sporotrichoid feature was noted after PAD infiltration of lesions situated on the limbs.[37,38]
Cure rate ranges from 60% (Tunisia) to 75% (Iran).[39] The number of infiltration almost 2 for L. major, little more for L. tropica. Relapses are rare if the treatment is well conducted.
One Tunisian retrospective analysis (2004) was carried out on affected children (L. major and L. tropica); 63% of patients received meglumine antimoniate intralesionnel: one local injection (1 ml/cm2) per week until recovery. This treatment is well tolerated with no side-effects.[40]
Cryotherapy
The sensitivity of the parasite to low temperatures was discovered for many years.
Cryotherapy has been only used in the old world CL particularly in small lesions. The procedure consists in a weekly regimen of liquid nitrogen applications using a cryospray (twice per session, 15-20 second freezing time, with a thaw of 1 minute) on non ulcerated lesions. Side effects include hypopigmentation noted in 68% in Turkey, but repigmentation occurred within 2-3 months.[41] New satellite lesions could be developed by some patients.
The efficiency of cryotherapy is confirmed in many studies. In fact, approximately 84% of the CL lesions were cured after 1 to 4 sessions in a Jordanian study (the remaining lesions (16%) were cured after an additional 1 to 3 session( s)),[42] 90% healing when cryotherapy is associated with Glucantime® infiltrations in an Iranian comparative study.[43]
Paromomycin (aminosidine) ointments
Paromomycin belongs to the aminoglycoside family of antibiotics. Two topical preparations are available for cutaneous leishmaniasis: 15% paromomycin sulphate dissolved in a soft white paraffin base, either with 12% methyl-benzothenium chloride or with 10% urea.[18] Side effects are rare and include itching erythema, edema, and tenderness.[35]
In L. major CL, the use of two ointments per day for 10-30 days leads to a recovery of about 80% of cases.[44] The efficiency of the regimen is less spectacular with L. tropica.[45]
Actually, new molecules are being tested: topical WR279,396 (paramomycin and gentamycin in a hydrophilic base) leading to more than 90% healing,[46] and paromomycin loaded liposomes.
Imiquimod
Imiquimod (Aldaras®) an imidazoquinoline amine is used in HPV-induced skin diseases, genital warts or premalignant conditions. Imiquimod 5% cream is applied in a thin layer every day for 20 days on CL lesion. The main problem with this treatment is its high cost which limits its use in such benign disease in poor countries.[48]
However, the topic is considered useless against Old World leishmania species according to Seeberger et al in a placebo-controlled prospective study (Syria, 2003).[49]
Thermotherapy
Experimental tests showed the efficiency of heat on the dermotrope parasite. Localized controlled heat (39-40°C) can be obtained thanks to devices like Lucas-championniere’s, Vapozone, or by the use of high energy waves. The procedure is painful and requires local anesthetic.[18]
In the Old World cutaneous leishmaniasis, a study conducted in Afghanistan showed the same previous cure rate with L. tropica.[51] In addition, 26 cases of CL due to L. major were successfully treated by local heat in Iraq.[52]
CO2 laser
CO2 Laser has been used on CL in Middle East (L. tropica and L. major). In an Iranian study, 53 a power of 30 watt and a pulse width of 0.5-5 s, until the ulcer bed turned brown and the hemostasis was performed, provided 94% healing of lesion caused by L. major. Lesions were previously anesthetized by local injection of 1-2% lidocaine. Recovery was obtained in 1 month versus 3 months with antimony. Another Iranian case report confirms the same result with L. tropica.[18]
Dynamic phototherapy
Photo-dynamic therapy is considered as on optional treatment of CL still under experimentation. After application of Δ amino-levulinic acid topic, a weekly regimen is conducted until the dermal smear turns negative.
Except local irritation and some scars, the technique is well tolerated.
In Iran, the photo-dynamic therapy was used to heal 5 L. major infected patients in 4 weeks without relapses.[54]
Electrotherapy
The parasiticidal effect of electricity on L. major, both in vitro and in vivo was confirmed. At 3 V, however, 3 weeks of electrotherapy in mice, for 10 min twice weekly, initially appeared to cure all the lesions and the therapy was then halted.[55]
In Iraq, the use of electric current seems promising as an antileishmanian agent.[56] Treatment by the Baghdadin device consisted of weekly sessions of 10 minutes of direct current electrical stimulation. The intensity of the direct current ranged between 5 and 15 milliamperes, and the voltage was kept below 40 volts. Among the 146 lesions, 135 (92.5%) showed total clearance or marked improvement in 4-6 weeks time. Approximately 67% of the lesions needed only one or two sessions.
Phytotherapy
Phytotherapy seems to be promising as a safety weapon against Leishmania. Thymus vulgaris, Achillea millefolium and propolis extracts were effective for the treatment of cutaneous leishmaniasis formice in a controlled study (Iran, 2008).[57]
In a Turkish study, the ethanolic, water and n-hexane extracts from the leaves of Arbutus unedo were tested in vitro against Leishmania tropica promastigotes. Those products were found to be more effective than the other extracts.[58] In Tunisia, vegetable oils (green argyle) were used with no response according to our experience (Tunisia, 2006).[59]
Others
In case of an isolated lesion, cryo-surgery or surgical excision may be indicated.
Immunotherapy
Leishmania infection usually induces a life-long immunological memory and protection against further infections. This infection may require generation of Th1 response represented by production of interferon (IFN)-γ in the absence of Th2 response, which is associated with interleukin (IL)- 4 or IL-10. Most Leishmania are easily cultured, and the production of vaccine using the parasite or its components is feasible.
Immunotherapy with a combined vaccine containing heatkilled Leishmania promastigotes and bacille Calmette-Guerin (BCG).
In a recent Iranian study,[60] the immune responses induced against Leishmania antigens in volunteers were studied. These volunteers were vaccinated in a double-blind, randomized field efficacy trial of a preparation of autoclaved Leishmania major mixed with a low dose of Bacille Calmette- Guerin vaccine (BCG). These volunteers developed either a cutaneous leishmaniasis (CL) lesion due to exposure to infected sandfly bite(s) or did not develop a lesion during the course of the trial were studied and compared with those of non-vaccinated controls. The results indicated that volunteers who developed CL in the vaccine arm showed a slightly higher in vitro proliferative response than cases who received BCG alone.[60]
Vaccination of C57BL/6 mice with live Leishmania major plus CpG DNA (Lm/CpG) prevents lesion development and provides long-term immunity.
Cytokines
Many cytokines are implicated in the immune response in case of CL. Basing on this fact; many studies especially in south America were conducted in order to show whether the use of such molecules as a treatment is useful or not.[61]
Used in association with Antimony, gamma interferon IFN-γ increases the immune response in CL cases allowing shorter treatment and more healing according to a controlled essay.[62]
IL-2 recombinant, used both as a systemic drug (3 cases report of diffuse CL, Akuffo et al. [63]) and in local application (Barral-Netto et al.[64]), seems to be efficiency. Recombinant Interleukin 12 cures mice infected with L. major (Heinzel, F.P et al).[65]
In an experimental study realized in mice, Leishmania major-infected IL-1α/β(-/-) mice were resistant to experimental CL compared to controls.[66]
A recent trial carried by Haruko Ota et al. showed that pretreatment of macrophages with the combination of IFN-γ and IL-12 induces resistance to L. major at the early phase of the infection.[67]
Immune prophylaxis
The development of a vaccine against CL which was one of the interesting ideas especially in endemic areas was tried for several years but with no evident results.
Leishmanization
It was noted that infra clinic repeated infestations by the parasite provides hosts in endemic areas with a premonition state against autochthones species, leading to a limited number and size of the lesion. Then, localized inoculation of low quantity of parasite has been used in middle and extreme east for several years.
The main limiting particularities of this technique are the possibility to develop a lesion similar to that which may occur in real infestation with the same indelible scar, and the restrained efficiency only on Leishmania species used in the vaccine. A case of dermatofibrosarcoma protuberans occurring in the site of prior leishmanization is reported.[68] Actually, this technique is forbidden by the WHO because of the virulence of some species.
Experimental vaccination
Irradied or mutant parasites were successfully used to prevent L. major infection for mice. More recently, essays using purified parasite antigens were conducted on mice (fraction 94-67 KDa, glucoproteine 63, glucoproteine 46, lipophosphoglycanes, cysteine proteinase,[69] Toll-like receptors)[70] showing a significant protection rate against both Old and New World species.
The treatment of the scare
The LC is a disease which can leave unaesthetic scars causing psychological damages. The scars can be pigmented, hypochromic, atrophic or hypertrophic. Some patients should be treated by topical depigmentant. Sometimes, in the grave cases, a psychological treatment is necessary.
Therapeutic strategy
Through our study we propose the following therapeutic strategy for old world cutaneous leishmaniasis:
A therapeutic abstention will be indicated in the case of simple and localized LC of a quick and a favorable evolution,
A local treatment will be indicated in case of simple hurts. It is based on Glucantime® on intralesional injection, and the cryotherapy or the local paromomycine. Intralesional injections should be avoided around the face and periorificial regions. This treatment allows to reduce the evolution duration and to ameliorate the scar quality,
Oral systematic treatment should be indicated in case of cutaneous multiple and not complicated leishmaniasis.
In the complicated forms (extended, recidivates, resistant for oral treatment), in facial localization, peri articular, peri orificiel: meglumine antimoniate intramuscular would be the treatment of choice. This old treatment proved its efficiency but it is toxic and its high cost have caused a limitation in its use.
For children, the local treatment should be indicated such as cryotherapy.
Prevention
The prevention is difficult because of the quantity and of the way of functioning of homes, big variety of the reservoirs and vectors.
The preventive treatment is collective and individual and it is based on: the eradication of the vector, the eradication of the reservoir and the protection against the bite of sand fly.
The eradication of the vector is based on:
the use of powerful insecticides,
the destruction of the localization of phlebotomus,
the vast plowing of lands around the human setting,
the digging of wide irrigation channels.
The eradication of the reservoir:
the diagnosis and the treatment of the affected humans,
killing the infected dogs in endemic zones.
The protection against the bite of sand fly:
The majority of the recommended precautionary measures aim at reducing the contact with phlebotomes.We should avoid outdoor activities between twilight and the dawn, a period during which phlebotomes are most active. For more protection, we recommend to wear defender clothes and to use an insecticide on the uncovered skin and on the edges of the clothes. To reduce the contact with phlebotomes, mechanical means can be used, for example: installing mosquito nets with very fine stitches around beds, doors and windows.
Conclusion
The diagnosis of Old World cutaneous leishmaniasis is based on the smear which is a simple investigation and remains used in endemic regions; the culture enables to identify the specimen. PCR has a high sensibility. A local treatment such as cryotherapy should be indicated in case of simple hurts. The systemic treatment is indicated in multiple and complicated infections.
References
- Goto H, Lindoso JA. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev Anti Infect Ther. 2010;8:419–433. doi: 10.1586/eri.10.19. [DOI] [PubMed] [Google Scholar]
- Postigo JA. Leishmaniasis in the World Health Organization Eastern Mediterranean Region. Int J Antimicrob Agents. 2010;36 Suppl 1:S62–65. doi: 10.1016/j.ijantimicag.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Clem A. A current perspective on leishmaniasis. J Glob Infect Dis. 2010;2:124–126. doi: 10.4103/0974-777X.62863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuon FF, Amato VS, Graf ME, Siqueira AM, Nicodemo AC, Amato Neto V. Treatment of New World cutaneous leishmaniasis--a systematic review with a meta-analysis. Int J Dermatol. 2008;47:109–124. doi: 10.1111/j.1365-4632.2008.03417.x. [DOI] [PubMed] [Google Scholar]
- Chaudhary RG, Bilimoria FE, Katare SK. Diffuse cutaneous leishmaniasis: co-infection with human immunodeficiency virus (HIV) Indian J Dermatol Venereol Leprol. 2008;74:641–643. doi: 10.4103/0378-6323.45111. [DOI] [PubMed] [Google Scholar]
- Guddo F, Gallo E, Cillari E, La Rocca AM, Moceo P, Leslie K, Colby T, Rizzo AG. Detection of Leishmania infantum kinetoplast DNA in laryngeal tissue from an immunocompetent patient. Hum Pathol. 2005;36:1140–1142. doi: 10.1016/j.humpath.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Lessa MM, Lessa HA, Castro TW, Oliveira A, Scherifer A, Machado P, Carvalho EM. Mucosal leishmaniasis: epidemiological and clinical aspects. Braz J Otorhinolaryngol. 2007;73:843–847. doi: 10.1016/S1808-8694(15)31181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis Lde C, Brito ME, Almeida EL, Félix SM, Medeiros AC, Silva CJ, Pereira VR. Clinical, epidemiological and laboratory aspects of patients with American cutaneous leishmaniasis in the State of Pernambuco. Rev Soc Bras Med Trop. 2008;41:439–443. doi: 10.1590/s0037-86822008000500001. [DOI] [PubMed] [Google Scholar]
- Faber WR, Oskam L, van Gool T, Kroon NC, Knegt-Junk KJ, Hofwegen H, van der Wal AC, Kager PA. Value of diagnostic techniques for cutaneous leishmaniasis. J Am Acad Dermatol. 2003;49:70–74. doi: 10.1067/mjd.2003.492. [DOI] [PubMed] [Google Scholar]
- Chargui N, Bastien P, Kallel K, Haouas N, Akrout FM, Masmoudi A, Zili J, Chaker E, Othman AD, Azaiez R, Crobu L, Mezhoud H, Babba H. Usefulness of PCR in the diagnosis of cutaneous leishmaniasis in Tunisia. Trans R Soc Trop Med Hyg. 2005;99:762–768. doi: 10.1016/j.trstmh.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Hajjaran H, Vasigheh F, Mohebali M, Rezaei S, Mamishi S, Charedar S. Direct diagnosis of Leishmania species on serosity materials punctured from cutaneous leishmaniasis patients using PCR-RFLP. J Clin Lab Anal. 2011;25:20–24. doi: 10.1002/jcla.20377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulet F, Botterel F, Buffet P, Morizot G, Rivollet D, Deniau M, Pratlong F, Costa JM, Bretagne S. Detection and identification of Leishmania species from clinical specimens by using a real-time PCR assay and sequencing of the cytochrome B gene. J Clin Microbiol. 2007;45:2110–2115. doi: 10.1128/JCM.02555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi F, Shahabi S, Kazemi B, Mohebali M, Abadi AR, Zare Z. Evaluation of PCR assay in diagnosis and identification of cutaneous leishmaniasis: a comparison with the parasitological methods. Parasitol Res. 2008;103:1159–1162. doi: 10.1007/s00436-008-1111-4. [DOI] [PubMed] [Google Scholar]
- Bensoussan E, Nasereddin A, Jonas F, Schnur LF, Jaffe CL. Comparison of PCR assays for diagnosis of cutaneous leishmaniasis. J Clin Microbiol. 2006;44:1435–1439. doi: 10.1128/JCM.44.4.1435-1439.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Narvaez FJ, Medina-Peralta S, Vargas-Gonzalez A, Canto-Lara SB, Estrada-Parra S. The histopathology of cutaneous leishmaniasis due to Leishmania (Leishmania) mexicana in the Yucatan peninsula, Mexico. Rev Inst Med Trop Sao Paulo. 2005;47:191–194. doi: 10.1590/s0036-46652005000400003. [DOI] [PubMed] [Google Scholar]
- Uthman MA, Satir AA, Tabbara KS. Clinical and histopathological features of zoonotic cutaneous leishmaniasis in Saudi Arabia. J Eur Acad Dermatol Venereol. 2005;19:431–436. doi: 10.1111/j.1468-3083.2005.01210.x. [DOI] [PubMed] [Google Scholar]
- Vidigal Cde P, Marcussi VM, Marcussi LM, Mikcha JM, Arraes SM, Lonardoni MV, Silveira TG. Enzyme immunoassay using Leishmania (Viannia) braziliensis antigens for laboratorial diagnosis of American cutaneous leishmaniasis. Acta Trop. 2008;107:208–212. doi: 10.1016/j.actatropica.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Minodier P, Parola P. Cutaneous leishmaniasis treatment. Travel Med Infect Dis. 2007;5:150–158. doi: 10.1016/j.tmaid.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Masmoudi A, Maalej N, Mseddi M, Souissi A, Turki H, Boudaya S, Bouassida S, Zahaf A. Glucantime injection: benefit versus toxicity. Med Mal Infect. 2005;35:42–45. doi: 10.1016/j.medmal.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Morizot G, Consign PH, Buffet PH. Leishmaniose cutanée: prise en charge. Réalité thérapeutique en dermato-vénérologie. 2005;152:5–8. [Google Scholar]
- Belazzoug S, Neal RA. Failure of meglumine antimoniate to cure cutaneous lesions due to Leishmania major in Algeria. Trans R Soc Trop Med Hyg. 1986;80:670–671. doi: 10.1016/0035-9203(86)90176-8. [DOI] [PubMed] [Google Scholar]
- Lai A Fat EJ, Vrede MA, Soetosenojo RM, Lai A Fat RF. Pentamidine, the drug of choice for the treatment of cutaneous leishmaniasis in Surinam. Int J Dermatol. 2002;41:796–800. doi: 10.1046/j.1365-4362.2002.01633.x. [DOI] [PubMed] [Google Scholar]
- Masmoudi A, Dammak A, Bouassida S, Elleuch N, Akrout F, Turki H, Zahaf A. Interest of metronidazole in the treatment of cutaneous leishmaniasis. Therapie. 2007;62:68–69. doi: 10.2515/therapie:2006088. [DOI] [PubMed] [Google Scholar]
- Belhadjali H, Elhani I, Youssef M, Babba H, Zili J. Cutaneous leishmaniasis treatment by metronidazole: study of 30 cases. Presse Med. 2009;38:325–326. doi: 10.1016/j.lpm.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Belehu A, Naafs B, Touw-Langendijk E. Failure of metronidazole treatment in Ethiopian mucocutaneous leishmaniasis. Br J Dermatol. 1978;99:421–422. doi: 10.1111/j.1365-2133.1978.tb06181.x. [DOI] [PubMed] [Google Scholar]
- Beltran F, Gutierrez M, Biagi F. Use of metronidazole in the treatment of Mexican cutaneous leishmaniasis. Bull Soc Pathol Exot Filiales. 1967;60:61–64. [PubMed] [Google Scholar]
- Dogra J, Aneja N, Lal BB, Mishra SN. Cutaneous leishmaniasis in India. Clinical experience with itraconazole (R51 211 Janssen) Int J Dermatol. 1990;29:661–662. doi: 10.1111/j.1365-4362.1990.tb02593.x. [DOI] [PubMed] [Google Scholar]
- al-Fouzan AS, al Saleh QA, Najem NM, Rostom AI. Cutaneous leishmaniasis in Kuwait. Clinical experience with itraconazole. Int J Dermatol. 1991;30:519–521. doi: 10.1111/j.1365-4362.1991.tb04878.x. [DOI] [PubMed] [Google Scholar]
- White JM, Salisbury JR, Jones J, Higgins EM, Vega-Lopez F. Cutaneous leishmaniasis: three children with Leishmania major successfully treated with itraconazole. Pediatr Dermatol. 2006;23:78–80. doi: 10.1111/j.1525-1470.2006.00177.x. [DOI] [PubMed] [Google Scholar]
- Cozzani E, Satta R, Fausti V, Cottoni F, Parodi A. Cutaneous sporotrichoid leishmaniasis resistant to pentavalent antimonial therapy: complete resolution with itraconazole. Clin Exp Dermatol. 2011;36:49–51. doi: 10.1111/j.1365-2230.2010.03855.x. [DOI] [PubMed] [Google Scholar]
- Mohebali M, Fotouhi A, Hooshmand B, Zarei Z, Akhoundi B, Rahnema A, Razaghian AR, Kabir MJ, Nadim A. Comparison of miltefosine and meglumine antimoniate for the treatment of zoonotic cutaneous leishmaniasis (ZCL) by a randomized clinical trial in Iran. Acta Trop. 2007;103:33–40. doi: 10.1016/j.actatropica.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Masmoudi A, Dammak A, Chaaben H, Maalej N, Akrout F, Turki H. Doxycycline for the treatment of cutaneous leishmaniasis. Dermatol Online J. 2008;14:22. [PubMed] [Google Scholar]
- Minodier P, Zambelli L, Mary C, Faraut F, Garnier JM, Berbis P. Cutaneous leishmaniasis treated with azithromycin in a child. Pediatr Infect Dis J. 2008;27:80–81. doi: 10.1097/INF.0b013e3181506683. [DOI] [PubMed] [Google Scholar]
- Silva-Vergara ML, Silva Lde S, Maneira FR, da Silva AG, Prata A. Azithromycin in the treatment of mucosal leishmaniasis. Rev Inst Med Trop Sao Paulo. 2004;46:175–177. doi: 10.1590/s0036-46652004000300011. [DOI] [PubMed] [Google Scholar]
- Lee SA, Hasbun R. Therapy of cutaneous leishmaniasis. Int J Infect Dis. 2003;7:86–93. doi: 10.1016/s1201-9712(03)90002-6. [DOI] [PubMed] [Google Scholar]
- Blum J, Desjeux P, Schwartz E, Beck B, Hatz C. Treatment of cutaneous leishmaniasis among travellers. J Antimicrob Chemother. 2004;53:158–166. doi: 10.1093/jac/dkh058. [DOI] [PubMed] [Google Scholar]
- Masmoudi A, Maalej N, Boudaya S, Turki H, Zahaf A. Adverse effects of intralesional Glucantime in the treatment of cutaneous leishmaniosis. Med Mal Infect. 2006;36:226–228. doi: 10.1016/j.medmal.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Masmoudi A, Ayadi N, Khabir A, Bouzid L, Bouassida S, Meziou TJ, Akrout F, Zahaf A, Boudawara T, Turki H. Sporotrichoid cutaneous leishmaniasis in Tunisia: a clinical and histological study. Ann Dermatol Venereol. 2008;135:63–67. doi: 10.1016/j.annder.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Beheshti M, Ghotbi Sh, Amirizade S. Thérapeutiques et effets néfastes de Glucantime utilisée pour le traitement de la leishmaniose cutanée. Shiraz E-Medical Journal. 2007;8:4. [Google Scholar]
- Kharfi M, Benmously R, El Fekih N, Daoud M, Fitouri Z, Mokhtar I, Ben Becher S, Kamoun MR. Childhood leishmaniasis: report of 106 cases. Dermatol Online J. 2004;10:6. [PubMed] [Google Scholar]
- Uzun S, Uslular C, Yücel A, Acar MA, Ozpoyraz M, Memişoğlu HR. Cutaneous leishmaniasis: evaluation of 3,074 cases in the Cukurova region of Turkey. Br J Dermatol. 1999;140:347–350. doi: 10.1046/j.1365-2133.1999.02673.x. [DOI] [PubMed] [Google Scholar]
- Mosleh IM, Geith E, Natsheh L, Schönian G, Abotteen N, Kharabsheh S. Efficacy of a weekly cryotherapy regimen to treat Leishmania major cutaneous leishmaniasis. J Am Acad Dermatol. 2008;58:617–624. doi: 10.1016/j.jaad.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Asilian A, Sadeghinia A, Faghihi G, Momeni A. Comparative study of the efficacy of combined cryotherapy and intralesional meglumine antimoniate (Glucantime) vs. cryotherapy and intralesional meglumine antimoniate (Glucantime) alone for the treatment of cutaneous leishmaniasis. Int J Dermatol. 2004;43:281–283. doi: 10.1111/j.1365-4632.2004.02002.x. [DOI] [PubMed] [Google Scholar]
- Moosavi Z, Nakhli A, Rassaii S. Comparing the efficiency of topical paromomycin with intralesional meglumine antimoniate for cutaneous leishmaniasis. Int J Dermatol. 2005;44:1064–1065. doi: 10.1111/j.1365-4632.2004.02597.x. [DOI] [PubMed] [Google Scholar]
- Faghihi G, Tavakoli-kia R. Treatment of cutaneous leishmaniasis with either topical paromomycin or intralesional meglumine antimoniate. Clin Exp Dermatol. 2003;28:13–16. doi: 10.1046/j.1365-2230.2003.01169.x. [DOI] [PubMed] [Google Scholar]
- Ben Salah A, Buffet PA, Morizot G, Ben Massoud N, Zâatour A, Ben Alaya N, Haj Hamida NB, El Ahmadi Z, Downs MT, Smith PL, Dellagi K, Grögl M. WR279,396, a third generation aminoglycoside ointment for the treatment of Leishmania major cutaneous leishmaniasis: a phase 2, randomized, double blind, placebo controlled study. PLoS Negl Trop Dis. 2009;3:e432. doi: 10.1371/journal.pntd.0000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro G, Santos DC, Oliveira MC, Fernandes AP, Ferreira LS, Ramaldes GA, Nunan EA, Ferreira LA. Topical delivery and in vivo antileishmanial activity of paromomycin-loaded liposomes for treatment of cutaneous leishmaniasis. J Liposome Res. 2010;20:16–23. doi: 10.3109/08982100903015025. [DOI] [PubMed] [Google Scholar]
- Miranda-Verástegui C, Llanos-Cuentas A, Arévalo I, Ward BJ, Matlashewski G. Randomized, double-blind clinical trial of topical imiquimod 5% with parenteral meglumine antimoniate in the treatment of cutaneous leishmaniasis in Peru. Clin Infect Dis. 2005;40:1395–1403. doi: 10.1086/429238. [DOI] [PubMed] [Google Scholar]
- Seeberger J, Daoud S, Pammer J. Transient effect of topical treatment of cutaneous leishmaniasis with imiquimod. Int J Dermatol. 2003;42:576–579. doi: 10.1046/j.1365-4362.2003.01955.x. [DOI] [PubMed] [Google Scholar]
- Velasco-Castrejon O, Walton BC, Rivas-Sanchez B, Garcia MF, Lazaro GJ, Hobart O, Roldan S, Floriani-Verdugo J, Munguia-Saldana A, Berzaluce R. Treatment of cutaneous leishmaniasis with localized current field (radio frequency) in Tabasco, Mexico. Am J Trop Med Hyg. 1997;57:309–312. doi: 10.4269/ajtmh.1997.57.309. [DOI] [PubMed] [Google Scholar]
- Reithinger R, Mohsen M, Wahid M, Bismullah M, Quinnell RJ, Davies CR, Kolaczinski J, David JR. Efficacy of thermotherapy to treat cutaneous leishmaniasis caused by Leishmania tropica in Kabul, Afghanistan: a randomized, controlled trial. Clin Infect Dis. 2005;40:1148–1155. doi: 10.1086/428736. [DOI] [PubMed] [Google Scholar]
- Willard RJ, Jeffcoat AM, Benson PM, Walsh DS. Cutaneous leishmaniasis in soldiers from Fort Campbell, Kentucky returning from Operation Iraqi Freedom highlights diagnostic and therapeutic options. J Am Acad Dermatol. 2005;52:977–987. doi: 10.1016/j.jaad.2005.01.109. [DOI] [PubMed] [Google Scholar]
- Asilian A, Sharif A, Faghihi G, Enshaeieh Sh, Shariati F, Siadat AH. Evaluation of CO laser efficacy in the treatment of cutaneous leishmaniasis. Int J Dermatol. 2004;43:736–738. doi: 10.1111/j.1365-4632.2004.02349.x. [DOI] [PubMed] [Google Scholar]
- Ghaffarifar F, Jorjani O, Mirshams M, Miranbaygi MH, Hosseini ZK. Photodynamic therapy as a new treatment of cutaneous leishmaniasis. East Mediterr Health J. 2006;12:902–908. [PubMed] [Google Scholar]
- Hejazi H, Eslami G, Dalimi A. The parasiticidal effect of electricity on Leishmania major, both in vitro and in vivo. Ann Trop Med Parasitol. 2004;98:37–42. doi: 10.1179/136485913X13789813917661. [DOI] [PubMed] [Google Scholar]
- Sharquie KE, al-Hamamy H, el-Yassin D. Treatment of cutaneous leishmaniasis by direct current electrotherapy: the Baghdadin device. J Dermatol. 1998;25:234–237. doi: 10.1111/j.1346-8138.1998.tb02387.x. [DOI] [PubMed] [Google Scholar]
- Nilforoushzadeh MA, Shirani-Bidabadi L, Zolfaghari-Baghbaderani A, Saberi S, Siadat AH, Mahmoudi M. Comparison of Thymus vulgaris (Thyme), Achillea millefolium (Yarrow) and propolis hydroalcoholic extracts versus systemic glucantime in the treatment of cutaneous leishmaniasis in balb/c mice. J Vector Borne Dis. 2008;45:301–306. [PubMed] [Google Scholar]
- Kivçak B, Mert T, Ertabaklar H, Balcioğlu IC, Ozensoy Töz S. In vitro activity of Arbutus unedo against Leishmania tropica promastigotes. Turkiye Parazitol Derg. 2009;33:114–115. [PubMed] [Google Scholar]
- Masmoudi A, Dammak A, Bouassida S. et al. The cutaneous leishmaniasis: comparative study between herbal essence preparation in the green clay and intralesional injecction of glucantime. Maghreb médical. 2007;27:383. [Google Scholar]
- Mutiso JM, Macharia JC, Mutisya RM, Taracha E. Subcutaneous immunization against Leishmania major - infection in mice: efficacy of formalin-killed promastigotes combined with adjuvants. Rev Inst Med Trop Sao Paulo. 2010;52:95–100. doi: 10.1590/s0036-46652010000200006. [DOI] [PubMed] [Google Scholar]
- Rocha FJ, Schleicher U, Mattner J, Alber G, Bogdan C. Cytokines, signaling pathways, and effector molecules required for the control of Leishmania (Viannia) braziliensis in mice. Infect Immun. 2007;75:3823–3832. doi: 10.1128/IAI.01335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcoff E, Giménez LA, Bernabó JG, Bottasso O. Interferon gamma as an immunological strategy for the treatment of human leishmaniasis. Medicina (BAires) 1994;54:265–271. [PubMed] [Google Scholar]
- Akuffo H, Kaplan G, Kiessling R, Teklemariam S, Dietz M, McElrath J, Cohn ZA. Administration of recombinant interleukin-2 reduces the local parasite load of patients with disseminated cutaneous leishmaniasis. J Infect Dis. 1990;161:775–780. doi: 10.1093/infdis/161.4.775. [DOI] [PubMed] [Google Scholar]
- Barral-Netto M, Brodskyn C, Carvalho EM, Barral A. Human_leishmaniasis/cytokines.bahia.br. Braz J Med Biol Res. 1998;31:149–155. doi: 10.1590/s0100-879x1998000100021. [DOI] [PubMed] [Google Scholar]
- Heinzel FP, Maier RA Jr. Interleukin-4-independent acceleration of cutaneous leishmaniasis in susceptible BALB/c mice following treatment with anti-CTLA4 antibody. Infect Immun. 1999;67:6454–6460. doi: 10.1128/iai.67.12.6454-6460.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautz-Neu K, Kostka SL, Dinges S, Iwakura Y, Udey MC, von Stebut E. IL-1 signalling is dispensable for protective immunity in Leishmania-resistant mice. Exp Dermatol. 2011;20:76–78. doi: 10.1111/j.1600-0625.2010.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota H, Takashima Y, Matsumoto Y, Hayashi Y, Matsumoto Y. Pretreatment of macrophages with the combination of IFN-gamma and IL-12 induces resistance to Leishmania major at the early phase of infection. J Vet Med Sci. 2008;70:589–593. doi: 10.1292/jvms.70.589. [DOI] [PubMed] [Google Scholar]
- Yazdanpanah MJ, Noorbakhsh SR, Kalantari MR, Maleki M, Kiafar B. Dermatofibrosarcoma protuberans occurring in the site of prior leishmanization. Int J Dermatol. 2006;45:1476–1477. doi: 10.1111/j.1365-4632.2006.03180.x. [DOI] [PubMed] [Google Scholar]
- Khoshgoo N, Zahedifard F, Azizi H, Taslimi Y, Alonso MJ, Rafati S. Cysteine proteinase type III is protective against Leishmania infantum infection in BALB/c mice and highly antigenic in visceral leishmaniasis individuals. Vaccine. 2008;26:5822–5829. doi: 10.1016/j.vaccine.2008.08.065. [DOI] [PubMed] [Google Scholar]
- Zhang WW, Matlashewski G. Immunization with a Toll-like receptor 7 and/or 8 agonist vaccine adjuvant increases protective immunity against Leishmania major in BALB/c mice. Infect Immun. 2008;76:3777–3783. doi: 10.1128/IAI.01527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


