Abstract
Background
Nocardia is an opportunistic pathogen that can cause disseminated infection in immunocompromised hosts. The most common type of skin lesion reported with disseminated Nocardia is a subcutaneous nodule; however, there are reports with unusual cutaneous presentations. Long term corticosteroid treatment is one of the largest risk factors for developing disseminated Nocardia. Initial treatment is empiric as each strain has unique susceptibilities and it takes weeks to speciate and test sensitivities.
Main observations
A 66-year-old female on long term corticosteroids for systemic lupus erythematosus (SLE) and antiphospholipid syndrome presented with a polymorphous skin eruption and systemic symptoms concerning for infection. Especially concerning were areas of hemorrhagic pustules on the lower legs, and two ecthymatous lesions on the thigh. Tissue culture Gram stain revealed Gram positive branching filamentous rods concerning for Nocardia. The patient improved with empiric treatment.
Conclusions
This case of Nocardiosis had unusual cutaneous findings that could have misguided the clinician, but the tissue culture and Gram stain proved to be useful for rapid diagnosis and proper treatment.
Keywords: antiphospholipid syndrome, infection, systemic lupus erythematosus
Introduction
When an immunosuppressed patient presents with disseminated infection, the differential for causative organism is extensive. In particular, disseminated Nocardia can have a polymorphous cutaneous presentation, which we highlight with the following case.
Case Report
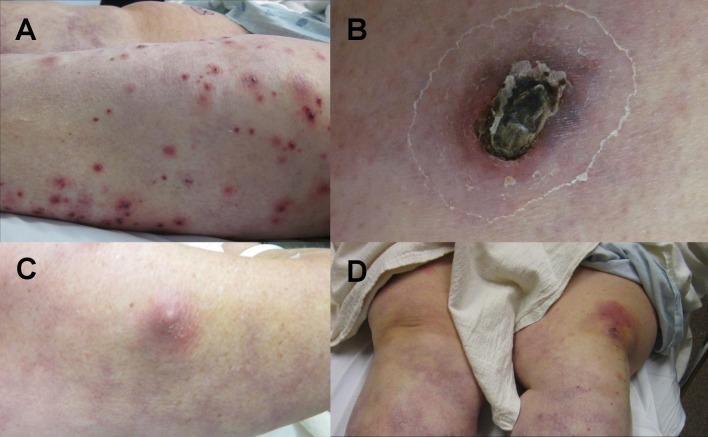
A 66-year-old female presented with a two week history of progressive new onset skin lesions on the upper and lower extremities in association with chills, malaise, anorexia, and shortness of breath. Her medical history included a 20 year history of SLE with antiphospholipid antibody syndrome, which had been controlled for more than 10 years on prednisone 20 mg daily. She was also taking warfarin for her antiphospholipid syndrome. Physical exam revealed numerous cutaneous findings including multiple hemorrhagic pustules on the left lower extremity and two ecthymatous lesions on the right thigh [Fig. 1]. There was also a firm, tender erythematous subcutaneous nodule on the right upper arm and a warm erythematous plaque on the left knee [Fig. 1]. The right hand was swollen and tender. An 8 mm punch biopsy was obtained from the right thigh and the wound drained gross purulent material. The tissue was bisected, sent for H&E and for tissue culture with Gram stain [Fig. 2]
Figure 1.
Physical exam revealed numerous cutaneous findings including A) multiple hemorrhagic pustules on the left lower extremity, B) a 4 cm ecthymatous lesion on the right thigh, C) an erythematous subcutaneous nodule on the right upper arm, D) and a warm erythematous plaque on the left knee.
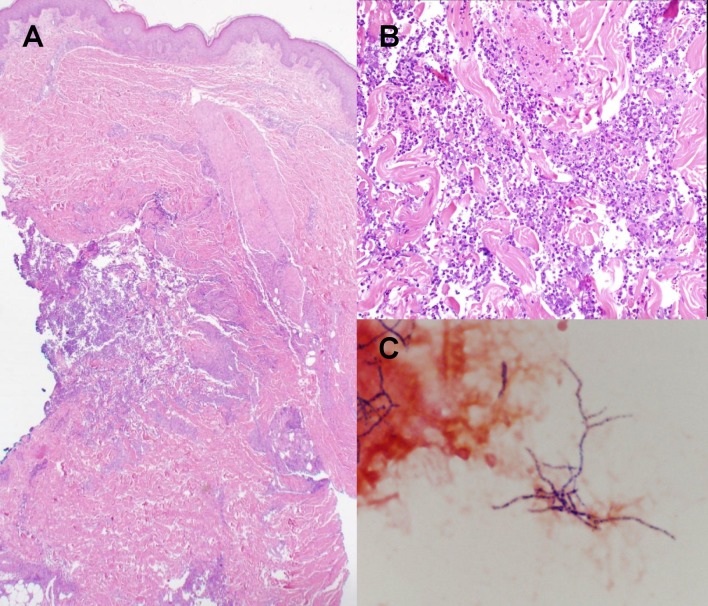
Figure 2.
The histologic findings were in favor of an infectious process A) low power view with an infiltrate in the mid and deep dermis, focally extending into the subcutaneous fat, B) high power view with a mostly neutrophilic infiltrate, C) Tissue culture Gram stain with Gram positive branching filamentous rods.
On histopathological examination, there was a mostly neutrophilic infiltrate in mid and deep dermis, focally extending into the subcutaneous fat. Special stains (PAS, GMS, AFB, Fite and Gram) were negative for microorganisms. The histologic findings of this biopsy were in favor of an infectious process. Tissue culture and clinical correlation were recommended. On initial Gram stain from tissue for culture, there were Gram positive branching filamentous rods associated with neutrophils, highly suggestive of Nocardia.
CT scan revealed a cavitary lesion in the right lung and subcutaneous soft tissue nodules. X-ray of the right hand showed a lytic lesion in the proximal phalanx of the index finger. The patient began treatment for presumptive disseminated Nocardiosis with trimethoprim/sulfamethoxazole (TMP-SMX) and meropenem. Due to a slow initial response, linezolid was added. The patient responded well with clearance of her skin findings. Later, linezolid and TMP-SMX were discontinued due to thrombocytopenia, but she continued to improve with treatment over the next two weeks with meropenem. Final speciation and sensitivities revealed Nocardia brasiliensis sensitive to TMP-SMX, linezolid, and ceftriaxone, but resistant to imipenem. Antibiotic therapy was then changed to ceftriaxone for long term treatment.
Discussion
Nocardia species are aerobic, Gram-positive, filamentous bacteria found in soil. They are opportunistic pathogens affecting immunocompromised hosts with the most common site of primary infection being the lung. They can disseminate to skin, brain, kidneys, joints, and eyes through hematogenous spread. Reported cutaneous manifestations of disseminated Nocardia include papules, pustules, bullae, subcutaneous nodules, and ulcers.[1-3] There has been a recent report of disseminated Nocardia mimicking cellulitis and erythema nodosum.[4] Our patient had a variety of skin lesions including hemorrhagic pustules, ecthyma, subcutaneous nodules, and erythematous plaques. Due to the hemorrhagic pustules, one may have suspected gonococcemia, infective endocarditis, or possibly vasculitis. Ecthyma is usually caused by pseudomonas sepsis, strep, or staph. To our knowledge, ecthymatous lesions have not been previously reported in disseminated Nocardia. Anticoagulation with warfarin may have contributed to the hemorrhage in the pustules. This case demonstrates that the cutaneous manifestations can be variable and widespread.
There have been previous case reports of disseminated Nocardia in lupus patients on corticosteroids as well as patients with primary anti-phospholipid syndrome on long term corticosteroids.[5-8] Corticosteroid use in association with Nocardia has also been reported with autoimmune blistering disease, ulcerative colitis, and other systemic autoimmune and inflammatory conditions.[9-12] A case control study in transplant recipients identified high dose steroids as the most significant independent risk factor for developing Nocardia infection.[13]
Initial treatment for presumed Nocardiosis must be empiric, as it takes three weeks to speciate and determine sensitivities to Nocardia. Each strain has individual unique susceptibility patterns, but most are susceptible to TMP/SMX and/or carbapenems. Most strains are also susceptible to linezolid.[13]
Finally, this case highlights the importance of obtaining tissue culture with stains for organisms on initial presentation when an infectious process is considered in the differential diagnosis, as pathogens such as Nocardia may be difficult to find on histopathologic analysis; and although the pattern of inflammation may be typical, the lack of identifiable organisms often prompts recommendation for tissue culture. Staining can be performed on tissue cultures immediately and may give the clinician a general idea of the identity of the organism. In this case, the identification of Grampositive branching rods narrowed the differential to presumed Nocardia infection and allowed for expedited speciation and sensitivity analysis (which can take weeks in Nocardia as well as atypical mycobacterium).
Conclusions
The cutaneous findings of disseminated Nocardia can be variable, as exemplified by our patient who presented with hemorrhagic pustules and ecthyma. Long term corticosteroid use is one of the largest risk factors for developing disseminated Nocardia, which is also demonstrated by our case. It is important to obtain cultures from tissue biopsies when disseminated infection is considered to allow for prompt organism identification and perhaps better patient care.
Acknowledgments
Photo contributors, Figure 2 (Gram stain): Nathan A. Ledeboer PhD and Neil W. Anderson PhD.
References
- Dodiuk-Gad R, Cohen E, Ziv M, Goldstein LH, Chazan B, Shafer J, Sprecher H, Elias M, Keness Y, Rozenman D. Cutaneous nocardiosis: report of two cases and review of the literature. Int J Dermatol. 2010;49:1380–1385. doi: 10.1111/j.1365-4632.2010.04554.x. [DOI] [PubMed] [Google Scholar]
- Wong KM, Chak WL, Chan YH, Choi KS, Chau KF, Lee KC, Li CS. Subcutaneous nodules attributed to nocardiosis in a renal transplant recipient on tacrolimus therapy. Am J Nephrol. 2000;20:138–141. doi: 10.1159/000013570. [DOI] [PubMed] [Google Scholar]
- Shapiro PE, Grossman ME. Disseminated Nocardia asteroides with pustules. J Am Acad Dermatol. 1989;20:889–892. doi: 10.1016/s0190-9622(89)70101-8. [DOI] [PubMed] [Google Scholar]
- George SJ, Rivera AM, Hsu S. Disseminated cutaneous nocardiosis mimicking cellulitis and erythema nodosum. Dermatol Online J. 2006;12:13. [PubMed] [Google Scholar]
- Hara H, Wakui F, Ochiai T. Disseminated Nocardia farcinica infection in a patient with systemic lupus erythematosus. J Med Microbiol. 2011;60:847–850. doi: 10.1099/jmm.0.025577-0. [DOI] [PubMed] [Google Scholar]
- Justiniano M, Glorioso S, Dold S, Espinoza LR. Nocardia brain abscesses in a male patient with SLE: successful outcome despite delay in diagnosis. Clin Rheumatol. 2007;26:1020–1022. doi: 10.1007/s10067-006-0262-x. [DOI] [PubMed] [Google Scholar]
- Leong KP, Tee NW, Yap WM, Chee TS, Koh ET. Nocardiosis in patients with systemic lupus erythematosus. The Singapore Lupus Study Group. J Rheumatol. 2000;27:1306–1312. [PubMed] [Google Scholar]
- Soy M, Oktun MT, Tunçbilek N, Ermantaş N, Okten O, Altinay G, Turgut B. Nocardiosis in a patient with primary anti-phospholipid syndrome. Rheumatol Int. 2006;26:461–464. doi: 10.1007/s00296-005-0014-2. [DOI] [PubMed] [Google Scholar]
- Carducci M, Nosotti L, Calcaterra R, Bonifati C, Mussi A, Pelagalli L, Di Emidio L, Laurenzi L, Russo A, Franco G, Toma L, Morrone A. Early development of disseminated nocardiosis during immunosuppressive treatment for pemphigus vulgaris. Eur J Dermatol. 2007;17:346–347. doi: 10.1684/ejd.2007.0221. [DOI] [PubMed] [Google Scholar]
- Kakurai M, Hiraga T, Yamada T, Usui K, Kiyosawa T, Nakagawa H. Subcutaneous nocardial abscesses in a patient with bullous pemphigoid during immunosuppressive therapy: report of a case and review of the Japanese literature. J Dermatol. 1999;26:829–833. doi: 10.1111/j.1346-8138.1999.tb02102.x. [DOI] [PubMed] [Google Scholar]
- Arora G, Friedman M, Macdermott RP. Disseminated Nocardia nova infection. South Med J. 2010;103:1269–1271. doi: 10.1097/SMJ.0b013e3181faec65. [DOI] [PubMed] [Google Scholar]
- Macfarlane DE, Bain B, Char G, La Grenade L, Williams E. Nocardiosis in patients treated with corticosteroids. A report on three cases. West Indian Med J. 1985;34:134–138. [PubMed] [Google Scholar]
- Peleg AY, Husain S, Qureshi ZA, Silveira FP, Sarumi M, Shutt KA, Kwak EJ, Paterson DL. Risk factors, clinical characteristics, and outcome of Nocardia infection in organ transplant recipients: a matched case-control study. Clin Infect Dis. 2007;44:1307–1314. doi: 10.1086/514340. [DOI] [PubMed] [Google Scholar]