Abstract
Background
Pyoderma gangrenosum is a rare neutrophilic dermatosis which leads to necrotic and painful skin ulceration. PG of the breast is extremely rare with 32 documented cases in the current literature. Delay in diagnosis worsens scarring as the ulcers are rapidly expanding, painful and usually slow to heal.
Case presentation
We present a case of pyoderma gangrenosum of the breast in a patient with associated rheumatoid arthritis which was initially diagnosed as an infected breast ulcer and later successfully treated with systemic steroids and intravenous immunoglobulin (IVIG).
Conclusion
Even though PG of the breast has been gaining increased recognition over the past two decades, this has been more common in the post-surgical setting. This case highlights the need to consider PG as a differential diagnosis when faced with unsual cases of breast ulceration and the importance of multidisplinary approach for effective treatment of this condition.
Keywords: breast, intreavenous immunoglobulins, mycobacterium ulcerans, pyoderma gangrenosum, rheumatoid arthritis, ulcer
Introduction
Pyoderma gangrenosum is a non-infectious ulcerative neutrophilic dermatosis that was initially described in 1916 by Brocq and later by Brunsting in 1930.[1-3] It can occur at any age but is commonly found in individuals aged 30-50 years and affects both genders. In approximately 50-70% of cases there is an association with autoimmune diseases such as inflammatory bowel disease (IBD), rheumatoid arthritis (RA) and haemopoeitic malignancies such as leukemias, lymphomas and myeloma.[3-4] Lesions typically appear in the lower extremities, but can also occur in extracutaneous sites such as eyes, spleen and lungs. PG can also occur in areas of trauma, a phenomenon known as pathegy.[3-4] Only 32 cases of PG involving the breast have been described in the current literature signifying the rare nature of this condition.
Case Report
A 61-year-old woman presented with a 3-month history of a rapidly growing, painful and ulcerated lesion on her right breast. She associated the ulcer's formation to her scratching a bite from a sandfly while visiting Sudan. Her past medical history included longstanding sero-negative rheumatoid arthritis involving her hips and knees, rheumatic valvular heart disease requiring triple valve replacement and pulmonary hypertension. Examination of the skin revealed a 13 cm x 7 cm, ulcerated lesion occupying the lower, outer right breast with partial sparing of the nipple-areolar complex [Fig. 1].
Figure 1.
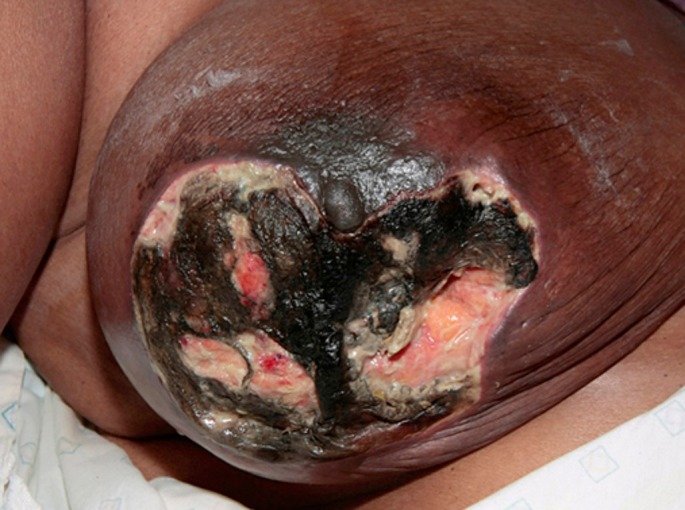
Extensive painful ulcer on right breast at initial presentation.
Ultrasound and mammographic correlation did not show any suspicious lesions, calcifications or lymph nodes. Routine bloods showed renal and liver function consistent with her initial baseline levels but an elevated white cell count at 14.5 x 109/L. Inflammatory markers were also raised with her Erythrocyte Sedimentation Rate and CRP measuring 100 mm/h and 104 mg/L. Speckled ANA titre was also elevated but rheumatoid factor was normal. Multiple punch biopsies were taken circumferentially from the wound which grew mixed enteric flora including Serratia macerans, E.coli and Pseudomonas aeruginosa. Fungal cultures were negative. Prior to formal diagnosis of the ulcer as PG the wound was debrided in an attempt to minimise the frequency of dressing changes which were distressing to the patient. During the debridement tissue biopsies were sent for histopathology and PCR. Histopathology revealed non specific inflammation and ulceration with an abundance of neutrophils but no evidence of malignancy [Fig. 2]. The apperance of infective causes of ulceration such as the confluent necrosis and abundant mycobacterium organisms seen in M. ulcerans or paratisized macrophages consistent with Leishmaniasis were absent. Both the PCR and quantiferon tests for M. ulcerans and M. tuberculosis were also negative.
Figure 2.
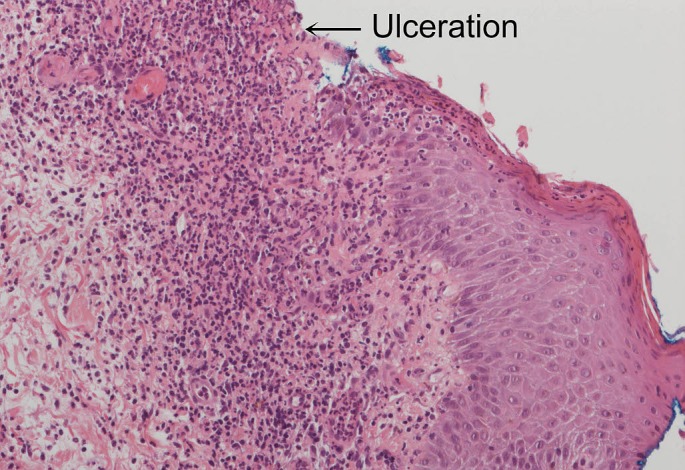
Histopathology showing ulceration and abundant neutrophils (Magnification x200 high power field).
The diagnosis of PG was considered due to the patient's known history of rheumatoid arthritis, and also because other causes of cutaneous ulcerations such as infectious (bacterial, viral or fungal), parasitic (leishmaniasis), malignancies (breast carcinoma, squamous cell carcinoma) and other exogenous tissue injury such as factitial ulcers were excluded. The patient also presented with classical features of PG including rapid enlargement of the lesion, pain and response to systemic steroid treatment. The lesion also occurred in an area of previous trauma, a sandfly bite which could be associated with a pathergy phenomenon as well.
Management consisted of a multidisciplinary team approach with input from surgical, medical and allied health teams. Her regular dose of Leflunomide for her RA was ceased on advice from rheumatologists to avoid additional immunossuppression. She received daily wound dressings which allowed non surgical wound debridement and encouranged granulation tissue. Oral prednisone 1 mg/kg was trialled initially which successfully halted progression of the breast ulcer. Intravenous antibiotics were also required as she developed E-coli septicemia. She was treated with timentin 60 mg/kg/8 hourly and gentamicin 4 mg/kg/day and was later changed to fosfomycin 1 g/6 hourly due to deterioration in her renal function. The patient developed significant peripheral neuropathy, which severely impaired her mobility and steroid induced psychosis, therefore steroid sparing agents were considered for her PG, including immunosuppressive medications (cyclosporine, mycophenolate mofetil), biological agents (infliximab, adulimab) or IVIG.
IVIG was considered to be the most suitable treatment given her intermittent infections, sepsis and impaired liver and kidney function. It was initiated primarily for her sepsis but she had a very good response to this therapy with granulation tissue formation within 2 weeks of therapy. She had monthly infusions of IVIG 0.4 g/kg for a total of 3 months with marked reduction in her wound and pain within 6 weeks [Fig. 3]. She was continued on 15 mg of prednisone for her rheumatoid arthritis and PG to allow complete resolution of her wound and discharged with ongoing wound care regime. At discharge her breast wound was 2 cm x 2 cm in size [Fig. 4].
Figure 3.
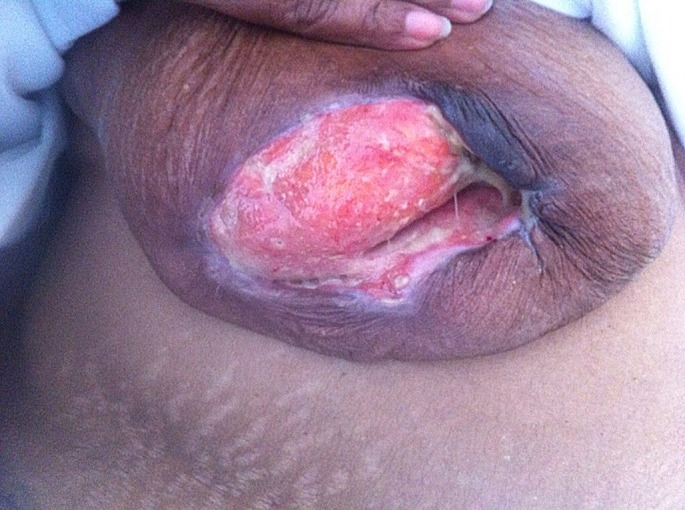
Massive reduction in the size of the ulcer 6 weeks post IVIG therapy.
Figure 4.
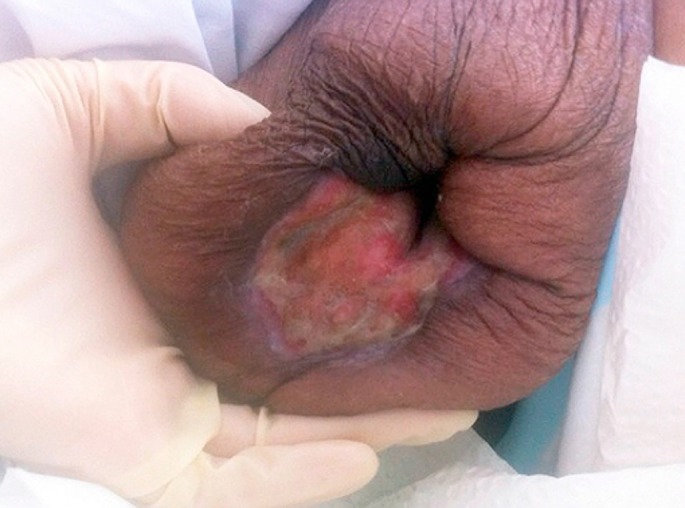
Near complete resolution of the ulcer with scarring.
Discussion
The breast is an uncommon site for PG with only 32 cases reported in the current literature [Table 1].[5-32] In 25 of these cases PG occurred post breast surgery, predominantly breast reduction mammoplasty.[7-8,11-14,16,18-19,21-32] 16 out of 32 cases described in the literature have been associated with conditions such as inflammatory bowel disease, rhematoid arthritis and breast malignancy but there are also cases with no pre-existing disease.[6-8,10-12,17,23,25-26,28,30-31] The age of presentation for PG is variable and ranges from 15 to 66 years old. All cases occurred in females. Treatment with systemic steroids occurred in 25 out of 32 cases, alone or in combination with cyclosporin, dapsone, vitamin A, topical tacrolimus and infliximab infusion. Oral cyclosporin was the second most common systemic agent used in treatment of PG with 10 out of 32 cases.[5-32] Topical steroid in one case was successful in controlling the condition.[22] There were 2 cases treated with infliximab infusion and only one case was also sufficiently treated with combination of predisone, cyclosporin and IVIG.[28,30,32] Vaccuum assisted closures and hyperbaric oxygen were used in 4 cases.[14,29-30,32] A useful observation made by us was that 5 out of the 32 cases in the current literature also underwent breast debridement upon initial presentation, as the diagnosis of PG was not initially considered.[6,9,17,19,32] This is likely due to lack of association of this rare condition and also because PG is hard to clinically distinguish from infective ulcers, which if left untreated can have a fatal outcome.
Table 1.
| Case | Age/ (Sex) |
Associated Disorders | Unilateral/ Bilateral |
Surgery (pre- admission) |
Surgery (post admission) | Treatment | Ref Article |
|---|---|---|---|---|---|---|---|
| 1 | 61 (F) | RA | Unilateral | none | Breast debridement | Prednisone, IVIG | |
| 2 | 51 (F) | none | Unilateral | none | none | Methylprednisolone, salicylazosulfapyridine | 5 |
| 3 | 61 (F) | RA | Unilateral | none | Breast debridement | Cyclosporin | 6 |
| 4 | 58 (F) | UC | Bilateral | Bilateral reduction Mammoplasty |
none | Prednisone | 7 |
| 5 | 66 (F) | breast cancer | Bilateral | Bilateral mastectomy and reconstruction | none | Prednisone, tacrolimus 0.03% cream | 7 |
| 6 | 28 (F) | none | Bilateral | Bilateral mastopexy | none | Prednisone | 8 |
| 7 | 50 (F) | AML | Bilateral | Left mastectomy | none | Prednisone | 8 |
| 8 | 15 (F) | none | Unilateral | none | Breast debridement | Prednisone, Vitamin A | 9 |
| 9 | 54 (F) | UC | Unilateral | none | none | Prednisolone, 0.1% betamethasone cream | 10 |
| 10 | 50 (F) | breast cancer | Unilateral | Bilateral mastectomy | none | Prednisone, cyclosporine | 11 |
| 11 | 59 (F) | breast cancer | Unilateral | Right mastectomy and reconstruction | none | Cyclosporine, prednisone | 11 |
| 12 | 51 (F) | breast cancer | Bilateral | Bilateral mastectomy and reconstruction | none | Cyclosporine | 12 |
| 13 | 21 (F) | none | Bilateral | Bilateral reduction Mammoplasty |
none | Prenisolone, cyclosporine | 13 |
| 14 | 57 (F) | none | Bilateral | Bilateral reduction Mammoplasty |
none | Prednisolone, hyperbaric oxygen therapy | 14 |
| 15 | 29 (F) | none | Bilateral | none | Cyclosporine | 15 | |
| 16 | 41 (F) | none | Bilateral | Bilateral mastopexy | none | Methylprednisolone | 16 |
| 17 | 58 (F) | UC | Unilateral | Breast debridement | Prednisolone, cyclosporine | 17 | |
| 18 | 54 (F) | none | Bilateral | Bilateral reduction Mammoplasty |
none | Cyclosporine | 18 |
| 19 | 40 (F) | none | Unilateral | Bilateral mastopexy | Right breast debridement | Steroid therapy (non specified) | 19 |
| 20 | 63 (F) | none | Unilateral | Fine Needle Aspiration | Systemic steroids | 20 | |
| 21 | 36 (F) | Fibroadenoma left breast | Unilateral | Wide local excision left breast tumour | none | Cyclosporine | 21 |
| 22 | 24 (F) | none | Unilateral | Bilateral reduction Mammoplasty |
none | Cyclosporine | 21 |
| 23 | 34 (F) | none | Unilateral | Bilateral reduction Mammoplasty |
none | Clobetasol propionate 0.05% cream | 22 |
| 24 | 35 (F) | UC | Unilateral | Bilateral reduction Mammoplasty |
none | Prednisone | 23 |
| 25 | 27 (F) | none | Bilateral | Bilateral reduction Mammoplasty |
none | Methylprednisolone and cyclosporin | 24 |
| 26 | 43 (F) | UC | Bilateral | Bilateral augmentation mammoplasty |
none | Prednisone, 6-metilprednisolone | 25 |
| 27 | - (F) | breast cancer | Unilateral | Mastectomy | none | Prednisone | 26 |
| 28 | 56 (F) | none | Bilateral | Bilateral augmentation mammoplasty |
none | Prednisone, Dapsone | 27 |
| 29 | 37 (F) | breast cancer | Unilateral | Modified right mastectomy and reconstruction | none | Infliximab infusion | 28 |
| 30 | 58 (F) | none | Bilateral | Bilateral reduction Mammoplasty |
none | Prednisone, hyperbaric oxygen | 29 |
| 31 | 45 (F) | breast cancer | Unilateral | Transverse rectus abdominis flap reconstruction | none | Prednisone, cyclosporin A, IVIG, Vaccuum assisted closure | 30 |
| 32 | 56 (F) | breast cancer | Unilateral | Left Mastectomy | none | Prednisone | 31 |
| 33 | 49 (F) | none | Bilateral | Bilateral reduction mammoplasty |
Breast debridement | Prednisone, Infliximab infusion, Vaccuum assisted closure | 32 |
| F = Female; Ref = Reference; RA = Rheumatoid Arthritis; AML = Acute Myeloid Leukemia; UC = Ulcerative Colitis | |||||||
PG remains a clinical diagnosis and a proposed clinical criteria has been developed to aid its diagnosis.[3] This includes presence of two major and at least two minor features. The major criteria includes i) rapid progression with marginal expansion of 1-2 cm per day or 50% increase in a month, ii) exclusion of other causes of cutaneous ulceration. The minor criteria includes i) history to suggest pathergy phenomenon, ii) presence of systemic disease associated with PG, iii) histopathological findings (dermal neutrophilia, inflammation and lymphocytic vasculitis), and iv) rapid response to systemic corticosteroid treatment.[34] In this case, the patient easily fulfilled three out of the four criteria. Steroid treated halted the progression of her disease and IVIG provided rapid wound healing.
Management of PG can be challenging and should include a multidisciplinary approach. The lesions are often very painful and a multimodal approach to analgesia is advocated. Regular reviews by wound care nurses is necessary, with the use of hydrogels and films for chronic wounds and hydrocolloid and more absorptive dressings for exudative wounds.[3] Any evidence of wound infection should be promptly treated in consultation with the hospital's infectious diseases team. Systemic therapy remains the mainstay of treatment and encompasses the use of corticosteroids, cyclosporine, antineutrophilic therapies, biologic therapies and other immunosuppressive agents. Corticosteroids such as prednisone or methylprednisolone 0.5-1 mg/kg and cyclosporine 3-5 mg/kg/day are generally proposed in literature as first line agents for treatment of PG and produce good response rates.[3-4,33,35-36] Both these agents have serious adverse side-effect profiles which makes them hazardous to use long term. Biologic therapies such as TNF-alpha inhibitors have shown success in treatment of PG associated with conditions such as Crohn's and the newer monclonal antibodies such as adalimumab and ustekinumab have also showed some promise in isolated case reports, but large scale studies are required to properly define their role in the future.[1,9] There only 25 documented cases of successful treatment with IVIG in current literature, but this therapy seems effective for long, severe and refractory cases of PG.[3,37] The role of surgery in management of PG has been controversial given the existence of pathergy phenomenon in a large number of patients.[3] There are encouraging reports in the current literature showing patients with stable PG who have benefited from gentle debridements, split skin grafts and vaccuum assisted closures, with reduction in wound related morbidity.[3,5]
Conclusion
PG of the breast is a rare condition and therefore remains a diagnostic challenge. This patient showed a very good initial response to systemic steroids but was not considered for long term use owing to the side-effect profile and her advancing age. Even though minimal literature is available on the efficacy of IVIG, she was trialled on this therapy and this proved a very successful alternative in her case. The aim of this case is to highlight that PG should be considered as a differential diagnosis, in addition to malignancy and infections aetiologies when presented with an unsual case of breast ulceration. Despite her prolonged inpatient admission the patient had resolution of her breast ulcer to a point where she was suitable for discharge after a total of 11 months. Emphasis is again placed on the role of prompt diagnosis in the prevention of an extended hospital stay and minimisation of scarring and it's associated psychological morbidity in these cases.
References
- Brocq L. Novel contribution a l'etude du phagedenisme geometrique. Ann Dermatol Syphil. 1916;1:1–39. [Google Scholar]
- Brunsting LA GW, O'Leary PA. Pyoderma (ecthyma) gangrenosum: Clinical and experimental observations in five cases occurring in adults. Arch Derm Syphilol. 1930;118:743–768. [Google Scholar]
- Ahronowitz I, Harp J, Shinkai K. Etiology and management of pyoderma gangrenosum: a comprehensive review. Am J Clin Dermatol. 2012;13:191–211. doi: 10.2165/11595240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Cox NH, Jorizzo JL, Bourke JF, Vasculitis and neutrophilic vascular reactions. In: Burns T Breathnach S, Cox N, Griffiths C (eds). Rook’s Textbook of Dermatology, Vol. 3, 8th edn. Oxford: Blackwell Science; 2010. pp. 64–73. [Google Scholar]
- Mansur AT, Balaban D, Göktay F, Takmaz S. Pyoderma gangrenosum on the breast: A case presentation and review of the published work. J Dermatol. 2010;37:107–110. doi: 10.1111/j.1346-8138.2009.00756.x. [DOI] [PubMed] [Google Scholar]
- Dolan OM, Burrows D, Walsh M. Pyoderma gangrenosum of the breast treated with low-dose cyclosporin A. Clin Exp Dermatol. 1997;22:92–95. [PubMed] [Google Scholar]
- Davis MD, Alexander JL, Prawer SE. Pyoderma gangrenosum of the breasts precipitated by breast surgery. J Am Acad Dermatol. 2006;55:317–320. doi: 10.1016/j.jaad.2006.02.066. [DOI] [PubMed] [Google Scholar]
- Duval A, Boissel N, Servant JM, Santini C, Petit A, Vignon-Pennamen MD. Pyoderma gangrenosum of the breast: a diagnosis not to be missed. J Plast Reconstr Aesthet Surg. 2011;64:e17–e20. doi: 10.1016/j.bjps.2010.07.022. [DOI] [PubMed] [Google Scholar]
- Havlik RJ, Giles PD, Havlik NL. Pyoderma Gangrenosum of the breast: sequential grafting. Plast Reconstr Surg. 1998;101:1909–1914. doi: 10.1097/00006534-199806000-00020. [DOI] [PubMed] [Google Scholar]
- Duke G, Al Samaraee A, Husain A, Meggitt S, Fasih T. Pyoderma gangrenosum: a rare cause of breast ulceration. Ochsner J. 2012;12:155–158. [PMC free article] [PubMed] [Google Scholar]
- Carrasco López C, López Martínez A, Roca Mas JO, Serra Payro JM, Viñals Viñals JM. Pyoderma gangrenosum in breast reconstruction. J Plast Surg Hand Surg. 2012;46:281–282. doi: 10.3109/2000656X.2011.636970. [DOI] [PubMed] [Google Scholar]
- MacKenzie D, Moiemen N, Frame JD. Pyoderma gangrenosum following breast reconstruction. Br J Plast Surg. 2000;53:441–443. doi: 10.1054/bjps.2000.3349. [DOI] [PubMed] [Google Scholar]
- Horner B, El-Muttardi N, Mercer D. Pyoderma gangrenosum complicating bilateral breast reduction. Br J Plast Surg. 2004;57:679–681. doi: 10.1016/j.bjps.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Gulyas K, Kimble FW. Atypical pyoderma gangrenosum after breast reduction. Aesthetic Plast Surg. 2003;27:328–331. doi: 10.1007/s00266-003-3017-y. [DOI] [PubMed] [Google Scholar]
- Harries MJ, McMullen E, Griffiths CE. Pyoderma gangrenosum masquerading as dermatitis artefacta. Arch Dermatol. 2006;142:1509–1510. doi: 10.1001/archderm.142.11.1509-b. [DOI] [PubMed] [Google Scholar]
- Poucke SV, Jorens PG, Peeters R, Jacobs W, de Beeck BO, Lambert J, Beaucourt L. Pyoderma gangrenosum: a challenging complication of bilateral mastopexy. Int Wound J. 2004;1:207–213. doi: 10.1111/j.1742-4801.2004.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterworth AS, Horgan K. Pyoderma gangrenosum – an unusual differential diagnosis for acute infection. Breast. 2004;13:250–253. doi: 10.1016/j.breast.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Berry MG, Tavakkolizadeh A, Sommerlad BC. Necrotizing ulceration after breast reduction. J R Soc Med. 2003;96:186–187. doi: 10.1258/jrsm.96.4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifchez SD, Larson DL. Pyoderma gangrenosum after reduction mammaplasty in an otherwise healthy patient. Ann Plast Surg. 2002;49:410–413. doi: 10.1097/00000637-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Swinson BD, Morrison CM, Sinclair JS. Pyoderma gangrenosum – a complication of breast biopsy. Ulster Med J. 2002;71:66–67. [PMC free article] [PubMed] [Google Scholar]
- Schöfer H, Baur S. Successful treatment of postoperative pyoderma gangrenosum with cyclosporin. J Eur Acad Dermatol Venereol. 2002;16:148–151. doi: 10.1046/j.1468-3083.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- Gudi VS, Julian C, Bowers PW. Pyoderma gangrenosum complicating bilateral mammaplasty. Br J Plast Surg. 2000;53:440–441. doi: 10.1054/bjps.2000.3350. [DOI] [PubMed] [Google Scholar]
- Simon AM, Khuthaila D, Hammond DC, Andres A. Pyoderma gangrenosum following reduction mammaplasty. Can J Plast Surg. 2006;14:37–40. doi: 10.1177/229255030601400109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau Salvat C, Miquel FJ, Pont V, Aliaga A. Pyoderma gangrenosum: unusual complication following mammoplasty reduction. Int J Dermatol. 1998;37:794–796. doi: 10.1046/j.1365-4362.1998.00486.x. [DOI] [PubMed] [Google Scholar]
- Sotillo-Gago I, Muñoz-Pérez MA, Camacho-Martinez F. Pyoderma gangrenosum after augmentation mammoplasty. Acta Derm Venereol. 1999;79:486. doi: 10.1080/000155599750010049. [DOI] [PubMed] [Google Scholar]
- Baruch J, Julien M, Touraine R, Auffret P. Postoperative pyoderma gangrenosum and cancer of the breast. Apropos of a case. Ann Chir Plast Esthet. 1990;35:73–75. [PubMed] [Google Scholar]
- Bonamigo RR, Behar PR, Beller C, Bonfá R. Pyoderma gangrenosum after silicone prosthesis implant in the breasts and facial plastic surgery. Int J Dermatol. 2008;47:289–291. doi: 10.1111/j.1365-4632.2008.03480.x. [DOI] [PubMed] [Google Scholar]
- Rietjens M, Cuccia G, Brenelli F, Manconi A, Martella S, De Lorenzi F. A pyoderma gangrenosum following breast reconstruction: a rare cause of skin necrosis. Breast J. 2010;16:200–202. doi: 10.1111/j.1524-4741.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- Grillo MA, Cavalheiro TT, da Silva Mulazani M, Rocha JL, Semchechen D, da Cunha CA. Postsurgical pyoderma gangrenosum complicating reduction mammaplasty. Aesthetic Plast Surg. 2012;36:1347–1352. doi: 10.1007/s00266-012-9981-3. [DOI] [PubMed] [Google Scholar]
- Schintler MV, Grohmann M, Donia C, Aberer E, Scharnagl E. Management of an unfortunate triad after breast reconstruction: pyoderma gangrenosum, full-thickness chest wall defect and Acinetobacter Baumannii Infection. J Plas Reconstr Aesthet Surg. 2010;63:e564–e567. doi: 10.1016/j.bjps.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Horgan K, Holt PJA. The management of acute pyoderma gangrenosum of the breast with a coincidental invasive breast cancer. J Derm Tr. 1994;5:215–217. [Google Scholar]
- Goshtasby PH, Chami RG, Johnson RM. A novel approach to the management of pyoderma gangrenosum complicating reduction mammaplasty. Aesthet Surg J. 2010;30:186–193. doi: 10.1177/1090820X10366011. [DOI] [PubMed] [Google Scholar]
- Hasselmann DO, Bens G, Tilgen W, Reichrath J. Pyoderma gangrenosum: clinical presentation and outcome in 18 cases and review of the literature. J Dtsch Dermatol Ges. 2007;5:560–564. doi: 10.1111/j.1610-0387.2007.0328.x. [DOI] [PubMed] [Google Scholar]
- Su WP, Davis MD, Weenig RH, Powell FC, Perry HO. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790–800. doi: 10.1111/j.1365-4632.2004.02128.x. [DOI] [PubMed] [Google Scholar]
- Saracino A, Kelly R, Liew D, Chong A. Pyoderma gangrenosum requiring inpatient management: a report of 26 cases with follow up. Australas J Dermatol. 2011;52:218–221. doi: 10.1111/j.1440-0960.2011.00750.x. [DOI] [PubMed] [Google Scholar]
- Reichrath J, Bens G, Bonowitz A, Tilgen W. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol. 2005;53:273–283. doi: 10.1016/j.jaad.2004.10.006. [DOI] [PubMed] [Google Scholar]
- de Zwaan SE, Iland HJ, Damian DL. Treatment of refractory pyoderma gangrenosum with intravenous immunoglobulin. Australas J Dermatol. 2009;50:56–59. doi: 10.1111/j.1440-0960.2008.00506.x. [DOI] [PubMed] [Google Scholar]


