Abstract
The approach-withdrawal model posits that depression and anxiety are associated with a relative right asymmetry in frontal brain activity. Most studies have tested this model using measures of cortical brain activity such as electroencephalography. However, neuropsychological tasks that differentially employ left vs. right frontal cortical regions can also be used to test hypotheses from the model. In two independent samples (Study 1 and 2), the present study investigated the performance of currently depressed individuals with or without a comorbid anxiety disorder and healthy controls on neuropsychological tasks tapping primarily left (verbal fluency) or right (design fluency) frontal brain regions. Across both samples, results indicated that comorbid participants performed more poorly than depressed only and control participants on design fluency, while all groups showed equivalent performance on verbal fluency. Moreover, comorbid participants showed “asymmetrical” performance on these two tasks (i.e., poorer design [right frontal] relative to verbal [left frontal] fluency), while depressed only and control participants showed approximately symmetrical profiles of performance. Results from these two samples suggest an abnormal frontal asymmetry in neurocognitive performance driven primarily by right frontal dysfunction among anxious-depressed individuals and highlight the importance of considering comorbid anxiety when examining frontal brain functioning in depression.
Keywords: depression, anxiety, asymmetry, fluency, neuropsychology
There are several theoretical models that attempt to explain the emotional and motivational deficits underlying depression and anxiety (Clark, Watson, & Mineka, 1994; Davidson, Pizzagalli, Nitschke, & Putnam, 2002; Gray, 1994; Shankman & Klein, 2003). One model that has received a great deal of interest is Davidson’s (1992; 1998) approach-withdrawal model, which posits two separate systems of emotion and motivation. The approach system controls appetitive behavior and sensitivity to reward, and is implemented by a neural circuit that incorporates left frontal regions. The withdrawal system underlies behavioral inhibition and avoidance, and is implemented by a neural circuit that incorporates right frontal regions. According to the approach-withdrawal model, depression and anxiety are associated with a hypoactive approach and hyperactive withdrawal system, respectively. As a result, the model hypothesizes that both conditions should be associated with an asymmetry in frontal brain activation due to reduced relative left activity (depression) and increased relative right activity (anxiety).
Using measures of brain activity such as electroencephalography (EEG), numerous studies have examined frontal brain asymmetry in depression. Consistent with expectations, several investigations have found frontal EEG asymmetries that differ from controls (characterized by reduced left relative to right frontal activation)1 in currently depressed individuals (Bruder et al., 1997; Henriques & Davidson, 1991), as well as in individuals at risk for (Tomarken, Dichter, Garber, & Simien, 2004) and in remission from depression (Gotlib, Ranganath, & Rosenfeld, 1998; Henriques & Davidson, 1990; although see Reid, Duke, & Allen, 1998). Similarly, anxiety has been associated with relative increased activity in right frontal regions (Blackhart, Minnix, & Kline, 2006; Kemp et al., 2010; Mathersul et al., 2008; Nitschke et al., 1999; Petruzzello & Landers, 1994; Wiedemann, Pauli, Dengler, Lutzenberger, Birbaumer, & Buchkremer, 1999; although see Heller, Nitschke, Etienne, & Miller, 1997). However, this association may only be present in anxiety disorders characterized by heightened anxious arousal (i.e., panic disorder), and not anxious apprehension (i.e., generalized anxiety disorder; Heller, Nitschke, Etienne, & Miller, 1997).
Given that depression and anxiety are each independently associated with a relative right frontal asymmetry, one would predict that those with both disorders should exhibit the same (or even greater) asymmetry. The few EEG studies that have examined asymmetries in those with comorbid depression and anxiety have yielded mixed results (Kentgen, Tenke, Pine, Fong, Klein, & Bruder, 2000; Mathersul, Williams, Hopkinson, & Kemp, 2008; Nitschke, Heller, Palmieri, & Miller, 1999; see Thibodeau et al., 2006 for review), although this may have been due to small sample sizes or the use of subthreshold symptomatology instead of full threshold-level DSM diagnoses. However, in the largest study of frontal brain asymmetry in comorbid depression and anxiety that used DSM-determined diagnoses, Bruder et al. (1997) found that those with comorbid depression and anxiety exhibited a more relative right frontal asymmetry than controls. Interestingly, the comorbid group also differed from those with non-anxious depression, while the latter group did not differ from controls. This suggests that the frontal asymmetry for depression may be specific to those with comorbid anxiety.
Besides direct measures of brain activity, another way to test hypotheses from the approach-withdrawal model is through the use of neuropsychological tests2 (Kolb & Whishaw, 1995). Neuropsychological tests are designed to provide objective measures of psychological functioning. Additionally, several neuropsychological tests have been shown to be associated with particular brain structures or pathways and can therefore be useful to understand the lateralization and localization of cerebral dysfunction. In relation to the present topic, performance on measures of verbal fluency (in which individuals verbally generate items from a particular cue or category) has been shown to be largely associated with left frontal regions in neuroimaging studies (Frith et al., 1991; Phelps et al., 1997) and differentiates those with left versus right frontal stroke lesions (Stuss et al., 1998). Similarly, performance on measures of design fluency (a non-verbal analogue to verbal fluency, in which individuals generate novel designs) has been shown to be associated with right frontal regions and differentiates those with right versus left frontal stroke lesions (Jones-Gotman, 1991; Ruff, Allen, Farrow, Niemann, & Wylie, 1994). Importantly, both verbal and design fluency have been shown to be associated with other regions in addition to left and right frontal cortices (Baldo & Shimamura, 1998; Elfgren & Risberg, 1998; Voets et al., 2006), respectively, and performance on each measure can be affected by dysfunction in other brain areas. Nonetheless, both measures may be particularly useful in identifying lateralized cerebral dysfunction in depression and anxiety.
The literature is mixed regarding the nature and severity of fluency deficits in depression and anxiety. Several studies have reported verbal fluency deficits in both depression (Henry & Crawford, 2005) and anxiety (Everhart & Harrison, 2002; Gass, Ansley, & Boyette, 1994; Horwitz & McCaffrey, 2008), while others have found no such deficits (Airaksinen, Larsson, & Forsell, 2005; Gladsjo et al., 1998; Kivircik, Yener, Alptekin, & Aydin, 2003). In addition, findings are also mixed regarding design fluency deficits in anxiety (Everhart & Harrison, 2002; Kivircik et al., 2003; Mataix-Cols, Barrios, Sànchez-Turet, Vallejo, & Junqué, 1999). To our knowledge, no studies have examined the effects of comorbidity on design fluency performance and the one study that examined the effects of comorbidity on verbal fluency (Basso et al., 2007) was with psychiatric inpatients, did not control for handedness (a critical variable in examinations of laterality differences), and determined diagnoses from chart review rather than semi-structured interview.
Present Study and Hypotheses
The present study investigated the effects of a comorbid anxiety disorder on neuropsychological measures of frontal brain functioning in individuals diagnosed with major depressive disorder (MDD). Specifically, data was examined from two independent samples (Study 1 and Study 2) in which neuropsychological tasks primarily associated with left (verbal fluency) and right (design fluency) frontal brain regions were administered to individuals with MDD only, comorbid MDD and an anxiety disorder, and healthy controls. In Study 1, independently derived measures of verbal (Benton & Hamsher, 1976) and design fluency (Jones-Gotman & Milner, 1977) were administered to examine potential group differences in each domain. The goal of study 2 was to replicate and extend the study 1 findings by improving upon some of its methodological shortcomings. Additionally, study 2 used co-normed measures of verbal and design fluency from the Delis-Kaplan Executive Function System (D-KEFS; Delis, Kaplan, & Kramer, 2001). This allowed for a group comparison of the relative difference in neuropsychological performance on tasks primarily associated with left (verbal fluency) versus right (design fluency) frontal brain regions (i.e., analogous to the ‘asymmetry index’ used in EEG studies).
In Study 1, given that EEG studies of depression have indicated reduced brain activity in left relative to right frontal regions (Henriques & Davidson, 1990, 1991; Gotlib et al., 1998) and verbal fluency is predominately associated with left frontal regions (Frith et al., 1991; Phelps et al., 1997; Stuss et al., 1998), it was hypothesized that participants with a current MDD diagnosis (both with and without a lifetime comorbid anxiety disorder) would perform worse on verbal fluency relative to controls. Second, since research has also shown that anxiety is associated with abnormal activity in right frontal regions (Blackhart et al., 2006; Mathersul et al., 2008; Wiedemann et al., 1999), it was hypothesized that participants with comorbid MDD and anxiety disorder would perform worse on design fluency relative to those without an anxiety disorder (i.e., MDD only and control participants).
Study 1
Method
Participants
The sample consisted of 64 individuals with current MDD as defined by the Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition (DSM-IV; American Psychiatric Association [APA], 1994), and 33 control participants. Diagnoses were made via the Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 1996). The assessments were conducted by S.A.S. and a master’s level diagnostician. The latter diagnostician has demonstrated high levels of interrater reliability in the past and has trained numerous diagnosticians on the SCID for 15 years (Keller et al., 1995; Klein, Schwartz, Rose, & Leader, 2000; Shankman et al., 2007). She trained S.A.S. to criterion, which included viewing the SCID 101 training videos, observing 2–3 joint SCID interviews, and completing 3 SCID interviews where diagnoses were in agreement. In addition, diagnoses were regularly discussed in best estimate meetings (Klein, Ouimette, Kelly, Ferro, & Riso, 1994).
Among the 64 MDD participants, 30 also met criteria for a lifetime anxiety disorder3, which included social phobia (n = 17), panic disorder (n = 14), specific phobia (n = 8), posttraumatic stress disorder (n = 5), and obsessive-compulsive disorder (n = 4). The control group was also interviewed using the SCID, and was required to have no current or past diagnoses of MDD, dysthymia, anxiety disorder, drug or alcohol dependence, or anorexia nervosa. The control group was also required to have a 24-item Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960) score of less than 8.
Participants were excluded from the study if they had a lifetime diagnosis of schizophrenia or other psychotic disorder, bipolar disorder, or dementia; were unable to read or write English; had a history of head trauma with loss of consciousness; or were left-handed (as confirmed by the Edinburgh Handedness Inventory; Oldfield, 1971). Participants were recruited through advertising in the community (e.g., flyers, Internet postings) and mental health clinics in the greater New York City/Long Island area. All participants gave informed consent and were paid for their participation.
Neuropsychological Tasks
Verbal fluency
Verbal Fluency was assessed using the Controlled Oral Word Association Test (COWAT; Benton & Hamsher, 1976), which contains both Letter (phonemic) and Category (semantic) Fluency conditions. For Letter Fluency, participants were asked to name as many words as they could think of that began with a specific letter of the alphabet within 60 seconds. Participants completed this task for three phonemic categories (F, A, S), with the final score calculated by summing the number of correct words produced across the three trials. For Category Fluency, participants were asked to name as many animals as they could think of within 60 seconds, with the final score equaling the total number of correct words produced. A total Verbal Fluency score was also calculated by adding scores from both the Letter and Category Fluency conditions. Test-retest reliability for both the Letter and Category Fluency conditions has been shown to be adequate, ranging from .60-.88 (des Rosiers & Kavanagh, 1987; Harrison, Buxton, Husain, & Wise, 2000; Sawrie, Chelune, Naugle, & Luders, 1996; Snow, Tierney, Zorzitto, Fisher, & Reid, 1988).
Design fluency
The Design Fluency task was developed by Jones-Gotman and Milner (1977), and contained both Free and Fixed Response conditions. In the Free Response condition, participants were instructed to draw as many novel designs as possible within 5 minutes. In the Fixed Response condition, participants were instructed to draw as many novel four-line designs as possible within 4 minutes. In both conditions, designs could not be nameable, scribbles, or minor variations of previous designs. The final score in each condition consisted of the total number of novel designs drawn. A total Design Fluency score was also calculated by adding the scores from both the Free and Fixed Response conditions. Test-retest reliability for both the Free and Fixed Response conditions has been shown to be adequate, ranging from .51-.91 (Harter, Hart, & Harter, 1999).
Results
Demographics and Clinical Characteristics
Participant demographics and clinical characteristics for Study 1 are presented on the left side of Table 1. The three groups were matched on age, education, ethnicity, and gender (all p’s > .31). In addition, the two depressed groups did not differ on medication use, age of onset of first affective disorder, lifetime alcohol abuse/dependence disorder, or lifetime substance abuse/dependence disorder (p’s > .13).
Table 1.
Demographics and Clinical Characteristics for Participants in Studies 1 and 2
| Study 1 | Study 2 | |||||
|---|---|---|---|---|---|---|
| Control (n= 33) |
MDD (n = 34) |
Comorbid (n= 30) |
Control (n = 50) |
MDD (n = 35) |
Comorbid (n= 43) |
|
| Demographic variables | ||||||
| Age | 33.8 yrs | 32.8 yrs | 34.9 yrs | 33.2 yrs | 32.4 yrs | 36.3 yrs |
| Sex (% female) | 72.7% | 70.6% | 56.7% | 66.0% | 51.4% | 72.1% |
| Race (% Caucasian) | 69.7% | 73.5% | 80.0% | 46.0% | 60.0% | 48.8% |
| Education | ||||||
| Grade 7 to 12 (without graduating high school) |
0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 7.0% |
| Graduated high school or high school equivalent |
18.2% | 20.6% | 16.7% | 8.0% | 0.0% | 4.7% |
| Part college | 27.3% | 32.4% | 53.3% | 32.0% | 42.9% | 37.2% |
| Graduated 2 year college | 15.2% | 2.9% | 6.7% | 0.0% | 8.6% | 4.7% |
| Graduated 4 year college | 12.1% | 20.6% | 13.3% | 32.0% | 31.4% | 23.3% |
| Part graduate/ professional school |
27.3% | 20.6% | 10.0% | 22.0% | 8.6% | 14.0% |
| Completed graduate/ professional school |
0.0% | 2.9% | 0.0% | 6.0% | 8.6% | 9.3% |
| Clinical variables | ||||||
| Global Assessment of Functioning (GAF; SD) |
84.4 (6.8) | 54.4 (7.4) | 51.5 (6.2) | 88.2 (7.0) | 53.5 (7.3) | 52.1 (6.5) |
| Hamilton Rating Scale of Depression (HRSD; SD) |
1.8 (1.9) | 24.8 (8.1) | 28.5 (7.2) | 2.8 (5.8) | 24.6 (7.3) | 26.5 (9.1) |
| Beck Anxiety Inventory (BAI: SD) |
- | - | - | 2.3 (3.4) | 12.2 (7.4) | 21.3 (14.2) |
| Age of onset of first affective disorder |
- | 18.7 yrs | 20.1 yrs | - | 13.1 yrs | 12.8 yrs |
| Currently taking psychiatric medication |
- | 41.2% | 60.0% | - | 28.6 % | 48.8 % |
| Lifetime alcohol abuse/dependence disorder |
- | 44.1% | 43.3% | - | 31.4% | 34.9% |
| Lifetime drug abuse/dependence disorder |
- | 26.5% | 20.0% | - | 17.1% | 27.9% |
| Current psychiatric medications |
||||||
| Any medication | - | 41.2% | 60.0% | - | 28.6 % | 48.8 % |
| SSRI/SNRI | - | 14.7% | 20.9% | |||
| Tricyclic/Tetracyclic Antidepressant |
- | 2.9% | 7.0% | |||
| Atypical Antidepressant | - | 0.0% | 9.3% | |||
| Atypical Antipsychotic | - | 0.0% | 6.0% | |||
| Benzodiazepine | - | 5.9% | 18.7% | |||
| Other | - | 2.9% | 14.0% | |||
Note. MDD = Major Depressive Disorder; SD = Standard deviation; SSRI = Selective Serotonin Reuptake Inhibitor; SNRI = Serotonin-Norepinephrine Reuptake Inhibitor. “Other” medications included stimulants (n = 2 comorbids), serotonin modulators (n = 2 comorbids), tryptophan (n = 1 comorbid), s-adenosylmethionine (SAMe, n = 1 comorbid), and hypnotics (n = 1 MDD, n = 1 comorbid). Information on specific medications was unavailable for Study 1.
As expected, the groups differed on their Global Assessment of Functioning (GAF) scores, F(2, 94) = 230.70, p < .001, ηp2 = .83, with control participants scoring higher than MDD only, F(1, 65) = 298.85, p < .001, and comorbid participants F(1, 61) = 398.00, p < .001, and the MDD only participants scoring higher than the comorbid participants at a trend level, F(1, 62) = 2.89, p = .09. In addition, groups differed on their 24-item HRSD scores, F(2, 94) = 169.11, p < .001, ηp2 = .78, with the MDD only and comorbid participants scoring higher than controls, F(1, 65) = 256.14, p < .001; F(1, 61) = 420.17, p < .001, respectively, and the comorbid participants scoring marginally higher than the MDD only participants, F(1, 62) = 3.65, p = .06.
Neuropsychological Performance
Table 2 displays means (and standard deviations) for each group on measures of Verbal and Design Fluency for Study 1. A separate one-way analysis of variance (ANOVA) with group (control vs. MDD only vs. comorbid) entered as the between-subjects factor was conducted for each performance variable (Verbal Fluency: Letter, Category, Total; Design Fluency: Free Response, Fixed Response, and Total).
Table 2.
Means and Standard Deviations of Neuropsychological Performance on Verbal and Design Fluency in Study 1
| Control (n = 33) |
MDD (n = 34) |
Comorbid (n = 30) |
Group differences |
||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD |
a = Control b = MDD c = Comorbid |
|
| Verbal total | 61.0 | 12.7 | 60.7 | 15.4 | 60.5 | 14.6 | ns |
| Letter fluency | 40.6 | 10.7 | 39.7 | 11.6 | 41.3 | 10.9 | ns |
| Category fluency | 20.5 | 4.5 | 21.0 | 5.1 | 19.2 | 5.1 | ns |
| Design total | 41.2 | 14.1 | 42.5 | 12.7 | 34.6 | 12.5 | a = b > c |
| Free response | 20.8 | 7.8 | 22.3 | 8.2 | 18.4 | 7.1 | b > c |
| Fixed response | 20.4 | 7.8 | 20.2 | 5.8 | 16.2 | 6.7 | a = b > c |
Note. MDD = Major Depressive Disorder; M = Mean; SD = Standard Deviation
Results indicated that groups did not differ on any of the measures of Verbal Fluency- Letter, F(2, 94) = 0.18, ns, ηp2 < .01, Category, F(2, 94) = 1.14, ns, ηp2 = .02, or Total, F(2, 94) = 0.01, ns, ηp2 < .01. However, the groups did differ on two of the three measures of Design Fluency, including the Fixed Response, F(2, 94) = 3.91, p < .05, ηp2 = .08, and Total score, F(2, 94) = 3.25, p < .05, ηp2 = .07, but not the (less constrained) Free Response, F(2, 94) = 1.97, p = .15, ηp2 = .04. Follow-up analyses indicated that, for both the Fixed Response and Total scores, comorbid participants produced fewer novel designs than control and MDD only participants, who did not differ (See Table 2). Given the present study’s a priori hypotheses regarding the group effect on Design Fluency, follow-up analyses were conducted on Free Response even though the overall ANOVA only approached significance. Follow-up comparisons indicated that comorbid participants produced fewer novel designs than MDD only participants (p = .05), but did not differ from controls (p = .23).
Given that the MDD only and comorbid groups differed on GAF and HRSD scores, the present study also examined whether individual differences on these variables contributed to the pattern of results. For these analyses, GAF and HRSD scores were entered into separate GLM models with mean-centered GAF (or HRSD) entered as continuous between-subjects factors. Results suggested that neither GAF nor HRSD were related to performance on any of the measures (all p’s > .60).
Discussion
Results from Study 1 indicated that participants with comorbid MDD and a lifetime anxiety disorder demonstrated poorer design fluency relative to those with MDD only and control participants, who did not differ. In contrast, the hypothesis for verbal fluency was not confirmed in that all three groups were comparable in their performance. In other words, comorbid participants demonstrated a hemisphere-specific deficit in neuropsychological performance on a task primarily associated with the right frontal cortex. These results are consistent with approach-withdrawal model’s hypothesis (Davidson, 1992; 1998) that anxiety is associated with right frontal dysfunction. Interestingly, this finding is also consistent with Bruder et al. (1997), who found that those with comorbid depression and anxiety differed from controls and those with non-anxious depression on frontal EEG asymmetry, but the latter two groups did not differ.
It is important to note that there were several limitations to Study 1. First, there was heterogeneity within the comorbid group, such that (1) the group consisted of a mixture of five different comorbid anxiety disorders, and (2) only some of the participants met criteria for a current anxiety disorder (see Footnote 2). Second, reliability of Axis I diagnoses were not calculated, because the SCID interviews were not audio recorded. Third, although the results are suggestive of an “asymmetrical” cognitive profile within the comorbid participants (characterized by better performance on verbal relative to design fluency), it is difficult to examine group differences in “within-person” cognitive profiles because the COWAT (Benton & Hamsher, 1976) and design fluency tasks (Jones-Gotman & Milner, 1977) were not designed to be directly compared and were normed on separate samples. These limitations were addressed in Study 2.
Study 2
In Study 2, verbal and design fluency were again examined in the three mutually exclusive groups, but improved upon Study 1 by (1) requiring that all comorbid participants meet criteria for current panic disorder (PD), (2) audio recording a subset of SCID interviews to calculate diagnostic reliability, and (3) using co-normed measures of verbal and design fluency from the Delis-Kaplan Executive Function System (D-KEFS; Delis, Kaplan, & Kramer, 2001). Using the D-KEFS allowed for a comparison of the groups on their relative performance on Verbal versus Design Fluency (e.g., Houston et al., 2005) and thus more directly test for group differences on ‘left versus right asymmetry.’ For Study 2, it was hypothesized that, similar to Study 1, participants would not differ on measures of Verbal Fluency. In addition, it was hypothesized that, similar to Study 1, comorbid participants would produce fewer novel designs relative to the MDD only and control participants, who would not differ. Finally, it was hypothesized that comorbid participants would exhibit a within-person asymmetry (characterized by greater Verbal relative to Design Fluency performance) that would differ from the MDD only and controls.
Method
Participants
The sample consisted of 35 individuals with current MDD, 43 individuals with current MDD and current PD (i.e., comorbids) and 50 control participants recruited from the greater Chicago area. Diagnoses were made via the SCID (First et al., 1996) and all interview assessments were conducted by S.A.S. and advanced clinical psychology doctoral students who were trained to criterion by S.A.S. Diagnosticians were trained to criterion by viewing the training videos, observing 2–3 joint SCID interviews with S.A.S., and completing 3 SCID interviews (observed by S.A.S.) where diagnoses were in agreement with S.A.S. Similar to Study 1, participants in the MDD only group were required to have no current or past history of anxiety disorder. Participants in the comorbid group were allowed to meet criteria for additional current and past anxiety disorders, which included social phobia (n = 13), specific phobia (n = 4), posttraumatic stress disorder (n = 7), and obsessive-compulsive disorder (n = 5). To determine reliability of diagnoses, 16 SCIDs were audio recorded and scored by a second rater blind to original diagnoses. The interrater reliability indicated perfect agreement for MDD and PD diagnoses (both Kappas = 1.00). As the neuropsychological measures were given as part of a larger study on early onset depression, both depressed groups were required to have an age of onset of first affective disorder (dysthymia or MDD) before 18. Overall exclusion criteria and the definition of the control group were identical to those of Study 1.
To validate group differences in anxiety, participants also completed the Beck Anxiety Inventory (BAI; Beck, Epstein, Brown, & Steer, 1988). The BAI is a 21-item self-report questionnaire designed as a general measure of anxiety symptom severity. Each item is rated according to how often the symptom has bothered the person over the previous week. Examples of symptoms assessed by the BAI include nervous, shaky, scared, unable to relax, and difficulty breathing. Items were rated on a on a four-point Likert scale ranging from 0 = ‘not at all’ to 3 = ‘a lot.’ Six participants (2 controls, 3 MDD only, 1 comorbid) did not complete the BAI.
Neuropsychological Tasks
Verbal and Design Fluency were assessed using subtests from the D-KEFS (Delis et al., 2001), a widely used neuropsychological battery of executive functioning. Each of these tests yields several scores as described below. Additionally, because the two tests were normed on the same nationally representative reference group (N=1750), direct within-person comparisons of Verbal versus Design Fluency performance could be made.
Verbal fluency
The Verbal Fluency test consisted of three conditions: Letter Fluency, Category Fluency, and Category Switching. During the Letter Fluency condition, participants were required to generate as many words as they could that begin with the letters F, A, and S within 60 seconds per letter. Participants were instructed that none of the words could be names of people, places, numbers, or a different conjugation of a previously generated word (e.g., “takes” and “taking”). During the Category Fluency condition, participants were required to generate as many exemplars of the categories animals and boys’ names within 60 seconds per category. During the Category Switching condition, participants were required to switch back and forth between generating as many exemplars of fruits and pieces of furniture(e.g., orange, bed, apple, chair…) as possible within 60 seconds.
The Letter Fluency score was the total number of unique appropriate responses across the three letter trials. Similarly, the Category Fluency score was the total number of unique appropriate responses across the two category trials. Finally, the Category Switching score condition was the total number of unique appropriate fruit and pieces of furniture responses.
Design fluency
The Design Fluency test consisted of three conditions of increasing difficulty – Basic, Filter, and Switch. In the Basic condition, participants were presented with a sheet of paper with 35 squares, each of which contained an identical array of five filled (black) dots. They were instructed to generate a different design in each square by connecting dots with four straight lines, ensuring that each line touches at least one other line at a dot. Participants were required to generate as many designs as they could within 60 seconds. During the Filter condition, participants were presented with 35 squares, each of which contained an identical array of five filled (black) and five empty (white) dots. They were again asked to generate as many designs as they could within 60 seconds in accordance with the same rules as the Basic condition, but by connecting only the empty dots (and thus ignoring the filled dots). Lastly, during the Switch condition, participants were presented with 35 squares containing arrays of five filled and five empty dots. They were required to generate as many four line designs as they could within 60 seconds by switching between an empty and a filled dot (or vice versa) with each line.
Each of these conditions yielded a total score–the number of appropriate unique designs generated within the time limit. Additionally, two types of errors were tabulated for each condition. Any design which was identical to one already produced in the same condition was scored as a perseveration. Any non-perseverative design that otherwise violated a rule (e.g., used only three lines; did not switch from an empty to a filled dot in the Switch condition) was scored as an inappropriate design. For each type of error (perseveration and inappropriate design), a total percent error score (i.e., total percentage errors across all three conditions) was calculated by taking the total number of errors and dividing it by the total number of designs generated during the Design Fluency task.
Discriminating power
Pioneering work by Chapman, Chapman, and colleagues (Chapman & Chapman, 1973, 1978; Miller, Chapman, Chapman, & Collins, 1995) indicated that it is important to match tasks on discriminating power when identifying a domain-specific cognitive deficit. Discriminating power represents the sensitivity of a test to individual differences, such that tests with greater discriminating power are better at differentiating the more from less competent. Discriminating power can be calculated by taking the product of the test’s observed-score variance and test-retest reliability (e.g., Melinder, Barch, Heydebrand, & Csernansky, 2005). The D-KEFS manual (Delis et al., 2001) provided individual subtest measures of observed-score variance ([standard deviation]2 = observed-score variance) and test-retest reliability. Thus, it was possible to calculate and compare the discriminating power of the D-KEFS’ Verbal and Design Fluency subtests.
Table 3 displays the standard deviation, variance, test-retest reliability coefficient, and discriminating power for each subtest of Verbal and Design Fluency. In general, the Verbal Fluency subtests had slightly better discriminating power relative to Design Fluency. This suggests that it may be more difficult to detect group differences in Design Fluency when using the D-KEFS version of the fluency tests.
Table 3.
Psychometric Properties and Discriminating Power for D-KEFS Verbal and Design Fluency Subtests in Study 2
| SD | Variance | Test-Retest Reliability |
Discriminating Power |
|
|---|---|---|---|---|
| Verbal Fluency | ||||
| Letter | 3.14 | 9.86 | .80 | 7.89 |
| Category | 3.25 | 10.56 | .79 | 8.34 |
| Switch | 3.39 | 11.49 | .52 | 5.98 |
| Design Fluency | ||||
| Basic | 2.74 | 7.51 | .58 | 4.35 |
| Filter | 2.98 | 8.88 | .57 | 5.06 |
| Switch | 2.95 | 8.70 | .32 | 2.79 |
Note. SD = Standard Deviation; SD and test-retest reliability coefficients reported from the D-KEFS normative sample (Delis et al., 2001) for all ages (8 – 89); Variance for each subtest was calculated by squaring the SD (i.e., variance = SD2); Discriminating power was calculated by taking the product of the variance and test-retest reliability (i.e., variance X test-retest reliability).
Results
Demographic and Clinical Characteristics
Participant demographics and clinical characteristics for Study 2 are presented on the right side of Table 1. Similar to Study 1, all three groups were matched on age, education, ethnicity, and gender (all p’s > .16). In addition, the two depressed groups did not differ on age of onset of first affective disorder, lifetime alcohol abuse/dependence disorder, or lifetime substance abuse/dependence disorder (p’s > .39), but did differ on psychiatric medication use at a trend level, χ2(2, N = 78) = 3.31, p = .07, with more comorbid participants taking medication compared to participants with MDD only.
As expected, the groups differed on their GAF scores, F(2, 125) = 400.60, p < .001, ηp2 = .87, with control participants scoring higher than the MDD only, F(1, 83) = 486.27, p < .001, and comorbid participants F(1, 91) = 660.32, p < .001, who did not differ, F(1, 76) = 0.86, ns. In addition, groups also differed on their HRSD scores, F(2, 125) = 143.66, p < .001, ηp2 = .70, with the MDD only and comorbid participants scoring higher than controls, F(1, 83) = 233.81, p < .001; F(1, 91) = 229.28, p < .001, respectively, but not differing from each other, F(1, 76) = 1.03, ns. Finally, groups differed on their BAI scores, F(2, 119) = 45.41, p < .001, ηp2 = .43, with MDD only participants reporting higher anxiety scores than controls, F(1, 78) = 64.37, p < .001, and comorbid participants reporting higher scores than controls, F(1, 88) = 79.92, p < .001, and MDD only participants, F(1, 72) = 10.83, p < .01.
Neuropsychological Performance
Table 4 displays means (and standard deviations) for each group on measures of Verbal and Design Fluency for Study 2. In order to compare performance across the Verbal and Design fluency tests, individual raw scores for each condition of the Verbal (Letter, Category, Switch) and Design (Basic, Filter, Switch) Fluency tests were converted to scaled scores (M = 10, SD = 3) according to the D-KEFS norms (Delis et al., 2001). Scaled scores for each condition were then averaged within each domain (Verbal or Design Fluency) to produce a mean scaled score. Comparison of group performance was conducted using a 3 (Group: Controls, MDD only, Comorbids) X 2 (Fluency: Verbal vs. Design) mixed-model ANOVA with Group as the between-subjects factor and Fluency as the within-subjects factors.
Table 4.
Means and Standard Deviations of Neuropsychological Performance on D-KEFS Verbal and Design Fluency in Study 2
| Control (n = 50) |
MDD (n= 35) |
Comorbid (n= 43) |
Group differences‡ | ||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD |
a = Control b = MDD c = Comorbid |
|
| Verbal Fluency total | 97.0 | 19.2 | 94.1 | 17.9 | 96.6 | 16.8 | ns |
| Letter total | 42.1 | 12.2 | 40.4 | 11.4 | 41.1 | 9.8 | ns |
| Category total | 40.2 | 7.8 | 39.5 | 7.0 | 40.8 | 8.2 | ns |
| Switching total | 14.8 | 2.4 | 14.2 | 3.0 | 14.7 | 2.3 | ns |
| Design Fluency total | 30.2 | 8.6 | 29.8 | 8.9 | 25.3 | 8.8 | a = b > c |
| Basic total | 10.4 | 3.3 | 10.5 | 3.5 | 9.0 | 4.1 | a = b > c |
| Filter total | 10.8 | 3.6 | 11.0 | 3.6 | 9.1 | 3.5 | a = b > c |
| Switch total | 9.0 | 3.2 | 8.3 | 3.0 | 7.2 | 2.4 | a = b > c * |
| Design fluency % perseverative errors | 10.4% | 9.1 | 12.6% | 12.2 | 7.9% | 7.8 | ns |
| Design fluency % inappropriate design errors |
1.8% | 3.3 | 2.1% | 3.1 | 3.9% | 5.3 | a = b > c** |
Note. MDD = Major Depressive Disorder; M = Mean; SD = Standard Deviation
= Group differences in Verbal and Design Fluency total and condition scores were examined using scaled scores
= Comorbid participants produced fewer novel designs relative to MDD only participants at a trend level (p < .09)
= Comorbid participants made more inappropriate design errors relative to MDD only participants at a trend level (p = .06).
Results indicated a Group X Fluency interaction, F(2, 125) = 3.36, p < .05, ηp2 = .05. Follow-up analyses indicated that groups did not differ on Verbal Fluency, F(2, 125) = 0.34, ns, but did differ on Design Fluency, F(2, 125) = 4.90, p < .01. Additionally, comorbid participants produced fewer novel designs relative to control and MDD only participants (p’s < .05), who did not differ (p = .79). Group comparisons were also conducted for each individual condition (i.e., Basic, Filter, Switch) of the Design Fluency test to determine whether the pattern of results was similar or different across conditions. As expected, within each Design Fluency condition, comorbid participants produced fewer novel designs relative to control and MDD only participants, who did not differ (See Table 4).
Since both tests were normed on the same nationally representative reference group (Delis et al., 2001), group differences on the relative performance (i.e., within-person difference) in Verbal and Design Fluency were also examined. For these analyses, the mean scaled score for Design Fluency was subtracted from the mean scaled score for Verbal Fluency (i.e., Verbal – Design), producing an asymmetry score with positive scores indicating better performance on Verbal relative to Design Fluency and negative scores indicating vice versa.
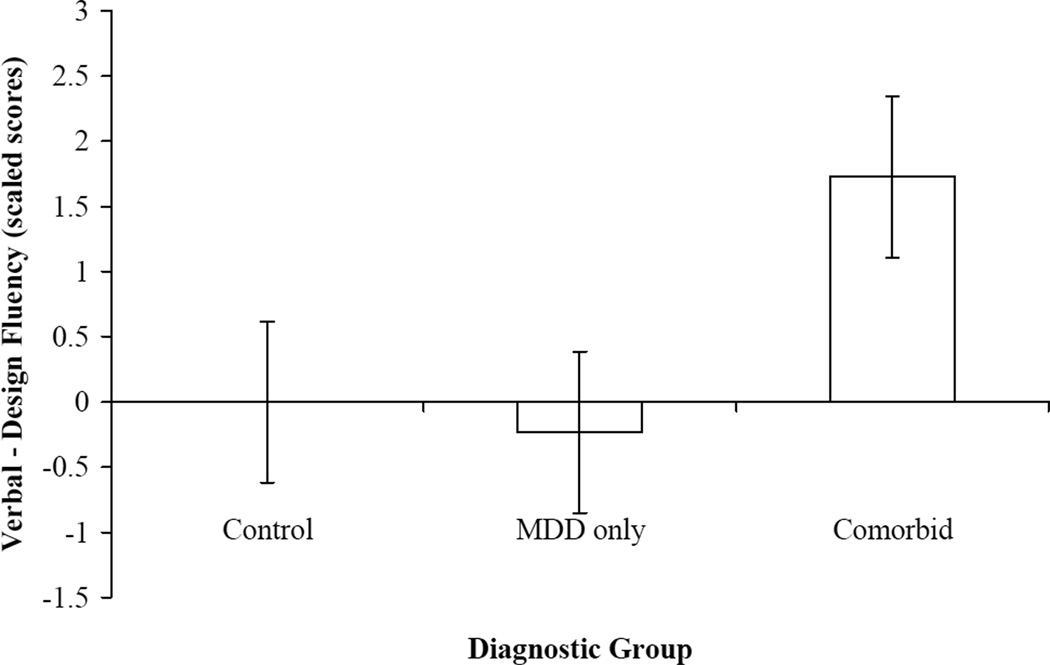
Figure 1 displays means (and standard errors) for the Verbal-Design Fluency asymmetry score for each group. Results indicated that the groups significantly differed on their Verbal-Design Fluency asymmetry score, F(2, 125) = 4.29, p < .05, ηp2 = .06, such that comorbid participants had greater asymmetrical performance on Verbal versus Design Fluency relative to MDD only and control participants, who did not differ. Further examination of the asymmetry scores indicated that control and MDD only participants were nearly symmetrical in their performance on Verbal and Design Fluency, while the comorbid participants performed better on Verbal relative to Design Fluency.
Figure 1.
Group means of within-person difference between total verbal fluency and total design fluency performance. MDD = Major Depressive Disorder. Error bars represent standard error.
It could be argued that comorbid participants produced fewer novel designs simply due to slow psychomotor speed, rather than deficits in Design Fluency per se. Error rates each group made on the Design Fluency task were therefore examined, because if the group differences were simply due to slow processing speed, then the groups should not differ on errors made during the task (see bottom of Table 3). Results indicated that the groups differed on the percentage of inappropriate design, F(2, 120) = 3.23, p < .05, ηp2 = .05, but not perseverative errors, F(2, 120) = 2.18, ns, ηp2 = .04. Follow-up analyses revealed that comorbid participants made more inappropriate design errors relative to control (p < .05) and MDD only participants at a trend level (p = .06), and the latter two groups did not differ (p = .78).
Given that MDD only and comorbid participants differed on psychiatric medication use, the present study also examined whether medication use differentiated Verbal and Design Fluency performance. Within the MDD only and comorbid participants, a one-way ANOVA was conducted with medication status (currently taking psychiatric medication vs. not currently taking psychiatric medication) entered as the between-subjects factor for each performance variable. Results were non-significant for all analyses (p’s > .60) suggesting that medication use did not account for the results.
Finally, the present study examined whether individual differences in depression or anxiety severity were associated with Verbal or Design Fluency performance in the MDD only and comorbid participants. Results indicated that HRSD (all p’s > .22) and BAI scores (all p’s > .21) were not correlated with Verbal or Design Fluency performance. In addition, BAI scores were not correlated with Design Fluency performance within the comorbid participants only (all p’s > .63).
Discussion
In Study 2, the findings from Study 1 were replicated–that there was a deficit in design fluency among participants with comorbid MDD and anxiety disorder–in a more homogeneous group of comorbid participants, all of whom met criteria for current PD. The use of co-normed measures of verbal and design fluency also allowed for the comparison of within-person differences in these two abilities. These analyses revealed that while verbal versus design fluency performance was near-symmetrical for both MDD only and control participants, comorbid participants showed an asymmetry characterized by better verbal than design fluency performance. Finally, the finding that comorbid participants made more inappropriate design errors relative to MDD only and control participants implies that the results described above stem at least partially from a specific deficit in design fluency ability, rather than from general psychomotor slowing.
General Discussion
The approach and withdrawal motivational systems are putatively associated with the left and right frontal cortices, respectively, and have been implicated in both mood and anxiety disorders (Davidson, 1992; 1998). Nonetheless, the impact of comorbid anxiety on asymmetrical frontal brain functioning in depression is not fully understood. While most studies of frontal asymmetry have used physiological measures of brain activity (i.e., EEG), the present study examined relative performance on neuropsychological tasks that are differentially associated with left vs. right frontal functioning. In two independent samples, the present study found that individuals with MDD and a comorbid anxiety disorder showed impaired design fluency, a task primarily associated with right frontal regions, relative to both MDD only and healthy individuals. The robustness of this finding is underscored by the replication using a different measure of design fluency in a more homogeneous sample, in which all comorbid participants met criteria for current PD.
Although results from Study 1 were suggestive of the hypothesized “frontal asymmetry,” the use of co-normed measures of verbal and design fluency in Study 2 provided the opportunity to confirm this pattern. Individuals with comorbid MDD and PD showed a within-person asymmetry characterized by poorer design relative to verbal fluency performance, whereas participants with MDD only and controls showed approximately symmetrical task performance4. Taken together, these findings suggest an abnormal frontal asymmetry in neurocognitive performance driven primarily by right frontal dysfunction among anxious-depressed individuals. Furthermore, the finding that comorbid individuals committed more errors during the design fluency task than the other groups suggests that this result is not a mere artifact of general psychomotor slowing, but instead reflects specific impairment in design fluency.
Results from the present study highlight the importance of considering comorbid anxiety when examining frontal brain asymmetries in depression. Within the EEG literature, there are numerous studies indicating an abnormal frontal brain asymmetry in depression (Henriques & Davidson, 1990, 1991; Gotlib et al., 1998; Tomarken et al., 2004). However, few of these studies have assessed comorbid anxiety symptomatology, which may contribute to the frontal asymmetry (see Thibodeau, Jorgensen, & Kim, 2006). Indeed, in the highest-quality examination to date of the effects of comorbid anxiety on frontal EEG asymmetry in depression, Bruder et al. (1997) found that only comorbid participants (and not those with depression only) differed from control participants. Results from the present study support the assertion of Bruder and colleagues that the presence of comorbid anxiety may act to heighten the asymmetry found in depression.
The present study also highlights the important distinction between measures of brain activity (e.g., EEG) and functioning (e.g., verbal and design fluency). The majority of research thus far supporting approach-withdrawal deficits in depression and anxiety have relied on the use of physiological measures of brain activity (e.g., EEG; Bruder et al., 1997; Gotlib et al., 1998; Henriques & Davidson, 1990; 1991; Tomarken et al., 2004). However, these findings do not directly address brain functioning, which can be assessed using neurocognitive measures, such as verbal and design fluency. Interestingly, the EEG literature has implicated right frontal hyperactivity in anxiety (Blackhart et al., 2006; Kemp et al., 2010; Mathersul et al., 2008; Nitschke et al., 1999; Petruzzello & Landers, 1994; Wiedemann et al., 1999), whereas the present study found poorer performance on a right frontal task (design fluency) in individuals with comorbid depression and anxiety. Therefore, results from the present study suggest that hyperactivation of the right frontal cortex may interfere with the cognitive processes that are associated with this region.
Furthermore, findings from the present study are consistent with Heller and colleagues’ valence-arousal model (Heller et al. 1997). The valence-arousal model distinguishes between two subtypes of anxiety disorders- those characterized by anxious arousal (e.g., panic disorder) and those characterized by anxious apprehension (e.g., generalized anxiety disorder). More specifically, the model hypothesizes that only anxious arousal disorders should be associated with right frontal hyperactivation. In both Study 1 and 2, comorbid participants were predominately characterized by anxious arousal disorders (i.e., panic disorder, specific phobia, social phobia, etc.), and demonstrated neurocognitive deficits on a right frontal task (i.e., design fluency). These findings therefore suggest that heightened anxious arousal may be associated with impaired right frontal cognitive functioning.
The approach-withdrawal model explains the lateralized cerebral deficits associated with depression and anxiety in the context of an affective framework. Yet, the present study utilized neurocognitive (i.e., non-affective) measures of brain functioning in support of the frontal brain asymmetry hypothesized by the approach-withdrawal model. It is important to clarify that the present study does not suggest that fluency deficits lead to motivational deficits (or vice versa). Instead, the results underscore that the brain regions hypothesized to implement approach and withdrawal motivation are not only associated with affect and emotion, but are critical to other cognitive processes (e.g., verbal and design fluency). While the present study was not designed to examine the mechanism through which affect and cognition relate, there are several potential explanations. For example, one hypothesis is that hypervigilance, a behavioral sequela of anxiety, may deplete attentional resources implemented by the right frontal hemisphere that are essential to visuospatial executive functioning. Consistent with this hypothesis, research has shown that anxiety selectively disrupts visuospatial (and not verbal) working memory (Lavric, Rippon, & Gray, 2003; Shackman, Sarinopoulos, Maxwell, Pizzagalli, Lavric, & Davidson, 2006). Future research is needed to explore these and other possible mechanisms that link these affective and neurocognitive constructs. In sum, given the fact that the aforementioned cognitive processes were impaired in a manner consistent with the approach-withdrawal model adds to the nomological network (Cronbach & Meehl, 1955) regarding the role of frontal brain asymmetry in internalizing disorders.
While the present study found no group differences in verbal fluency, this does not necessarily suggest that there is no left hemisphere dysfunction in depression or comorbid depression and anxiety. There is a substantial literature, including EEG (Henriques & Davidson, 1990, 1991; Gotlib et al., 1998), fMRI (Grimm et al., 2008; Herrington et al., 2010), and stroke studies (Robinson, Kubos, Starr, Rao, & Price, 1983; 1984), implicating an association between left frontal hypoactivation and depression. In addition, while design fluency is primarily associated with right frontal regions, several studies have shown that it also associated with left frontal regions (although to a lesser degree than right frontal regions; Baldo, Shimamura, Delis, Kramer, & Kaplan, 2001; Elfgren & Risberg, 1998; Tucha, Smely, & Lange, 1999). Therefore, if design fluency relies on both left and right frontal brain regions, then consistent with the approach-withdrawal model, the finding of design fluency deficits in comorbid participants may have been due to dysfunction in both the left and right frontal hemispheres (though right frontal regions to a greater degree).
There are several explanations for why the present study did not find group differences in verbal fluency. First, while verbal fluency has been shown to be primarily associated with left frontal regions (Phelps, Hyder, Blamire, & Shulman, 1997; Warburton et al., 1996), it may rely on different structures or neural pathways than those associated with approach motivation. Second, the literature on executive functioning deficits in depression is relatively mixed (Basso & Bornstein, 1999; Christensen, Griffiths, MacKinnon, & Jacomb, 1997; Sweeney, Kmiec, & Kupfer, 2000; Veiel, 1997), and several factors have been shown to moderate this relationship. For example, executive functioning has been shown to be negatively associated with the severity of depressive symptomatology (McClintock, Husain, Greer, & Cullum, 2010; McDermott, & Ebmeier, 2009), and several studies that have shown verbal fluency dysfunction used inpatient samples (e.g., Degl’Innocenti, Ågren, & Bäckman, 1998; Fossati, Amar, Raoux, Ergis, & Allilaire, 1999). Therefore, the present study may not have found group differences in verbal fluency because of the use of non-inpatient, community-based samples.
An important issue when comparing performance on different neuropsychological tests is potential differences in psychometric properties (Chapman & Chapman, 1973, 1978; Strauss, 2001). There are several features of any psychological test (e.g., reliability, validity, discriminating power) that can impact the ability to detect group differences in a psychological function. For example, in Study 1 the measures of verbal and design fluency were relatively matched on the known psychometric properties, such as test-retest reliability. In Study 2, the D-KEFS measure of verbal fluency had slightly better discriminating power relative to design fluency. However, despite the slightly poorer discriminability power, comorbid participants still differed in design fluency, highlighting the validity of the finding.
The present study had several strengths. The finding of decreased design fluency in comorbid relative to MDD only and control participants was replicated across two independent samples that were collected in different regions of the United States. In addition, this result was replicated using different measures of verbal and design fluency (Delis et al., 2001; Jones-Gotman & Milner, 1977). Finally, all three groups from both studies were matched on age, education, ethnicity, and gender– critical variables in neurocognitive performance.
The study also had several limitations. First, performance on measures of verbal and design fluency are not uniquely associated with the left and right frontal cortex, but rely on other brain regions as well. Nonetheless, numerous neuroimaging and stroke studies have converged on the finding that verbal fluency is primarily associated with the left frontal cortex (Frith et al., 1991; Jurado & Roselli, 2007; Phelps et al., 1997), while right frontal cortex plays a comparatively larger role in design fluency (Baldo et al., 2001; Elfgren & Risberg, 1998; Tucha et al., 1999). Second, in both Study 1 and 2, participants in the comorbid group were allowed to meet criteria for other current anxiety disorders, adding heterogeneity to the sample. However, only requiring one anxiety disorder may have resulted in a less representative sample given the large comorbidity among anxiety disorders (Kessler, Chiu, Demler, & Walters, 2005). Finally, both Study 1 and Study 2 did not include a measure of psychomotor speed. Although the results suggest that group differences were not due to processing speed deficits (as both verbal and design fluency draw on processing speed), the present study cannot rule out the possibility that the group differences in design fluency were partially caused by differences in psychomotor speed. On the other hand, the Study 2 analysis of error commission during this task suggests that motor speed is unlikely to fully account for the results.
In summary, in two independent samples the present study demonstrated a specific deficit in design fluency among individuals with MDD and a comorbid anxiety disorder compared to those with MDD only and healthy controls. Additionally, comorbid individuals exhibited asymmetrical task performance, characterized by poorer design (i.e., right frontal) relative to verbal (i.e., left frontal) fluency, while MDD only and healthy individuals exhibited approximately symmetrical performance. These results support several hypotheses from the approach-withdrawal model and highlight the importance of considering comorbid anxiety when examining asymmetrical frontal brain functioning in depression.
Acknowledgments
This study was supported by NIMH Grants F31 MH67309 and R21 MH080689, as well as the American Psychological Foundation/Council of Graduate Departments of Psychology Clarence J. Rosecrans Scholarship awarded to S.A.S. We would also like to thank Daniel N. Klein, Gerard E. Bruder, Craig E. Tenke, and Neil H. Pliskin for their assistance on the project.
Footnotes
Most studies examining frontal EEG asymmetry in depression have utilized alpha power as an inverse measure of brain activity. Thus, increased alpha power over left relative to right frontal regions is inferred as decreased brain activation over left relative to right frontal regions. While the use of alpha power as an inverse measure of brain activity has been controversial (Allen, Coan, & Nazarian, 2004; Tenke & Kayser, 2005), several studies have shown that alpha power is inversely correlated with other measures of brain activity, such as functional magnetic resonance imaging (fMRI; Goldman, Stern, Engel, & Cohen, 2002) and positron emission tomography (PET; Oakes et al., 2004). In addition, alpha power has been shown to be inversely associated with performance on neuropsychological tasks known to be mediated by specific cortical regions (e.g., Davidson, Chapman, Chapman, & Henriques, 1990).
There has also been controversy over the use of an asymmetry index compared to examining activity in particular hemispheres. The majority of the research on frontal EEG asymmetry has computed an asymmetry index (i.e., right alpha power minus left alpha power), which has been reliably related to depression. While there have been some studies that have “unpacked” which hemisphere drives the asymmetry index, these findings have been inconsistent (i.e., Bruder et al., 1997; Kentgen et al., 2000). That is, for many studies the relationship between the frontal EEG asymmetry and depression is only seen with the asymmetry index and not with alpha power over a specific hemisphere. Furthermore, Allen and colleagues (2004) have suggested that earlier methods for calculating alpha power at individual electrodes may have magnified individual hemisphere effects. Thus, the relative relationship as indexed by the asymmetry index may be a more reliable metric.
The literature on frontal brain asymmetry in depression and comorbid anxiety has relied heavily on EEG, and has neglected traditional neuropsychological testing. In addition, fMRI and PET studies have been relatively unsuccessful in supporting the frontal EEG asymmetry literature in healthy individuals and those with internalizing disorders (Spielberg, Stewart, Levin, Miller, & Heller, 2008; Spielberg et al., 2011).
The asymmetry controversy discussed in footnote 1 is also relevant within research utilizing neurocognitive indices of frontal brain functioning. The majority of research within this domain has made specific hemispheric predictions, and has generally resisted the use of an asymmetry index due to challenges in matching tasks on psychometric properties (i.e., discriminating power; Chapman & Chapman, 1978). Therefore, as discussed below, task selection is critical when computing an asymmetry index using neurocognitive measures as the tasks need to be matched on important psychometric properties in order to make strong inferences about the relative contribution of left versus right hemisphere functioning.
Twenty of the comorbid MDD and lifetime anxiety disorder participants also met criteria for a current anxiety disorder, including panic disorder (n = 7), social phobia (n = 13), specific phobia (n = 6), obsessive-compulsive disorder (n = 1), and posttraumatic stress disorder (n = 1). The effects of a lifetime anxiety disorder diagnosis were examined as the smaller number of participants with a current anxiety disorder diagnosis (n = 20) would have reduced statistical power. When the comorbid group was limited to only those with a current anxiety disorder, the pattern of results were nearly the same but statistically non-significant.
Control participants exhibited “balanced symmetry” between verbal and design fluency scaled scores. To our knowledge, no studies have reported on the normative difference between verbal and design fluency measures from the D-KEFS. However, the finding of “balanced symmetry” in control participants suggests equally developed verbal and design fluency abilities relative to the D-KEFS’ national reference sample. In addition, this finding supports the use of the D-KEFS measures of verbal and design fluency when examining frontal brain asymmetry in depression and anxiety, as the reference point of comparison (i.e., healthy controls) is 0.Table 1.
References
- Airaksinen E, Larsson M, Forsell Y. Neuropsychological functions in anxiety disorders in population-based samples: Evidence of episodic memory dysfunction. Journal of Psychiatric Research. 2005;39:207–214. doi: 10.1016/j.jpsychires.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Allen JJB, Coan JA, Nazarian M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology. 2004;67:183–218. doi: 10.1016/j.biopsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP. Letter and category fluency in patients with frontal lobe lesions. Neuropsychology. 1998;12:259–267. doi: 10.1037//0894-4105.12.2.259. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP, Delis DC, Kramer J, Kaplan E. Verbal and design fluency in patients with frontal lobe lesions. Journal of the International Neuropsychological Society. 2001;7:586–596. doi: 10.1017/s1355617701755063. [DOI] [PubMed] [Google Scholar]
- Basso MR, Bornstein RA. Relative memory deficits in recurrent versus first-episode major depression on a word-list learning task. Neuropsychology. 1999;13:557–563. doi: 10.1037//0894-4105.13.4.557. [DOI] [PubMed] [Google Scholar]
- Basso MR, Lowery N, Ghormley C, Combs D, Purdie R, Neel J, Bornstein RA. Comorbid anxiety corresponds with neuropsychological dysfunction in unipolar depression. Cognitive Neuropsychiatry. 2007;12:437–456. doi: 10.1080/13546800701446517. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher KdeS. Multilingual Aphasia Examination. Iowa City: University of Iowa; 1976. [Google Scholar]
- Blackhart GC, Minnix JA, Kline JP. Can EEG asymmetry patterns predict future development of anxiety and depression? A preliminary study. Biological Psychology. 2006;72:46–50. doi: 10.1016/j.biopsycho.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JE, Quitkin FM. Regional brain asymmetries in major depression with or without an anxiety disorder: A quantitative electroencephalographic study. Biological Psychiatry. 1997;41:939–948. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. Problems in the measurement of cognitive deficit. Psychological Bulletin. 1973;79:380–385. doi: 10.1037/h0034541. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of differential deficit. Journal of Psychiatric Research. 1978;14:303–311. doi: 10.1016/0022-3956(78)90034-1. [DOI] [PubMed] [Google Scholar]
- Christensen H, Griffiths K, MacKinnon A, Jacomb P. A quantitative review of cognitive deficits in depression and alzheimer-type dementia. Journal of the International Neuropsychological Society. 1997;3:631–651. [PubMed] [Google Scholar]
- Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology. 1994;103:103–116. [PubMed] [Google Scholar]
- Cronbach LJ, Meehl PE. Construct validity in psychological tests. Psychological Bulletin. 1955;52:281–301. doi: 10.1037/h0040957. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain and Cognition. 1992;20:125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition and Emotion. Special Issue: Neuropsychological Perspectives on Affective and Anxiety Disorders. 1998;12:307–330. [Google Scholar]
- Davidson RJ, Chapman JP, Chapman LJ, Henriques JB. Asymmetrical brain electrical activity discriminates between psychometrically-matched verbal and spatial cognitive tasks. Psychophysiology. 1990;27:528–543. doi: 10.1111/j.1469-8986.1990.tb01970.x. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annual Review of Psychology. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- des Rosiers G, Kavanagh D. Cognitive assessment in closed head injury: Stability, validity and parallel forms for two neuropsychological measures of recover. International Journal of Clinical Neuropsychology. 1987;9:162–173. [Google Scholar]
- Degl'Innocenti A, Ågren H, Bäckman L. Executive deficits in major depression. Acta Psychiatrica Scandinavica. 1998;97:182–188. doi: 10.1111/j.1600-0447.1998.tb09985.x. [DOI] [PubMed] [Google Scholar]
- Elfgren CI, Risberg J. Lateralized frontal blood flow increases during fluency tasks: Influence of cognitive strategy. Neuropsychologia. 1998;36:505–512. doi: 10.1016/s0028-3932(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Everhart DE, Harrison DW. Heart rate and fluency performance among high- and low-anxious men following autonomic stress. International Journal of Neuroscience. 2002;112:1149–1171. doi: 10.1080/00207450290026120. [DOI] [PubMed] [Google Scholar]
- Fossati P, Amar G, Raoux N, Ergis AM, Allilaire JF. Executive functioning and verbal memory in young patients with unipolar depression and schizophrenia. Psychiatry Research. 1999;89:171–187. doi: 10.1016/s0165-1781(99)00110-9. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston KJ, Liddle PF, Frackowiak RS. A PET study of word finding. Neuropsychologia. 1991;29:1137–1148. doi: 10.1016/0028-3932(91)90029-8. [DOI] [PubMed] [Google Scholar]
- Gass CS, Ansley J, Boyette S. Emotional correlates of fluency test and maze performance. Journal of Clinical Psychology. 1994;50:586–590. doi: 10.1002/1097-4679(199407)50:4<586::aid-jclp2270500414>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Gladsjo JA, Rapaport MH, McKinney R, Lucas JA, Rabin A, Oliver T, ….Judd LL. A neuropsychological study of panic disorder: Negative findings. Journal of Affective Disorders. 1998;49:123–131. doi: 10.1016/s0165-0327(98)00006-8. [DOI] [PubMed] [Google Scholar]
- Goldman RI, Stern JM, Engel J, Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002;13:2487–2492. doi: 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Ranganath C, Rosenfeld JP. Frontal EEG alpha asymmetry, depression, and cognitive functioning. Cognition & Emotion. 1998;12:449–478. [Google Scholar]
- Gray JA. In: Framework for a taxonomy of psychiatric disorder. van Goozen SHM, Van de Poll NE, Sergeant JA, editors. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1994. pp. 29–59. [Google Scholar]
- Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, Northoff G. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: An fMRI study in severe major depressive disorder. Biological Psychiatry. 2008;63:369–376. doi: 10.1016/j.biopsych.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–61. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JE, Buxton P, Husain M, Wise R. Short test of semantic and phonological fluency: normal performance, validity and test-retest reliability. British Journal of Clinical Psychology. 2000;39:181–191. doi: 10.1348/014466500163202. [DOI] [PubMed] [Google Scholar]
- Harter SL, Hart CC, Harter GW. Expanded scoring criteria for the design fluency test: reliability and validity in neuropsychological and college samples. Archives of Clinical Neuropsychology. 1999;14:419–432. [PubMed] [Google Scholar]
- Heller W, Nitschke JB, Etienne MA, Miller GA. Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology. 1997;106:376–385. doi: 10.1037//0021-843x.106.3.376. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. Journal of Abnormal Psychology. 1990;99:22–31. doi: 10.1037//0021-843x.99.1.22. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. Journal of Abnormal Psychology. 1991;100:535–545. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR. A meta-analytic review of verbal fluency deficits in depression. Journal of Clinical and Experimental Neuropsychology. 2005;27:78–101. doi: 10.1080/138033990513654. [DOI] [PubMed] [Google Scholar]
- Herrington JD, Heller W, Mohanty A, Engels AS, Banich MT, Webb AG, Miller GA. Localization of asymmetric brain function in emotion and depression. Psychophysiology. 2010;47:442–454. doi: 10.1111/j.1469-8986.2009.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz JE, McCaffrey RJ. Effects of a third party observer and anxiety on tests of executive function. Archives of Clinical Neuropsychology. 2008;23:409–417. doi: 10.1016/j.acn.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Houston WS, Delis DC, Lansing A, Jacobson MW, Cobell KR, Salmon DP, Bondi MW. Executive function asymmetry in older adults genetically at-risk for Alzheimer's disease: Verbal versus design fluency. Journal of the International Neuropsychological Society. 2005;11:863–870. doi: 10.1017/s1355617705051015. [DOI] [PubMed] [Google Scholar]
- Jones-Gotman M. Localization of lesions by neuropsychological testing. Epilepsia. 1991;32:S41–S52. [PubMed] [Google Scholar]
- Jones-Gotman M, Milner B. Design fluency: The invention of nonsense drawings after focal cortical lesions. Neuropsychologia. 1977;15:653–674. doi: 10.1016/0028-3932(77)90070-7. [DOI] [PubMed] [Google Scholar]
- Jurado MB, Rosselli M. The elusive nature of executive functions: A review of our current understanding. Neuropsychology Review. 2007;17:213–233. doi: 10.1007/s11065-007-9040-z. [DOI] [PubMed] [Google Scholar]
- Keller MB, Klein DN, Hirschfeld RMA, Kocsis JH, Mccullough JP, Miller I, Marin DB. Results of the DSM-IV mood disorders field trial. American Journal of Psychiatry. 1995;152:843–849. doi: 10.1176/ajp.152.6.843. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Griffiths K, Felmingham KL, Shankman SA, Drinkenburg W, Arns M, Bryant RA. Disorder specificity despite comorbidity: Resting EEG alpha asymmetry in major depressive disorder and post-traumatic stress disorder. Biological Psychology. 2010;85:350–354. doi: 10.1016/j.biopsycho.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Kentgen LM, Tenke CE, Pine DS, Fong R, Klein RG, Bruder GE. Electroencephalographic asymmetries in adolescents with major depression: Influence of comorbidity with anxiety disorders. Journal of Abnormal Psychology. 2000;109:797–802. doi: 10.1037//0021-843x.109.4.797. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivircik BB, Yener GG, Alptekin K, Aydin H. Event-related potentials and neuropsychological tests in obsessive-compulsive disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2003;27:601–606. doi: 10.1016/S0278-5846(03)00047-2. [DOI] [PubMed] [Google Scholar]
- Klein DN, Ouimette PC, Kelly HS, Ferro T. Test-retest reliability of team consensus best-estimate diagonses of axis I and II disorders in a family study. The American Journal of Psychiatry. 1994;151:1043–1047. doi: 10.1176/ajp.151.7.1043. [DOI] [PubMed] [Google Scholar]
- Klein DN, Schwartz JE, Rose S, Leader JB. Five-year course and outcome of dysthymic disorder: A prospective, naturalistic follow-up study. The American Journal of Psychiatry. 2000;157:931–939. doi: 10.1176/appi.ajp.157.6.931. [DOI] [PubMed] [Google Scholar]
- Lavric A, Rippon G, Gray JR. Threat-evoked anxiety disrupts spatial working memory performance: An attentional account. Cognitive Therapy and Research. 2003;27:489–504. [Google Scholar]
- Mataix-Cols D, Barrios M, Sànchez-Turet M, Vallejo J, Junqué C. Reduced design fluency in subclinical obsessive-compulsive subjects. The Journal of Neuropsychiatry and Clinical Neurosciences. 1999;11:395–397. doi: 10.1176/jnp.11.3.395. [DOI] [PubMed] [Google Scholar]
- Mathersul D, Williams LM, Hopkinson PJ, Kemp AH. Investigating models of affect: Relationships among EEG alpha asymmetry, depression, and anxiety. Emotion. 2008;8:560–572. doi: 10.1037/a0012811. [DOI] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Greer TL, Cullum CM. Association between depression severity and neurocognitive function in major depressive disorder: A review and synthesis. Neuropsychology. 2010;24:9–34. doi: 10.1037/a0017336. [DOI] [PubMed] [Google Scholar]
- McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. Journal of Affective Disorders. 2009;119:1–8. doi: 10.1016/j.jad.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Melinder MRD, Barch DM, Heydebrand G, Csernansky JG. Easier tasks can have better discriminating power: The case for verbal fluency. Journal of Abnormal Psychology. 2005;114:385–391. doi: 10.1037/0021-843X.114.3.383. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Palmieri PA, Miller GA. Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 1999;36:628–637. [PubMed] [Google Scholar]
- Oakes TR, Pizzagalli DA, Hendrick AM, Horras KA, Larson CL, Abercrombie HC, Davidson RJ. Functional coupling of simultaneous electrical and metabolic activity in the human brain. Human Brain Mapping. 2004;21:257–270. doi: 10.1002/hbm.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Petruzzello SJ, Landers DM. State anxiety reduction and exercise: Does hemispheric activation reflect such changes? Medicine & Science in Sports & Exercise. 1994;26:1028–1035. [PubMed] [Google Scholar]
- Phelps EA, Hyder F, Blamire AM, Shulman RG. FMRI of the prefrontal cortex during overt verbal fluency. Neuroreport. 1997;8:561–565. doi: 10.1097/00001756-199701200-00036. [DOI] [PubMed] [Google Scholar]
- Reid SA, Duke LM, Allen JJB. Resting frontal electroencephalographic asymmetry in depression: Inconsistencies suggest the need to identify mediating factors. Psychophysiology. 1998;35:389–404. [PubMed] [Google Scholar]
- Robinson RG, Kubos KL, Starr LB, Rao K, Price TR. Mood changes in stroke patients - relationship to lesion location. Comprehensive Psychiatry. 1983;24:555–566. doi: 10.1016/0010-440x(83)90024-x. [DOI] [PubMed] [Google Scholar]
- Robinson RG, Kubos KL, Starr LB, Rao K, Price TR. Mood disorders in stroke patients - importance of location of lesion. Brain. 1984;107:81–93. doi: 10.1093/brain/107.1.81. [DOI] [PubMed] [Google Scholar]
- Ruff RM, Allen CC, Farrow CE, Niemann H. Figural fluency: Differential impairment in patients with left versus right frontal lobe lesions. Archives of Clinical Neuropsychology. 1994;9:41–55. [PubMed] [Google Scholar]
- Sawrie SM, Chelune GJ, Naugle RI, Luders HO. Empirical methods for assessing meaningful change following epilepsy surgery. Journal of the International Neuropsychological Society. 1996;2:556–564. doi: 10.1017/s1355617700001739. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Sarinopoulos I, Maxwell JS, Pizzagalli DA, Lavric A, Davidson RJ. Anxiety selectively disrupts visuospatial working memory. Emotion. 2006;6:40–61. doi: 10.1037/1528-3542.6.1.40. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Klein DN. The relation between depression and anxiety: An evaluation of the tripartite, approach-withdrawal and valence-arousal models. Clinical Psychology Review. 2003;23:605–637. doi: 10.1016/s0272-7358(03)00038-2. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Klein DN, Tenke CE, Bruder GE. Reward sensitivity in depression: A biobehavioral study. Journal of Abnormal Psychology. 2007;116:95–104. doi: 10.1037/0021-843X.116.1.95. [DOI] [PubMed] [Google Scholar]
- Snow WG, Tierney MC, Zorzitto ML, Fisher RH, Reid DW. One-year test-retest reliability of selected tests in older adults. New Orleans. Paper presented at the meeting of the International Neuropsychological Society.1988. [Google Scholar]
- Spielberg JM, Miller GA, Engels AS, Herrington JD, Sutton BP, Banich MT, Heller W. Trait approach and avoidance motivation: Lateralized neural activity associated with executive function. NeuroImage. 2011;54:661–670. doi: 10.1016/j.neuroimage.2010.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Stewart JL, Levin RL, Miller GA, Heller W. Prefrontal cortex, emotion, and approach/withdrawal motivation. Social and Personality Psychology Compass. 2008;2:135–153. doi: 10.1111/j.1751-9004.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss ME. Demonstrating specific cognitive deficits: A psychometric perspective. Journal of Abnormal Psychology. 2001;110:6–14. doi: 10.1037//0021-843x.110.1.6. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Hamer L, Palumbo C, Dempster R, Binns M, Izukawa D. The effects of focal anterior and posterior brain lesions on verbal fluency. Journal of the International Neuropsychological Society. 1998;4:265–278. [PubMed] [Google Scholar]
- Sweeney JA, Kmiec JA, Kupfer DJ. Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biological Psychiatry. 2000;48:674–684. doi: 10.1016/s0006-3223(00)00910-0. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. Reference-free quantification of EEG spectra: Combining current source density (CSD) and frequency principal components analysis (fPCA) Clinical Neurophysiology. 2005;116:2826–2846. doi: 10.1016/j.clinph.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. Journal of Abnormal Psychology. 2006;115:715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Keener AD. Frontal brain asymmetry and depression: A self-regulatory perspective. Cognition and Emotion.Special Issue: Neuropsychological Perspectives on Affective and Anxiety Disorders. 1998;12:387–420. [Google Scholar]
- Tucha O, Smely C, Lange KW. Verbal and figural fluency in patients with mass lesions of the left or right frontal lobes. Journal of Clinical and Experimental Neuropsychology. 1999;21:229–236. doi: 10.1076/jcen.21.2.229.928. [DOI] [PubMed] [Google Scholar]
- Veiel HOF. A preliminary profile of neuropsychological deficits associated with major depression. Journal of Clinical and Experimental Neuropsychology. 1997;19:587–603. doi: 10.1080/01688639708403745. [DOI] [PubMed] [Google Scholar]
- Voets NL, Adcock JE, Flitney DE, Behrens TEJ, Hart Y, Stacey R, … Matthews PM. Distinct right frontal lobe activation in language processing following left hemisphere injury. Brain: A Journal of Neurology. 2006;129:754–766. doi: 10.1093/brain/awh679. [DOI] [PubMed] [Google Scholar]
- Warburton E, Wise RJS, Price CJ, Weiller C, Hadar U, Ramsay S, Frackowiak RS. Noun and verb retrieval by normal subjects studies with PET. Brain. 1996;119:159–179. doi: 10.1093/brain/119.1.159. [DOI] [PubMed] [Google Scholar]
- Wiedemann G, Pauli P, Dengler W, Lutzenberger W, Birbaumer N, Buchkremer G. Frontal brain asymmetry as a biological substrate of emotions in patients with panic disorders. Archives of General Psychiatry. 1999;56:78–84. doi: 10.1001/archpsyc.56.1.78. [DOI] [PubMed] [Google Scholar]