Abstract
Infection of mice with murine gammaherpesvirus 68 (MHV-68) is a well-characterized small animal model for the study of gammaherpesvirus infection. MHV-68 belongs to the same herpesvirus family as herpesvirus saimiri (HVS) of New World squirrel monkeys and human herpesvirus 8 (HHV-8) (also referred to as Kaposi's sarcoma-associated herpesvirus [KSHV]). The open reading frame ORF74 of HVS, KSHV, and MHV-68 encodes a protein with homology to G protein-coupled receptors and chemokine receptors in particular. ORF74 of KSHV (human ORF74 [hORF74]) is highly constitutively active and has been implicated in the pathogenesis of Kaposi's sarcoma. MHV-68-encoded ORF74 (mORF74) is oncogenic and has been implicated in viral replication and reactivation from latency. Here, we show that mORF74 is a functional chemokine receptor. Chemokines with an N-terminal glutamic acid-leucine-arginine (ELR) motif (e.g., KC and macrophage inflammatory protein 2) act as agonists on mORF74, activating phospholipase C, NF-κB, p44/p42 mitogen-activated protein kinase, and Akt signaling pathways and inhibiting formation of cyclic AMP. Using 125I-labeled CXCL1/growth-related oncogene α as a tracer, we show that murine CXCL10/gamma interferon-inducible protein 10 binds mORF74, and functional assays show that it behaves as an antagonist for this virally encoded G protein-coupled receptor. Profound differences in the upstream activation of signal transduction pathways between mORF74 and hORF74 were found. Moreover, in contrast to hORF74, no constitutive activity of mORF74 could be detected.
Murid herpesvirus 4 strain 68 (MHV-68) belongs to the same family of herpesviruses as the prototype gamma-2-herpesvirus herpesvirus saimiri (HVS) and human Kaposi's sarcoma (KS)-associated herpesvirus (KSHV) (also called human herpesvirus 8 [HHV-8]) and is genetically related to the human gamma-1-herpesvirus Epstein-Barr virus (EBV) (13, 44). Herpesviruses establish a lifelong infection of the host, are involved in lymphoproliferative diseases, and are associated with several types of tumors. For example, EBV is associated with Burkitt's lymphoma (32), and KSHV is found in virtually all cases of KS, which is the most common AIDS-related malignancy (11). KSHV and EBV are highly species-specific viruses, for which no fully permissive cell lines or animal models are available. In contrast, MHV-68 readily infects a variety of cell lines (25) and laboratory mice (29). Therefore, MHV-68 has been widely adopted as a model for the study of gammaherpesviruses.
Infection of murid rodents with MHV-68 via the respiratory system leads to lytic infection of lung epithelial cells followed by latent infection in these cells (41); in the spleens of these rodents, mainly B lymphocytes, but also macrophages and dendritic cells, are infected (14, 42, 43, 47). Early after infection, a splenomegaly characterized by a transient expansion of mononuclear cells is observed, followed by a peripheral infectious mononucleosis-like syndrome. Extended periods of infection are characterized by the development of a lymphoproliferative disease in about 10% of the mice (29).
All members of the gamma-2-herpesvirus family encode homologs of cellular proteins. Several of these homologs have retained their cellular functions and properties and are likely to interfere with cellular events. Among these proteins is ORF74, encoded by, for example, HVS (30), KSHV (8), and MHV-68 (44, 45), which shows homology to human chemokine receptors and to CXCR2 in particular. ORF74 encoded by KSHV (human ORF74 [hORF74]) has gained a lot of interest, since this viral G protein-coupled receptor (vGPCR) is constitutively active and can be positively and negatively regulated by endogenous chemokines (2, 16-18, 34). Initially, hORF74 was shown to transform NIH 3T3 cells and to be tumorigenic when these cells were injected into nude mice (2, 3). Moreover, transgenic mice expressing hORF74 under the control of human CD2 promoter or simian virus 40 early promoter develop angioproliferative lesions in multiple organs that morphologically resemble KS lesions (21, 49). This tumorigenic property appears to be a function of both constitutive signaling and regulation by chemokines (22). Similarly, transgenic mice expressing hORF74 exclusively in endothelial cells developed symptoms closely resembling KS, implicating hORF74 in the initiation of KSHV-induced tumor development (26).
At the moment, little is known about ORF74 encoded by MHV-68 (mORF74). mORF74 shows 23% identity with hORF74 (44). In contrast to hORF74, which has been characterized as a lytic gene (9, 23), mORF74 shows early-late, latency-associated expression kinetics, with minimal transcription in lytically infected cells (12, 33, 45). mORF74 has been shown to be involved in viral replication and reactivation of MHV-68 from latency (24, 28). Similar to hORF74, mORF74 was recently shown to transform NIH 3T3 cells (45), suggesting that mORF74 might also act as a constitutively active chemokine receptor. However, so far, no details on the ligand binding characteristics or intracellular signaling have been reported for mORF74.
In this study, we show that mORF74, in contrast to hORF74, is not constitutively signaling in transiently transfected COS-7 cells. However, upon stimulation with a distinct subset of chemokines, mORF74 activates phospholipase C (PLC), p44/p42 mitogen-activated protein kinase (MAPK), Akt, and to a lesser extent, NF-κB, and inhibits cyclic AMP (cAMP) formation. Additionally, we identify murine gamma interferon inducible protein 10 (IP-10) as an antagonist and show that 125I-labeled CXCL1/growth-related oncogene α (GROα) can be used as a radioligand for this virally encoded GPCR.
MATERIALS AND METHODS
Materials.
Chloroquine diphosphate, DEAE-dextran (chloride form), o-phenylenediamine (OPD), and pertussis toxin (PTX) were obtained from Sigma (St. Louis, Mo.). Bovine serum albumin fraction V (BSA) and Nonidet P-40 were obtained from Roche (Mannheim, Germany). d-Luciferin was purchased from Duchefa Biochemie B.V. (Haarlem, The Netherlands). Cell culture media, penicillin, and streptomycin were obtained from Life Technologies (Gaithersburg, Md.), and fetal bovine serum was purchased from Integro B.V. (Dieren, The Netherlands). [myo-2-3H]inositol (17 Ci/mmol) and 125I-labeled CXCL1/GROα (2,200 Ci/mmol) were obtained from Perkin-Elmer Life Sciences (Boston, Mass.). CXCL11/IP-9 was purchased from R&D Systems, Inc. (Minneapolis, Minn.), and other chemokines were obtained from PeproTech (Rocky Hill, N.J.).
DNA constructs.
The cDNA containing MHV-68-encoded ORF74 has been previously described (45) and was subcloned into pcDEF3 (a gift from J.A. Langer) (19). mORF74 was tagged at the N terminus with the influenza virus hemagglutinin (HA) epitope (HA-mORF74) using PCR. The cDNA of the HHV-8-encoded ORF74 (GenBank accession number U71368 with a silent G→T mutation at position 927) was a gift from T. Schwartz and inserted in pcDEF3 after PCR amplification. The reporter plasmid pNF-κB-Luc was obtained from Stratagene (La Jolla, Calif.), and pTLNC-21CRE was obtained from W. Born.
Cell culture and transfection.
COS-7 cells were grown at 5% CO2 at 37°C in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum, 50 IU of penicillin per ml, and 50 mg of streptomycin per ml. COS-7 cells were transiently transfected using DEAE-dextran (106 cells per indicated amount of cDNA). The total amount of cDNA transfected per experiment was kept constant by the addition of the empty vector (pcDEF3).
Enzyme-linked immunosorbent assay (ELISA).
Transfected COS-7 cells were seeded in 48-well plates (Costar). Forty-eight hours after transfection, cells were washed with Tris-buffered saline (TBS), fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS), and permeabilized with 0.5% Nonidet P-40 in TBS where indicated. After the cells were blocked with 1% skim milk in 0.1 M NaHCO3 (pH 8.6) for 4 h at room temperature, they were incubated overnight at 4°C with mouse monoclonal anti-HA antibody (a gift from J. van Minnen) in TBS containing 0.1% BSA. Cells were washed three times with TBS and incubated for 2 h at room temperature with goat anti-mouse horseradish peroxidase-conjugated secondary antibody (Bio-Rad). Subsequently, cells were incubated with 150 μl of OPD substrate solution (2.2 mM OPD, 35 mM citric acid, 66 mM Na2HPO4, 0.015% H2O2 [pH 5.6]). The reaction was stopped with 75 μl of 1 M H2SO4, and absorption at 490 nm was determined.
[3H]inositol phosphate production.
Transfected COS-7 cells were seeded in 24-well plates (Costar), and 24 h after transfection, they were labeled overnight in Earle's inositol-free minimal essential medium supplemented with [myo-2-3H]inositol (1 μCi/ml) in the presence or absence of PTX (100 ng/ml). Subsequently, the medium was aspirated, and cells were washed for 10 min with Dulbecco's modified Eagle's medium containing 25 mM HEPES (pH 7.4) and 20 mM LiCl and incubated for 2 h in the same medium in the presence or absence of the indicated chemokines. The incubation was stopped by aspiration of the medium and addition of ice-cold 10 mM formic acid. After incubation on ice for 90 min, inositol phosphates were isolated by anion-exchange chromatography (Dowex AG1-X8 columns; Bio-Rad) and counted by liquid scintillation.
Reporter gene assays.
COS-7 cells were transfected with pNF-κB-Luc (NF-κB assay) or pTLNC-21CRE (cAMP-responsive element [CRE] assay), and indicated plasmids. Transfected cells were seeded in 96-well white plates (Costar) in serum-free culture medium in the presence or absence of PTX (100 ng/ml). For the NF-κB assay, the cells were incubated with the indicated chemokines for 48 h, after which NF-κB-driven luciferase (Luc) expression was measured by aspiration of the medium and addition of 25 μl of luciferase assay reagent (0.83 mM ATP, 0.83 mM d-luciferin, 18.7 mM MgCl2, 0.78 μM Na2H2P2O7, 38.9 mM Tris [pH 7.8], 0.39% [vol/vol] glycerol, 0.03% [vol/vol] Triton X-100, 2.6 μM dithiothreitol). Luminescence was measured for 3 s in a Wallac Victor2 instrument. For the CRE assay, the indicated chemokines were added together with forskolin (10−5 M) 18 h after transfection, and cells were assayed for luminescence 24 h after transfection as described above.
Western blot analysis.
Transfected COS-7 cells were seeded in 12-well plates (Costar). Forty-eight hours after transfection, cells were stimulated with chemokines (10−7 M, 5 min) and lysed in radioimmunoprecipitation assay buffer (phosphate-buffered saline containing 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 2 μg of aprotinin per ml, 2 μg of leupeptin per ml), sonicated, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and blotted onto a polyvinylidene difluoride membrane. Antibodies recognizing p44/42 MAPK, Akt, and phospho-Akt (S473) (New England Biolabs, Inc., Beverly, Mass.) were used in combination with a goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (Bio-Rad). The antibody recognizing phospho-p44/42 MAPK (T202/Y204) (New England Biolabs, Inc.) was used in combination with a goat anti-mouse horseradish peroxidase-conjugated secondary antibody (Bio-Rad). Protein bands were detected by an enhanced-chemiluminescence assay and directly quantified with an Image station (NEN Life Science Products, Inc., Boston, Mass.).
Binding experiments.
Transfected COS-7 cells were seeded in 48-well plates. Forty-eight hours after transfection, binding was performed on whole cells for 4 h at 4°C using 125I-labeled CXCL1/GROα (approximately 80 pM) in binding buffer (50 mM HEPES [pH 7.4], 1 mM CaCl2, 5 mM MgCl2, 0.5% BSA) containing increasing concentrations of unlabeled chemokine. After incubation, cells were washed three times with ice-cold binding buffer supplemented with 0.5 M NaCl. Subsequently, cells were lysed and counted in a Wallac Compugamma counter.
RESULTS
Expression of mORF74 in COS-7 cells.
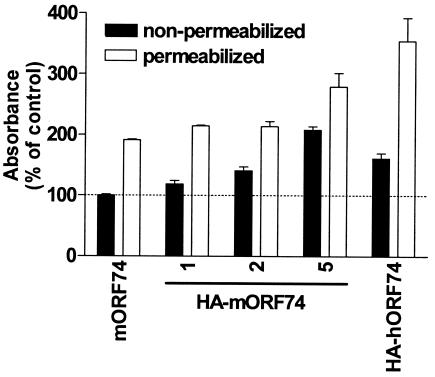
To determine whether mORF74 was expressed at the cell surface of transiently transfected COS-7 cells, mORF74 was tagged with the influenza virus HA epitope. Transfection of COS-7 cells with increasing amounts of cDNA encoding HA-mORF74 resulted in increased cell surface and intracellular expression of HA-mORF74 as determined by an ELISA with a mouse monoclonal anti-HA antibody (Fig. 1). HA-mORF74 was mainly expressed on the cell surface (nonpermeabilized cells), whereas a considerable proportion of HA-hORF74 was also found inside the cells (permeabilized cells). Since transfection of 2 μg of cDNA encoding HA-mORF74 resulted in a cell surface expression level comparable to the level with transfection of 2 μg of the cDNA encoding HA-hORF74, further experiments were performed using 2 μg of cDNA encoding mORF74 per 106 COS-7 cells unless indicated otherwise.
FIG. 1.
Expression of HA-tagged mORF74. COS-7 cells were transfected with increasing amounts (1, 2, and 5 μg) of cDNA encoding HA-mORF74, 2 μg of cDNA encoding untagged mORF74, or 2 μg of cDNA encoding HA-hORF74. Forty-eight hours after transfection, vGPCR expression was determined in an ELISA. A representative experiment performed in triplicate is shown. The experiment was repeated two times.
Comparison of constitutive signaling capacities of mORF74 and hORF74. (i) PLC activation.
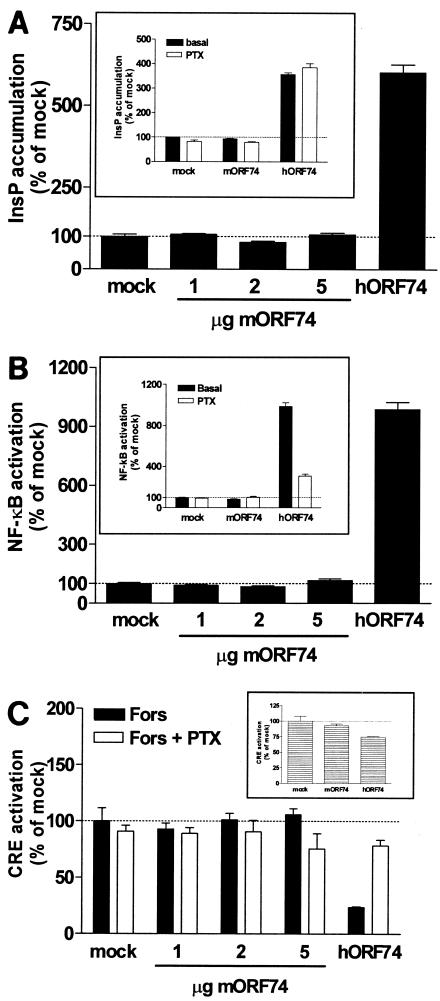
hORF74 has been shown to constitutively activate a variety of signal transduction pathways, including inositol phosphate (InsP) formation through activation of PLC (2, 34, 38). Transient transfection of COS-7 cells with increasing amounts of cDNA encoding mORF74 did not result in an increase of InsP (Fig. 2A), whereas in the same set of experiments, hORF74 expression constitutively enhanced InsP production via a PTX-insensitive pathway (Fig. 2A, insert).
FIG. 2.
Absence of constitutive activity of mORF74. (A) Activation of PLC. COS-7 cells were transfected with increasing amounts of cDNA encoding mORF74, 2 μg of cDNA encoding hORF74, or 5 μg of empty vector (mock). Forty-eight hours after transfection, InsP accumulation was determined. (Insert) Activation of PLC in the presence of PTX. COS-7 cells were transfected with 2 μg of cDNA encoding vGPCR or empty vector and incubated in the presence or absence of PTX. Forty-eight hours after transfection, InsP accumulation was determined. (B) Activation of NF-κB. COS-7 cells were transfected with increasing amounts of cDNA encoding mORF74, 2 μg of cDNA encoding hORF74, or 5 μg of empty vector (mock) and 5 μg of pNFκB-Luc. Forty-eight hours after transfection, NF-κB-driven luciferase expression was determined. (Insert) Activation of NF-κB in the presence of PTX. COS-7 cells were transfected with 2 μg of cDNA encoding vGPCR or empty vector and 5 μg of pNFκB-Luc and incubated in the presence or absence of PTX. Forty-eight hours after transfection, NF-κB-driven luciferase expression was determined. (C) Inhibition of cAMP. COS-7 cells were transfected with increasing amounts of cDNA encoding mORF74, 2 μg of cDNA encodinghORF74, or 5 μg of empty vector (mock) and 5 μg of pTLNC-21CRE and incubated in the presence or absence of PTX. Eighteen hours after transfection, forskolin (Fors) (10−5 M) was added. Twenty-four hours after transfection, CRE-driven luciferase expression was determined. (Insert) CRE modulation in the absence of forskolin. In all panels, a representative experiment performed in triplicate is shown. Each experiment was repeated at least two times.
(ii) NF-κB activation.
Constitutive activation of the transcription factor NF-κB by hORF74 has also been shown in several cell lines (6, 10, 27, 31, 36, 37, 39). We used a luciferase reporter gene with NF-κB-responsive elements to determine whether mORF74 activates NF-κB as well. Transfection of COS-7 cells with increasing amounts of cDNA encoding mORF74 did not activate NF-κB, in contrast to hORF74, which potently stimulated NF-κB-driven luciferase expression (Fig. 2B). The constitutive signaling of hORF74 was inhibited for approximately 70% upon PTX treatment (Fig. 2B, insert), indicating the involvement of Gi proteins as well as PTX-insensitive G proteins.
(iii) Inhibition of cAMP.
Since mORF74 did not show constitutive signaling in the InsP and NF-κB pathways, we sought other pathways that could have been activated in an agonist-independent manner. Most chemokine receptors signal through G proteins of the Gi class, which results in an inhibition of the formation of intracellular cAMP. Consequently, we studied the inhibition of forskolin-induced cAMP levels using a reporter gene assay. Expression of increasing amounts of mORF74 had no effect on forskolin-induced CRE activation. In contrast, hORF74 constitutively inhibited cAMP formation (Fig. 2C). As expected, PTX treatment had no significant effect on mORF74-expressing cells, but it reversed the effect of hORF74, showing that the inhibition of cAMP formation by hORF74 is indeed mediated by Gi proteins. mORF74 also did not modulate CRE activity in the absence of forskolin, whereas hORF74 inhibited CRE in the absence of forskolin (Fig. 2C, insert).
Chemokine regulation of mORF74.
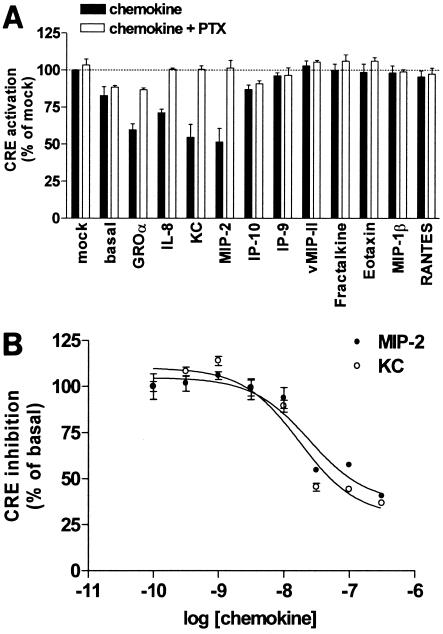
To explore the ability of chemokines to activate mORF74, mORF74-expressing COS-7 cells were incubated with a panel of human CXC chemokines (CXCL1/GROα, CXCL10/IP-10, CXCL11/IP-9, and CXCL8/interleukin 8 [IL-8]), the murine CXC chemokines KC and macrophage inflammatory protein (MIP-2), human CC chemokines (CCL4/MIP-1β, CCL5/RANTES, and CCL11/eotaxin), the HHV-8-encoded CC chemokine vCCL2/vMIP-II, and the CX3C chemokine CX3CL1/fractalkine. The effects of the various chemokines were evaluated in the CRE reporter gene assay.
All of the CXC chemokines containing the glutamic acid-leucine-arginine (ELR) motif (CXCL1/GROα, CXCL8/IL-8, KC, and MIP-2) inhibited forskolin-induced CRE activation in a PTX-sensitive manner, whereas the other CXC, CC, and CX3C chemokines had no effect (Fig. 3A). None of the tested chemokines had an effect on forskolin-induced cAMP levels in mock-transfected cells (data not shown).
FIG. 3.
Activation of mORF74 by chemokines. (A) mORF74-mediated inhibition of cAMP formation. COS-7 cells were transfected with 2 μg of cDNA encoding mORF74 or with 2 μg of empty vector (mock) and 5 μg of pTLNC-21CRE and incubated in the presence or absence of PTX. Eighteen hours after transfection, forskolin (10−5 M) and chemokines (10−7 M) were added. Twenty-four hours after transfection, CRE-driven luciferase expression was determined. (B) Dose-dependent activation of mORF74 by KC and MIP-2. COS-7 cells were transfected with 2 μg of cDNA encoding mORF74 and 5 μg of pTLNC-21CRE. Eighteen hours after transfection, forskolin (10−5 M) and chemokines were added. Twenty-four hours after transfection, CRE-driven luciferase expression was determined. In both panels, a representative experiment performed in triplicate is shown. Each experiment was repeated at least two times.
The agonistic properties of the murine chemokines KC and MIP-2 were further explored, since they are the most relevant, considering the fact that mORF74 is encoded by a murine herpesvirus. KC and MIP-2 both inhibited forskolin-induced CRE activation in a dose-dependent way with pEC50s of 7.6 ± 0.3 and 7.3 ± 0.5, respectively (pEC50 = the negative log of the drug concentration that provides a response halfway between baseline and maximum) (Fig. 3B).
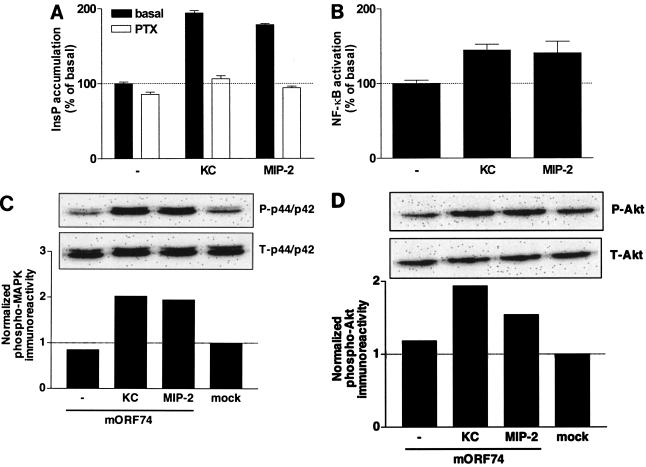
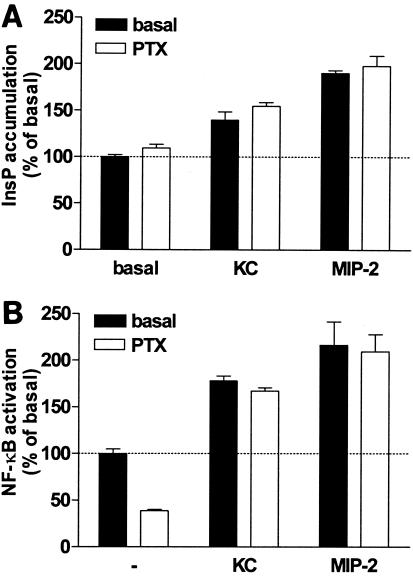
Subsequently, we determined the mORF74-mediated effects of KC and MIP-2 on the activation of PLC and NF-κB. Incubation of mORF74-transfected COS-7 cells with KC or MIP-2 resulted in an increase in InsP formation (Fig. 4A). The agonist-induced PLC activation was sensitive to treatment with PTX, indicative of the involvement of Gi proteins (Fig. 4A). Activation of mORF74 by KC and MIP-2 resulted in a moderate activation of NF-κB (Fig. 4B).
FIG. 4.
mORF74-mediated signal transduction. (A) mORF74-mediated activation of PLC by KC and MIP-2. COS-7 cells were transfected with 2 μg of cDNA encoding mORF74 and incubated in the presence or absence of PTX. Forty-eight hours after transfection, InsP accumulation was determined in the presence or absence (−) of chemokines (10−7 M). A representative experiment performed in triplicate is shown. (B) mORF74-mediated activation of NF-κB by KC and MIP-2. COS-7 cells were transfected with 2 μg of cDNA encoding mORF74 and 5 μg of pNFκB-Luc and incubated in the presence or absence (−) of chemokines (10−7 M). Forty-eight hours after transfection, NF-κB-driven luciferase expression was determined. A representative experiment performed in triplicate is shown. (C) mORF74-mediated activation of p44/p42 MAPK by KC and MIP-2. COS-7 cells were transfected with 2 μg of cDNA encoding mORF74 or 2 μg of empty vector. Forty-eight hours after transfection, cells were stimulated with chemokine (10−7 M) (5 min), and phosphorylation of p44/p42 MAPK was determined by Western blot analysis using specific anti-phospho-p44/p42 MAPK (P-p44/p42) antibodies. Phosphorylation was quantified by chemiluminescence and corrected for total MAPK (T-p44/p42) expression on stripped blots. Data are presented as fold increase over control values (mock-transfected cells). A representative experiment is shown. (D) mORF74-mediated activation of Akt by KC and MIP-2. COS-7 cells were transfected with 2 μg of cDNA encoding mORF74 or 2 μg of empty vector. Forty-eight hours after transfection, cells were stimulated with chemokine (10−7 M) (5 min), and phosphorylation of Akt was determined by Western blot analysis using specific anti-phospho-Akt (P-Akt) antibodies. Phosphorylation was quantified by chemiluminescence and corrected for total Akt (T-Akt) expression on stripped blots. Data are presented as fold increase over control values (mock-transfected cells). A representative experiment is shown. All experiments were repeated at least two times.
Recently, it was suggested that the p44/p42 MAPK and Akt signaling pathways were involved in mORF74-mediated replication of MHV-68, using inhibitors of their upstream kinases MEK and phosphatidylinositol 3-kinase (24). Previously, we and others have shown the constitutive activation of p42/p44 MAPK and Akt by hORF74 in COS-7 cells, which can be positively and negatively regulated by CXCL1/GROα and CXCL10/IP-10, respectively (27, 38, 40). In agreement with our findings in other assays, mORF74 did not constitutively phosphorylate p42/p44 MAPK in COS-7 cells but activated p42/p44 MAPK when stimulated with KC or MIP-2 (Fig. 4C). We also did not detect constitutive signaling for mORF74 to Akt in COS-7 cells, but Akt was activated after incubation with KC or MIP-2 (Fig. 4D).
Differential signaling of mORF74 and hORF74 upon stimulation.
Recently, transgenic mice expressing the wild-type (WT) hORF74 and mutant hORF74 proteins have been generated, and several murine chemokines were found to stimulate or inhibit the constitutive activity of hORF74 in COS-7 cells (22, 26, 49). In view of these mouse models, we compared the upstream signal transduction mechanisms of hORF74 with those of mORF74 upon stimulation with murine chemokines. MIP-2 has been reported to activate hORF74-mediated InsP formation in COS-7 cells, whereas KC was found to be inactive (22). COS-7 cells transfected with hORF74 were incubated with KC and MIP-2 in the presence or absence of PTX. MIP-2 activated hORF74 as expected, and KC increased InsP production to a lesser extent (Fig. 5A). Constitutive InsP formation by hORF74 was insensitive to PTX (Fig. 2A and 5A). In contrast to MIP-2- or KC-induced stimulation of mORF74 (Fig. 4A), the agonist-induced InsP production by hORF74 was insensitive to PTX (Fig. 5A) and thus likely to be mediated by the Gαq/11 class of G proteins. Similar to the findings for hORF74-mediated InsP production, MIP-2 was more potent than KC in activating NF-κB through hORF74 (Fig. 5B). Interestingly, neither KC- nor MIP-2-induced NF-κB activation via hORF74 was sensitive to PTX (Fig. 5B), whereas constitutive NF-κB activation by hORF74 was PTX sensitive (Fig. 2B and 5B).
FIG. 5.
Agonist-induced signaling of hORF74. (A) hORF74-mediated activation of PLC. COS-7 cells were transfected with 2 μg of cDNA encoding hORF74 and incubated in the presence or absence of PTX. Forty-eight hours after transfection, InsP accumulation was determined in the presence or absence of chemokines (10−7 M). (B) hORF74-mediated activation of NF-κB. COS-7 cells were transfected with 2 μg of cDNA encoding hORF74 and incubated in the presence or absence of PTX and chemokines (10−7 M). Forty-eight hours after transfection, NF-κB-driven luciferase expression was determined. In both panels, a representative experiment performed in triplicate is shown. Each experiment was repeated at least two times.
Binding of murine chemokines to mORF74.
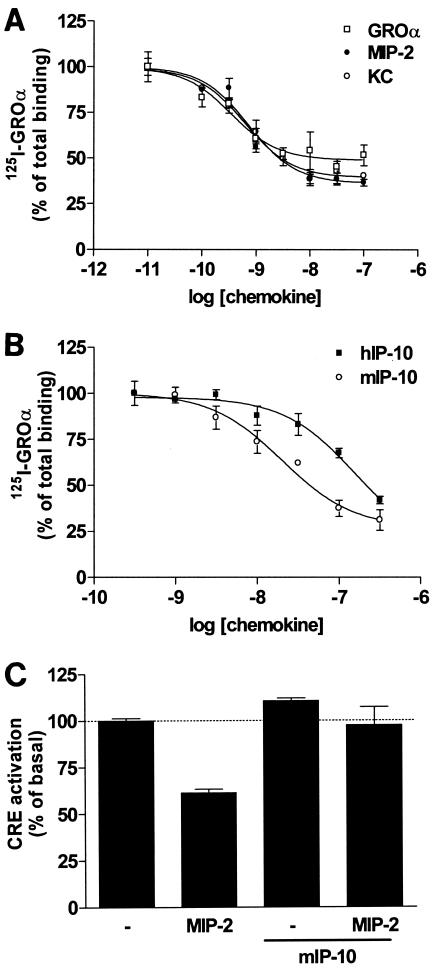
Since human CXCL1/GROα acts as an agonist on mORF74 in the CRE reporter gene assay (Fig. 3A), we used human 125I-labeled CXCL1/GROα as a radioligand to determine the affinities of KC and MIP-2 for mORF74. CXCL1/GROα, KC, and MIP-2 all bound mORF74 with high affinity, with pIC50 values of 9.5 ± 0.02, 9.3 ± 0.1, and 9.1 ± 0.4, respectively (pIC50 = the negative log of the drug concentration that provides an inhibition halfway between maximum and baseline) (Fig. 6A).
FIG. 6.
Binding and antagonism of chemokines. (A) Displacement of 125I-labeled CXCL1/GROα (125I-GRO) by agonists. COS-7 cells were transfected with 5 μg of cDNA encoding mORF74. Forty-eight hours after transfection, 125I-labeled CXCL10/GROα binding was determined in the presence of increasing concentrations of unlabeled CXCL1/GROα, KC, or MIP-2. (B) Displacement of 125I-labeled CXCL1/GROα (125I-GRO) by human and murine IP-10 (hIP-10 and mIP-10, respectively). COS-7 cells were transfected with 5 μg of cDNA encoding mORF74. Forty-eight hours after transfection, 125I-labeled CXCL10/GROα binding was determined in the presence of increasing concentrations of unlabeled hCXCL10/IP-10 or mCXCL10/mIP-10. (C) Antagonism of mIP-10 on mORF74. COS-7 cells were transfected with 2 μg of cDNA encoding mORF74 and 5 μg of pTLNC-21CRE. Eighteen hours after transfection, forskolin (10−5 M), MIP-2 (10−7 M), and mIP-10 (3 × 10−7 M) were added. Twenty-four hours after transfection, CRE-driven luciferase expression was determined. In all panels, a representative experiment performed in triplicate is shown. Each experiment was repeated at least two times.
Recently, KC and MIP-2 were shown to be involved in mORF74-mediated viral replication (24). After infection of NIH 3T3 cells with WT or mORF74 deletion mutants of MHV-68, no difference was found in viral replication in the absence of ligands. However, addition of KC or MIP-2 resulted in an increase in viral replication in cells infected with WT virus but not with mORF74 deletion mutants, indicating a role for mORF74 in MHV-68 replication. Interestingly, Crg-2/mouse IFN-γ (gamma interferon)-inducible protein 10 (mIP-10) was found to block KC-induced MHV-68 replication in NIH 3T3 cells, whereas it had no effect when added alone (24).
Both mIP-10 and human CXCL10/IP-10 could displace 125I-labeled CXCL1/GROα in a dose-dependent manner (Fig. 6B). mORF74 seems to have been optimized for the recognition of mIP-10, since this murine chemokine exhibits a higher pIC50 value (7.5 ± 0.1) for mORF74 than human CXCL10/IP-10 (6.5 ± 0.4).
To determine whether mIP-10 could block agonist-induced CRE inhibition, mORF74-transfected cells were incubated with MIP-2, mIP-10, or MIP-2 and mIP-10 together. As shown in Fig. 6C, mIP-10 reversed the inhibitory effect of MIP-2 on forskolin-induced CRE activation, whereas mIP-10 alone had no effect. Together, these findings support the hypothesis that the inhibitory effects of mIP-10 on agonist-induced viral replication are through antagonism of mORF74.
DISCUSSION
Recently, several GPCRs encoded by various members of the herpesvirus family have been shown to signal in a ligand-independent fashion. Constitutive activity was shown for human cytomegalovirus-encoded US28 (7) and UL33 (46), murine cytomegalovirus-encoded M33 (46), rat cytomegalovirus-encoded R33 (20) and HHV-8-encoded ORF74 (2). On the basis of these observations, it is tempting to speculate that constitutive activity is a general feature of vGPCRs and is important for viral pathogenesis (39).
In view of the transforming potential of MHV-68-encoded mORF74 in NIH 3T3 cells (45), we compared the signaling capacities of mORF74 with the highly constitutively active vGPCR hORF74, encoded by HHV-8. Although HA-mORF74 was expressed at the cell surface at the same level as HA-hORF74 as determined in an ELISA, we could not detect any degree of constitutive signaling by mORF74 to PLC, NF-κB, CRE, MAPK, or Akt in COS-7 cells. Also HA-mORF74 was not signaling constitutively, in contrast to HA-ORF74 (data not shown). However, we could detect ligand-dependent activation of these pathways by mORF74. The human chemokines CXCL1/GROα, CXCL8/IL-8, murine KC, and MIP-2, which all contain the N-terminal motif glutamic acid-leucine-arginine (ELR), inhibited forskolin-induced CRE activation. Inhibition of CRE activation was sensitive to PTX, indicating the involvement of Gi proteins. Similar to hORF74, only chemokines containing the ELR motif could activate mORF74. The signaling capabilities of the murine chemokines KC and MIP-2 were further investigated. Besides inhibiting CRE activation, KC and MIP-2 activated PLC, MAPK, Akt, and, to a lesser extent, NF-κB through mORF74. Activation of PLC by KC and MIP-2 was also PTX sensitive and thus mediated through G proteins of the Gi class. Cellular chemokine receptors, including CXCR2 and CXCR3, activate PLC through a similar classical PTX-sensitive mechanism in COS-7 cells upon stimulation with CXCL8/IL-8 and CXCL11/IP-9, respectively (data not shown). It is likely that this response is mediated by the release of βγ subunits from Gαi proteins which can stimulate PLC isoforms, such as PLC-β2, as was shown for example for CXCR1 and CXCR2 (48).
mORF74 activated NF-κB upon stimulation with KC and MIP-2. However, compared to hORF74, mORF74-induced NF-κB activation was only moderate. It therefore was difficult to address the involvement of Gi proteins in mORF74-mediated NF-κB activation. Of interest is a recent paper showing that overexpression of NF-κB inhibits gammaherpesvirus lytic promoter activation and MHV-68 replication (5). Chemokine-induced activation of NF-κB by mORF74 might inhibit MHV-68 lytic replication and therefore contribute to the establishment or maintenance of viral latency. This would be in agreement with the expression of mORF74 during latency.
MHV-68 is used as a model for infection with human gammaherpesviruses, such as HHV-8, for which no well-defined in vitro and in vivo assays are available. Aside from this, only transgenic mouse models for hORF74-induced KS have been developed (22, 26, 49). Therefore, we investigated whether mORF74 uses signal transduction cascades similar to those used by hORF74 upon activation with murine chemokines. The G proteins through which mORF74 stimulates PLC are different from those used by hORF74. Besides the lack of constitutive signaling of mORF74, activation of PLC by KC or MIP-2 through mORF74 is mediated by Gi proteins, in contrast to hORF74. It has previously been reported that KC does not activate hORF74 in COS-7 cells (22), but we find a small but significant stimulation of hORF74 by KC. Therefore, KC can be considered a partial agonist for hORF74, which might be of importance for the proper evaluation of transgenic mouse models. We confirm the findings that hORF74 constitutively activates NF-κB (27, 36) in a Gi-dependent manner. However, PTX did not fully inhibit constitutive activation of NF-κB, suggesting that constitutive activation of other G proteins, e.g., Gαq/11, contributes to the remaining signal (10, 37). Interestingly, hORF74-mediated activation of NF-κB by KC and MIP-2 is completely independent from Gi activation in COS-7 cells, in contrast to its constitutive activity. Our data suggest that hORF74 constitutively couples to at least two classes of G proteins in COS-7 cells, since hORF74 constitutively activates PLC in a PTX-insensitive manner and NF-κB in a PTX-sensitive manner. Agonists induce a switch in G protein coupling by hORF74 to a PTX-insensitive G protein.
hORF74 has been shown to induce a KS-like disease in transgenic mice (26, 49). Using hORF74 mutants, it was concluded that hORF74-mediated induction of the KS-like disease in transgenic mice requires not only constitutive signaling but also modulation of constitutive activity by endogenous chemokines (22). Our findings suggest that constitutive and ligand-induced signaling of hORF74 may be two, at least partly, separated phenomena due to preferential coupling to different G proteins. This would explain why constitutive activity alone is not sufficient to induce a KS-like disease in transgenic mice. The lack of constitutive activity, the different G protein preference, and the predominant expression of mORF74 during latency, whereas hORF74 is mainly transcribed during lytic infection, indicate that care must be taken in the use of MHV-68 infection as a model system for hORF74-mediated pathogenesis.
We identified the human chemokine 125I-labeled CXCL1/GROα as a suitable and commercially available radioligand for mORF74. KC and MIP-2 displaced 125I-labeled CXCL1/GROα with high affinities. CXCL10/IP-10 and mIP-10, which do not contain the ELR motif, were also able to displace 125I-labeled CXCL1/GROα, with the murine variant having the highest affinity. Although mIP-10 does not inhibit forskolin-induced cAMP formation, it could block the effect of MIP-2. Together with our binding data, this identifies mIP-10 as an antagonist for mORF74, explaining the findings of Lee et al., who found that mIP-10 could block KC-induced replication of MHV-68 (24). Recently, increased mRNA expression of mIP-10 was observed in the lungs of mice and moderate levels of KC were found in bronchoalveolar lavage fluid specimens after infection with MHV-68, both peaking at day 7 postinfection (35). Therefore, it is not unlikely that these chemokines compete for binding to mORF74 in vivo.
In addition to PLC and NF-κB, mORF74 stimulated both p44/p42 MAPK and Akt upon addition of KC or MIP-2. p44/p42 MAPK, known to be involved in cellular proliferation and Akt, an inhibitor of apoptosis, are also activated by hORF74 (27, 38, 40). Interestingly, specific inhibitors of MEK and phosphatidylinositol 3-kinase, two kinases directly upstream of p44/p42 MAPK and Akt, respectively, were able to block KC-enhanced replication of MHV-68 in NIH 3T3 cells (24), further corroborating the importance of these pathways in mORF74-mediated viral replication. A recombinant MHV-68 with a disrupted mORF74 gene did not affect virus replication in vitro or acute replication in vivo compared to WT MHV-68 (28). However, disruption of mORF74 appeared to lead in time to a diminished efficiency of virus reactivation from peritoneal exudate cells. Also, Lee et al. reported no differences in MHV-68 replication in vitro after disruption of mORF74 (24). However, they found increased viral replication in NIH 3T3 cells after addition of KC or MIP-2 for WT MHV-68 but not for virus with disrupted mORF74. In addition, they also showed impaired reactivation from latency for the mutant virus. These findings imply that mORF74 is involved in both viral replication and reactivation from latency. Persistent replication during latency might be a direct consequence of reactivation from latency. Moreover, reactivation from latency and replication during acute infection appear to be two distinct processes (15). It is unclear in which stage of MHV-68 replication mORF74 is important. mORF74 might be involved in suppression of lytic replication, maintenance of latency, and avoiding detection by the immune response through activation of NF-κB (5). On the other hand, mORF74 was shown to be involved in reactivation from latency and persistent replication, thereby allowing the virus to infect other cells or spread through the population. The activity of mORF74 can be regulated in a dose-dependent manner by chemokines and be blocked by antagonists, thereby acting as an environmental sensor. This suggests that the effect on viral replication might be either way, depending on the strength and duration of the chemokine signal.
Although mORF74 displays transforming activity in NIH 3T3 cells (45), we did not detect constitutive signaling activity by mORF74. A possible explanation for the observed transformation of mORF74-expressing NIH 3T3 cells is activation of mORF74 via autocrine secreted chemokines of NIH 3T3 cells, such as KC (4). This might result in activation of transcription factors and subsequent expression and secretion of factors, such as chemokines, cytokines, and VEGF, which in turn can activate mORF74 itself or cellular receptors, resulting in transformation. An autocrine loop, however, would be in conflict with the findings of Lee et al. (24) who found no differences in virus growth between WT and mORF74 deletion mutants after infection of NIH 3T3 cells in the absence of exogenously added KC. One cannot exclude the possibility that mORF74 is constitutively activating pathways other than the ones investigated here and that these pathways are involved in transformation but not virus growth. Also, for the human cytomegalovirus-encoded US27, no constitutive activity could be detected (46), and ECRF3/ORF74 from HVS does not appear to display constitutive activity either, although it responds to chemokines (1). Therefore, constitutive activity might not be a prominent feature for all vGPCRs.
Taken together, we found that mORF74 does not display constitutive activity in transiently transfected COS-7 cells. Nevertheless, the murine chemokines KC and MIP-2 activate NF-κB and the kinase pathways p44/p42 MAPK and Akt. Moreover, KC and MIP-2 activate PLC and inhibit forskolin-induced CRE activation via G proteins of the Gi class. Murine IP-10 acts as an antagonist on mORF74. The identification of 125I-labeled CXCL1/GROα as the first radioligand for mORF74 will facilitate further research of this vGPCR.
Acknowledgments
This work was funded in part by The Netherlands Organization for Scientific Research (Chemische Wetenschappen) (C.P.F. and D.V.), the Royal Netherlands Academy of Arts and Sciences (M.J.S.), and by a Royal Society (London) University Research Fellowship (J.P.S.).
REFERENCES
- 1.Ahuja, S. K., and P. M. Murphy. 1993. Molecular piracy of mammalian interleukin-8 receptor type B by herpesvirus saimiri. J. Biol. Chem. 268:20691-20694. [PubMed] [Google Scholar]
- 2.Arvanitakis, L., E. Geras-Raaka, A. Varma, M. C. Gershengorn, and E. Cesarman. 1997. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 385:347-350. [DOI] [PubMed] [Google Scholar]
- 3.Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. Geras-Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gershengorn, E. A. Mesri, and M. C. Gershengorn. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86-89. [DOI] [PubMed] [Google Scholar]
- 4.Bosio, A., C. Knorr, U. Janssen, S. Gebel, H. J. Haussmann, and T. Muller. 2002. Kinetics of gene expression profiling in Swiss 3T3 cells exposed to aqueous extracts of cigarette smoke. Carcinogenesis 23:741-748. [DOI] [PubMed] [Google Scholar]
- 5.Brown, H. J., M. J. Song, H. Deng, T. T. Wu, G. Cheng, and R. Sun. 2003. NF-κB inhibits gammaherpesvirus lytic replication. J. Virol. 77:8532-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon, M., N. J. Philpott, and E. Cesarman. 2003. The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor has broad signaling effects in primary effusion lymphoma cells. J. Virol. 77:57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casarosa, P., R. A. Bakker, D. Verzijl, M. Navis, H. Timmerman, R. Leurs, and M. J. Smit. 2001. Constitutive signaling of the human cytomegalovirus-encoded chemokine receptor US28. J. Biol. Chem. 276:1133-1137. [DOI] [PubMed] [Google Scholar]
- 8.Cesarman, E., R. G. Nador, F. Bai, R. A. Bohenzky, J. J. Russo, P. S. Moore, Y. Chang, and D. M. Knowles. 1996. Kaposi's sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. J. Virol. 70:8218-8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiou, C. J., L. J. Poole, P. S. Kim, D. M. Ciufo, J. S. Cannon, C. M. ap Rhys, D. J. Alcendor, J. C. Zong, R. F. Ambinder, and G. S. Hayward. 2002. Patterns of gene expression and a transactivation function exhibited by the vGCR (ORF74) chemokine receptor protein of Kaposi's sarcoma-associated herpesvirus. J. Virol. 76:3421-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couty, J. P., E. Geras-Raaka, B. B. Weksler, and M. C. Gershengorn. 2001. Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor signals through multiple pathways in endothelial cells. J. Biol. Chem. 276:33805-33811. [DOI] [PubMed] [Google Scholar]
- 11.Dourmishev, L. A., A. L. Dourmishev, D. Palmeri, R. A. Schwartz, and D. M. Lukac. 2003. Molecular genetics of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) epidemiology and pathogenesis. Microbiol. Mol. Biol. Rev. 67:175-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebrahimi, B., B. M. Dutia, K. L. Roberts, J. J. Garcia-Ramirez, P. Dickinson, J. P. Stewart, P. Ghazal, D. J. Roy, and A. A. Nash. 2003. Transcriptome profile of murine gammaherpesvirus-68 lytic infection. J. Gen. Virol. 84:99-109. [DOI] [PubMed] [Google Scholar]
- 13.Efstathiou, S., Y. M. Ho, S. Hall, C. J. Styles, S. D. Scott, and U. A. Gompels. 1990. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J. Gen. Virol. 71:1365-1372. [DOI] [PubMed] [Google Scholar]
- 14.Flano, E., S. M. Husain, J. T. Sample, D. L. Woodland, and M. A. Blackman. 2000. Latent murine γ-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 165:1074-1081. [DOI] [PubMed] [Google Scholar]
- 15.Gangappa, S., L. F. van Dyk, T. J. Jewett, S. H. Speck, and H. W. Virgin IV. 2002. Identification of the in vivo role of a viral bcl-2. J. Exp. Med. 195:931-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geras-Raaka, E., A. Varma, I. Clark-Lewis, and M. C. Gershengorn. 1998. Kaposi's sarcoma-associated herpesvirus (KSHV) chemokine vMIP-II and human SDF-1α inhibit signaling by KSHV G protein-coupled receptor. Biochem. Biophys. Res. Commun. 253:725-727. [DOI] [PubMed] [Google Scholar]
- 17.Geras-Raaka, E., A. Varma, H. Ho, I. Clark-Lewis, and M. C. Gershengorn. 1998. Human interferon-γ-inducible protein 10 (IP-10) inhibits constitutive signaling of Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor. J. Exp. Med. 188:405-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gershengorn, M. C., E. Geras-Raaka, A. Varma, and I. Clark-Lewis. 1998. Chemokines activate Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor in mammalian cells in culture. J. Clin. Investig. 102:1469-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman, L. A., E. C. Cutrone, S. V. Kotenko, C. D. Krause, and J. A. Langer. 1996. Modifications of vectors pEF-BOS, pcDNA1 and pcDNA3 result in improved convenience and expression. BioTechniques 21:1013-1015. [DOI] [PubMed] [Google Scholar]
- 20.Gruijthuijsen, Y. K., P. Casarosa, S. J. Kaptein, J. L. Broers, R. Leurs, C. A. Bruggeman, M. J. Smit, and C. Vink. 2002. The rat cytomegalovirus R33-encoded G protein-coupled receptor signals in a constitutive fashion. J. Virol. 76:1328-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo, H. G., M. Sadowska, W. Reid, E. Tschachler, G. Hayward, and M. Reitz. 2003. Kaposi's sarcoma-like tumors in a human herpesvirus 8 ORF74 transgenic mouse. J. Virol. 77:2631-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holst, P. J., M. M. Rosenkilde, D. Manfra, S. C. Chen, M. T. Wiekowski, B. Holst, F. Cifire, M. Lipp, T. W. Schwartz, and S. A. Lira. 2001. Tumorigenesis induced by the HHV8-encoded chemokine receptor requires ligand modulation of high constitutive activity. J. Clin. Investig. 108:1789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirshner, J. R., K. Staskus, A. Haase, M. Lagunoff, and D. Ganem. 1999. Expression of the open reading frame 74 (G-protein-coupled receptor) gene of Kaposi's sarcoma (KS)-associated herpesvirus: implications for KS pathogenesis. J. Virol. 73:6006-6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, B. J., U. H. Koszinowski, S. R. Sarawar, and H. Adler. 2003. A gammaherpesvirus G protein-coupled receptor homologue is required for increased viral replication in response to chemokines and efficient reactivation from latency. J. Immunol. 170:243-251. [DOI] [PubMed] [Google Scholar]
- 25.Mistrikova, J., H. Raslova, M. Mrmusova, and M. Kudelova. 2000. A murine gammaherpesvirus. Acta Virol. 44:211-226. [PubMed] [Google Scholar]
- 26.Montaner, S., A. Sodhi, A. Molinolo, T. H. Bugge, E. T. Sawai, Y. He, Y. Li, P. E. Ray, and J. S. Gutkind. 2003. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi's sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell 3:23-36. [DOI] [PubMed] [Google Scholar]
- 27.Montaner, S., A. Sodhi, S. Pece, E. A. Mesri, and J. S. Gutkind. 2001. The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res. 61:2641-2648. [PubMed] [Google Scholar]
- 28.Moorman, N. J., H. W. Virgin IV, and S. H. Speck. 2003. Disruption of the gene encoding the γHV68 v-GPCR leads to decreased efficiency of reactivation from latency. Virology 307:179-190. [DOI] [PubMed] [Google Scholar]
- 29.Nash, A. A., B. M. Dutia, J. P. Stewart, and A. J. Davison. 2001. Natural history of murine γ-herpesvirus infection. Philos. Trans. R. Soc. Lond. B 356:569-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholas, J., K. R. Cameron, and R. W. Honess. 1992. Herpesvirus saimiri encodes homologues of G protein-coupled receptors and cyclins. Nature 355:362-365. [DOI] [PubMed] [Google Scholar]
- 31.Pati, S., M. Cavrois, H. G. Guo, J. S. Foulke, Jr., J. Kim, R. A. Feldman, and M. Reitz. 2001. Activation of NF-κB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi's sarcoma pathogenesis. J. Virol. 75:8660-8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2628. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott Williams and Wilkins, New York, N.Y. [Google Scholar]
- 33.Rochford, R., M. L. Lutzke, R. S. Alfinito, A. Clavo, and R. D. Cardin. 2001. Kinetics of murine gammaherpesvirus 68 gene expression following infection of murine cells in culture and in mice. J. Virol. 75:4955-4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenkilde, M. M., T. N. Kledal, H. Brauner-Osborne, and T. W. Schwartz. 1999. Agonists and inverse agonists for the herpesvirus 8-encoded constitutively active seven-transmembrane oncogene product, ORF-74. J. Biol. Chem. 274:956-961. [DOI] [PubMed] [Google Scholar]
- 35.Sarawar, S. R., B. J. Lee, M. Anderson, Y. C. Teng, R. Zuberi, and S. Von Gesjen. 2002. Chemokine induction and leukocyte trafficking to the lungs during murine gammaherpesvirus 68 (MHV-68) infection. Virology 293:54-62. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz, M., and P. M. Murphy. 2001. Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor constitutively activates NF-κB and induces proinflammatory cytokine and chemokine production via a C-terminal signaling determinant. J. Immunol. 167:505-513. [DOI] [PubMed] [Google Scholar]
- 37.Shepard, L. W., M. Yang, P. Xie, D. D. Browning, T. Voyno-Yasenetskaya, T. Kozasa, and R. D. Ye. 2001. Constitutive activation of NF-κB and secretion of interleukin-8 induced by the G protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus involve Gα13 and RhoA. J. Biol. Chem. 276:45979-45987. [DOI] [PubMed] [Google Scholar]
- 38.Smit, M. J., D. Verzijl, P. Casarosa, M. Navis, H. Timmerman, and R. Leurs. 2002. Kaposi's sarcoma-associated herpesvirus-encoded G protein-coupled receptor ORF74 constitutively activates p44/p42 MAPK and Akt via Gi and phospholipase C-dependent signaling pathways. J. Virol. 76:1744-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smit, M. J., C. Vink, D. Verzijl, P. Casarosa, C. A. Bruggeman, and R. Leurs. 2003. Virally encoded G protein-coupled receptors: targets for potentially innovative anti-viral drug development. Curr. Drug Targets 4:431-441. [DOI] [PubMed] [Google Scholar]
- 40.Sodhi, A., S. Montaner, V. Patel, M. Zohar, C. Bais, E. A. Mesri, and J. S. Gutkind. 2000. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1α. Cancer Res. 60:4873-4880. [PubMed] [Google Scholar]
- 41.Stewart, J. P., E. J. Usherwood, A. Ross, H. Dyson, and T. Nash. 1998. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J. Exp. Med. 187:1941-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sunil-Chandra, N. P., S. Efstathiou, and A. A. Nash. 1992. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 73:3275-3279. [DOI] [PubMed] [Google Scholar]
- 43.Usherwood, E. J., J. P. Stewart, K. Robertson, D. J. Allen, and A. A. Nash. 1996. Absence of splenic latency in murine gammaherpesvirus 68-infected B cell-deficient mice. J. Gen. Virol. 77:2819-2825. [DOI] [PubMed] [Google Scholar]
- 44.Virgin, H. W., IV, P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wakeling, M. N., D. J. Roy, A. A. Nash, and J. P. Stewart. 2001. Characterization of the murine gammaherpesvirus 68 ORF74 product: a novel oncogenic G protein-coupled receptor. J. Gen. Virol. 82:1187-1197. [DOI] [PubMed] [Google Scholar]
- 46.Waldhoer, M., T. N. Kledal, H. Farrell, and T. W. Schwartz. 2002. Murine cytomegalovirus (CMV) M33 and human CMV US28 receptors exhibit similar constitutive signaling activities. J. Virol. 76:8161-8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weck, K. E., S. S. Kim, H. I. Virgin IV, and S. H. Speck. 1999. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 73:3273-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu, D., G. J. LaRosa, and M. I. Simon. 1993. G protein-coupled signal transduction pathways for interleukin-8. Science 261:101-103. [DOI] [PubMed] [Google Scholar]
- 49.Yang, T. Y., S. C. Chen, M. W. Leach, D. Manfra, B. Homey, M. Wiekowski, L. Sullivan, C. H. Jenh, S. K. Narula, S. W. Chensue, and S. A. Lira. 2000. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma. J. Exp. Med. 191:445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]