Abstract
Purpose
We characterized the presence of hemangiogenesis (HA) and lymphangiogenesis (LA) in human corneal specimens exhibiting 13 underlying pathologies.
Methods
Human corneal specimens were obtained from consenting subjects (n = 2 or n = 3 for each pathology; total sample size, n = 35). The pathological specimens were stained with hematoxylin and eosin (H&E) to determine the presence or absence of corneal neovascularization (NV) and superficial or deep stromal distribution of NV. Immunohistochemical staining was then performed to differentiate HA (positive for CD31) from LA (positive for lymphatic vessel endothelial hyaluronan receptor-1 [LYVE-1]).
Results
The double-negative (CD31−/LYVE-1−) immunostaining, indicating the absence of NV, was exhibited by 21 specimens (60%). CD31+/LYVE-1−, indicating the presence of HA and absence of LA, was exhibited by 12 specimens (34%). The double-positive (CD31+/LYVE-1+) phenotype, indicating both HA and LA, was exhibited by 2 specimens (6%). Notably, the CD31−/LYVE-1+ phenotype, indicating the presence of LA and absence of HA, was not detected among the specimens. Deep stromal NV was exhibited in a 4:3 ratio to superficial stromal NV. The double-negative immunostaining was more prevalent in noninflammatory pathologies, particularly in comparison with combined neovascular phenotypes (ie, CD31+ or LYVE-1+). Among the neovascular phenotypes, HA was 7 times more common than LA. Specimens exhibiting LA presented only with the double-positive phenotype.
Conclusions
HA is the predominant component of NV in corneal pathologies. LA accompanies HA; however, isolated LA (from lymphatics in the conjunctiva) does not occur in these corneal pathologies. Our results suggest the potential therapeutic utility of targeting antineovascular therapies specifically for corneal HA and/or LA pathology.
Keywords: corneal neovascularization, hemangiogenesis, lymphangiogenesis, LYVE-1, CD31
Corneal avascularity, which reflects a balance between competing proangiogenic and antiangiogenic mediators, has been characterized as “angiogenic privilege.”1–5 Corneal neovascularization (NV), also referred to as angiogenesis or hemangiogenesis (HA), is the formation of ectopic corneal vasculature from preexisting vasculature.6,7 Corneal NV is thus antagonistic to angiogenic privilege and mediates a number of visual morbidities,8 such as blindness,3 general visual impairment, and abnormal wound healing.1,5,9 Corneal NV is promoted by a wide variety of proangiogenic factors—vascular endothelial growth factor, basic fibroblast growth factor, stromal derived factor-1, CXC chemokines, interleukin-6, platelet-derived growth factor, hepatocyte growth factor, and metalloproteinases (MMP2, MMP9, and MMP14).5,6,10–13 In addition, numerous pathological insults are known to promote corneal NV, including various infectious, inflammatory, and chemical traumas.14–16 Antiangiogenic factors include angiostatin (produced by the proteolytic cleavage of plasminogen) and endostatin (a type XVIII collagen proteolytic product).5
The proposed mechanisms of neovascular visual impairment consist of lipid keratopathy, corneal scarring, and edema.17–20 In addition, preoperative corneal NV is associated with increased incidence of graft failure secondary to penetrating keratoplasty,21,22 the most common form of allotransplantation.23 Several conditions may cause graft failure, such as increased intraocular pressure, infection excluding endophthalmitis, and surface problems that are not immune mediated. However, graft rejection usually indicates the specific immunologic response of the host to the donor corneal tissue. A corneal graft with immunologic response may or may not cause corneal graft failure. Similarly, Regenfuss et al3 report that the presence of lymphatic vasculature suggests a high-risk status of a recipient bed, and Cursiefen et al1 report that HA concomitant with lymphangiogenesis (LA) poses an appreciable threat for graft rejection.
Corneal HA is the neovascular proliferation of blood vessels, whereas LA is the proliferation of lymphatic endothelial cells.24,25 The characterization of LA has historically lagged behind that of HA because of a comparative dearth of known lymphatic vessel protein markers.5,26 Recently, however, a number of LA-specific protein markers (vascular endothelial growth factor receptor-3 [VEGFR-3], podoplanin, prox1, and lymphatic vessel endothelial hyaluronan receptor-1 [LYVE-1]) have been identified.27–29
In the present study, we determined the presence of HA and LA in 13 underlying ocular pathologies by immunoassay with anti-LYVE-1 and anti-CD31 antibodies. Our findings demonstrated that these markers were spatially dispersed in stereotypical neovascular patterns.30–32 We also reported that (1) a neovascular phenotype was detected in 40% of pathological specimens, (2) HA was detected 7 times more frequently than LA (LA did not occur in the absence of HA, whereas 86% of specimens with HA did not exhibit LA), and (3) the ratio of deep stromal NV to superficial stromal NV was 4:3. These findings suggest potential therapeutic utility of clinical interventions targeted specifically to HA and/or LA pathology.
MATERIALS AND METHODS
Specimen Selection
This was a descriptive observational study characterizing the presence of NV in corneal specimen exhibiting 13 underlying ocular pathologies. A total of 35 corneal buttons were selected from the archives of the Ophthalmic Pathology Laboratory at the University of Illinois at Chicago. Two or 3 examples of each of the following diagnoses were retrieved: Acanthamoeba keratitis, bullous keratopathy, chronic keratitis, corneal scarring, Fuchs dystrophy, fungal keratitis, graft failure, graft rejection, herpes simplex virus (HSV) keratitis, inflammatory pannus, keratoconus, pterygium, and ulcerative keratitis with perforation. Subjects provided informed consent for removal of corneal specimens in accordance with the guidelines for experimental investigations required by the Institutional Review Board at the University of Illinois at Chicago (Chicago, IL).
Immunohistochemistry
Formalin-fixed paraffin-embedded blocks were cut into 4-µm consecutive sections. Consecutive sections were individually stained with a hematoxylin and eosin (H&E) stain, an immunohistochemical stain using antibodies against CD31, and an immunohistochemical stain using antibodies against LYVE-1. H&E–stained sections were photographed under an Axioscope II bright field microscope (Carl Zeiss Micro-Imaging, Thornwood, NY) and examined for corneal NV.
For deparaffinization and antigen retrieval, the corneal sections were boiled at 95 to 100°C in 10 mM sodium citrate solution (pH 6.0) for 30 minutes. The sections were then incubated for 16 hours at 4°C with primary antibodies against the vascular endothelial cell marker CD31 (clone JC70A; Dako, Carpinteria, CA) and the lymphatic endothelial cell marker LYVE-1 (Abcam, Cambridge, MA). Sections were then incubated for 1 hour with the appropriate secondary antibody (fluorescein isothiocyanate-conjugated [FITC]-donkey anti-mouse IgG or Cy5 donkey anti-rabbit IgG; Jackson Immunologicals, West Grove, PA). The nucleus was stained with propidium iodide. Confocal microscopic analysis was performed on a Leica SP2 confocal system (Leica Microsystems, Bannockburn, IL) using sequential scanning to minimize bleed-through. Finally, the Cy5 chromophore was activated by 633-nm laser, and the signal was shown as a blue pseudocolor. Omission of primary antibodies in the immunofluorescence stains was used as the control.
Cases were characterized as either HA-only (CD31+/LYVE-1−) or LA-only (CD31−/LYVE-1+), double positive (CD31+/LYVE-1+) showing both HA and LA, and double negative (CD31−/LYVE-1−) showing neither HA nor LA. NV was confirmed via immunohistochemical visualization of vascular lumens peripherally bounded by either CD31+ or LYVE-1+ cells. Immunohistochemical and H&E assessments were used to differentiate superficial stromal NV from deep stromal NV. Propidium iodide counterstaining was used to discriminate nonspecific staining from neovascular immunohistochemical staining. A principal methodological complexity of our investigations, which has been well documented in the literature,14,33 is the fact that lymphatic vasculature may seem disorganized and edematous, sometimes showing luminal collapse; thus occasionally making unambiguous immunohistochemical classification difficult.
RESULTS
Table 1 summarizes the results of immunohistochemical and H&E staining. Figure 1 presents representative corneal NV from 9 pathological buttons. NV (presence of either HA or LA) was seen in 14 cases (40%). Twelve cases (34%) exhibited an HA-only phenotype (CD31+/LYVE-1−; Fig. 2), and none showed LA-only phenotype (CD31−/LYVE-1+). The 12 HA-only cases represented the following diagnoses: bullous keratopathy, chronic keratitis, fungal keratitis, graft failure, graft rejection, HSV keratitis, inflammatory pannus, pterygium, and ulcerative keratitis with perforation. Two cases (6%), both with pterygium, exhibited the double-positive (CD31+/LYVE-1+) phenotype (Fig. 3). The double-negative (CD31−/LYVE-1−) immunostaining was the most prevalent, observed in 21 cases (60%; Fig. 4).
TABLE 1.
Results of Immunohistochemical and H&E Staining
| Pathology | Subject | HA CD31 | LA LYVE-1 | Neovascular Distribution (Superficial Stroma or Deep Stroma) |
|---|---|---|---|---|
| Acanthamoeba keratitis | 1 | − | − | N/A |
| 2 | − | − | N/A | |
| 3 | − | − | N/A | |
| Bullous keratopathy | 4 | + | − | Deep stroma |
| 5 | − | − | N/A | |
| 6 | − | − | N/A | |
| Chronic keratitis | 7 | − | − | N/A |
| 8 | + | − | Deep stroma | |
| 9 | − | − | N/A | |
| Corneal scarring | 10 | − | − | N/A |
| 11 | − | − | N/A | |
| Fuchs dystrophy | 12 | − | − | N/A |
| 13 | − | − | N/A | |
| 14 | − | − | N/A | |
| Fungal keratitis | 15 | − | − | N/A |
| 16 | + | − | Deep stroma | |
| Graft failure | 17 | − | − | N/A |
| 18 | + | − | Deep stroma | |
| Graft rejection | 19 | − | − | N/A |
| 20 | + | − | Deep stroma | |
| 21 | + | − | Deep stroma | |
| HSV keratitis | 22 | − | − | N/A |
| 23 | − | − | N/A | |
| 24 | + | − | Deep stroma | |
| Inflammatory pannus | 25 | + | − | Deep stroma |
| 26 | + | − | Superficial stroma | |
| 27 | + | − | Superficial stroma | |
| Keratoconus | 28 | − | − | N/A |
| 29 | − | − | N/A | |
| 30 | − | − | N/A | |
| Pterygium | 31 | + | − | Superficial stroma |
| 32 | + | + | Superficial stroma | |
| 33 | + | + | Superficial stroma | |
| Ulcerative keratitis | 34 | − | − | N/A |
| With perforation | 35 | + | − | Superficial stroma |
N/A, not applicable.
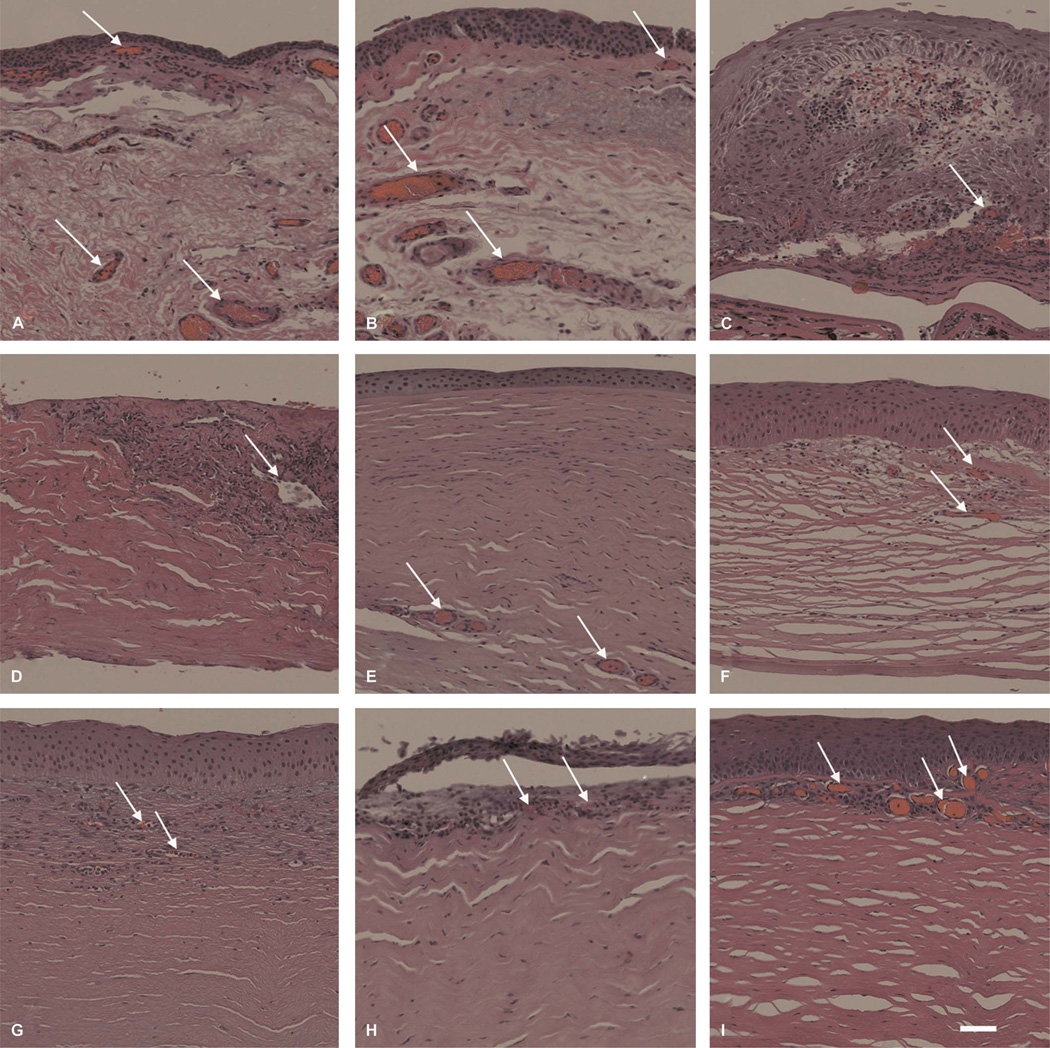
FIGURE 1.
Characterization of corneas exhibiting NV in various underlying ocular pathologies. H&E–stained specimens were assessed for histopathological characteristics of corneal vasculature. Arrows indicate neovascular formations. Pterygium presented with superficial stromal NV in subject 33 (A) and subject 32 (B). C, Ulcerative keratitis (subject 35) was also associated with superficial stromal NV. Specimens with (D) fungal keratitis (subject 16), (E) HSV keratitis (subject 24), (F) graft rejection (subject 21), and (G) chronic keratitis (subject 8) presented with deep stromal NV. Specimens (H and I) with inflammatory pannus in these images show NV distributed to superficial stroma (subjects 26 and 27); scale bar, 47.62 µm.
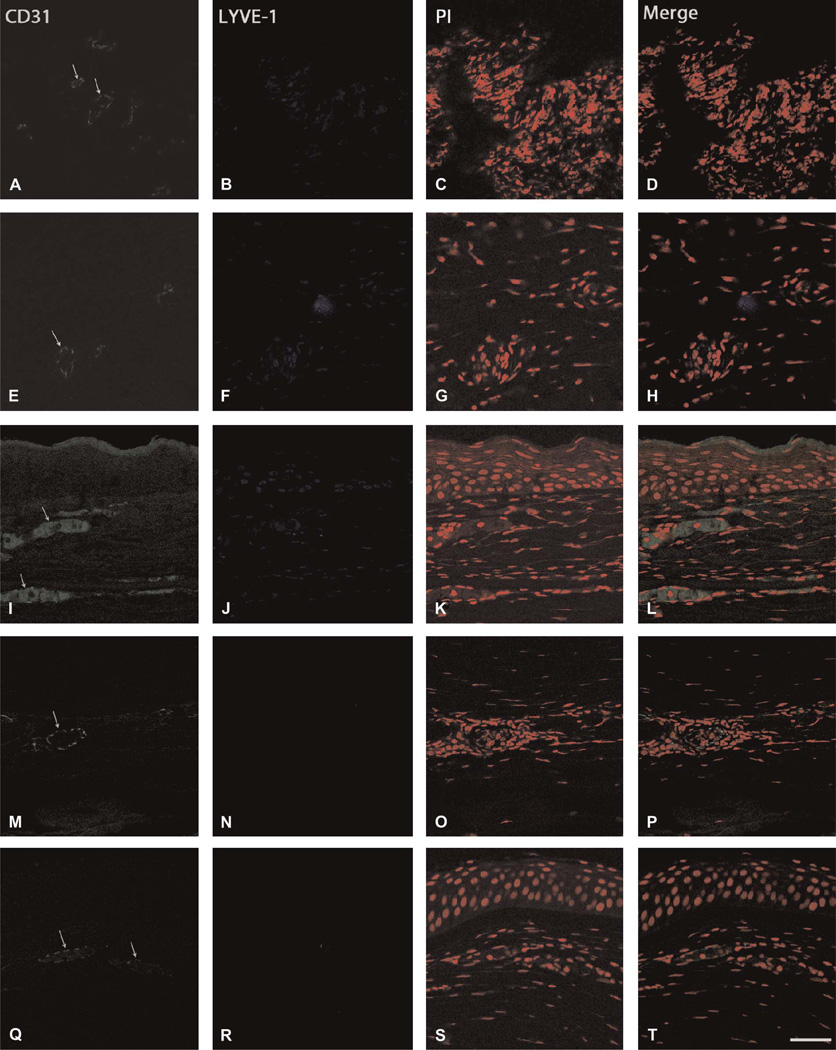
FIGURE 2.
Immunohistochemical visualization of the CD31+/LYVE-1− phenotype. Specimens exhibiting vascular structures were immunostained for CD31 (green), LYVE-1 (blue), and propidium iodide (PI, red) to differentiate between HA and LA. Arrows indicate neovascular formations. Representative specimens showing (A–D) fungal keratitis (subject 16), (E–H) HSV keratitis (subject 24), (I–L) inflammatory pannus (subject 27), (M–P) inflammatory pannus (subject 25), and (Q–T) chronic keratitis (subject 8); scale bar, 47.62 µm.
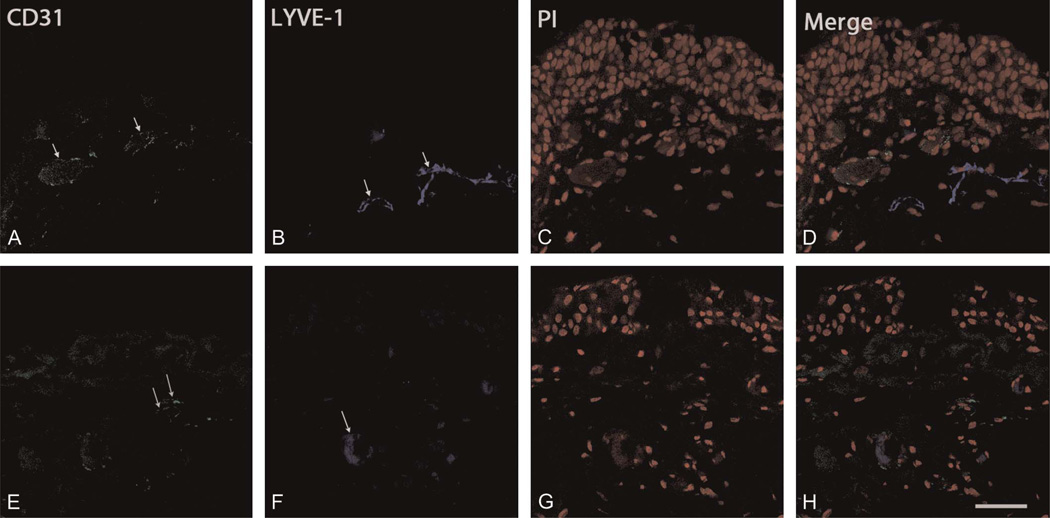
FIGURE 3.
Immunohistochemical visualization of the CD31+/LYVE-1+ phenotype. The specimens with pterygium exhibiting vascular structures were immunostained for CD31 (green), LYVE-1 (blue), and propidium iodide (PI, red). Arrows indicate neovascular formations. Immunostaining shows specimens positive for both CD31 and LYVE-1. Representative specimens showing pterygium (A–D) in subject 33 and (E–H) subject 32; scale bar, 47.62 µm.
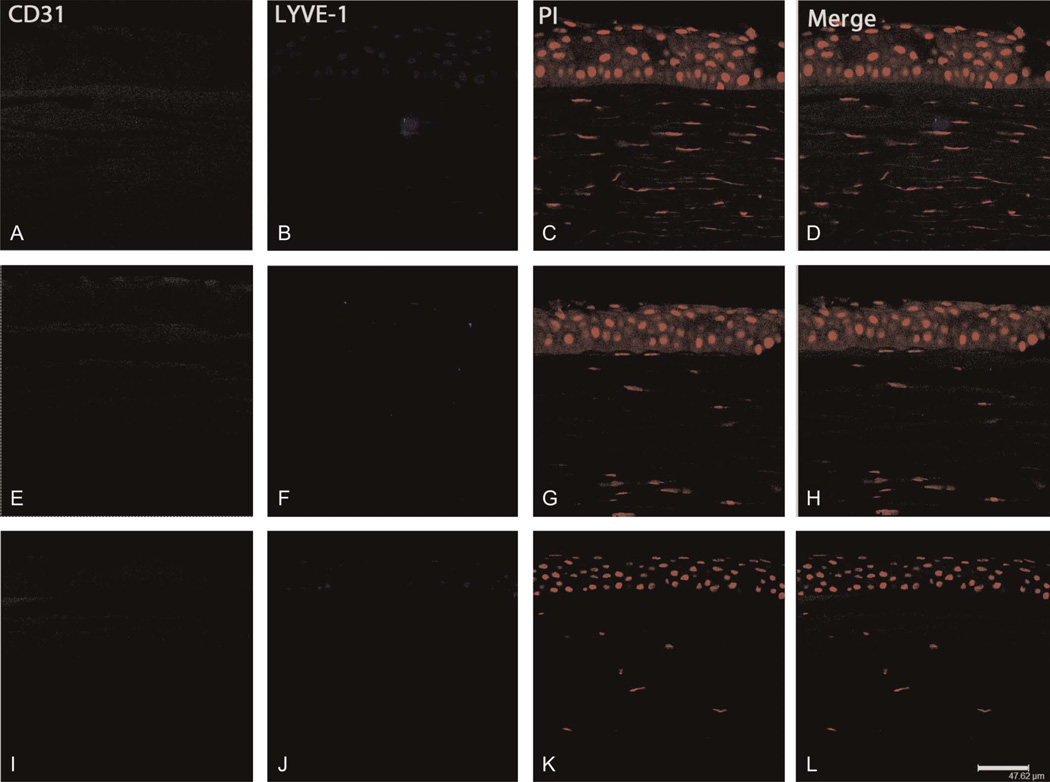
FIGURE 4.
Immunohistochemical visualization of the CD31−/LYVE-1− phenotype. Specimens exhibiting no vascular structures were immunostained for CD31 (green), LYVE-1 (blue), and propidium iodide (PI, red). Representative specimens showing keratoconus (A–D) in subject 30, Fuchs dystrophy (E–H) in subject 14, and corneal scarring (I–L) in subject 11; scale bar, 47.62 µm.
To characterize distribution of neovascular vessels as either superficial stromal or deep stromal, we subjected pathological corneal specimens to H&E staining and assessed for stereotypical presentation of neovascular structures. We then immunostained specimens presenting positively for NV for the presence of CD31 or LYVE-1, as described previously, and noted either superficial or deep stromal distribution. Deep stromal NV was exhibited in 8 of 14 corneal specimens (57% of NV) and was associated with CD31+/LYVE-1− and CD31+/LYVE-1+ phenotypes; bullous keratopathy, chronic keratitis, fungal keratitis, graft failure, graft rejection, HSV keratitis, and inflammatory pannus demonstrated NV (Figs. 1–4). Superficial stromal NV was exhibited in 6 of 14 corneal specimens (43% of NV) and was associated with the CD31+/LYVE-1− phenotype; inflammatory pannus, pterygium, and ulcerative keratitis with perforation demonstrated superficial stromal NV (Figs. 1–4).
DISCUSSION
The association of corneal HA and LA is not completely understood. Some models of NV suggest that HA and LA, which may be induced by similar pathophysiological processes,8,34 develop in parallel8,34 but regress along different time courses.1 An alternative framework focuses on the spatial distribution of HA and LA in vascularized corneas; this approach characterizes, for example, distinct patterns of polarization of HA and LA in murine corneal models.14 Consistent with previous studies,35–37 we detected HA 7 times more frequently than LA. However, because our study explicitly differentiated corneal HA and LA, it is complementary to those studies that only grossly characterize NV for epidemiological purposes.2,38
Our results also demonstrated a notable asymmetry in the frequency of HA and LA across our corneal buttons. Only 14% of CD31+ specimens were also LYVE-1+, whereas 100% of LYVE-1+ specimens were also CD31+. This datum highlights the question of whether LA is necessarily associated with HA in corneal NV—a historically fertile area of research inquiry.8
Additionally, we found that the double-negative immunostaining was more prevalent than HA-only neovascular phenotypes (CD31+ and LYVE-1−) and was particularly common in noninflammatory pathologies (eg, Fuchs dystrophy, keratoconus). This finding is consistent with published models of corneal NV that associate NV with inflammation.14 In contrast, the double-positive phenotype was associated only with pterygium in the present study. Considering that pterygium is a lesion of the perilimbal conjunctiva that extends onto the cornea—characterized by elastotic degeneration of collagen and fibrovascular proliferation—and given the presence of lymphatics in the normal conjunctiva, it is, therefore, not surprising that pterygium would show the CD31+/LYVE-1+ phenotype.
Our final subject of investigation was the discrimination of superficial stromal NV from deep stromal NV. Here, we observed a 4:3 ratio of deep stromal distribution to superficial stroma distribution. In accordance with published models of NV,39–42 we observed that specimens with bullous keratopathy, chronic keratitis, fungal keratitis, graft failure, graft rejection, HSV keratitis, and inflammatory pannus exhibited deep stromal NV, whereas specimens with inflammatory pannus, pterygium, and ulcerative keratitis with perforation exhibited superficial stromal NV. By permitting segmentation of corneal HA and LA distribution via CD31 and LYVE-1 immunohistochemistry, our results complement those corneal NV studies.
In sum, in the present study, the double-negative immunostaining was more prevalent among the pathological corneal specimens than were the neovascular phenotypes. HA-positive specimens predominated, and LA was strongly associated with the presence of HA (although the reverse was not observed). Deep stromal vascular distribution was more common than superficial stromal vascular distribution.
Our results suggest the potential therapeutic utility of targeting antineovascular therapies specifically to corneal HA and/or LA pathology. Further study is needed to elucidate patterns of HA and LA in other corneal diseases.
Acknowledgments
Supported by EY10101 (D.T.A.) and EY01792 (D.T.A.) and an unrestricted grant from the Research to Prevent Blindness, New York, NY.
Footnotes
The authors state that they have no financial or conflicts of interest to disclose.
REFERENCES
- 1.Cursiefen C, Maruyama K, Jackson DG, et al. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea. 2006;25:443–447. doi: 10.1097/01.ico.0000183485.85636.ff. [DOI] [PubMed] [Google Scholar]
- 2.Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- 3.Regenfuss B, Bock F, Parthasarathy A, et al. Corneal (lymph)angiogenesis—from bedside to bench and back: a tribute to Judah Folkman. Lymphat Res Biol. 2008;6:191–201. doi: 10.1089/lrb.2008.6348. [DOI] [PubMed] [Google Scholar]
- 4.Chang JH, Gabison EE, Kato T, et al. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12:242–249. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Ellenberg D, Azar DT, Hallak JA, et al. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res. 2010;29:208–248. doi: 10.1016/j.preteyeres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong Y, Koh DR. Neutrophils promote inflammatory angiogenesis via release of preformed VEGF in an in vivo corneal model. Cell Tissue Res. 2010;339:437–448. doi: 10.1007/s00441-009-0908-5. [DOI] [PubMed] [Google Scholar]
- 7.Chung ES, Chauhan SK, Jin Y, et al. Contribution of macrophages to angiogenesis induced by vascular endothelial growth factor receptor-3-specific ligands. Am J Pathol. 2009;175:1984–1992. doi: 10.2353/ajpath.2009.080515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regina M, Zimmerman R, Malik G, et al. Lymphangiogenesis concurrent with haemangiogenesis in the human cornea. Clin Experiment Ophthalmol. 2007;35:541–544. doi: 10.1111/j.1442-9071.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- 9.Onguchi T, Han KY, Chang JH, et al. Membrane type-1 matrix metalloproteinase potentiates basic fibroblast growth factor-induced corneal neovascularization. Am J Pathol. 2009;174:1564–1571. doi: 10.2353/ajpath.2009.080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ono M. Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci. 2008;99:1501–1506. doi: 10.1111/j.1349-7006.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebrahem Q, Minamoto A, Hoppe G, et al. Triamcinolone acetonide inhibits IL-6- and VEGF-induced angiogenesis downstream of the IL-6 and VEGF receptors. Invest Ophthalmol Vis Sci. 2006;47:4935–4941. doi: 10.1167/iovs.05-1651. [DOI] [PubMed] [Google Scholar]
- 12.Bourcier T, Berbar T, Paquet S, et al. Characterization and functionality of CXCR4 chemokine receptor and SDF-1 in human corneal fibroblasts. Mol Vis. 2003;9:96–102. [PubMed] [Google Scholar]
- 13.Zhang J, Cao R, Zhang Y, et al. Differential roles of PDGFR-alpha and PDGFR-beta in angiogenesis and vessel stability. FASEB J. 2009;23:153–163. doi: 10.1096/fj.08-113860. [DOI] [PubMed] [Google Scholar]
- 14.Ecoiffier T, Yuen D, Chen L. Differential distribution of blood and lymphatic vessels in the murine cornea. Invest Ophthalmol Vis Sci. 2010;51:2436–2440. doi: 10.1167/iovs.09-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi W, Liu J, Li M, et al. Expression of MMP, HPSE, and FAP in stroma promoted corneal neovascularization induced by different etiological factors. Curr Eye Res. 2010;35:967–977. doi: 10.3109/02713683.2010.502294. [DOI] [PubMed] [Google Scholar]
- 16.Wuest TR, Carr DJ. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med. 2010;207:101–115. doi: 10.1084/jem.20091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conrad TJ, Chandler DB, Corless JM, et al. In vivo measurement of corneal angiogenesis with video data acquisition and computerized image analysis. Lab Invest. 1994;70:426–434. [PubMed] [Google Scholar]
- 18.Qazi Y, Maddula S, Ambati BK. Mediators of ocular angiogenesis. J Genet. 2009;88:495–515. doi: 10.1007/s12041-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.BenEzra D, Griffin BW, Maftzir G, et al. Topical formulations of novel angiostatic steroids inhibit rabbit corneal neovascularization. Invest Ophthalmol Vis Sci. 1997;38:1954–1962. [PubMed] [Google Scholar]
- 20.Gordon YJ, Mann RK, Mah TS, et al. Fluorescein-potentiated argon laser therapy improves symptoms and appearance of corneal neovascularization. Cornea. 2002;21:770–773. doi: 10.1097/00003226-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Inoue K, Amano S, Oshika T, et al. Risk factors for corneal graft failure and rejection in penetrating keratoplasty. Acta Ophthalmol Scand. 2001;79:251–255. doi: 10.1034/j.1600-0420.2001.790308.x. [DOI] [PubMed] [Google Scholar]
- 22.Bachmann B, Taylor RS, Cursiefen C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: an evidence-based meta-analysis. Ophthalmology. 2010;117:1300–1305. e1307. doi: 10.1016/j.ophtha.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 23.Vassileva PI, Hergeldzhieva TG. Avastin use in high risk corneal transplantation. Graefes Arch Clin Exp Ophthalmol. 2009;247:1701–1706. doi: 10.1007/s00417-009-1170-y. [DOI] [PubMed] [Google Scholar]
- 24.Chung ES, Saban DR, Chauhan SK, et al. Regulation of blood vessel versus lymphatic vessel growth in the cornea. Invest Ophthalmol Vis Sci. 2009;50:1613–1618. doi: 10.1167/iovs.08-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho WG, Albuquerque RJ, Kleinman ME, et al. Small interfering RNA-induced TLR3 activation inhibits blood and lymphatic vessel growth. Proc Natl Acad Sci U S A. 2009;106:7137–7142. doi: 10.1073/pnas.0812317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dana MR. Angiogenesis and lymphangiogenesis-implications for corneal immunity. Semin Ophthalmol. 2006;21:19–22. doi: 10.1080/08820530500509358. [DOI] [PubMed] [Google Scholar]
- 27.Baluk P, McDonald DM. Markers for microscopic imaging of lymphangiogenesis and angiogenesis. Ann N Y Acad Sci. 2008;1131:1–12. doi: 10.1196/annals.1413.001. [DOI] [PubMed] [Google Scholar]
- 28.Jackson DG. Immunological functions of hyaluronan and its receptors in the lymphatics. Immunol Rev. 2009;230:216–231. doi: 10.1111/j.1600-065X.2009.00803.x. [DOI] [PubMed] [Google Scholar]
- 29.Jackson DG, Prevo R, Clasper S, et al. LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol. 2001;22:317–321. doi: 10.1016/s1471-4906(01)01936-6. [DOI] [PubMed] [Google Scholar]
- 30.Niederkorn JY. Immune mechanisms of corneal allograft rejection. Curr Eye Res. 2007;32:1005–1016. doi: 10.1080/02713680701767884. [DOI] [PubMed] [Google Scholar]
- 31.Cosar CB, Sridhar MS, Sener B. Late onset of deep corneal vascularization: a rare complication of intrastromal corneal ring segments for keratoconus. Eur J Ophthalmol. 2009;19:298–300. doi: 10.1177/112067210901900222. [DOI] [PubMed] [Google Scholar]
- 32.Madigan MC, Penfold PL, Holden BA, et al. Ultrastructural features of contact lens-induced deep corneal neovascularization and associated stromal leukocytes. Cornea. 1990;9:144–151. [PubMed] [Google Scholar]
- 33.Maruyama K, Ii M, Cursiefen C, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cursiefen C, Cao J, Chen L, et al. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Invest Ophthalmol Vis Sci. 2004;45:2666–2673. doi: 10.1167/iovs.03-1380. [DOI] [PubMed] [Google Scholar]
- 35.Ling S, Lin H, Xiang D, et al. Clinical and experimental research of corneal lymphangiogenesis after keratoplasty. Ophthalmologica. 2008;222:308–316. doi: 10.1159/000144030. [DOI] [PubMed] [Google Scholar]
- 36.Cursiefen C, Schlotzer-Schrehardt U, Kuchle M, et al. Lymphatic vessels in vascularized human corneas: immunohistochemical investigation using LYVE-1 and podoplanin. Invest Ophthalmol Vis Sci. 2002;43:2127–2135. [PubMed] [Google Scholar]
- 37.Watari K, Nakao S, Fotovati A, et al. Role of macrophages in inflammatory lymphangiogenesis: enhanced production of vascular endothelial growth factor C and D through NF-kappaB activation. Biochem Biophys Res Commun. 2008;377:826–831. doi: 10.1016/j.bbrc.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 38.Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998;43:245–269. doi: 10.1016/s0039-6257(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 39.Espana EM, Di Pascuale MA, He H, et al. Characterization of corneal pannus removed from patients with total limbal stem cell deficiency. Invest Ophthalmol Vis Sci. 2004;45:2961–2966. doi: 10.1167/iovs.03-1397. [DOI] [PubMed] [Google Scholar]
- 40.Naib-Majani W, Breipohl W, Shazli EE, et al. The Ets-1 transcription factor is involved in pterygial angiogenesis. Anat Histol Embryol. 2007;36:107–110. doi: 10.1111/j.1439-0264.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 41.Saravia M, Zapata G, Ferraiolo P, et al. Anti-VEGF monoclonal antibody-induced regression of corneal neovascularization and inflammation in a rabbit model of herpetic stromal keratitis. Graefes Arch Clin Exp Ophthalmol. 2009;247:1409–1416. doi: 10.1007/s00417-009-1101-y. [DOI] [PubMed] [Google Scholar]
- 42.Cursiefen C, Kuchle M, Naumann GO. Angiogenesis in corneal diseases: histopathologic evaluation of 254 human corneal buttons with neovascularization. Cornea. 1998;17:611–613. doi: 10.1097/00003226-199811000-00008. [DOI] [PubMed] [Google Scholar]