Abstract
Cell-cell and cell-matrix mechanical interactions through membrane receptors direct a wide range of cellular functions and orchestrate the development of multicellular organisms. To define the single molecular forces required to activate signaling through a ligand-receptor bond, we developed the Tension Gauge Tether (TGT) approach in which the ligand is immobilized to a surface through a rupturable tether before receptor engagement. TGT serves as an autonomous gauge to restrict the receptor-ligand tension. Using a range of tethers with tunable tension tolerances, we show that cells apply a universal peak tension of ~40 pN to single integrin-ligand bonds during initial adhesion. We find that less than 12 pN is required to activate Notch receptors. TGT can also provide a defined molecular mechanical cue to regulate cellular functions.
Cells sense and respond to the mechanical properties of the surrounding extracellular matrix (ECM) and neighboring cells. Reciprocally, cells also apply force on the ECM and transmit mechanical signals to neighboring cells. These mechanical interactions activate intracellular signaling pathways and regulate such diverse processes as cell adhesion, polarization, migration, proliferation and differentiation (1, 2). As a result, by tuning bulk mechanical properties like stiffness, texture, and geometry of the substrate, researchers have gained insight into processes such as stem cell differentiation (3) and tumor metastasis (4). Single-molecule force spectroscopy has been used to study various mechano-sensitive membrane receptors including integrin, cadherin and Notch (5–8). However, these approaches cannot reveal the single molecule forces required for physiological functions because they either measure collective forces exerted through many molecules or probe molecular unbinding or unfolding forces only. More recently, FRET-based force sensors were developed and inserted to target sites to monitor cellular forces (9, 10), but great efforts must be taken to prepare the sensors and to track and interpret the fluorescence signal. Here, we describe a platform termed Tension Gauge Tether (TGT) that allows us to determine the single molecule forces required for mechanical signaling in cells.
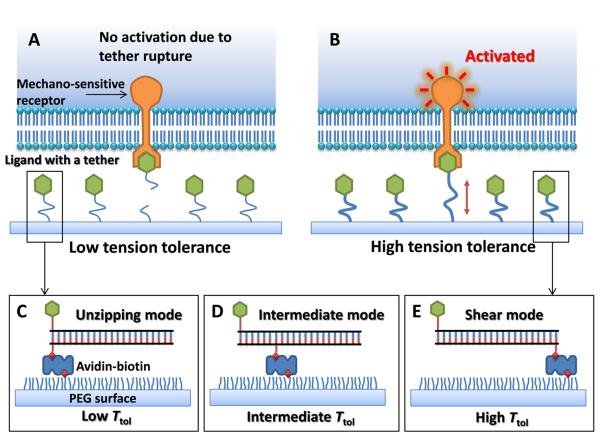
In TGT, a ligand is covalently conjugated to a tether that ruptures at a critical force, which we term `tension tolerance' or Ttol, and is immobilized on a solid surface through the tether (Fig. 1). Cells are plated on the surface and membrane receptors engage with and apply tension to the ligands. If signal activation through the receptor requires a molecular tension larger than Ttol, the tether will rupture, abolishing signal activation. In contrast, if the required tension is smaller than Ttol, the tether will endure, activating the receptor-mediated signaling. By engineering a series of tethers with different Ttol values, the tension required for signal activation can be determined by observing receptor-regulated cell activities which are usually much easier to detect than single molecule events. Because each ligand is equipped with an individual tension gauge, the measurement is independent of the receptor or ligand density.
Fig. 1.
Principle of Tension Gauge Tether (TGT). A ligand for a membrane receptor is immobilized on the surface through a tether which ruptures if the tension applied by the cell through the receptor is larger than its tension tolerance Ttol. Signaling through the receptor is not activated if the tension required for activation exceeds Ttol and ruptures the tether (A) and is activated if Ttol is larger than the required tension (B). (C, D, E). DNA duplex helix as a tether with tunable Ttol values.
DNA is a good candidate for use as rupturable tether in TGT because force application geometry (Fig. S1) strongly affects its rupture force with an unzipping force rupturing the DNA at a much lower force than a shearing force (11, 12). Albrecht et al have exploited this feature to estimate the rupture force of an antigen-antibody bond relative to the rupture forces of dsDNA in unzipping or shear geometry (13). The estimated rupture force of a 21 base pair DNA is about 12 pN in the unzipping geometry and is about 56 pN in the shear geometry (14). Intermediate rupture forces can be obtained by applying forces through an internal position on the DNA duplex and the theoretical Ttol values of the resulting tethers can be estimated (Figs. 1D and S2). Ttol represents the force required to rupture the tether in less than 2 seconds when the force is applied at a constant level (12) (see Supplementary Discussion in (14)).
Integrins are membrane receptors that mediate cell adhesion, sense and transduce mechanical information from the ECM into cells. Bulk traction forces applied through a collection of integrin-ligand bonds have been extensively examined (15–18). Here we apply the TGT platform to probe the tension on a single integrin-ECM ligand bond required for cell adhesion (specifically, integrin αVβ3 and its ligand, cyclic RGDfK peptide). To reduce non-specific adhesion, ligands with the DNA tether are immobilized through an Avidin-biotin linker on a glass surface passivated with polyethylene glycol (PEG). The Avidin-biotin unbinding force, ~160 pN (19), is much larger than the tether rupture forces used here (≤56 pN).
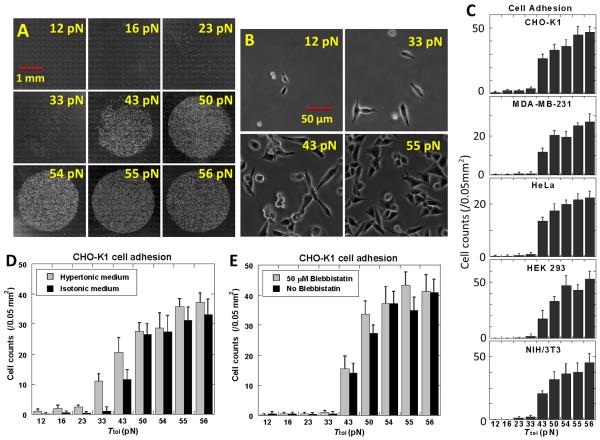
We conjugated RGDfK to nine different DNA tethers with estimated Ttol values of 12, 16, 23, 33, 43, 50, 54, 55 and 56 pN (Fig. S2). These DNA tethers have various force application geometries but share the same length, sequence and thermal stability. A TGT array was created by depositing the nine constructs as 2 mm diameter spots at a surface ligand density of 600 molecules/μm2 (Fig. 2A. See calibration in fig. S3). CHO-K1 cells were incubated on this surface for 30 min at a density of 1×106 cells/mL. The surface was gently rinsed and unbound cells were removed through media exchange. Phase contrast images were taken to measure the cell density.
Fig. 2.
Cell adhesion on TGT with RGDfK. (A) Phase contrast images of adherent CHO-K1 cells (white dots) on circular regions coated with nine TGT constructs of RGDfK-conjugated dsDNA with the indicated Ttol values. (B) Zoomed in phase contrast images of CHO-K1 cells on four of the regions. (C). Cell density (counts per 0.05 mm2) as a function of Ttol for five different cell lines as indicated. (D) Cell density vs. Ttol for CHO-K1 cells in hypertonic vs. isotonic medium. (E) Cell density vs. Ttol for CHO-K1 cells with and without 50 μM Blebbistatin. All error bars denote the standard deviation (n=8).
We observed little cell adhesion for DNA tethers with Ttol ≤33 pN and significant adhesion for Ttol ≥ 43 pN with the cell counts increasing slightly with increasing Ttol (Figs. 2B and 2C), suggesting that the peak tension CHO-K1 cells generate on a single RGDfK-integrin αVβ3 bond during adhesion lies mostly in the range of 33~43 pN. To examine the effect of ligand density, we created the same TGT surface with lower ligand densities and arrived at the same tension threshold for cell adhesion (Fig. S4). Other cell lines were also tested: MDA-MB-231, HeLa, HEK 293 and NIH/3T3 cells. Remarkably, all of the measured molecular tension thresholds fall within the same range, with the largest increase in cell counts occurring between a Ttol of 33 pN and 43 pN (Figs. 2D and S5). In contrast, bulk traction forces normally vary among different cell lines (20–22). Our observation suggests that there exists a common tension threshold value for the molecular tension generated by cells on an active integrin αVβ3 during adhesion. The same tension threshold was observed for cell seeding times as short as 5 min (Figs. S6 and S7).
Using constructs that cannot be ruptured but have similar ligand accessibility as the TGTs and additional constructs with similar ligand accessibility but with different force application geometries, we could ensure that the close proximity of the ligand and biotin in the low Ttol constructs does not reduce ligand accessibility and cause poor cell adhesion (Fig. S8). We also confirmed that the tether indeed ruptures in low Ttol constructs by labeling RGDfK-conjugated DNA strand with Cy3 fluorophore and observing the loss of fluorescence under the cell plating sites (Figs. S9 and S10).
Since integrins are membrane proteins that are anchored to the cytoskeleton, the cell membrane and the cytoskeleton are two potential determinants of the tension threshold. We used a hypertonic medium to reduce the membrane tension and the myosin II inhibitor blebbistatin to reduce the cytoskeleton tension. The tension threshold shifted to 23~33 pN for cells in the hypertonic medium (150 mM sucrose) as compared to 33~43 pN in an isotonic medium (Figs. 2E and S11). In contrast, the tension threshold remained unchanged with 0–100 μM blebbistatin (Figs. 2F, S12 and S13). Cell morphology changes confirmed the efficacy of the blebbistatin treatment (Fig. S14). The data suggests that molecular tension on integrins during the initial stages of cell adhesion is primarily regulated by the membrane tension, reminiscent of a recent study that found the membrane tension to play a dominant role in restricting actin assembly and Rac activation in the leading-edge protrusion of migrating cells (23).
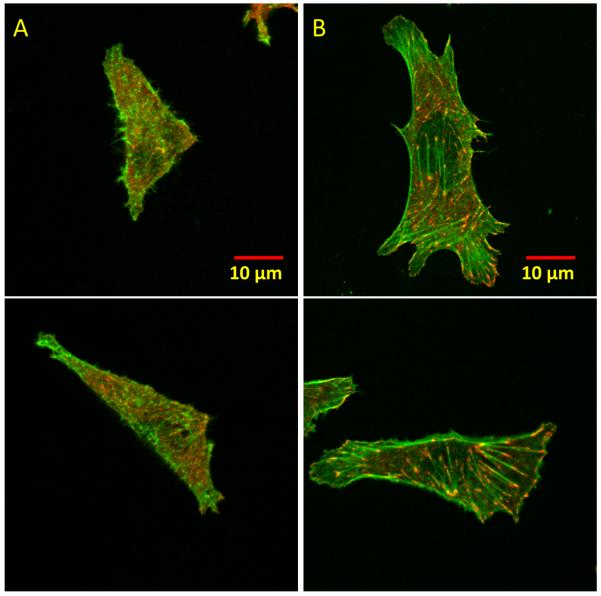
We next examined the formation of focal adhesions (FA) which can occur following initial cell adhesion and spreading. FAs consist of clusters of integrins and other adaptor proteins such as vinculin, talin, and paxillin, and their formation requires a mechanical stimulus (24, 25). We asked whether the measured ~40 pN tension threshold reflects initial cell adhesion or FA formation. On a regular petridish surface, CHO-K1 cells formed FAs and well-organized stress fibers, as detected through vinculin immunostaining and F-actin staining by phalloidin-TRITC, respectively (Fig. S15). We seeded CHO-K1 cells for 30 min, 1 hour and 2 hours on two TGT surfaces, one with the highest Ttol=56 pN and the other with Ttol=43 pN, which is just above the tension threshold for adhesion. At the 30 min seeding time, cells adhered and spread on both surfaces, but vinculin remained diffusive and stress fibers did not form on either surface (Fig. S16), confirming that the initial stages of cell adhesion and FA formation are two separate stages. Together with the observation that the threshold tension for adhesion takes effect at an early time point (5 min) of initial cell spreading which typically lasts 15 min and is independent of talin (26), we conclude that ~40 pN tension required for initial cell adhesion acts prior to FA formation. We speculate that integrins may work alone without clustering in the initial stages of cell adhesion, resulting in our observed common tension threshold for different cell lines. At the 1 hour seeding time, FAs and stress fibers started to form on the 56 pN surface but not on the 43 pN surface (Fig. S16). After 2 hours, FAs and stress fibers were well formed on the 56 pN surface but still not on the 43 pN surface (Fig. 3). Thus FA formation requires a molecular tension larger than that required for initial cell adhesion.
Fig. 3.
Confocal fluorescent images of CHO-K1 cells. (A) 43 pN TGT surface. (B) 56 pN TGT surface. Actin in green and vinculin in red. Images were obtained after 2-hour cell plating. Bright green lines in (B) are stress fibers that terminate in focal adhesion complexes marked in red.
Next, we examined the molecular tension required for Notch receptor activation using a fluorescence reporter, H2B-YFP. The Notch signaling pathway is responsible for intercellular communication, cell differentiation, and cell fate determination. The Notch receptor, a transmembrane protein, is activated by binding to its ligands on the neighboring cell's membrane (27). Several studies have implicated mechanical forces in Notch activation. For example, a force applied to a Notch-ligand bond may expose a cleavage site of Notch to initiate activation (28–30).
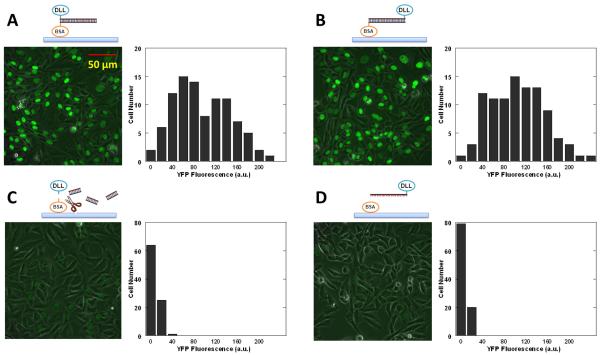
To determine the force required, we conjugated a Notch ligand DDL1 (Delta-like protein 1) to a DNA strand and Bovine serum albumin (BSA) to the complementary strand. 25 nM of DLL1-DNA was annealed to BSA-DNA immobilized on a glass coverslip treated with Fibronectin. After blocking the surface with free BSA, we seeded CHO-K1 cells stably expressing human NOTCH1 with its intracellular domain replaced by the activator Gal4esn. This cell line was also transfected with Gal4esn-controlled H2B-YFP. After notch activation, H2B-YFP fluorescence was detected in the nucleus, reaching an optimal level after two days (31). We tested 24 bp DNA tethers, one in unzipping and the other in shear geometry with an estimated Ttol of 12 and 58 pN, respectively. Cells on both surfaces expressed H2B-YFP indicating Notch activation (Figs. 4A and 4B). As a control, a surface with TGT in the unzipping configuration was further treated with DNase I which cleaves the DNA tether. As another control, DLL1-DNA conjugate was incubated with a surface coated with free BSA only (i.e. no complementary strand on the surface). On both surfaces, the YFP fluorescence level was dramatically lower (Figs. 4C and 4D), ruling out the possibility that Notch is activated by nonspecifically adsorbed DLL1. Other tethers with Ttol=45, 50, and 53 pN all resulted in Notch activation (Fig. S17). We conclude that Notch activation requires either no tension or a tension below 12 pN.
Fig. 4.
H2B-YFP expression in the nucleus as a reporter of Notch activation. (A–D) YFP fluorescence images of CHO-K1 cells show nuclear fluorescence of H2B-YFP when Notch is activated. Histograms of fluorescence intensities of single cells are also shown. (a.u.: arbitrary unit) (A) Ttol=12 pN. (B) Ttol=58 pN. (C) 12 pN TGT with DNase I digestion. (D) A control with unconjugated BSA. Here, we used BSA for TGT immobilization and surface passivation because PEG surface did not survive two days period required for our Notch reporter system.
We used TGT mainly as a measurement tool here but it can also provide a defined single molecule mechanical cue to the cells as we demonstrated by showing that Ttol values control the formation of stress fibers and FAs. It is well known that mechanical cues stemming from substrate stiffness or micro-patterning can affect cancer cell or stem cell behaviors (1, 32). Tunable mechanical properties that have been examined were bulk features such as compliance, texture and geometry. However, the sensors of mechanical cues are normally comprised of single molecules and questions remain about how a receptor as a single molecule can sense bulk properties such as stiffness. We suggest that the TGT platform provides a viable means to address these questions.
Supplementary Material
Acknowledgements
We would like to thank Irwin D. Bernstein for the generous gift of DLL1. We also appreciate the genetically modified CHO-K1 cell-line for Notch study provided by Michael B. Elowitz. We thank Qian Xu, Ning Wang and Yingxiao Wang for carefully reading the manuscript. Funding was provided by National Science Foundation through the Physics Frontiers Center Program (0822613). T.H. is an investigator with the Howard Hughes Medical Institute.
References
- 1.Discher DE, Janmey P, Wang YL. Science. 2005 Nov;310:1139. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman BD, Grashoff C, Schwartz MA. Nature. 2011 Jul 21;475:316. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engler AJ, Sen S, Sweeney HL, Discher DE. Cell. 2006 Aug 25;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 4.Levental KR, et al. Cell. 2009 Nov 25;139:891. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merkel R, Nassoy P, Leung A, Ritchie K, Evans E. Nature. 1999 Jan 7;397:50. doi: 10.1038/16219. [DOI] [PubMed] [Google Scholar]
- 6.Wang N, Butler JP, Ingber DE. Science. 1993 May 21;260:1124. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 7.Wang YX, et al. Nature. 2005 Apr 21;434:1040. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- 8.Shergill B, Meloty-Kapella L, Musse AA, Weinmaster G, Botvinick E. Dev Cell. 2012 Jun 12;22:1313. doi: 10.1016/j.devcel.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grashoff C, et al. Nature. 2010 Jul 8;466:263. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stabley DR, Jurchenko C, Marshall SS, Salaita KS. Nat Methods. 2011 Oct 30; doi: 10.1038/nmeth.1747. [DOI] [PubMed] [Google Scholar]
- 11.Essevazroulet B, Bockelmann U, Heslot F. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:11935. doi: 10.1073/pnas.94.22.11935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatch K, Danilowicz C, Coljee V, Prentiss M. Physical Review E. 2008 Jul;78 doi: 10.1103/PhysRevE.78.011920. [DOI] [PubMed] [Google Scholar]
- 13.Albrecht C, et al. Science. 2003 Jul 18;301:367. doi: 10.1126/science.1084713. [DOI] [PubMed] [Google Scholar]
- 14.Materials and methods and additional information are available as supplementary materials on Science Online.
- 15.Harris AK, Stopak D, Wild P. Nature. 1981;290:249. doi: 10.1038/290249a0. 1981. [DOI] [PubMed] [Google Scholar]
- 16.Harris AK, Wild P, Stopak D. Science. 1980;208:177. doi: 10.1126/science.6987736. 1980. [DOI] [PubMed] [Google Scholar]
- 17.Balaban NQ, et al. Nat Cell Biol. 2001 May;3:466. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 18.Tan JL, et al. Proceedings of the National Academy of Sciences of the United States of America. 2003 Feb 18;100:1484. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moy VT, Florin EL, Gaub HE. Science. 1994;266:257. doi: 10.1126/science.7939660. [DOI] [PubMed] [Google Scholar]
- 20.Li Z, et al. Nano Lett. 2009 Oct;9:3575. doi: 10.1021/nl901774m. [DOI] [PubMed] [Google Scholar]
- 21.Munevar S, Wang YL, Dembo M. Biophys J. 2001 Apr;80:1744. doi: 10.1016/s0006-3495(01)76145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen JX, Li HX, SundarRaj N, Wang JHC. Cell Motil Cytoskel. 2007 Apr;64:248. doi: 10.1002/cm.20178. [DOI] [PubMed] [Google Scholar]
- 23.Houk AR, et al. Cell. 2012 Jan 20;148:175. doi: 10.1016/j.cell.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riveline D, et al. Journal of Cell Biology. 2001 Jun 11;153:1175. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oakes PW, Beckham Y, Stricker J, Gardel ML. Journal of Cell Biology. 2012 Feb 6;196:363. doi: 10.1083/jcb.201107042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, et al. Nat Cell Biol. 2008 Sep;10:1062. doi: 10.1038/ncb1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Artavanis-Tsakonas S, Rand MD, Lake RJ. Science. 1999 Apr 30;284:770. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 28.Gordon WR, et al. Nature Structural & Molecular Biology. 2007 Apr;14:295. doi: 10.1038/nsmb1227. [DOI] [PubMed] [Google Scholar]
- 29.Varnum-Finney B, et al. Journal of Cell Science. 2000 Dec;113:4313. doi: 10.1242/jcs.113.23.4313. [DOI] [PubMed] [Google Scholar]
- 30.Parks AL, Klueg KM, Stout JR, Muskavitch MA. Development. 2000 Apr;127:1373. doi: 10.1242/dev.127.7.1373. [DOI] [PubMed] [Google Scholar]
- 31.Sprinzak D, et al. Nature. 2010 May 6;465:86. doi: 10.1038/nature08959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salaita K, et al. Science. 2010 Mar 12;327:1380. doi: 10.1126/science.1181729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.