Abstract
BACKGROUND
MicroRNAs are important regulators of gene expression, including those involving electrical remodeling in atrial fibrillation (AF). Recently, KCNN3, the gene that encodes the small conductance calcium-activated potassium channel 3 (SK3), was found to be strongly associated with AF.
OBJECTIVES
This study sought to evaluate the changes in atrial myocardial microRNAs in patients with permanent AF and to determine the role of microRNA on the regulation of cardiac SK3 expression.
METHODS
Atrial tissue obtained during cardiac surgery from patients (4 sinus rhythm and 4 permanent AF) was analyzed by microRNA arrays. Potential targets of microRNAs were predicted by software programs. The effects of specific microRNAs on target gene expression were evaluated in HL-1 cells from a continuously proliferating mouse hyperplastic atrial cardiomyocyte cell line. Interactions between microRNAs and targets were further evaluated by luciferase reporter assay and by Argonaute pull-down assay.
RESULTS
Twenty one microRNAs showed significant, greater than two-fold changes in AF. miR-499 was upregulated by 2.33 fold (P<0.01) in AF atria, whereas SK3 protein expression was down-regulated by 46% (P<0.05). Transfection of miR-499 mimic in HL-1 cells resulted in the downregulation of SK3 protein expression, while that of miR-499 inhibitor upregulated SK3 expression. Binding of miR-499 to the 3′UTR of KCNN3 was confirmed by luciferase reporter assay and by the enhanced presence of SK3 mRNA in Argonaute pulled-down microRNA-induced silencing complexes (mRISC) after transfection with miR-499.
CONCLUSION
Atrial miRNA-499 is significantly upregulated in AF, leading to SK3 downregulation and possibly contributing to the electrical remodeling in AF.
Keywords: atrial fibrillation, microRNA, SK3 channel, electrical remodeling, small-conductance calcium-activated potassium channel
Introduction
Atrial fibrillation (AF) begets AF, and this process is associated with substantial cardiac electrical and structural remodeling 1–3. A number of cardiac ion channels have been found to undergo profound changes as a result of AF, including the L-type Ca2+ channels, the transient outward K+ channels, the strong inward rectifier K+ channel (IK1), the acetylcholine-activated K+ channel (IK,ACh), and the ultra-rapid delayed rectifier K+ channel (IKur) 3–7. However, the underlying mechanisms that lead to these changes remain unclear.
Recently, genome-wide association studies (GWAS) identified KCNN3, which encodes the small conductance calcium-activated potassium channel 3 (SK3), as a new locus strongly associated with AF 8,9. SK channels are widely distributed and mRNAs of all three SK isoforms have been detected in mouse atria and ventricles 10, and atrial myocytes from the SK2 null mouse exhibited action potential prolongation with inducible atrial fibrillation 11. These and other reports suggest that SK channels may participate in the development or maintenance of AF. However, very little is known about the alteration of SK3 channels in AF and the molecular mechanisms regulating SK3 expression have not been thoroughly delineated.
MicroRNAs are known regulators of gene expression and recent reports have shown that microRNAs are involved with the regulation of cardiac electrophysiology 12. Various microRNAs have been shown to participate in the electrical and structural remodeling of AF 13–15. In this study, we sought to determine whether SK3 expression is altered in AF and to delineate the role of microRNA in the regulation of SK3 expression.
Methods
Patients
This study was approved by the Mayo Clinic Institutional Review Board. All patients provided informed consent. Tissues were obtained from the right atrial appendage during cardiac surgery and were immediately snap-frozen and stored at −80°C. Four patients had no history of AF (SR) and four had permanent AF (>1 year of AF). Patient characteristics are shown in Table 1. Patients with dilated or hypertrophic cardiomyopathy, congenital heart disease, uncontrolled hypertension (>160/90 mmHg), type 1 diabetes mellitus, or untreated obstructive sleep apnea were excluded.
Table 1.
Clinical characteristics of the patients used in our study
| Rhythm | Age | Sex | Surgery | LVEF | Co-morbidities |
|---|---|---|---|---|---|
| SR | 48 | M | AVR | 68% | AS, hypertension, aortic dissection |
| SR | 84 | M | AVR, CABG | 70% | AS, βlipids, prostate cancer, MR, TIA |
| SR | 60 | M | CABG | 71% | MI, hypertension, βlipids |
| SR | 77 | F | AVR, myomectomy | 78% | HCM, AS, MV papilloma, hypertension, hypercholesterolemia, smoking |
| AF | 59 | M | MV repair | 70% | MR, CVA, endocarditis |
| AF | 74 | F | AVR | 65% | AS, DM II, hypertension, βlipids, ulcerative colitis, hyperuricemia |
| AF | 74 | M | MV repair, CABG | 45–50% | MR, CAD, hypertension, hip replacement, smoking, BPH |
| AF | 77 | F | MV repair | 70% | MR |
AF = permanent atrial fibrillation; AS = aortic stenosis; AVR = aortic valve replacement; BPH = benign prostatic hypertrophy; CABG = coronary artery bypass grafting surgery; CAD = coronary artery disease; CVA = cerebrovascular accident; DM = diabetes mellitus; EF = left ventricular ejection fraction; F = female; HCM = hypertrophic cardiomyopathy; M = male; MV = mitral valve; SR = normal sinus rhythm; MI = myocardial infarction; MR = mitral valve regurgitation; TIA = transient ischemic attack.
Microarray analysis
MicroRNA array analysis was performed by the Mayo Clinic Advanced Genomic Technology Center Gene Expression Core using the Affymetrix GeneChip miRNA2.0 array (Affymetrix, Santa Clara, CA). Partek software (Partek Incorporated, St. Louis, Missouri) was used for data preprocessing, principal components analysis (PCA), and differential expression detection.
microRNA target prediction
Potential targets of microRNA regulation were predicted using miRwalk 16 with the following algorithms: Targetscan, miRanda, DIANA-microT, PITA, and miRwalk. The microRNA best predicted by these algorithms to regulate the KCNN3 gene was selected for further analysis.
HL-1 cell culture
HL-1 cells from a continuously proliferating mouse atrial hyperplastic cardiomyocyte cell line were kindly provided by Dr. William C. Claycomb 17. Transfection of miR-499 mimic, mimic negative control, miR-499 inhibitor, and inhibitor negative control (100 nM) (Qiagen) in HL-1 cells was performed using Lipofectamine 2000 (Invitrogen, Grand Island, NY). Cells were used for experiments 48 hours after transfection. The miR-499 mimic consisted of chemically synthesized double-stranded RNAs that “mimic” native miR-499. The miR-499 inhibitor consisted of single-stranded 2′-O-methylated (for stability against degradation) oligonucleotides complementary to the miR-499 and inhibits endogenous miR-499 from regulating its targets. For the miR-499 mimic negative control, we used the AllStars Negative Control siRNA, which consisted of a scrambled RNA sequence, that has been thoroughly tested and validated and has no homology to any known mammalian gene. The miScript Inhibitor Negative Control was used as the miR-499 inhibitor negative control and it has no homology to any known mammalian gene.
Western blot analysis
Western blotting was performed using polyclonal anti-SK3 (1:200 dilution; Alomone Labs, Jerusalem, Israel), polyclonal anti-GAPDH (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), monoclonal anti-actin (1:2500 dilution; Oncogene Research Products, Boston, MA), polyclonal anti-KCNQ5 (1:100 dilution; Abcam Inc, Cambridge, MA), polyclonal anti-KCNH8 (1 μg/ml, Abcam Inc), polyclonal anti-SCN2B (1:200 dilution; Santa Cruz Biotechnology), polyclonal anti-SCN4A (1:200 dilution; Santa Cruz Biotechnology,), polyclonal anti-SCN7A (1:200 dilution; Novus Biologicals, Littleton, CO), or polyclonal anti-TRPA1 (1:200 dilution; Santa Cruz Biotechnology) antibodies. Results were normalized against the expression of loading controls of GAPDH or actin.
Real-time PCR analysis
Real-time PCR was performed as previously described 18. The primer pairs for SK3 were:
Forward: 5′-ACT TGC AGC TTC ATG CGT TT-3′
Reverse: 5′-TCG AGG GGG CGA TCA AGA-3′
The primer pairs for GAPDH were:
Forward: 5′-CCA CCT TCG ATG CCG GGG CT-3′
Reverse: 5′-GGG GCC GAG TTG GGA TAG GG-3′
The reaction had initial denaturation at 95°C for 3 min, 50 cycles of denaturation at 95°C for 40 s, annealing at 58°C for 40 s, and extension at 72°C for 45 s. Fluorescence was measured at the end of each extension step and the data were normalized to GAPDH from the same preparation.
Luciferase reporter assay
The luciferase report assay was performed as previously described 15,19. A fragment of the 3′ untranslated region (3′UTR) of KCNN3 containing the predicted binding site for miR-499 was subcloned into the luciferase reporter vector (Switchgear Genomics, Menlo Park, CA). The vector was co-transfected with miR-499 mimic or mimic negative control into HL-1 cells using Lipofectamine 2000. Twenty-four hours after transfection, cells were lysed and assayed using the LightSwitch Luciferase Assay Reagent LS010 and the luciferase reporter signal was measured using a plate luminometer (Synergy 2, BioTek, Winooski, VT).
Co-immunoprecipitation and Argonaute pull-down assay
Immunoprecipitation was performed as previously described 19. HL-1 cells were washed with PBS, lysed, sonicated, and then centrifuged at 10,000 rpm for 10 minutes at 4°C. The supernatant (500 μg in 300 μl) from each sample was incubated with Argonaute 1 or Argonaute 2 antibodies (1:50, Cell Signaling Technology, Danvers, MA) with rotation overnight at 4°C. The immune complexes were pulled down by incubation with 40 μl Protein A/G Plus-Agarose (Santa Cruz) at 4°C for 4 hours with rotation. Immune complexes were collected by centrifugation at 3000 rpm for 3 min and washed with lysis buffer. One ml of TRIzol reagent (Invitrogen) was added into each sample to isolate RNA and the purified RNA was reverse transcribed to cDNA. SK3 mRNA levels were measured using quantitative real-time PCR.
Statistical analysis
Data were presented as means ± SEM. Unpaired t-test was used to compare data between groups. P < 0.05 was considered to be statistically significant.
Results
Atrial microRNA expression profiles are different in permanent AF patients compared with SR patients
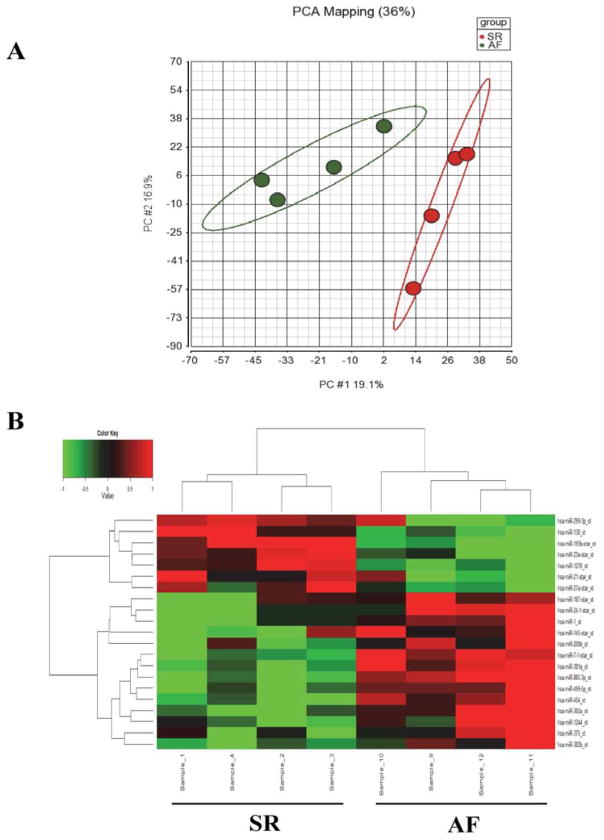
The quality of the total RNA and microRNA isolated from human tissue had RNA Integrity Number >7.5. The PCA plots demonstrated that the results from SR and permanent AF were each independently clustered, with clear separation between the two populations (Figure 1A). Of the 1,100 mature human microRNAs examined in the array, 194 (17.64%) were found to be differentially expressed with a P value <0.05, of which 21 had at least a two-fold difference in expression, with 14 upregulations and 7 downregulations, in the AF group compared to SR group. Dendrogram and heat-map of these 21 microRNAs showed distinct differences between SR and AF groups (Figure 1B and Table 2).
Figure 1.
MicroRNA expression patterns. A: Principal components analysis (PCA) of microRNA expression profiles, encompassing all human microRNA probes in the array, shows that SR (red) and permanent AF (green) patients cluster into two groups. The horizontal axis corresponds to principal component 1 (PC#1), which explains 19.1% of the total variance in these datasets. The vertical axis corresponds to PC#2, which explains 16.9% of the variance. B: Dendrogram and heat-map overview of the 21 microRNAs differentially expressed with ≥2-fold changes and P<0.05 (vertical axis) between the SR (left 4 columns, horizontal axis) and the AF groups (right 4 columns). Red indicates enhanced levels and green indicates reduced levels of microRNA expression in AF patients compared to SR patients.
Table 2.
MiRNA expression in SR versus permanent AF patients
| miRNA | Fold-change (AF vs SR)* | p value | Sequence |
|---|---|---|---|
| hsa-miR-208b | 3.6480 | 0.0398 | 5′-AUAAGACGAACAAAAGGUUUGU-3′ |
| hsa-miR-499 | 2.3261 | 0.0069 | 5′-UUAAGACUUGCAGUGAUGUUU-3′ |
| hsa-miR-885-3p | 2.2863 | 0.0003 | 5′-AGGCAGCGGGGUGUAGUGGAUA-3′ |
| hsa-miR-24-1-star | 2.2728 | 0.0082 | 5′-UGCCUACUGAGCUGAUAUCAGU-3′ |
| hsa-miR-454 | 2.2671 | 0.0032 | 5′-UAGUGCAAUAUUGCUUAUAGGGU-3′ |
| hsa-miR-375 | 2.1342 | 0.0346 | 5′-UUUGUUCGUUCGGCUCGCGUGA-3′ |
| hsa-miR-1244 | 2.0705 | 0.0205 | 5′-AAGUAGUUGGUUUGUAUGAGAUGGUU-3′ |
| hsa-miR-187-star | 2.0642 | 0.0404 | 5′-GGCUACAACACAGGACCCGGGC-3′ |
| hsa-miR-302b | 2.0592 | 0.0489 | 5′-UAAGUGCUUCCAUGUUUUAGUAG-3′ |
| hsa-miR-7-1-star | 2.0424 | 0.0003 | 5′-CAACAAAUCACAGUCUGCCAUA-3′ |
| hsa-miR-1 | 2.0277 | 0.0189 | 5′-UGGAAUGUAAAGAAGUAUGUAU-3′ |
| hsa-miR-301a | 2.0181 | 0.0005 | 5′-CAGUGCAAUAGUAUUGUCAAAGC-3′ |
| hsa-miR-302a | 2.0143 | 0.0029 | 5′-UAAGUGCUUCCAUGUUUUGGUGA-3′ |
| hsa-miR-145-star | 2.0046 | 0.0145 | 5′-GGAUUCCUGGAAAUACUGUUCU-3′ |
| hsa-miR-23a-star | −2.0400 | 0.0034 | 5′-GGGGUUCCUGGGGAUGGGAUUU-3′ |
| hsa-miR-138 | −2.1212 | 0.0037 | 5′-AGCUGGUGUUGUGAAUCAGGCCG-3′ |
| hsa-miR-27a-star | −2.1417 | 0.0220 | 5′-AGGGCUUAGCUGCUUGUGAGCA-3′ |
| hsa-miR-21-star | −2.1631 | 0.0367 | 5′-CAACACCAGUCGAUGGGCUGU-3′ |
| hsa-miR-299-3p | −2.1797 | 0.0151 | 5′-UAUGUGGGAUGGUAAACCGCUU-3′ |
| hsa-miR-1270 | −2.2206 | 0.0058 | 5′-CUGGAGAUAUGGAAGAGCUGUGU-3′ |
| hsa-miR-193b-star | −2.3089 | 0.0002 | 5′-CGGGGUUUUGAGGGCGAGAUGA-3′ |
Positive values indicate that microRNAs were upregulated in the permanent AF group compared with the SR group. Negative values indicate that miRNAs were downregulated in the permanent AF group compared with the SR group.
Atrial SK3 protein expression was downregulated in permanent AF
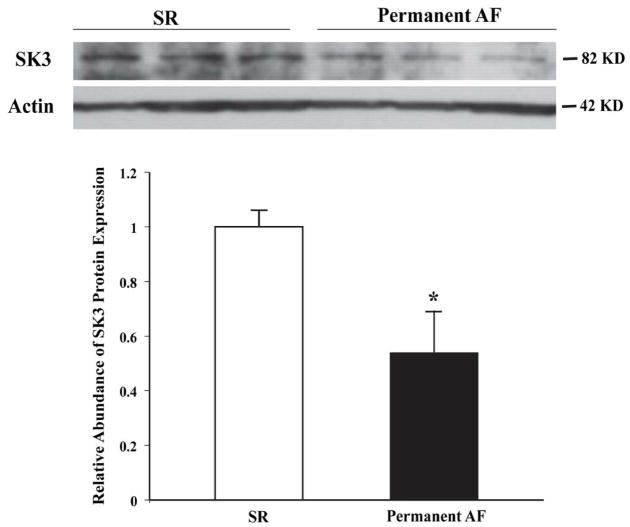
Through Western blot analysis of three pairs of atrial samples, we found that the protein levels of SK3 were downregulated by 46% in permanent AF (0.54±0.15 vs. 1.0±0.06 in SR, n=3 for both, P<0.05) (Figure 2).
Figure 2.
Downregulation of SK3 protein expression in the atrial tissues from patients with permanent AF patients compared to those with SR. *P<0.05 vs. SR, n=3 for each group.
MiR-499 was upregulated by 2.33-fold in permanent AF and had a higher predicted score for targeting KCNN3 than other microRNAs according to the predicting tools used. Hence, miR-499 was selected for further examination for its role on SK3 regulation.
MiR-499 targets the 3′UTR of KCNN3
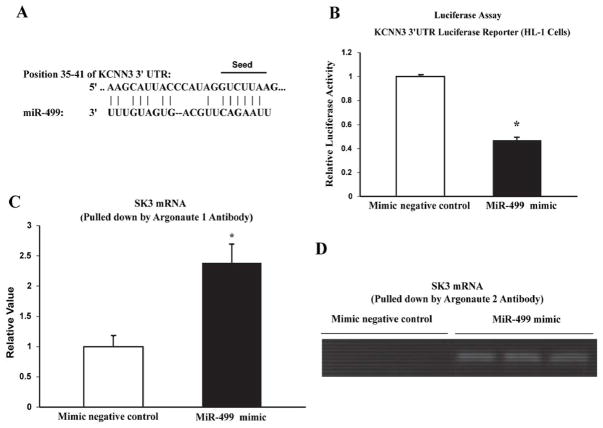
KCNN3 contains a conserved sequence in its 3′UTR, which is complementary to the seed site of miR-499 (Figure 3A). To further confirm the direct binding of miR-499 to the 3′UTR of KCNN3, we used a luciferase reporter assay in HL-1 cells 19. Co-transfection of miR-499 mimic (100 nM) with the luciferase reporter vector resulted in significantly diminished luciferase activity compared to cells co-transfected with the miR-499 negative control (0.47±0.02 relative units with miR-499 mimic vs. 1.0±0.01 relative units with control, n=4, P <0.001). These results strongly indicate that miR-499 binds directly to the mRNA of KCNN3, resulting in reduced expression of SK3.
Figure 3.
MiR-499 targets KCNN3. A: Alignment of the sequences of miR-499 with its target site in the 3′ UTRs of KCNN3. The miR-499 sequence is identical between human and mouse, and the binding site of KCNN3 is conserved among mammals. The nucleotides marked represent the miR-499 “seed site” and its paired sequence. B: Luciferase reporter assay in HL-1 cells showing co-transfection of miR-499 mimic significantly reduced luciferase activity with the 3′UTR of KCNN3 subcloned into the luciferase reporter vector. *P<0.05 vs. control, n=4 for each group. C and D: Argonaute 1 and 2 pull-down assays in HL-1 cells. The levels of KCNN3 transcripts were determined by quantitative real-time RT-PCR (C) and agarose gel electrophoresis (D).
It is known that microRNAs target mRNAs by the association of the microRNA/mRNA with Argonaute (Ago) proteins to form microRNA-inducing silencing complexes (miRISCs), leading to post-transcriptional repression 20. In HL-1 cells, the Ago 1 pulled-down miRISCs contained KCNN3 mRNA at levels 2.38-fold higher in cells transfected with miR-499 mimic compared with those transfected with the mimic negative control (2.38 ±0.03 relative units in miR-499 mimic vs. 1.0±0.18 relative units in control, n=3, P=0.017) (Figure 3C). In the Ago 2 pull-down, KCNN3 mRNA was clearly recruited to the miRISCs in cells transfected with miR-499 mimic, but the mimic negative control failed to recruit KCNN3 mRNA to the miRISC and the experiment was repeated with the same results (Figure 2D). These findings indicate that miR-499 targets KCNN3 mRNA and facilitates its recruitment into miRISCs, resulting in transcriptional repression of SK3 expression.
MiR-499 negatively regulates SK3 protein expression in cardiac myocytes
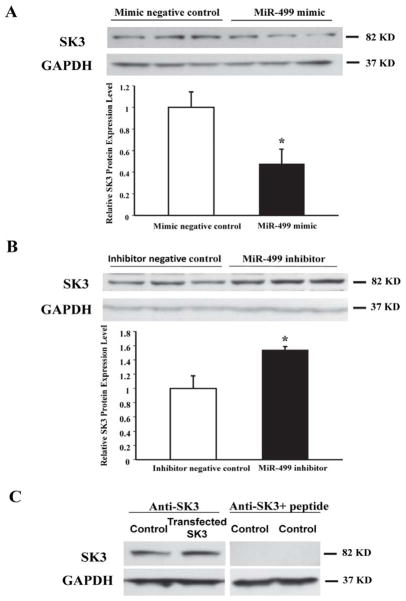
Western blot analysis of HL-1 cells showed that transfection with miR-499 mimic significantly downregulated the expression of SK3 (0.48±0.13 vs. 1.0±0.14 relative units in control, n=5, P<0.01) (Figure 4A), while that with the miR-499 inhibitor upregulated the expression of SK3 (1.54±0.04 vs. 1.0±0.18 relative units, n=3, P<0.05) (Figure 4B). These results indicate that the levels of SK3 expression are regulated by miR-499 in cardiac myocytes. Identity of the SK3 band on Western blot was further characterized by overexpressing SK3 in HL-1 cells using a plasmid construct containing the KCNN3 gene (Figure 4C). Specificity of the anti-SK3 antibody was demonstrated by blockade of antibody immunoreactivity against the SK3 band after preincubation with the SK3 antigenic peptide (Figure 4C).
Figure 4.
Western blot analysis showing the modulation of SK3 expression by miR-499 in cultured HL-1 cells. A: Protein expression of SK3 was significantly reduced in HL-1 cells transfected with miR-499 mimic compared with mimic negative control (*P<0.05 vs. control, n=5 for each group). B: Protein expression of SK3 was increased in HL-1 cells transfected with miR-499 inhibitor compared to those transfected with inhibitor negative control. (*P<0.05 vs. control, n=3 for each group). C: Specificity of the SK3 protein band on Western blots was validated by overexpressing SK3 in HL-1 cells and blockade of antibody immunoreactivity by preincubation with the antigenic peptide.
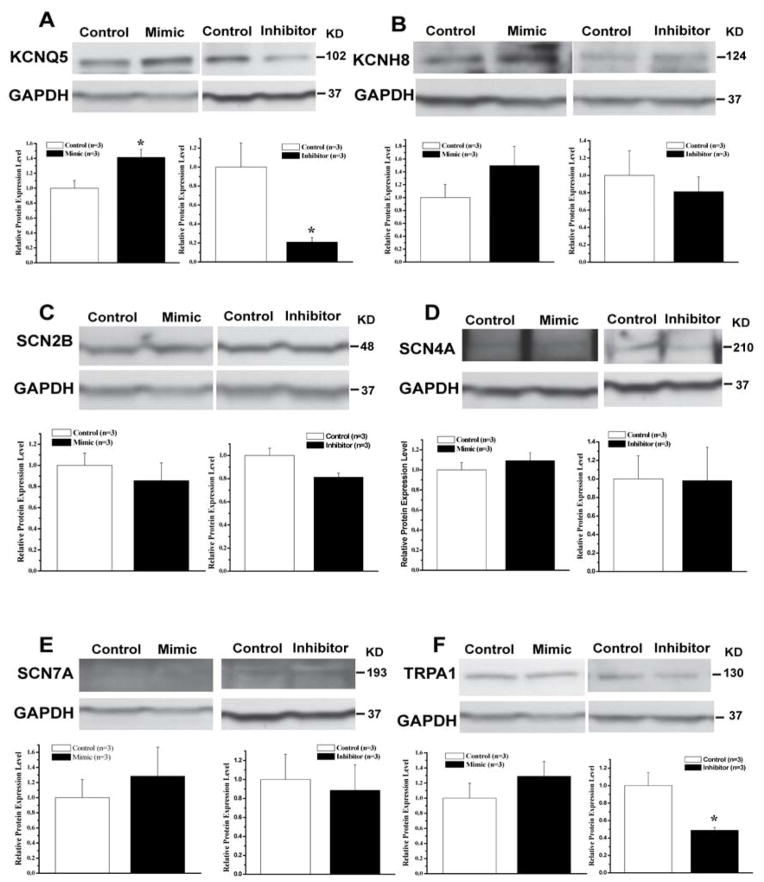
Effects of miR-499 on other cardiac ion channels
In addition to the KCNN3 gene, the potential cardiac ion channel targets of miR-499 regulation include those of other potassium channels/subunits (KCNQ5 and KCNH8), sodium channels/subunits (SCN2B, SCN4A, and SCN7A), and cation channels (TRPA1). We further determined the effects miR-499 mimic and inhibitor on the levels of expression of these ion channels/subunits in HL-1 cells. Interestingly, the protein expression of KCNQ5 was significantly upregulated by miR-499 mimic (1.41±0.11 vs. 1.0±0.10 relative units in control, n=3, P<0.05) and downregulated by miR-499 inhibitor (0.21±0.05 vs. 1.0±0.25 relative units in control, n=3, P<0.05) (Figure 5A), suggesting that miR-499 does not directly regulate KCNQ5 expression but may be regulating a negative inhibitor of KCNQ5 expression. In addition, miR-499 inhibitor significantly downregulated TRPA1 expression while miR-499 mimic had no significant effect (Figure 5F). Expression of KCNH8, SCN2B, SCN4A, and SCN7A, was not significantly altered by changes in miR-499 expression (Figure 5). These results suggest that regulation of KCNN3 expression by miR-499 is quite unique.
Figure 5.
Effects of miR-499 mimic and inhibitor on the levels of protein expression of potential miR-499 target genes that include KCNQ5 (A), KCNH8 (B), SCN2B (C), SCN4A (D), SCN7A (E), and TRPA1 (F). * represents P <0.05 vs. control, n=3 for all samples.
KCNN3 mRNA expression was downregulated by miR-499
It is widely accepted that microRNAs act as negative regulators of gene expression by inhibiting translation of mRNA or by promoting mRNA degradation 20. Quantitative RT-PCR showed that the level of SK3 mRNA expression was significantly downregulated by 39% in HL-1 cells 48 hours after transfection with miR-499 mimic (0.61±0.09 vs. 1.0±0.04 relative units, n=3, P=0.015) (Figure 6). These results suggest that miR-499 significantly reduced the steady state levels of KCNN3 mRNA, thereby reducing SK3 expression in cardiac cells. It is noted that the seed sequence of miR-499 is highly conserved between mouse KCNN3 and the three transcripts of human KCNN3. Hence, all three transcripts of human KCNN3 may be targets of miR-499 based on miR-499 seed sequence.
Figure 6.
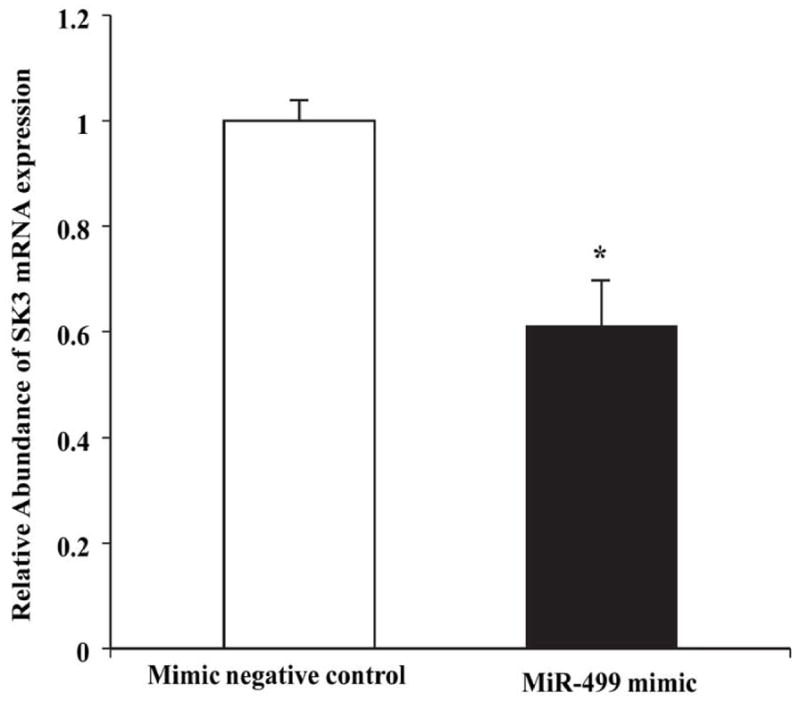
Real-time PCR analysis showing decreased SK3 mRNA expression by miR-499 in HL-1 cells. * represents P<0.05 vs. control, n=3 for each group.
Discussion
In this study, we have made several important observations regarding the regulation of SK3 channels by miR-499. First, we found that atrial microRNA expression profiles were different in patients with permanent AF compared to those with SR. Second, miR-499 was significantly upregulated while SK3 was down-regulated in AF atria. Third, we verified experimentally that miR-499 binds to the 3′UTR of KCNN3 mRNA using the luciferase reporter assay. In addition, Ago protein pull-down assays showed that miR-499 effectively enriched KCNN3 mRNA in the miRISCs. Fourth, in cultured atrial cells, the miR-499 mimic significantly reduced SK3 protein expression, while the miR-499 inhibitor significantly enhanced the level of SK3 expression. Fifth, KCNN3 mRNA expression level was also downregulated by transfection of the miR-499 mimic into HL-1 cells. These observations provide compelling evidence that miR-499 regulates the expression of atrial SK3 channels, which may contribute to the electrical remodeling in AF.
Electrical remodeling in atrial fibrillation and SK3 channel
Electrical remodeling is a maladaptation that renders the atria susceptible to the initiation and maintenance of AF. Studies using human tissues have shown that the L-type Ca2+ currents (ICa,L) were significantly downregulated by 50 to 60% 4,5,7 while the strong inward rectifier K+ current IK1 4,7,21 and the acetylcholine-activated K+ current IK,ACh 4,22 were significantly upregulated in the fibrillating human atria. These changes lead to shortening of the atrial action potential and atrial refractoriness, promoting arrhythmogenesis by reentry. In addition, the downregulation of the ICa,L results in the loss of rate adaptation which is a prominent feature in electrical remodeling of AF 5. On the other hand, the Ito and the IKUR were found to be downregulated by more than 50% as a result of AF in humans 4,5,7, similar to what we observed in SK3 channels in this study. The relevance of repolarizing current downregulation in the pathogenesis of AF was underscored by the finding that a loss-of-function mutation in Kv1.5 is associated with familial AF 23. The role of SK3 in AF is not entirely clear, but the strong association between KCNN3 and AF from GWAS studies suggests that it warrants further investigation 8,9. The results of our study help to elucidate the role of SK3 in the electrical remodeling of AF.
SK channels and cardiac electrophysiology
Very little is known about the regulation of cardiac SK channels. Cardiac SK channels contribute to the repolarization of cardiac action potentials 10,11. Rapid burst pacing in the rabbit atrium for 3 hours resulted in shortening of the pulmonary vein action potential that was associated with upregulation of SK2 expression but no change in SK3 expression 24. A microarray study comparing ion channel gene expression in patients with valvular heart disease showed that the SK2 mRNA expression was not altered in AF compared with those in sinus rhythm 21. In animal models of AF induced by cholinergic and electrical stimulation, inhibitors of SK channels were found to be antiarrhythmic 25. Similar findings were reported on AF induced by burst pacing in aging male spontaneously hypertensive rats 26. On the other hand, atrial myocytes from mice with genetic ablation of SK2 exhibited action potential prolongation and inducible atrial fibrillation 11. Whether the downregulation of SK3 produces the same electrophysiological effects is unknown, although similar properties would be expected. Apart from AF, SK2 has been shown to participate in the regulation of AV nodal function 27 and SK channel upregulation is implicated as the mechanism that underlies the recurrent ventricular fibrillation in failing rabbit ventricles 28. Results of the current study may provide important new information on the role of SK3 in AF, and if these findings are substantiated in a larger population of patients, they may guide our future approaches in the management of AF.
Role of microRNA in AF and miR-499
Recent studies support the hypothesis that microRNAs play an important role in the electrical remodeling of AF 29. The greatly reduced miR-1 expression in human AF contributes to the upregulation of Kir2.1 channel, leading to increased IK1 14. MiR-328 was elevated by 3.5-fold in AF patients with rheumatic heart disease and was responsible for the downregulation of the L-type Ca2+ channel genes CACNA1C and CACNB1 15. miR-499 has been reported to regulate myocardial function with dysregulations in heart failure 30 but has not been shown to participate in electrical remodeling. Our novel finding that miR-499 regulates cardiac SK3 channels provides further evidence that microRNAs are closely involved with cardiac remodeling in AF.
Regulation of SK3 channel by miR-499
SK channels have been shown to contribute to the configuration of the cardiac action potential 10,24,31, but the molecular mechanism through which these channels are regulated is unknown. Our findings that SK3 expression is regulated by miR-499, therefore, provide novel insights on the mechanisms that underlie the changes in expression of these channels.
KCNN3 was a highly scored target of miR-499 by several computational algorithms. The significant increase in miR-499 expression in the fibrillating human atrium was accompanied by a significant reduction of SK3 protein expression. Our findings were further substantiated by the expression of miR-499 mimic and inhibitor in HL-1 cells, with results confirming that miR-499 targets KCNN3 and negatively regulates both SK3 mRNA and protein expression (Figures 4 and 6). Moreover, the direct interaction between miR-499 and its binding site at the 3′UTR of KCNN3 was demonstrated by the luciferase reporter assay, and such targeting induced enrichment of SK3 transcripts in miRISCs in HL-1 cells (Figure 3). Our results suggest that downregulation of SK3 expression by miR-499 is due to enhanced degradation of SK3 mRNA. These changes likely contribute to the electrical remodeling in AF.
Potential limitations
We could only obtain biopsies from the right atrial appendage. There could be heterogeneity in microRNA expression in different parts of the heart but we were unable to obtain tissues from the left atria for examination. The number of patients in this study is small and we did not examine the microRNA changes in paroxysmal and persistent AF. There is no known successful technique to culture human atrial myocytes and our in vitro studies were performed in HL-1 cells. Electrophysiology studies in HL-1 cells were difficult for determination of a phenotype from changes in miR-499 expression. In this study, we only focused on microRNAs that showed >2-fold changes with P < 0.05 and we could have missed important microRNAs that are pertinent to the electrical remodeling in AF.
Despite these limitations, our results consistently indicate the role of miR-499 in the regulation SK3. The clinical relevance of the relationship between miR-499 and SK3 channels in human AF warrants further studies in the future.
Acknowledgments
Dr. Lee received support from the National Institutes of Health (HL74180 and HL080118). Dr. Cha received support from the Mayo Clinic Foundation. The authors thank the Mayo Gene Expression Core for assistance in microRNA identification.
ABBREVIATIONS
- AF
atrial fibrillation
- miR-499
microRNA 499
- miRISC
miR-inducing silencing complex
- PCR
polymerase chain reaction
- RT
reverse transcription
- SK3
small-conductance calcium-activated potassium channel 3
- SR
sinus rhythm
Footnotes
The authors have declared no conflicts of interest.
Justification of authorship:
Tian-You Ling, Xiao-Li Wang, Qiang Chai, and Tin-Wah Lau conducted the experiments, analyzed the results, and wrote the manuscript. Celeste M. Koestler participated in study design, recruited and consented patients. Soon J. Park, Richard C. Daly, and Kevin L. Greason participated in study design, secured patient atrial tissue, and participated in writing the manuscript. Jin Jen provided advice and supervised the work on microRNA microarray and helped analyze the microarray results. Li-Qun Wu, Wei-Feng Shen, and Win-Kuang Shen provided advice and helped with manuscript writing. Yong-Mei Cha designed and supervised the studies, provided funding support, and participated in manuscript writing. Hon-Chi Lee designed and supervised the study, analyzed data, wrote the manuscript, and provided funding support for the studies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Corradi D, Callegari S, Maestri R, Benussi S, Alfieri O. Structural remodeling in atrial fibrillation. Nature Clinical Practice Cardiovascular Medicine. 2008;5:782–796. doi: 10.1038/ncpcardio1370. [DOI] [PubMed] [Google Scholar]
- 2.Nattel S, Maguy A, Le Bouter S, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 3.Van Wagoner DR, Nerbonne JM. Molecular basis of electrical remodeling in atrial fibrillation. Journal of Molecular and Cellular Cardiology. 2000;32:1101–1117. doi: 10.1006/jmcc.2000.1147. [DOI] [PubMed] [Google Scholar]
- 4.Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kuhlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovascular Research. 1999;44:121–131. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 5.Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circulation Research. 1999;85:428–436. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- 6.Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1. 5 expression are reduced in chronic human atrial fibrillation. Circulation Research. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 7.Workman AJ, Kane KA, Rankin AC. The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovascular Research. 2001;52:226–235. doi: 10.1016/s0008-6363(01)00380-7. [DOI] [PubMed] [Google Scholar]
- 8.Ellinor PT, Lunetta KL, Glazer NL, et al. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet. 2010;42:240–244. doi: 10.1038/ng.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olesen MS, Jabbari J, Holst AG, et al. Screening of KCNN3 in patients with early-onset lone atrial fibrillation. Europace. 2011;13:963–967. doi: 10.1093/europace/eur007. [DOI] [PubMed] [Google Scholar]
- 10.Tuteja D, Xu D, Timofeyev V, et al. Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol. 2005;289:H2714–H2723. doi: 10.1152/ajpheart.00534.2005. [DOI] [PubMed] [Google Scholar]
- 11.Li N, Timofeyev V, Tuteja D, et al. Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol. 2009;587:1087–1100. doi: 10.1113/jphysiol.2008.167718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latronico MV, Condorelli G. RNA silencing: small RNA-mediated posttranscriptional regulation of mRNA and the implications for heart electropathophysiology. J Cardiovasc Electrophysiol. 2009;20:230–237. doi: 10.1111/j.1540-8167.2008.01357.x. [DOI] [PubMed] [Google Scholar]
- 13.Cardin S, Guasch E, Luo X, et al. Role for MicroRNA-21 in Atrial Profibrillatory Fibrotic Remodeling Associated With Experimental Postinfarction Heart Failure. Circulation. 2012;5:1027–1035. doi: 10.1161/CIRCEP.112.973214. [DOI] [PubMed] [Google Scholar]
- 14.Girmatsion Z, Biliczki P, Bonauer A, et al. Changes in microRNA-1 expression and IK1 up-regulation in human atrial fibrillation. Heart Rhythm. 2009;6:1802–1809. doi: 10.1016/j.hrthm.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 15.Lu Y, Zhang Y, Wang N, et al. MicroRNA-328 contributes to adverse electrical remodeling in atrial fibrillation. Circulation. 2010;122:2378–2387. doi: 10.1161/CIRCULATIONAHA.110.958967. [DOI] [PubMed] [Google Scholar]
- 16.Dweep H, Sticht C, Pandey P, Gretz N. miRWalk--database: prediction of possible miRNA binding sites by “walking” the genes of three genomes. J Biomed Inform. 2011;44:839–847. doi: 10.1016/j.jbi.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Claycomb WC, Lanson NA, Jr, Stallworth BS, et al. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou W, Wang XL, Kaduce TL, Spector AA, Lee HC. Impaired arachidonic acid-mediated dilation of small mesenteric arteries in Zucker diabetic fatty rats. American journal of physiology Heart and circulatory physiology. 2005;288:H2210–H2218. doi: 10.1152/ajpheart.00704.2004. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J, Wang KC, Wu W, et al. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci U S A. 2011;108:10355–10360. doi: 10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaborit N, Steenman M, Lamirault G, et al. Human atrial ion channel and transporter subunit gene-expression remodeling associated with valvular heart disease and atrial fibrillation. Circulation. 2005;112:471–481. doi: 10.1161/CIRCULATIONAHA.104.506857. [DOI] [PubMed] [Google Scholar]
- 22.Dobrev D, Friedrich A, Voigt N, et al. The G protein-gated potassium current I(K,ACh) is constitutively active in patients with chronic atrial fibrillation. Circulation. 2005;112:3697–3706. doi: 10.1161/CIRCULATIONAHA.105.575332. [DOI] [PubMed] [Google Scholar]
- 23.Olson TM, Alekseev AE, Liu XK, et al. Kv1. 5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006;15:2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 24.Ozgen N, Dun W, Sosunov EA, et al. Early electrical remodeling in rabbit pulmonary vein results from trafficking of intracellular SK2 channels to membrane sites. Cardiovascular Research. 2007;75:758–769. doi: 10.1016/j.cardiores.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diness JG, Sorensen US, Nissen JD, et al. Inhibition of small-conductance Ca2+-activated K+ channels terminates and protects against atrial fibrillation. Circulation. 2010;3:380–390. doi: 10.1161/CIRCEP.110.957407. [DOI] [PubMed] [Google Scholar]
- 26.Diness JG, Skibsbye L, Jespersen T, et al. Effects on atrial fibrillation in aged hypertensive rats by Ca2+-activated K+ channel inhibition. Hypertension. 2011;57:1129–1135. doi: 10.1161/HYPERTENSIONAHA.111.170613. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q, Timofeyev V, Lu L, et al. Functional roles of a Ca2+-activated K+ channel in atrioventricular nodes. Circulation Research. 2008;102:465–471. doi: 10.1161/CIRCRESAHA.107.161778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chua SK, Chang PC, Maruyama M, et al. Small-conductance calcium-activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circulation Research. 2011;108:971–979. doi: 10.1161/CIRCRESAHA.110.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Lu Y, Yang B. MicroRNAs and atrial fibrillation: new fundamentals. Cardiovascular Research. 2011;89:710–721. doi: 10.1093/cvr/cvq350. [DOI] [PubMed] [Google Scholar]
- 30.Matkovich SJ, Hu Y, Eschenbacher WH, Dorn LE, Dorn GW., 2nd Direct and indirect involvement of microRNA-499 in clinical and experimental cardiomyopathy. Circulation Research. 2012;111:521–531. doi: 10.1161/CIRCRESAHA.112.265736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Y, Tuteja D, Zhang Z, et al. Molecular identification and functional roles of a Ca2+-activated K+ channel in human and mouse hearts. The Journal of Biological Chemistry. 2003;278:49085–49094. doi: 10.1074/jbc.M307508200. [DOI] [PubMed] [Google Scholar]