SUMMARY
Setting
The Republic of Moldova, Eastern Europe, 2007–2010. Moldova has among the highest reported nationwide proportions of TB patients with multidrug-resistant tuberculosis (MDR-TB) worldwide.
Objective
To assess risk factors and timing of default from treatment for non-MDR-TB. Default has been associated with increased mortality and amplification of drug resistance and may contribute to the high MDR-TB rates in Moldova.
Design
A retrospective analysis of routine surveillance data on all non-MDR-TB patients reported.
Results
14.7% of non-MDR-TB patients defaulted from treatment during the study period. Independent risk factors for default included sociodemographic factors (i.e. homelessness, living alone, less formal education and spending substantial time outside Moldova in the year prior to diagnosis) and health-related factors (i.e. HIV-coinfection, greater lung pathology, and increasing TB drug resistance). TB treatment is usually initiated within an institutional setting in Moldova and the default risk was highest in the month following the hospitalized treatment phase (among civilians) and after leaving prison (among those diagnosed while incarcerated).
Conclusions
Targeted interventions to increase treatment adherence for patients at highest risk of default and improving the continuity of care for patients transitioning from institutional to community care may substantially reduce the default risk.
Keywords: Eastern Europe, hospitalization, prisons, drug resistance
INTRODUCTION
The Republic of Moldova has a high estimated TB incidence (182/100,000 people1 in 2010) and among the highest published nationwide percentages of cases with multidrug-resistant TB (MDR-TB) globally; a 2006 survey reported 19.5% of treatment-naïve cases and 50.8% of previously treated cases had MDR-TB2.
One barrier to TB control is treatment default, defined as treatment interruption for at least two consecutive months1. Default can undermine effective TB control because patients with sustained non-adherence to treatment may remain infectious3 and suffer an increased risk of TB recurrence4 and TB-related mortality5. Of particular relevance in Moldova, default and sub-optimal adherence may increase the probability of acquired drug resistance3,6. Of TB cases specifically returning after treatment default (a subset of all those that have previously received treatment) in Moldova (2007–2010), 64% had MDR-TB7.
Studies in other settings have documented several risk factors for default including alcoholism8,9, substance abuse10, unemployment8,11,12, previous incarceration12 and homelessness8,11.
Given the importance of reducing treatment default for preventing acquired resistance and minimizing the prevalence of untreated drug-resistant TB, we sought to identify risk factors and temporal patterns of treatment default among TB cases without MDR-TB in Moldova. Using national data collected from 2007 to 2010, we identify individual-level risk factors for default among non-MDR-TB cases. We also examine the time at which individuals defaulted to permit better insight into potential causes of treatment default. Identifying host- and time-related risks of default may facilitate the deployment of targeted interventions.
METHODS
Study setting
Moldova is a former Soviet Union (FSU) country of around 4 million population13, however, an estimated 25% of the workforce migrate internationally for employment14. All TB treatment is based on the DOTS strategy15 involving 6- and 8-month treatment regimens for new and previously treated cases without MDR-TB, respectively. Some patients receive treatment for longer depending on their drug resistance profile or at the discretion of the treating physician16. In contrast with many countries, although in common with many FSU countries, most TB patients in Moldova receive the first two months of treatment (the intensive phase) as inpatients in specialized TB hospitals; the remaining continuation phase of treatment is received in an ambulatory setting.
Substantial investment has been made toward improving TB control in Moldova and addressing highly drug-resistant disease17,18. In particular, drug susceptibility testing (DST) is now offered to all culture-positive patients. Since 2007, DST has been performed for 94% of all eligible cases (estimated directly from this Moldovan database7) and detailed demographic, medical and laboratory data on all notified TB cases are collected in real-time in an online database.
Moldova has four culture and DST laboratories (including one national reference laboratory) that have passed external quality assurance from the Supranational Reference Laboratory Network in Borstel, Germany. DST is done on both solid culture using the absolute concentration method19 and liquid culture (BACTEC MGIT 960).
Study design and data collection
We conducted standard analyses of national TB surveillance data extracted from the cohort of TB cases notified in Moldova between January 2007 and December 2010 to achieve several aims: 1) to estimate the percentage of non-MDR-TB cases that defaulted on treatment, 2) to document high risk times for default during treatment and 3) to identify risk factors for treatment default. Data used was as downloaded on 14 September 2011.
In Moldova, suspected TB cases are tested with sputum microscopy and culture; the national policy is to perform DST on all culture-positive samples. At diagnosis, detailed demographic information on each individual is entered into the national database and laboratory results are added when available. Outcome definitions are recorded as per WHO1 recommendations and specifically, default is defined as treatment interruption for at least two consecutive months (the date of default is recorded as the date of last uptake of medication). All data are verified by the National Tuberculosis Programme (NTP) and the National Centre of Health Management. The NTP and local TB facilities resolve apparent inconsistencies by comparing the online data with hand-written paper records maintained at the TB facilities. Additionally, for our analyses, a small fraction of cases with inconsistent dates were excluded (see below).
Statistical analysis
Our analysis was restricted to TB patients with pulmonary and/or extra-pulmonary TB confirmed through DST to be sensitive to isoniazid and/or rifampin (i.e. “non-MDR”) at baseline and in whom MDR-TB was not detected prior to treatment default (Supplementary Figure S1, group G).
We calculated the percentage of cases that defaulted on treatment overall and by year among cases with confirmed outcomes or still on <1 year of treatment on 14 September 2011. We used a least squares regression to detect linear trends in the fraction of cases that defaulted by year weighting each data point by the number of TB cases included.
We also investigated the timing of default separately for new and previously treated cases, including only those cases that defaulted within the first year of treatment (since guidelines for successful outcomes specify that treatment should be completed within 12 months of diagnosis20). We excluded observations that were missing diagnosis or treatment result date and observations where recorded treatment result date preceded diagnosis date (Supplementary Figure S1, group L, default cases only).
We used proportional hazards regression models21 for new and for previously treated cases to identify risk factors associated with treatment default. We considered time from initial diagnosis to treatment result date as the period at risk since treatment is initiated at diagnosis and this approach allowed us to include primary defaulters. We included outcomes occurring within one year after diagnosis and, for cases that were recorded as cured/completed treatment, at least 6 months after diagnosis since these outcomes could not have been properly recorded earlier (Supplementary Figure S1, group L).
We developed a full model including all potential explanatory variables for which <10% of individuals were missing data to obtain fully adjusted hazard ratios and used backwards elimination to identify factors independently associated with default and other variables that adjusted for probable confounding. When explanatory variables could be represented by different forms (e.g. linear or categorical), we compared alternatives in univariable models via likelihood ratio tests and used the best form in the multivariable model. We tested the proportional-hazards assumption with Schoenfeld’s global test22. If the proportional-hazards assumption was violated for a particular covariate, a time-by-covariate interaction was added to the model21.
Alcoholism and drug use data were missing for >10% of cases and physicians designated these conditions in the absence of formal definitions, which limits our confidence in the consistency of these classifications. However, since substance abuse has previously been linked with default8,10–12,23, we did a sub-analysis by including these variables in our final model to assess the impact of these likely imperfect classifications. Given concerns of data completeness and quality for these variables, our main results reported are from models that exclude alcoholism or drug use.
This analysis was done using a subset of non-identifiable clinical and laboratory variables extracted from the Moldovan SIME-TB database which includes data collected during routine care; the analyses was deemed exempt by Partners IRB, Boston MA.
RESULTS
There were 4,890 non-MDR-TB cases included in our estimates of the percentages of non-MDR-TB cases that defaulted. Only 66 (1.3%) of these cases had documented extra-pulmonary involvement. After exclusions for missing or inaccurately recorded dates, 4,021 cases were included in our analysis of timing of and risk factors for default. See Supplementary Figure S1 for a full breakdown.
Percentage of non-MDR-TB cases defaulting on treatment
Of non-MDR-TB cases notified between 2007 and 2010, 14.7% defaulted on treatment (11.5% of new cases and 25.7% of previously treated cases) (Table 1). Among the categories of previously treated cases, those returning for treatment after a previous default had the highest default risk (41.8%). There was no secular trend in the percentage of patients defaulting on treatment 2007–2010 (Supplementary Table S1).
Table 1.
Treatment outcome among patients confirmed to have non-MDR-TB at initial diagnosis and throughout treatment in Moldova, 2007–2010
| Case type
|
|||||||
|---|---|---|---|---|---|---|---|
| New, n (%) | Previously treated
|
Total*, n (%) | |||||
| Relapse, n (%) | Return from default, n (%) | After failure, n (%) | Chronic, n (%) | All previously treated, n (%) | |||
| Cured | 1987 (52.4) | 298 (45.5) | 64 (22.9) | 57 (41.9) | 1 (6.3) | 420 (38.6) | 2409 (49.3) |
| Completed | 795 (21.0) | 93 (14.2) | 19 (6.8) | 9 (6.6) | 2 (12.5) | 123 (11.3) | 919 (18.8) |
| Failed treatment | 182 (4.8) | 44 (6.7) | 20 (7.1) | 22 (16.2) | 0 (0.0) | 86 (7.9) | 270 (5.5) |
| Defaulted | 437 (11.5) | 135 (20.6) | 117 (41.8) | 24 (17.6) | 3 (18.8) | 279 (25.7) | 719 (14.7) |
| Died | 249 (6.6) | 62 (9.5) | 45 (16.1) | 17 (12.5) | 7 (43.8) | 131 (12.1) | 382 (7.8) |
| Still on treatment as of 14 Sept 2011 | 141 (3.7) | 23 (3.5) | 15 (5.4) | 7 (5.1) | 3 (18.8) | 48 (4.4) | 191 (3.9) |
| Total | 3791 | 655 | 280 | 136 | 16 | 1087 | 4890 |
| Excluding those still on treatment as of 14 Sept 2011 | |||||||
| Total | 3650 | 632 | 265 | 129 | 13 | 1039 | 4699 |
| Percentage defaulted | 12.0 | 21.4 | 44.2 | 18.6 | 23.1 | 26.9 | 15.3 |
Includes an additional 12 cases that initiated treatment abroad
Timing of treatment default
The median time to default was 110 days for new cases and 125 days for previously treated cases (Figure 1). The greatest default risk in any single month occurred in the month immediately following the intensive phase of treatment.
Figure 1.
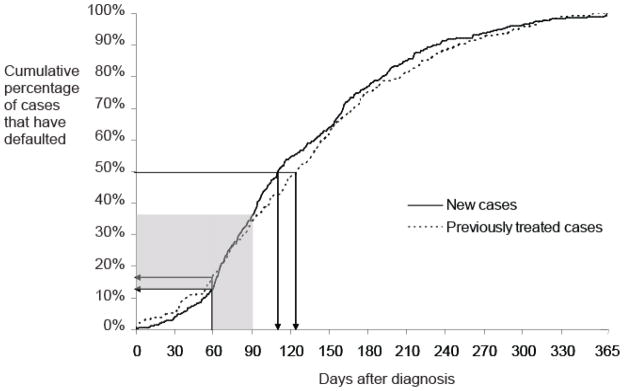
Cumulative percentage of tuberculosis (TB) cases without multidrug-resistant TB (MDR-TB) in Moldova that defaulted by days after diagnosis. Diagnoses included from 2007 to 2010. Separate lines are shown for new and previously treated cases. Previously treated cases includes: relapse cases, returns from default, treatment failures and chronic cases. Horizontal arrows show the percentage that had defaulted by the end of the intensive phase of treatment (2 months) which is usually spent in hospital. Vertical arrows show the median time of default. The grey shaded area shows the 30 day period during which the highest percentage of new cases defaulted. This coincides with the month directly following the intensive (hospitalized) treatment phase.
Individual-level risk factors for treatment default
We identified several individual-level baseline characteristics that were associated with the hazard of treatment default (Table 2). The numbers of cases and hazard rates of defaulting per person-year by characteristic are shown in Supplementary Table S2. Both models satisfied the assumption of proportional hazards (p=0.19 and p=0.46 for the new and the previously treated cases models respectively). In particular, we noted the following:
Table 2.
Individual-level risk factors for default from treatment among non-MDR-TB cases in new and previously treated TB cases in Moldova for TB cases diagnosed between 2007 and 2010. Results from univariable and multivariable models are presented. Cells left blank indicate that that variable was not included in the multivariable model.
| New TB cases | Previously treated TB cases | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Univariable model results | Multivariable model results | Univariable model results | Multivariable model results | |||||
|
| ||||||||
| Variable | Hazard ratio (95% CI§) | P-value | Hazard ratio (95% CI§) | P-value | Hazard ratio (95% CI§) | P-value | Hazard ratio (95% CI§) | P-valu |
| Living in an urban or rural area | ||||||||
| Rural | Ref | Ref | ||||||
| Urban | 1.31 (1.07, 1.60) | 0.008 | 1.36 (1.06, 1.75) | 0.015 | ||||
| Homeless | ||||||||
| No | Ref | Ref | Ref | |||||
| Yes | 3.56 (2.61, 4.84) | <0.0001 | 2.33 (1.64, 3.29) | <0.0001 | 1.50 (0.96, 2.36) | 0.074 | ||
| Gender | ||||||||
| Female | Ref | Ref | ||||||
| Male | 1.29 (1.00, 1.66) | 0.046 | 1.31 (0.89, 1.92) | 0.17 | ||||
| Citizenship | ||||||||
| Moldovan | Ref | Ref | Ref | |||||
| Other | 3.21 (1.33, 7.75) | 0.010 | 0.00 (0.00, >100) | 0.98 | 0.00 (0.00, >100) | 0.99 | ||
| Occupation | ||||||||
| Employed/retired/disabled | Ref | Estimates varied by time# | Ref | Ref | ||||
| Student | 0.28 (0.07, 1.16) | 0.080 | 1.79 (0.43, 7.40) | 0.42 | 1.94 (0.40, 9.37) | 0.41 | ||
| Unemployed | 2.62 (1.98, 3.46) | <0.0001 | 2.19 (1.56, 3.09) | <0.0001 | 2.18 (1.00, 4.79) | 0.051 | ||
| Salaried | ||||||||
| Yes | Ref | Ref | Ref | |||||
| No | 2.46 (1.85, 3.27) | <0.0001 | 2.13 (1.49, 3.04) | <0.0001 | 1.07 (0.47, 2.43) | 0.86 | ||
| Education (linear) | ||||||||
| For each increase in education level† | 0.72 (0.63, 0.83) | <0.0001 | 0.77 (0.66, 0.91) | 0.002 | 0.73 (0.61, 0.88) | 0.0009 | 0.80 (0.65, 1.00) | 0.046 |
| Spent >3 months outside Moldova during previous 12 months | ||||||||
| No | Ref | Ref | Ref | |||||
| Yes | 1.26 (1.00, 1.59) | 0.054 | 1.29 (0.91, 1.83) | 0.15 | 1.55 (1.06, 2.27) | 0.025 | ||
| Was previously in detention | ||||||||
| No | Ref | Ref | Ref | Ref | ||||
| Yes | 1.54 (1.14, 2.08) | 0.005 | 1.28 (0.91, 1.80) | 0.16 | 1.72 (1.28, 2.33) | 0.0005 | 1.68 (1.18, 2.39) | 0.004 |
| In detention at the time of diagnosis | ||||||||
| No and did not go into detention during treatment | Ref | Ref | Ref | Ref | ||||
| No but went into detention during treatment | 1.86 (0.46, 7.50) | 0.38 | 1.00 (0.24, 4.21) | 0.99 | 0.00 (0.00, >100) | 0.99 | 0.00 (0.00, >100) | 0.99 |
| Yes and remained there throughout treatment | 0.16 (0.04, 0.64) | 0.010 | 0.04 (0.01, 0.33) | 0.002 | 0.00 (0.00, >100) | 0.97 | 0.00 (0.00, >100) | 0.98 |
| Yes and was released during treatment | 5.73 (2.96, 11.09) | <0.0001 | 2.04 (0.89, 4.67) | 0.096 | 1.48 (0.37, 5.95) | 0.58 | 0.67 (0.14, 3.09) | 0.60 |
| Household size | ||||||||
| Living with others | Ref | Ref | Ref | Ref | ||||
| Living alone | 1.73 (1.39, 2.16) | <0.0001 | 1.57 (1.20, 2.04) | 0.0008 | 1.30 (1.00, 1.70) | 0.048 | 1.35 (0.98, 1.86) | 0.064 |
| Number of children in the household | ||||||||
| None | Ref | Ref | Ref | |||||
| At least one | 1.30 (1.05, 1.60) | 0.017 | 1.06 (0.80, 1.40) | 0.70 | 0.54 (0.30, 0.96) | 0.032 | ||
| Lives with someone with diagnosed TB | ||||||||
| No | Ref | Ref | Ref | |||||
| Yes | 1.27 (0.87, 1.85) | 0.22 | 0.86 (0.55, 1.37) | 0.53 | 1.54 (1.19, 1.99) | 0.001 | ||
| Degree of lung pathology | ||||||||
| Infiltration | Ref | Ref | Ref | |||||
| Destruction | 1.70 (1.36, 2.12) | <0.0001 | 1.59 (1.25, 2.02) | 0.0002 | 1.45 (1.08, 1.95) | 0.014 | ||
| Smear microscopy result | ||||||||
| Negative/untested/result unknown | Ref | Ref | ||||||
| Positive | 1.19 (0.96, 1.48) | 0.12 | 1.36 (0.98, 1.88) | 0.067 | ||||
| Culture positivity (linear, graded 1–3) | ||||||||
| For each increase in grade | 1.02 (0.90, 1.16) | 0.72 | 1.11 (0.95, 1.31) | 0.19 | ||||
| HIV status | ||||||||
| Negative | Ref | Ref | Ref | |||||
| Positive/untested/result unknown | 1.68 (1.31, 2.15) | <0.0001 | 1.55 (1.17, 2.05) | 0.002 | 1.27 (0.91, 1.77) | 0.16 | ||
| Presence of resistance to first line drugs at baseline (linear)* | ||||||||
| Each additional drug | 1.26 (1.11, 1.44) | 0.0005 | 1.27 (1.10, 1.46) | 0.001 | 1.03 (0.88, 1.21) | 0.71 | ||
| Age (years) | ||||||||
| < 30 | Ref | Ref | ||||||
| 30–39 | 1.44 (1.12, 1.86) | 0.005 | 1.20 (0.80, 1.78) | 0.38 | ||||
| ≥ 40 | 0.85 (0.67, 1.08) | 0.19 | 0.81 (0.56, 1.17) | 0.27 | ||||
| Region of residence¶ | <0.0001 | <0.0001 | 0.056 | 0.11 | ||||
Categories in increasing order: No education, primary, secondary, specialized secondary, higher;
CI=Confidence Interval;
Region of residence has 45 levels therefore we are presenting the p-value for the statistical significance of the entire variable (from a likelihood ratio test), Figure 3 shows a map of the default rates by region of residence,
These parameter estimates varied significantly by time; students were less likely than all other occupation categories to default and unemployed people were more likely to default although estimated hazard ratios varied by time on treatment (Supplementary Table S4),
A full breakdown of resistance profiles is shown in Supplementary Table S3
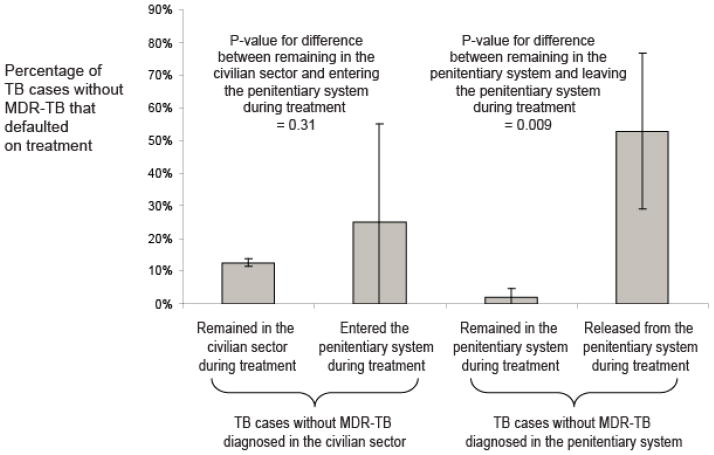
Among new cases, patients diagnosed and completing treatment in prison had a significantly lower default hazard than those diagnosed outside prison (Table 2, Figure 2). New TB cases diagnosed in prison and released during treatment had a substantially higher default hazard than those diagnosed and treated entirely in prison (Table 2, Figure 2). Other significant risk factors for default among new cases included HIV co-infection, extensive lung destruction and resistance to first-line TB drugs at baseline (Supplementary Table S3). There were substantial differences in default risk between geographic regions among new cases (p<0.0001) and previously treated cases (p=0.11) (Figure 3).
Figure 2.
Percentage of new tuberculosis (TB) incident cases without multidrug-resistant TB (MDR-TB) notified between 2007 and 2010 in Moldova that defaulted on treatment. Results are stratified by diagnosis location (civilian sector or penitentiary system) and further stratified by whether or not the patients remained in that location or were transferred in or out from the penitentiary system. Binomial confidence intervals and p-values for differences between groups are shown.
Figure 3.
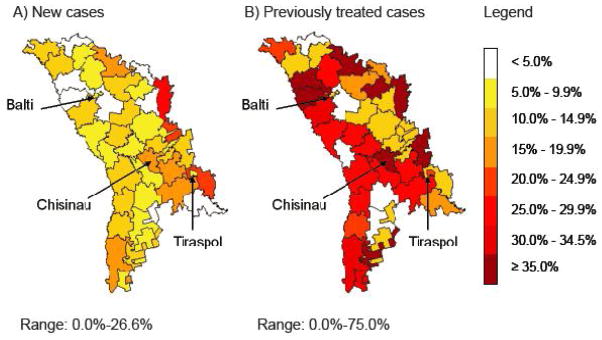
Maps of the percentage of tuberculosis (TB) cases without multidrug-resistant TB (MDR-TB) that defaulted on treatment by region of residence in Moldova (2007–2010). Data are stratified by (A) new and (B) previously treated cases. Note that numbers in some regions for previously treated cases are small (<10) and thus estimated regional rates should be interpreted with caution. The major cities (Chisinau (capital city), Balti and Tiraspol) are shown.
Sub-analysis of risk factors related to alcoholism and drug abuse
In our sub-analysis, new cases suffering from alcoholism were at an 83% (95% Confidence Interval (CI): 31%, 155%) increased default hazard (p=0.0004). Previously treated cases with baseline alcoholism had a 115% (95% CI: 46%, 217%) increased hazard of defaulting (p=0.0001). New cases with drug abuse/addiction problems had a 159% (95% Confidence Interval (CI): 1%, 562%) increased default hazard (p=0.048). Drug abuse/addiction was not significantly associated with default among previously treated cases.
DISCUSSION
During 2007–2010, 14.7% of non-MDR-TB patients defaulted on treatment. This percentage is relatively high compared with other studies of default in other FSU countries8,10–12.
We found that the default risk is substantially increased for non-MDR-TB cases that transferred out of institutional settings during treatment. Among non-MDR-TB cases diagnosed in prison, there was a stark contrast between the default rates among those treated exclusively in prison (2% default rate) and those released during treatment (53% default rate). Movement out of prison was also identified in a study in California24 as a default risk factor. A study in Tomsk, Russia, found that improved co-ordination of TB care between the civilian and prison sectors reduced overall default rates23 indicating potential models of intervention for use in Moldova.
The timing of default often coincides with the time at which patients transfer care settings in Moldova. The WHO recommends that tuberculosis care is delivered outside of hospital settings unless patients are severely ill, or have conditions that require close monitoring15. However, Moldovans receive their intensive treatment phase in hospital and the continuation phase in the community and therefore most patients must transfer between care settings. The month following discharge from hospital was highest default risk period for all patients. The high risk period we identified may result from either an administrative failure to link care between settings or because patients experiencing clinical improvement and returning to work may feel less motivated or able to continue care.
Continuity of care between penitentiary/in-patient and outpatient settings relies on the transfer of TB records to the receiving TB treatment center (nearest one to their stated address after release/discharge) prior to release from incarceration/hospital. If patients do not report to their local TB service within a week, they are actively sought out. Recently, financial and other support methods (e.g. food supplements) are being used to try to improve treatment outcomes and reduce defaults among prisoners after release.
Consistent with our findings, a systematic review of default timing found that the majority of patients defaulted after the intensive phase25, although in this review, the intensive phase was not necessarily occurring in hospital. A study in Uzbekistan, where the first two months of treatment was also delivered in hospital, found that the majority of default events occurred during the hospitalization phase, but that a substantial proportion completed the intensive phase and failed to start the continuation phase8.
We found that alcoholism was associated with an increased default risk concurring with other studies in the region8,10–12,23. While this association is plausible, our findings should be viewed cautiously due to the imperfect classification of alcoholism and the substantial amount of missing data for this sub-analysis (approximately 50%). Intervention studies in Tomsk, Russia are evaluating the effect of naltrexone or monthly counseling interventions for TB patients with alcohol use disorders as part of routine care with an aim of reducing poor treatment outcomes including default26,27.
Our finding of an association between an increased default risk and baseline drug resistance among new cases is concerning. Potential reasons for this observation include 1) greater difficulty with adherence to longer treatment regimens sometimes necessary for additional drug resistance, 2) additional side effects resulting from more toxic drugs, and 3) lack of clinical improvement leading to less motivation for treatment adherence. Alternatively, if some retreatment patients were misclassified as new patients, this could potentially explain an association between baseline resistance and probability of default since both resistance and defaults are concentrated among the retreatment group. Further studies to ascertain whether baseline drug resistance is truly associated with default in other settings would be useful since treatment defaults among those with poly-resistant (non-MDR) TB may facilitate the amplification of resistance.
While this study made use of a comprehensive clinical and laboratory database, the nature of these data imposes several limitations. First, these data do not allow us to identify the causes of treatment default for individuals within the cohort. In the future, qualitative studies involving interviews with defaulters will allow for a clearer understanding of how and why defaults occur and what interventions may be most effective. Additionally, further studies of the substantial geographic variation in default risk might uncover potential drivers of such patterns including spatial differences in healthcare provision or patient characteristics. Despite these limitations, our approach for evaluating risk factors and timing of default provides information that may help identify the most vulnerable individuals and the riskiest times for treatment default.
Our analysis is also limited by missing and misclassified data, problems frequently encountered when analyzing surveillance datasets. However, it seems unlikely that there should be systematic differences in quality of data collected at baseline and while on treatment for defaulters and non-defaulters and therefore our central conclusions should be unaffected. Note that since we focused on culture-confirmed non-MDR-TB cases, our results do not necessarily apply to non-culture-confirmed TB cases.
While default rates were lower in Moldova during the hospitalized treatment phase, other studies have found that obligatory admission to TB hospitals may increase treatment default during that time8,28. Additionally, hospitalization of TB patients has been associated with nosocomial TB transmission29,30, increased MDR-TB risk10 and, in Moldova, an increased default risk once patients are transferred to ambulatory care. Further work should focus on how low default rates can be achieved through provision of ambulatory care from treatment initiation. Additionally, improved co-ordination between prison and civilian TB services may help to reduce default rates in Moldova.
Acknowledgments
We thank all those involved in surveillance, laboratory testing and treatment for tuberculosis in the Republic of Moldova. This work was supported by Award Number U54GM088558 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest
The authors have no conflicts of interest
References
- 1.World Health Organization. Global Tuberculosis Control 2011. Geneva: 2011. Report No.: WHO/HTM/TB/2011.16 http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf. [Google Scholar]
- 2.World Health Organization. Multidrug and extensively drug-resistant (M/XDR-TB). 2010 Global Report on Surveillance and Response. Geneva: 2010. Report No.: WHO/HTM/TB/2010.3 http://whqlibdoc.who.int/publications/2010/9789241599191_eng.pdf. [Google Scholar]
- 3.Pablos-Mendez A, Knirsch CA, Barr RG, Lerner BH, Frieden TR. Nonadherence in tuberculosis treatment: predictors and consequences in New York City. Am J Med. 1997;102:164–70. doi: 10.1016/s0002-9343(96)00402-0. [DOI] [PubMed] [Google Scholar]
- 4.Verver S, Warren RM, Beyers N, et al. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med. 2005;171:1430–5. doi: 10.1164/rccm.200409-1200OC. [DOI] [PubMed] [Google Scholar]
- 5.Kolappan C, SR, Karunakaran K, Narayanan PR. Mortality of tubercuolsis patients in Chennai, India. Bulletin of the World Health Organization. 2006;84:555–60. doi: 10.2471/blt.05.022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weis SE, Slocum PC, Blais FX, et al. The effect of directly observed therapy on the rates of drug resistance and relapse in tuberculosis. N Engl J Med. 1994;330:1179–84. doi: 10.1056/NEJM199404283301702. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins HE, Plesca V, Ciobanu A, et al. Assessing spatial heterogeneity of MDR-TB in a high burden country. European Respiratory Journal. 2012 doi: 10.1183/09031936.00111812. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasker E, Khodjikhanov M, Usarova S, et al. Default from tuberculosis treatment in Tashkent, Uzbekistan; who are these defaulters and why do they default? BMC Infect Dis. 2008;8:97. doi: 10.1186/1471-2334-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller AC, Gelmanova IY, Keshavjee S, et al. Alcohol use and the management of multidrug-resistant tuberculosis in Tomsk, Russian Federation. Int J Tuberc Lung Dis. 2012;16:891–6. doi: 10.5588/ijtld.11.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelmanova IY, Keshavjee S, Golubchikova VT, et al. Barriers to successful tuberculosis treatment in Tomsk, Russian Federation: non-adherence, default and the acquisition of multidrug resistance. Bull World Health Organ. 2007;85:703–11. doi: 10.2471/BLT.06.038331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakubowiak WM, Bogorodskaya EM, Borisov SE, Danilova ID, Kourbatova EV. Risk factors associated with default among new pulmonary TB patients and social support in six Russian regions. Int J Tuberc Lung Dis. 2007;11:46–53. [PubMed] [Google Scholar]
- 12.Kliiman K, Altraja A. Predictors and mortality associated with treatment default in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2010;14:454–63. [PubMed] [Google Scholar]
- 13.National Bureau of Statistics of the Republic of Moldova. [Accessed 2 February 2012];2004 Population Census Results. 2004 at http://www.statistica.md/pageview.php?l=en&idc=295.
- 14.International Organization for Migration. Migration in Moldova: A Country Profile 2008. Geneva: 2008. http://publications.iom.int/bookstore/free/Moldova_Profile2008.pdf. [Google Scholar]
- 15.World Health Organization. Geneva: 2010. Treatment of tuberculosis guidelines, fourth edition. Report No.: WHO/HTM/TB/2009.420. http://whqlibdoc.who.int/publications/2010/9789241547833_eng.pdf. [PubMed] [Google Scholar]
- 16.Cu privire la implementarea progamului national de control si profilaxie a tuberculozei in Republica Moldova, pentru anii 2006–2010 (TB treatment guidelines, in Moldovan) Ministry of Health and Social Protection; Chisinau: 2006. Order 180, Annex 11. [Google Scholar]
- 17.Dara M, Kluge H. Roadmap to prevent and combat drug-resistant tuberculosis. Copenhagen: WHO Regional Office for Europe; 2011. http://www.euro.who.int/__data/assets/pdf_file/0014/152015/e95786.pdf. [Google Scholar]
- 18.Soltan V, Henry AK, Crudu V, Zatusevski I. Increasing tuberculosis case detection: lessons from the Republic of Moldova. Bull World Health Organ. 2008;86:71–6. doi: 10.2471/BLT.06.038265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crudu V. Anti-tuberculosis drug resistance surveillance, Republic of Moldova, 2006. Chisinau: 2009. Available from valeriu.crudu@gmail.com. [Google Scholar]
- 20.Ditah IC, Reacher M, Palmer C, et al. Monitoring tuberculosis treatment outcome: analysis of national surveillance data from a clinical perspective. Thorax. 2008;63:440–6. doi: 10.1136/thx.2006.073916. [DOI] [PubMed] [Google Scholar]
- 21.Cox DR. Regression models and life tables. Journal of the Royal Statistical Society Series B - Methodolgical. 1972;34:187–220. [Google Scholar]
- 22.Schoenfeld D. Partial residuals for the proportional hazards regression-model. Biometrika. 1982;69:239–41. [Google Scholar]
- 23.Shin SS, Pasechnikov AD, Gelmanova IY, et al. Treatment outcomes in an integrated civilian and prison MDR-TB treatment program in Russia. Int J Tuberc Lung Dis. 2006;10:402–8. [PubMed] [Google Scholar]
- 24.Cummings KC, Mohle-Boetani J, Royce SE, Chin DP. Movement of tuberculosis patients and the failure to complete antituberculosis treatment. Am J Respir Crit Care Med. 1998;157:1249–52. doi: 10.1164/ajrccm.157.4.9708058. [DOI] [PubMed] [Google Scholar]
- 25.Kruk ME, Schwalbe NR, Aguiar CA. Timing of default from tuberculosis treatment: a systematic review. Trop Med Int Health. 2008;13:703–12. doi: 10.1111/j.1365-3156.2008.02042.x. [DOI] [PubMed] [Google Scholar]
- 26.Greenfield SF, Shields A, Connery HS, et al. Integrated Management of Physician-delivered Alcohol Care for Tuberculosis Patients: Design and Implementation. Alcohol Clin Exp Res. 2010;34:317–30. doi: 10.1111/j.1530-0277.2009.01094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin SS, Livchits V, Nelson AK, et al. Implementing evidence-based alcohol interventions in a resource-limited setting: novel delivery strategies in Tomsk, Russia. Harv Rev Psychiatry. 2012;20:58–67. doi: 10.3109/10673229.2012.649121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nyirenda TE, Harries AD, Gausi F, et al. Decentralisation of tuberculosis services in an urban setting, Lilongwe, Malawi. Int J Tuberc Lung Dis. 2003;7:S21–8. [PubMed] [Google Scholar]
- 29.Crudu V, Rusch-Gerdes S, Niemann S, Moraru N, Carchilan L, Lesan V, Straten E, Soltan V. Failure of tuberculosis therapy by nosocomial transmission of multidrug resistant M. tuberculosis strains in a high incidence setting. 41st Union World Conference; Berlin. 2010. [Google Scholar]
- 30.Nodieva A, Jansone I, Broka L, Pole I, Skenders G, Baumanis V. Recent nosocomial transmission and genotypes of multidrug-resistant Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2010;14:427–33. [PubMed] [Google Scholar]