Abstract
Pre-B-cell transformation by Abelson virus (Ab-MLV) is a multistep process in which primary transformants are stimulated to proliferate but subsequently undergo crisis, a period of erratic growth marked by high levels of apoptosis. Inactivation of the p53 tumor suppressor pathway is an important step in this process and can be accomplished by mutation of p53 or down-modulation of p19Arf, a p53 regulatory protein. Consistent with these data, pre-B cells from either p53 or Ink4a/Arf null mice bypass crisis. However, the Ink4a/Arf locus encodes both p19Arf and a second tumor suppressor, p16Ink4a, that blocks cell cycle progression by inhibiting Cdk4/6. To determine if p16Ink4a plays a role in Ab-MLV transformation, primary transformants derived from Arf−/− and p16Ink4a−/− mice were compared. A fraction of those derived from Arf−/− animals underwent crisis, and even though all p16Ink4a−/− primary transformants experienced crisis, these cells became established more readily than cells derived from +/+ mice. Analyses of Ink4a/Arf−/− cells infected with a virus that expresses both v-Abl and p16Ink4a revealed that p16Ink4a expression does not alter cell cycle profiles but does increase the level of apoptosis in primary transformants. These results indicate that both products of the Ink4a/Arf locus influence Ab-MLV transformation and reveal that in addition to its well-recognized effects on the cell cycle, p16Ink4a can suppress transformation by inducing apoptosis.
Abelson murine leukemia virus (Ab-MLV) transforms pre-B cells in vivo and in vitro (34). Expression of the v-Abl protein tyrosine kinase, the single protein product of Ab-MLV, provides a strong growth stimulus that is countered by cellular tumor suppressor pathways. Thus, Ab-MLV-mediated transformation is a multistep process (11, 49, 54). In vitro, Ab-MLV infection induces pre-B-cell proliferation and formation of primary transformants. As these cells proliferate, they enter crisis, a period characterized by widespread apoptosis and erratic growth. Only a fraction of primary transformants survive to emerge as highly malignant, established cell lines (29, 51). Escape from crisis correlates with inactivation of the p53 pathway, either by acquiring a p53 mutation or by down-modulating the p53 regulatory protein, p19Arf (15, 29, 49). Consistent with the importance of these proteins in transformation, bone marrow cells from either p53 or Ink4a/Arf−/− mice fail to undergo crisis (29, 51).
The Ink4a/Arf locus encodes two tumor suppressor proteins, p19Arf and p16Ink4a, both of which play important roles in suppressing tumor development in humans and mice (22, 35, 40, 43, 44). p19Arf affects p53 by blocking Mdm2-mediated inhibition, thereby allowing p53 to interfere with cell cycle progression and promote apoptosis; p16Ink4a inhibits Rb phosphorylation through effects on cdk4 and cdk6 and causes G1 arrest (46). Mutations or epigenetic changes affecting the Ink4a/Arf locus occur frequently in human tumors, and while some changes affect both products, others affect p16Ink4a or p19Arf alone (35, 44). Consistent with the importance of this locus in tumorigenesis, mice lacking these products are tumor prone (17, 22, 41, 43).
Although the way in which p19Arf affects transformation has been studied extensively (46), less is known about the ways in which p16Ink4a influences oncogenesis. Animals lacking only p16Ink4a are tumor prone (22, 43), and the protein influences resistance to chemotherapy in a mouse lymphoma model (40). In vitro, loss of p16Ink4a enhances the growth of a variety of cells, including fibroblasts, macrophages, keratinocytes, and glia (3, 10, 14, 30, 43). In addition, p16Ink4a inhibits integrin-mediated cell spreading on vitronectin (1, 8) and induces apoptosis in some tumor cells (4, 36), suggesting that this protein can affect cell growth in multiple ways. Interestingly, BALB/c, a mouse strain that is highly susceptible to Ab-MLV in vivo and in vitro (32, 33), expresses a hypomorphic p16Ink4a allele, and inheritance of this allele correlates with susceptibility to carcinogen-induced tumors (9, 13, 56), raising the possibility that p16Ink4a contributes to susceptibility to Ab-MLV transformation.
Here we tested the role of p16Ink4a in Ab-MLV transformation by comparing the susceptibility of bone marrow cells from Ink4a/Arf−/−, Arf−/−, and p16Ink4a−/− mice to all phases of transformation. These analyses revealed that a fraction of primary transformants from Arf−/− mice exhibit crisis and that primary transformants derived from p16Ink4a−/− mice became established more readily than those from +/+ mice. Coexpression of p16Ink4a and v-Abl in transforming pre-B cells increased apoptosis, suggesting that p16Ink4a can influence survival of cells stimulated by an oncogenic signal. These data reveal that both p19Arf and p16Ink4a affect the ability of Ab-MLV to induce transformation.
MATERIALS AND METHODS
Cells, viruses, and mice.
Ab-MLV-transformed pre-B cell lines were maintained as described previously (29). Ab-MLV-P120 stocks were prepared by using the pMIG vector (12, 52) and the pSV-Ψ−-E-MLV retroviral packaging plasmid (27) and titrated as described elsewhere (24). To prepare viruses expressing both v-Abl and p16Ink4a, v-abl coding sequences were used to replace Gfp sequences 3′ of the internal ribosome entry site (IRES) in the pMIG vector (12, 52), and the coding sequences for p16Ink4a from pKS-mp16 (28) were inserted into the EcoRI site upstream of the IRES. This virus and a control virus in which v-abl sequences were inserted downstream of the IRES were titrated by infecting bone marrow cells with dilutions of virus stock. DNA was prepared from the cells 24 h later, and real-time PCR was used to quantitate the number of Ab-MLV copies. Amplification of sequences within the Rag1 gene was used to standardize the number of copies of cellular sequence. Bone marrow transformation assays were carried out as described elsewhere (33). To monitor the frequency with which primary transformants became established, the cells were explanted from agar and plated in liquid medium (29, 51). Growth was monitored on a daily basis, and when the cells could be subcultured on a regular and predictable basis and levels of apoptosis were less than 10%, the cells were considered established. Ink4a/Arf−/− mice (41) backcrossed with C57BL/6 mice for seven generations, Arf−/− mice from a breeding pair on a mixed C57BL/6-129 background obtained from C. J. Sherr (St. Jude's Children's Hospital), and p16Ink4a−/− animals (43) on a mixed 129Sv-FvB/n background were studied. Genotypes were assessed by PCR amplification of the allele of interest.
Apoptosis and cell cycle analysis.
Cells were suspended in 1.12% sodium citrate containing 2 mg of RNase A/ml and then mixed with an equal volume of 0.2% Triton X-100-0.1% sodium citrate containing 100 μg of propidium iodide/ ml (6). The samples were analyzed by using a FACScan instrument (Becton Dickinson) and ModFit LT software (Verity Software). To monitor apoptosis, cells were stained with propidium iodide or merocyanin 540, a dye that specifically stains apoptotic cells (31); comparable results were obtained with each procedure.
Protein analysis.
Cell lysates were prepared and quantitated as described previously (6) and fractionated on sodium dodecyl sulfate-polyacrylamide gels. Proteins were electrotransferred to polyvinylidene difluoride membranes (Millipore) and probed with anti-p19Arf (Novus), anti-p16Ink4a (RDI), anti-Gag/v-Abl (39), or anti-β-actin antibody (Sigma). The bands were visualized by developing the blots with a chemiluminescence kit (Tropix) according to the manufacturer's instructions. To quantitate levels of p16Ink4a, multiple exposures were evaluated by densitometry using the amount of p16Ink4a in the L1-2 cell line as a standard. This level of p16Ink4a was set at 1.
RESULTS
Absence of p16Ink4a or p19Arf does not affect pre-B-cell primary transformation frequency.
The first stage of Ab-MLV transformation, called primary transformation, involves Ab-MLV-mediated stimulation of pre-B-cell growth (29, 51). To determine if loss of p16Ink4a or p19Arf affects this phase of transformation, bone marrow from Ink4a/Arf−/−, Arf−/−, and p16Ink4a−/− mice and littermate controls was infected and plated in soft agar. Ten days later, macroscopic colonies of primary transformants were counted. Primary transformants were recovered in all infected samples, and although the numbers varied between the different +/+ and −/− animals, no difference that correlated with genotypes was observed (Table 1). Indeed, variation in primary transformation frequencies of as much as fourfold can be observed between genetically identical animals (15, 33, 51). Similar transformation frequencies indicate that loss of Ink4a/Arf locus products does not significantly alter Ab-MLV target cell populations or the ability of Ab-MLV to stimulate pre-B-cell growth.
TABLE 1.
Transformation frequencies are similar in the different Ink/Arf locus knockout micea
| Genotype | No. of primary transformants per 106 nucleated cells
|
|
|---|---|---|
| Expt 1 | Expt 2 | |
| Ink4a/Arf−/− | 59 ± 3.5 | 72 ± 3.1 |
| Ink4a/Arf+/+ | 95 ± 4.5 | 28 ± 2.1 |
| Arf−/− | 54 ± 5.0 | 31 ± 2.5 |
| Arf+/− | 21 ± 1.9 | 23 ± 2.9 |
| p16Ink4a−/− | 162 ± 7.0 | 115 ± 5.0 |
| p16Ink4a+/+ | 146 ± 4.0 | 159 ± 6.0 |
Bone marrow cells were infected with matched titers of Ab-MLV-P120 and plated in agar; macroscopic colonies of primary transformants were counted 10 days later (33). One +/+ or heterozygous animal and one −/− animal were used in each experiment. The transformation frequencies for p19Arf+/− mice are similar to those obtained for +/+ mice from the p19Arf colony. The values given represent the average number of colonies obtained per 106 nucleated bone marrow cells ± the standard error of the mean. In each experiment, at least 6 × 106 cells were evaluated in 72 independently plated cultures. The same virus stock was used to compare +/+ and −/− mice of each genotype, but different virus stocks were used with different groups of mice. Uninfected cultures did not contain macroscopic colonies.
Some Arf−/− Ab-MLV-transformed pre-B cells undergo crisis.
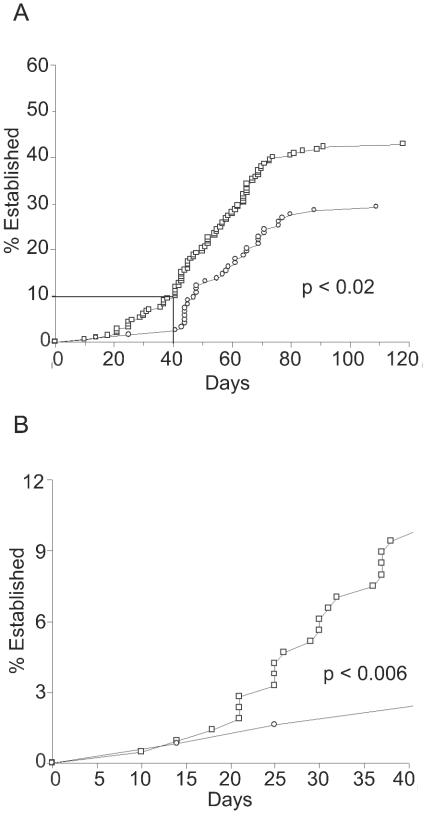
The second phase of the transformation process involves expansion of primary transformants. During this phase, cells from +/+ mice enter a period of crisis, characterized by erratic growth and high levels of apoptosis (49, 51, 54). Cells from p53−/− and Ink4a/Arf−/− mice bypass the crisis phase (29, 51). To test whether p19Arf is the only Ink4a/Arf locus product involved in crisis, primary transformants derived from Ink4a/Arf−/−, Arf−/−, and control mice were plated in liquid medium and their growth and survival were monitored. As expected (29, 49, 51), all of the primary transformants derived from +/+ mice entered crisis within 2 to 5 days, and 10% of them became established in 46 to 67 days (Fig. 1). Consistent with previous results (29), all of the primary transformants from Ink4a/Arf−/− mice bypassed crisis. In contrast, even though all Arf−/− primary transformants became established, 39% (28 of 72) displayed a variable period of crisis lasting 20 to 50 days (P < 0.001). These data suggest that p16Ink4a contributes to crisis.
FIG. 1.
Arf−/− primary transformants exhibit crisis. Primary transformants derived by infecting bone marrow from Ink4a/Arf−/− (•), Arf−/− (⋄), Ink4a/Arf+/+ (▪), and Arf+/+ (▴) mice with Ab-MLV were monitored for establishment. The Arf+/+ animals are wild-type mice from our Arf−/− breeding colony. Cells were considered established when they displayed <10% apoptotic cells and grew regularly (15, 29). These data represent analyses of at least 70 primary transformants per genotype. The P value represents comparison of the curves obtained for cells from Ink4a/Arf−/− and Arf−/− mice using a log-rank test.
Arf−/− Ab-MLV transformants that bypass crisis express low levels of p16Ink4a.
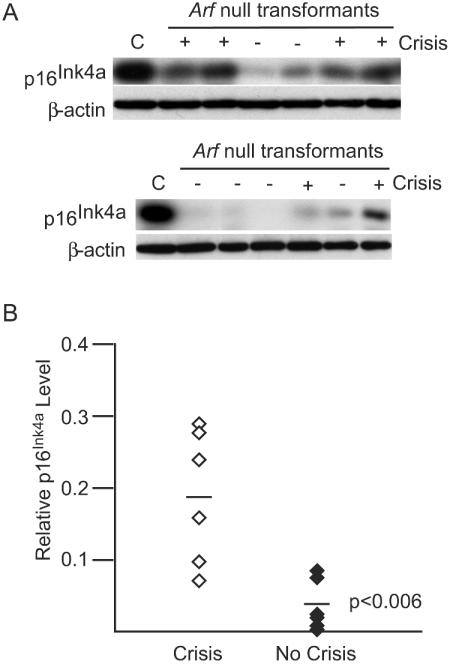
Both Ink4a/Arf locus products are expressed during crisis (29), and previous work has suggested that expression of p19Arf is particularly important in modulating the p53 response (29). However, p16Ink4a expression, perhaps particularly in the absence of p19Arf, may also be important during crisis. To explore this question, p16Ink4a levels were analyzed in Arf−/− transformants undergoing crisis. All seven of the transformants tested that exhibited crisis, including the representatives shown (Fig. 2A), expressed readily detectable levels of p16Ink4a, and levels increased with the onset of crisis. This pattern is consistent with involvement of p16Ink4a in crisis. Although some cells that did not experience crisis also expressed detectable levels of p16Ink4a, most of these cell lines expressed lower levels of the protein than cells undergoing crisis (P < 0.006) (Fig. 2B). Although the precise amounts of p16Ink4a that are needed for effects on crisis are not known, the strong proliferative signals provided by v-Abl may allow cells expressing lower levels to circumvent these effects. Consistent with this idea, cells downregulate p16Ink4a levels after becoming established (29; also our unpublished data), indicating that loss of p16Ink4a expression may contribute to crisis resolution.
FIG. 2.
p16Ink4a expression correlates with crisis in Arf−/− transformants. (A) Primary transformants from Arf−/− bone marrow cells were analyzed during the outgrowth period by Western blotting using anti-p16Ink4a and anti-β-actin antibodies. Representative samples are shown. The designations above the lanes indicate the presence (+) or absence (−) of crisis. C, L1-2, a fully established Ab-MLV transformant that expresses abundant p16Ink4a (29). (B) Densitometry was used to compare levels of p16Ink4a in primary transformants undergoing crisis to that in those that did not display crisis. Each point represents an individual cell line; the P value was obtained by using an unpaired, two-tailed t test.
Lack of p16Ink4a facilitates establishment.
Analyses of Ab-MLV-infected cells derived from Arf−/− null mice indicate that p16Ink4a contributes to crisis. To examine this possibility more fully, primary transformants from p16Ink4a+/+ and p16Ink4a−/− mice were studied. These analyses revealed that both types of cells experienced crisis. However, 43% (91 of 213) of the p16Ink4a−/− cells recovered and became established, while only 29% (36 of 123) of those derived from p16Ink4a+/+ animals survived (Fig. 3A). In addition, although the overall crisis period for both types of primary transformants was similar in duration, 9.4% (20 of 213) of those from p16Ink4a primary transformants became established in less than 40 days while only 1.6% (2 of 123) of those derived from +/+ littermates became established in the same rapid time frame (Fig. 3B). Thus, the absence of p16Ink4a enhances the ability of primary transformants to survive crisis and can shorten the crisis period significantly. However, the loss of p16Ink4a has a greater impact on some transformants than others and mirrors the results observed with the p19Arf null cells, where only a fraction of all primary transformants undergo crisis. These data suggest that the mechanism by which individual primary transformants recover from crisis is not identical and that several pathways influence the transformation process.
FIG. 3.
p16Ink4a−/− colonies become established more readily than +/+ colonies. (A) Primary transformants from p16Ink4a−/− (□) and +/+ littermates (○) were monitored for establishment. The data shown represent analysis of 213 and 123 primary transformants from −/− and +/+ mice, respectively. The boxed area identifies transformants that became established within the first 40 days and is illustrated in an enlarged form in panel B. The P values represent comparison of each of the curves using a log-rank test.
p19Arf levels are similar in p16Ink4a null and wild-type transformants.
Mice lacking p16Ink4a usually retain normal expression of p19Arf (3, 43). However, to exclude the possibility that the enhanced survival of p16Ink4a−/− transformants reflects aberrant expression of p19Arf in Ab-MLV-infected cells, the levels of p19Arf in primary transformants derived from p16Ink4a−/− were analyzed early in the crisis phase. Consistent with our earlier studies (29), most primary transformants analyzed expressed detectable p19Arf as the crisis phase began (representatives shown in Fig. 4A). As cells recovered from crisis, 4 of 17 transformants derived from p16Ink4a−/− cells expressed readily detectable p19Arf, while 3 of 15 derived from +/+ cells showed a similar pattern (representatives shown in Fig. 4B). Thus, the establishment advantage of the p16Ink4a−/− transformants is not due to differences in p19Arf expression. When considered along with the results of the establishment experiment, these data suggest that p16Ink4a contributes to crisis induction in a p19Arf-independent fashion but is not necessary for crisis to occur.
FIG. 4.
p19Arf is expressed normally in p16Ink4a null transformants. Lysates were prepared from different cell populations and analyzed by Western blotting using anti-p19Arf and anti-β-actin antibodies. (A) Analysis of lysates from p16Ink4a−/− and a control p16Ink4a+/+ primary transformant prepared 8 days post-explant from agar, near the onset of crisis. (B) Analysis of lysates from p16Ink4a−/− and p16Ink4a+/+ transformants prepared at the end of the crisis period. In both panels, an established cell line expressing abundant p19Arf (+) and an established cell line from a p19Arf null animal (−) are included as controls. The experiments shown are representative of analyses of 14 null and 6 wild-type transformants at crisis onset and 17 null and 15 wild-type transformants at the end of crisis. α-p19Arf, anti-p19Arf antibody; α-β-actin, anti-β-actin antibody.
p16Ink4a-v-Abl infection induces transformation.
p16Ink4a expression is classically associated with effects on cell growth (3, 10, 14, 30, 43) but has also been shown to influence other cellular processes (1, 4, 8, 36). To examine the mechanism by which p16Ink4a contributes to crisis, a retroviral vector expressing v-Abl and p16Ink4a in cis (p16Ink4a-v-Abl) was prepared. Matched titers of this virus and a control virus in which v-Abl was expressed in a similar fashion were used to infect Ink4a/Arf−/− bone marrow. To facilitate the isolation of a large number of cells, the infected cells were plated in liquid medium and monitored for expansion of pre-B cells (37). These types of cultures are scored as transformed when the density of rapidly growing pre-B cells exceeds 2 × 106 cells per ml (24), a process that requires between 10 and 15 days. All (18 of 18) of the cultures infected with the v-Abl control virus and 17 of 18 cultures infected with the p16Ink4a-v-Abl virus became transformed within a similar time frame (Fig. 5A), reinforcing our earlier result that the presence of Ink4a/Arf locus products does not influence the primary phase of the transformation process (Table 1). Cells did not expand in the absence of virus infection. Analyses of cultures by using Western blotting revealed that all of the transformants infected with the v-Abl-p16Ink4a virus expressed both proteins (Fig. 5B). In addition, the levels of v-Abl protein expressed in cells infected with the v-Abl-p16Ink4a virus were similar to those found in cells infected with the control, v-Abl virus, indicating that the presence of p16Ink4a sequences did not interfere with expression of v-Abl.
FIG. 5.
p16Ink4a-v-Abl transforms cells. Ink4a/Arf−/− bone marrow was infected with a virus expressing either v-Abl alone or both p16Ink4a and v-Abl and plated in liquid medium. Dishes were scored as transformed when they contained more than 2 × 106 pre-B cells per ml. (A) The day of transformation is indicated for p16Ink4a-v-Abl- and v-Abl-transformed cells. The asterisk indicates the average day of transformation. All 18 cultures infected with the v-Abl virus and 17 of 18 cultures infected with the p16Ink4a-v-Abl virus became transformed. (B) Lysates of primary transformants were analyzed by Western blotting with anti-p16Ink4a, anti-Gag/v-Abl (39), and anti-β-actin antibodies. The experiment shown is representative of experiments in which a total of 17 v-Abl-infected transformants and 18 v-Abl-p16-infected transformants were tested.
Apoptosis is increased in the primary transformants expressing p16Ink4a.
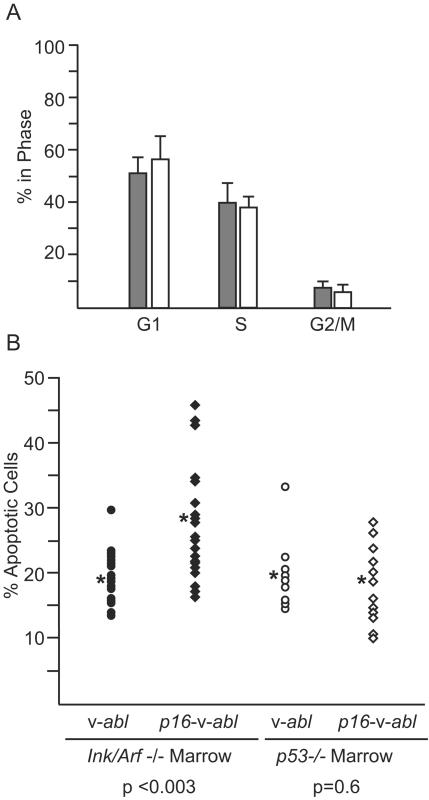
To understand the mechanism by which p16Ink4a contributes to crisis, cell cycle parameters and the level of apoptosis in Ink4a/Arf−/− primary transformants expressing the p16Ink4a-v-Abl and v-Abl viruses were compared. Analyses of propidium iodide-stained cells by flow cytometry revealed that the percentage of cells in the G1, S, and G2/M phases did not differ among the cultures (Fig. 6A). In contrast, primary transformants expressing the p16Ink4a-v-Abl virus had higher levels of apoptosis than those expressing v-Abl alone (P < 0.003) (Fig. 6B). The levels of apoptosis seen in v-Abl-infected Ink4a/Arf−/− cells were low and not apparent by visual inspection of the cells, in accord with a previous report of low levels of apoptosis in such cultures (29). These data indicate that cells expressing p16Ink4a undergo more apoptosis than cells lacking this protein and suggest that p16Ink4a contributes to the apoptotic response that characterizes crisis. Despite differences in the apoptotic response, primary transformants expressing the p16Ink4a-v-Abl virus could be established with kinetics that are similar to those observed for cells expressing v-Abl alone.
FIG. 6.
Expression of p16Ink4a in primary transformants leads to apoptosis. (A) Ink4a/Arf−/− cells infected with p16Ink4a-v-Abl (open bar) or v-Abl (filled bar) were harvested 24 to 72 h after transformation, stained with propidium iodide, and analyzed by flow cytometry to determine the percentage of cells in the G1, G2/M, and S phases of the cell cycle. The data are averages obtained from analyses of 18 independent cultures infected with v-Abl and 17 independent cultures infected with v-Abl-p16; the error bars represent standard deviations. (B) The percentage of apoptotic cells in the cultures analyzed in panel A and cultures transformed with either v-Abl or v-Abl-p16 derived from p53−/− mice were stained with propidium iodide and analyzed for the frequency of apoptotic cells by flow cytometry. Each point represents an individual clone derived from one of three independent experiments. The asterisk indicates the mean; the P values were obtained by using an unpaired, two-tailed t test.
To determine if the apoptotic effects of p16Ink4a require p53, bone marrow from p53−/− mice was infected with either the p16Ink4a-v-Abl or the v-Abl virus and plated in liquid medium. All of the infected cultures became transformed within 10 to 13 days. Analyses of apoptosis in these primary transformants revealed that the p53−/− cells transformed by p16Ink4a-v-Abl displayed levels of apoptosis similar to those found in cells transformed with the v-Abl virus (Fig. 6B). These levels were similar to those seen in the Ink4a/Arf−/− cells infected with the v-Abl virus, indicating that the p53−/− cells infected with the p16Ink4a-v-Abl virus exhibit a baseline level of apoptosis. These data indicate that p16Ink4a induces apoptosis only in cells with an intact p53 gene, suggesting that p53 may be required for this response.
DISCUSSION
Our results demonstrate that Ab-MLV-induced pre-B-cell transformation is influenced by both products of the Ink4a/Arf locus. Thus, oncogenic signals from v-Abl stimulate a cellular response that is orchestrated by both p19Arf and p16Ink4a. Consistent with this idea, recent evidence indicates that both of these proteins influence melanoma development stimulated by activated Ras (45); the central role of Ras activation in Ab-MLV transformation is well documented (38, 57), and it is likely that Ras is involved in activating expression of both Ink4a/Arf locus products in Ab-MLV-infected cells. Once activated, both products influence the apoptosis that characterizes the crisis phase of transformation. Although p16Ink4a expression is usually associated with effects on the cell cycle (44, 46, 47), p16Ink4a may influence apoptosis more commonly than originally thought. p16Ink4a expression is associated with apoptosis in some human carcinoma and leukemia cells (2, 4, 20, 36), and transgene-mediated expression of p16Ink4a in normal T cells causes enhanced apoptosis and differentiation arrest at an immature stage (23).
Analyses of primary transformants expressing the p16Ink4a-v-Abl virus derived from p53−/− mice indicate that a functional p53 protein is important for the apoptotic effects of p16Ink4a during crisis. Consistent with these data, among human tumors evaluated for p53 status, the subset that underwent apoptosis in response to p16Ink4a expression did so in a p53-dependent manner (20, 36). However, detectable changes in p53 levels are not observed during crisis even in wild-type cells (our unpublished data), probably because stabilization of p53 leads to rapid induction of apoptosis. Nonetheless, in many human tumors both p53 and p16Ink4a function is lost, suggesting that these products also act independently to influence tumor development (26, 44). In addition, spontaneous tumors arising in p16Ink4a−/− mice often lose p53 function, and p53/p16Ink4a double −/− mice are more tumor prone than either singly deficient animal (42). Taken together, these data indicate that the response to each of these tumor suppressors is influenced by the cell type and that the relationship between p16Ink4a and p53 is complex. A likely model predicts that an intact p53 pathway is important for some p16Ink4a-mediated responses, including the apoptotic response, but not for others (e.g., cell cycle arrest). Perhaps functional p53 sensitizes cells to the effects of p16Ink4a in a fashion that does not require direct cross talk between the two pathways.
Although p16Ink4a contributes to crisis during Ab-MLV-induced transformation, the effects appear to be more subtle than those of the second product of the Ink4a/Arf locus, p19Arf, in this transformation model. However, the response observed in the p16Ink4a null mice used here may be partly influenced by the strain background, one that is more permissive to all phases of Ab-MLV transformation than that used for the Ink4a/Arf and Arf null mice. Indeed, a much higher frequency of primary transformants from normal mice on 129/FvB backgrounds become transformed than on 129/C57BL/6 backgrounds (our unpublished data). Presumably this difference reflects the effects of unknown genes that differ in these strain combinations. Thus, it is possible that effects of p16Ink4a loss might be enhanced if the null allele were expressed on a C57BL/6 background.
p19Arf exerts a strong influence on the establishment phase of the Ab-MLV transformation process compared to the effects documented for p16Ink4a. Upregulation of p19Arf leads to p53 activation via effects on Mdm2 (46) and also affects growth and survival of cells by p53-independent pathways (53, 55). The dominant role of p19Arf in vitro is best revealed in primary transformants derived from Arf−/− mice. More than half of all the primary transformants bypass crisis, and all of those that undergo crisis recover and do so more rapidly than transformants from +/+ mice. In addition, even though primary transformants from p16Ink4a−/− animals have a survival advantage, crisis still occurs. Consistent with these effects, some fully established transformed cell lines continue to express p16Ink4a, and apoptosis was not observed following overexpression of the protein in several of these cell lines (29). Such cells have probably acquired additional mutations that circumvent the effects of p16Ink4a and will be a useful resource for probing the pathways involved.
p16Ink4a does not affect the cell cycle profile of Ab-MLV-transformed pre-B cells even though analyses of many human malignancies have linked the tumor suppressor function of p16Ink4a to its effects on Rb-mediated G1 progression (44, 46). Interestingly, analyses of Ink4a/Arf−/− bone marrow cells have revealed that p16Ink4a affects the growth of myeloid cells but not interleukin 7-dependent pre-B cells (30), the cells that are the primary target of Ab-MLV transformation (21). However, these cells were expanded under the influences of interleukin 7, a cytokine that normally influences their growth and differentiation, not under the influences of a strong oncogenic signal, and such a signal may be needed to reveal p16Ink4a function. Indeed, analyses of several different tumor models highlight the cell type specificity of p16Ink4a-mediated effects (3, 19, 22, 43).
Although the role of p16Ink4a is subtle in some in vitro transformation systems, studies of the Eμ-Myc lymphoma model have shown that p16Ink4a expression can have dramatic effects on the response of tumors to chemotherapy (40). In this situation, cells containing both functional p16Ink4a and p53 become cytostatic for extended periods of time. Because these lymphoma cells express markers associated with senescence pathways, p16Ink4a appears to activate this program in these tumors. These data, and our results that p16Ink4a affects a late stage in transformation, emphasize that studying only the induction phase of oncogenesis does not reveal a complete picture of the effects of p16Ink4a.
Our experiments demonstrate an underappreciated role for p16Ink4a in blocking oncogenesis by inducing apoptosis. p16Ink4a expression is strongly selected against in many types of human tumors by deletion, mutation, or epigenetic silencing (26, 35, 44), and its loss is a negative prognostic indicator for several tumors (5, 7, 16, 18, 48, 50). Loss of p16Ink4a has also been associated with tumor progression in human hematological tumors, where p16Ink4a gene deletions have been documented in late-stage leukemias and lymphomas that retained the gene at diagnosis (7, 25). Thus, loss of p16Ink4a can be an important late event in the multistage process of malignant transformation, allowing an indolent disease to become more aggressive. Escape from apoptosis may be one mechanism that is critical for acquisition of a more aggressive, malignant phenotype. Additional studies using the well-defined and simple model of Ab-MLV pre-B-cell transformation should help to uncover the mechanism by which this response is orchestrated.
Acknowledgments
We are grateful to Chris Schmidt for assistance with statistical analysis and Caleb Lee for comments on the manuscript.
This work was supported by CA 33771 (N.R.) from the National Cancer Institute and the Howard Hughes Medical Institute (N.E.S. and R.A.D.). R.A.D. is an American Cancer Society Professor.
REFERENCES
- 1.Adachi, Y., S. S. Lakka, N. Chandrasekar, N. Yanamandra, C. S. Gondi, S. Mohanam, D. H. Dinh, W. C. Olivero, M. Gujrati, T. Tamiya, T. Ohmoto, G. Kouraklis, B. Aggarwal, and J. S. Rao. 2001. Down-regulation of integrin αvβ3 expression and integrin-mediated signaling in glioma cells by adenovirus-mediated transfer of anti-sense urokinase-type plasminogen activator receptor (uPAR) and sense p16 genes. J. Biol. Chem. 276:47171-47177. [DOI] [PubMed] [Google Scholar]
- 2.Ausserlechner, M. J., P. Obexer, G. J. Wiegers, B. L. Hartmann, S. Geley, and R. Kofler. 2001. The cell cycle inhibitor p16(INK4A) sensitizes lymphoblastic leukemia cells to apoptosis by physiologic glucocorticoid levels. J. Biol. Chem. 276:10984-10989. [DOI] [PubMed] [Google Scholar]
- 3.Bachoo, R. M., E. A. Maher, K. L. Ligon, N. E. Sharpless, S. S. Chan, M. J. You, Y. Tang, J. DeFrances, E. Stover, R. Weissleder, D. H. Rowitch, D. N. Louis, and R. A. DePinho. 2002. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell 1:269-277. [DOI] [PubMed] [Google Scholar]
- 4.Calbo, J., M. Marotta, M. Cascallo, J. M. Roig, J. L. Gelpi, J. Fueyo, and A. Mazo. 2001. Adenovirus-mediated transfer of wt-p16 induces cell cycle arrest or apoptosis in pancreatic cancer. Cancer Gene Ther. 8:740-750. [DOI] [PubMed] [Google Scholar]
- 5.Calero Moreno, T. M., G. Gustagsson, S. Garwicz, D. Grander, G. K. Jonmundsson, B. M. Frost, A. Makipernaa, O. Rasool, E. R. Savolainen, K. Schmiegelow, S. Soderhall, K. Vettenranta, F. Wesenberg, S. Einhorn, and M. Heyman. 2002. Deletion of the Ink4-locus (the p16Ink4a, p14ARF and p15Ink4b genes) predicts relapse in children with ALL treated according to the Nordic protocols NOPHO-86 and NOPHO-92. Leukemia 16:2037-2045. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Y. Y., and N. Rosenberg. 1992. Lymphoid cells transformed by Abelson virus require the v-abl protein tyrosine kinase only during early G1. Proc. Natl. Acad. Sci. USA 89:6683-6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elenitoba-Johnson, K. S. J., R. D. Gascoyne, M. S. Lim, M. Chhanabai, E. S. Jaffe, and M. Raffeld. 1998. Homozygous deletions at chromosome 9p21 involving p16 and p15 are associated with histologic progression in follicle center lymphoma. Blood 91:4677-4685. [PubMed] [Google Scholar]
- 8.Fahraeus, R., and D. P. Lane. 1999. The p16(INK4a) tumour suppressor protein inhibits αvβ3 integrin-mediated cell spreading on vitronectin by blocking pCK-dependent localization of focal αvβ3 contacts. EMBO J. 18:2106-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Festing, M. F. W., L. Lin, T. R. Devereux, F. Gao, A. Yang, C. H. Anna, C. M. White, A. M. Malkinson, and M. You. 1998. At least four loci and gender are associated with susceptibility to the chemical induction of lung adenomas in A/J × BALB/c mice. Genomics 53:129-136. [DOI] [PubMed] [Google Scholar]
- 10.Foster, S. A., D. J. Wong, M. T. Barrett, and D. A. Galloway. 1998. Inactivation of p16 in human mammary epithelial cells by CpG island methylation. Mol. Cell. Biol. 18:1793-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green, P. L., D. A. Kaehler, and R. Risser. 1987. Clonal dominance and progression in Abelson murine leukemia virus lymphomagenesis. J. Virol. 61:2192-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawley, R. G., F. H. Lieu, A. Z. Fong, and T. S. Hawley. 1994. Versatile retroviral vectors for potential use in gene therapy. Gene Ther. 1:136-138. [PubMed] [Google Scholar]
- 13.Herzog, C. R., T. R. Devereux, B. Pittman, and M. You. 2002. Carcinogenic induction directs the selection of allelic losses in mouse lung tumorigenesis. Cancer Res. 62:6424-6429. [PubMed] [Google Scholar]
- 14.Hout, T. J., J. Rowe, M. Harland, S. Drayton, S. Brookes, C. Gooptu, P. Purkis, M. Fried, V. Bataille, E. Hara, J. Newton-Bishop, and G. Peters. 2002. Biallelic mutations in p16INK4a confer resistance to Ras- and Ets-induced senescence in human diploid fibroblasts. Mol. Cell. Biol. 22:8135-8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenab-Wolcott, J., D. Rodriguez-Correa, A. Reitmair, T. Mak, and N. Rosenberg. 2000. The absence of Msh2 alters Abelson virus pre-B cell transformation by influencing p53 mutation. Mol. Cell. Biol. 20:8373-8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin, M., S. Inoue, T. Umemura, J. Moriya, M. Arakawa, K. Nagashimia, and H. Kato. 2001. Cyclin D1, p16 and retinoblastoma gene product expression as a predictor for prognosis in non-small cell lung cancer at stages I and II. Lung Cancer 34:207-218. [DOI] [PubMed] [Google Scholar]
- 17.Kamijo, T., F. Zindy, M. F. Roussel, D. E. Quelle, J. R. Downing, R. A. Ashmun, G. Grosveld, and C. J. Sherr. 1997. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91:649-659. [DOI] [PubMed] [Google Scholar]
- 18.Kamiryo, T., K. Tada, S. Shiraishi, N. Shinojima, H. Nakamura, M. Kochii, J. Kuratsu, H. Saya, and Y. Ushio. 2002. Analysis of homozygous deletion of the p16 gene and correlation with survival in patients with glioblastoma multiforme. J. Neurosurg. 96:815-822. [DOI] [PubMed] [Google Scholar]
- 19.Kannan, K., N. E. Sharpless, X. Xu, R. C. O'Hagan, M. Rosenberg, and L. Chin. 2003. Components of the Rb pathway are critical targets of UV mutagenesis in a murine melanoma model. Proc. Natl. Acad. Sci. USA 100:1221-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawabe, S., J. A. Roth, D. R. Wilson, and R. E. Meyn. 2000. Adenovirus-mediated p16INK4a gene expression radiosensitizes non-small cell lung cancer cells in a p53-dependent manner. Oncogene 19:5359-5366. [DOI] [PubMed] [Google Scholar]
- 21.Kelliher, M. A., D. J. Weckstein, A. G. Knott, H. H. Wortis, and N. Rosenberg. 1993. ABL oncogenes directly stimulate two distinct target cells in bone marrow from 5-fluorouracil-treated mice. Oncogene 8:1249-1256. [PubMed] [Google Scholar]
- 22.Krimpenfort, P., K. C. Quon, W. J. Mooi, A. Loonstra, and A. Berns. 2001. Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature (London) 413:83-86. [DOI] [PubMed] [Google Scholar]
- 23.Lagresle, C., B. Gardie, S. Eyquem, M. Fasseu, J. C. Vieville, M. Pal, F. Sigaur, and J. C. Bories. 2002. Transgenic expression of the p16(INK4a) cyclin dependent kinase inhibitor leads to enhanced apoptosis and differentiation arrest of CD4-CD8-negative immature thymocytes. J. Immunol. 168:2325-2331. [DOI] [PubMed] [Google Scholar]
- 24.Mainville, C., K. Parmar, I. Unnikrishnan, G. D. Raffel, L. Gong, and N. Rosenberg. 2001. Alteration of the FLVRES motif in the v-Abl SH2 domain confers a temperature-sensitive transformation phenotype. J. Virol. 75:1816-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maloney, K. W., L. McGavran, L. F. Odom, and S. P. Hunger. 1999. Acquisition of p16INK4A and p15INK4B gene abnormalities between initial diagnosis and relapse in children with acute lymphoblastic leukemia. Blood 93:2380-2385. [PubMed] [Google Scholar]
- 26.Malumbres, M., and M. Barbacid. 2001. To cycle or not to cycle: a critical decision in cancer. Nat. Rev. Cancer 1:222-231. [DOI] [PubMed] [Google Scholar]
- 27.Muller, A. J., J. C. Young, A. M. Pendergast, M. Pondel, N. R. Landau, D. R. Littman, and O. N. Witte. 1991. BCR first exon sequences specifically activate the BCR/ABL tyrosine kinase oncogene of Philadelphia chromosome-positive human leukemias. Mol. Cell. Biol. 11:1785-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quelle, D. E., M. Cheng, R. A. Ashmun, and C. J. Sherr. 1997. Cancer-associated mutations at the INK4a locus cancel cell cycle arrest by p16INK4a but not by the alternative reading frame protein p19ARF. Proc. Natl. Acad. Sci. USA 94:669-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radfar, A., I. Unnikrishnan, H.-W. Lee, R. A. DePinho, and N. Rosenberg. 1998. p19Arf induces p53-dependent apoptosis during Abelson virus-mediated pre-B cell transformation. Proc. Natl. Acad. Sci. USA 95:13194-13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Randle, D. H., F. Zindy, C. J. Sherr, and M. F. Roussel. 2001. Differential effects of p19Arf and p16Ink4a loss on senescence of murine bone marrow-derived pre-B cells and macrophages. Proc. Natl. Acad. Sci. USA 96:9654-9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reid, S., R. Cross, and E. Snow. 1996. Combined Hoechst 33342 and merocyanin 540 staining to examine murine B cell cycle stage, viability and apoptosis. J. Immunol. Methods 192:43-54. [DOI] [PubMed] [Google Scholar]
- 32.Risser, R., M. Potter, and W. P. Rowe. 1978. Abelson virus-induced lymphomagenesis in mice. J. Exp. Med. 148:714-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg, N., and D. Baltimore. 1976. A quantitative assay for transformation of bone marrow cells by Abelson murine leukemia virus. J. Exp. Med. 143:1453-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenberg, N., and O. N. Witte. 1988. The viral and cellular forms of the Abelson (abl) oncogene. Adv. Virus Res. 35:39-81. [DOI] [PubMed] [Google Scholar]
- 35.Ruas, M., and G. Peters. 1998. The p16Ink4a/CDKN2A tumor suppressor and its relatives. Biochim. Biophys. Acta 1378:F115-F177. [DOI] [PubMed] [Google Scholar]
- 36.Sandig, V., K. Brand, S. Herwig, J. Lukas, J. Bartek, and M. Strauss. 1997. Adenovirally transferred p16INK4/DCKN2 and p53 genes cooperate to induce apoptotic tumor cell death. Nat. Med. 3:313-319. [DOI] [PubMed] [Google Scholar]
- 37.Sawyers, C. L., W. Callahan, and O. N. Witte. 1992. Dominant negative myc blocks transformation by abl oncogenes. Cell 70:901-910. [DOI] [PubMed] [Google Scholar]
- 38.Sawyers, C. L., J. McLaughlin, and O. N. Witte. 1995. Genetic requirement for ras in the transformation of fibroblasts and hematopoietic cells by the bcr-abl oncogene. J. Exp. Med. 181:307-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiff-Maker, L., M. C. Burns, J. B. Konopka, S. Clark, O. N. Witte, and N. Rosenberg. 1986. Monoclonal antibodies specific for v-abl- and c-abl-encoded molecules. J. Virol. 57:1182-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitt, C. A., J. S. Fridman, M. Yang, S. Lee, E. Baranov, R. M. Hoffman, and S. W. Lowe. 2002. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell 109:335-346. [DOI] [PubMed] [Google Scholar]
- 41.Serrano, M., H.-W. Lee, L. Chin, C. Cordon-Cardo, D. Beach, and R. A. DePinho. 1996. Role of the INK4a locus in tumor suppression and cell mortality. Cell 85:27-37. [DOI] [PubMed] [Google Scholar]
- 42.Sharpless, N. E., S. Alson, S. S. Chan, D. P. Silver, D. H. Castrillon, and R. A. DePinho. 2002. p16(INK4a) and p53 deficiency cooperate in tumorigenesis. Cancer Res. 62:2761-2765. [PubMed] [Google Scholar]
- 43.Sharpless, N. E., N. Bardeesy, K. H. Lee, D. Carrasco, D. H. Castrillon, A. J. Aguirre, E. A. Wu, J. W. Horner, and R. A. DePinho. 2001. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature (London) 413:86-91. [DOI] [PubMed] [Google Scholar]
- 44.Sharpless, N. E., and R. A. DePinho. 1999. The Ink4A/Arf locus and its two gene products. Curr. Opin. Genet. Dev. 9:22-30. [DOI] [PubMed] [Google Scholar]
- 45.Sharpless, N. E., K. Kannan, J. Xu, M. W. Bosenberg, and L. Chin. 2003. Both products of the mouse Ink4a/Arf locus suppress melanoma formation in vivo. Oncogene 22:5055-5059. [DOI] [PubMed] [Google Scholar]
- 46.Sherr, C. J. 2001. The Ink4a/ARF network in tumour suppression. Nat. Rev. Mol. Cell Biol. 2:731-737. [DOI] [PubMed] [Google Scholar]
- 47.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G-1 phase progression. Genes Dev. 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 48.Straume, O., I. Sviland, and L. A. Akslen. 2000. Loss of nuclear p16 protein expression correlates with increased tumor cell proliferation (Ki-67) and poor prognosis in patients with vertical growth phase melanoma. Clin. Cancer Res. 6:1845-1853. [PubMed] [Google Scholar]
- 49.Thome, K., A. Radfar, and N. Rosenberg. 1997. Mutation of Tp53 contributes to the malignant phenotype of Abelson virus transformed lymphoid cells. J. Virol. 77:8149-8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tutor, O., M. A. Diaz, M. Ramirez, P. Algara, L. Madero, and P. Martiniz. 2002. Loss of heterozygosity of p16 correlates with minimal residual disease at the end of the induction therapy in non-high risk childhood B-cell precursor acute lymphoblastic leukemia. Leuk. Res. 26:817-820. [DOI] [PubMed] [Google Scholar]
- 51.Unnikrishnan, I., A. Radfar, J. Jenab-Wolcott, and N. Rosenberg. 1999. p53 mediates apoptotic crisis in primary Ab-MLV-transformed pre-B cells. Mol. Cell. Biol. 19:4825-4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Parijs, L., Y. Refaeli, J. D. Lord, B. H. Nelson, A. K. Abbas, and D. Baltimore. 1999. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation induced cell death. Immunity 11:281-288. [DOI] [PubMed] [Google Scholar]
- 53.Weber, J. D., J. R. Jeffers, J. E. Rehg, D. H. Randle, G. Lozano, M. F. Roussel, C. J. Sherr, and G. P. Zambetti. 2000. p53-independent functions of the p19Arf tumor suppressor. Genes Dev. 14:2358-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whitlock, C. A., S. F. Ziegler, and O. N. Witte. 1983. Progression of the transformed phenotype in clonal lines of Abelson virus-infected lymphocytes. Mol. Cell. Biol. 3:596-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yarbrough, W. G., M. Bessho, A. Zanation, J. E. Bisi, and Y. Xiong. 2002. Human tumor suppressor ARF impedes S-phase progression independent of p53. Cancer Res. 62:1171-1177. [PubMed] [Google Scholar]
- 56.Zhang, S. L., W. DuBois, E. S. Ramsay, V. Bliskovski, H. C. Morse, L. Taddesse-Heath, W. C. Vass, R. A. DePinho, and B. A. Mock. 2001. Efficiency alleles of the Pctr1 modifier locus for plasmacytomas susceptibility. Mol. Cell. Biol. 21:310-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zou, X., S. Rudchenko, K.-K. Wong, and K. Calame. 1997. Induction of c-myc transcription by the v-Abl tyrosine kinase requires Ras, Raf1, and cyclin-dependent kinases. Genes Dev. 11:654-662. [DOI] [PubMed] [Google Scholar]