Abstract
Objectives
To evaluate the impact of tumor histology on clinicopathologic outcomes for patients with renal cell carcinoma (RCC) and venous tumor thrombus (VTT).
Methods
We identified 807 patients with RCC and VTT who underwent nephrectomy at our institution between 1970–2008. All pathologic specimens were re-reviewed by a single urologic pathologist. Patients with non-clear cell RCC (non-ccRCC) (n=56) were matched 1:2 to patients with clear cell RCC (ccRCC) VTT based onsymptoms at presentation, regional lymph node involvement, distant metastases, tumor thrombus level, nuclear grade and sarcomatoid differentiation. Survival was estimated using the Kaplan-Meier method and compared with the log-rank test.
Results
The 56 patients with non-ccRCC VTT included 26 papillary, 11 chromophobe, 5 collecting duct tumors, and 14 RCC not otherwise specified. Compared to unmatched patients with ccRCC VTT (n=751), patients with non-ccRCC VTT presented with larger tumor size (p=0.02), higher nuclear grade (p=0.04), and more frequent sarcomatoid differentiation (p<0.001) and lymph node invasion (p<0.001). However, when patients with non-ccRCC were matched to patients with cc-RCC, no significant differences were noted with regard to 5-year metastases-free survival (41% versus 34%; p=0.24) or cancer-specific survival (25% versus 27%; p=0.97).
Conclusions
Non-ccRCC VTT is associated with a high rate of adverse pathologic features. Nevertheless, when matched to patients with ccRCC, patients with non-ccRCC VTT did not have increased rates of recurrence or adverse survival. Aggressive surgical resection represents the mainstay of treatment in these cases, while continued efforts to optimize a multimodal management approach to such patients remain necessary.
Keywords: renal cell carcinoma, tumor thrombus, kidney cancer, histology
INTRODUCTION
The incidence of renal cell carcinoma (RCC) in the United States in 2012 is expected to be 64,770.1 Overall, 4–10% of newly diagnosed RCC patients have been found to have a venous tumor thrombus (VTT).2 In the absence of metastases, an aggressive surgical approach with curative intent for patients with RCC and VTT has been associated with 5-year survival rates between 40%–68%.3, 4 Established prognostic features for patients with RCC and VTT include tumor stage, grade, presence of necrosis and sarcomatoid differentiation, lymph node involvement, and metastatic status.5, 6 Notably, however, the importance of tumor histology in this setting has not been well studied to date.
In fact, histologic subtype has been extensively evaluated for patients with clinically-localized RCC, and of note clear cell RCC (ccRCC) has been associated with adverse outcomes compared to non-clear cell RCC (non-ccRCC) in this setting.7–10 Interestingly, in albeit smaller numbers, for patients with metastatic kidney cancer, non-ccRCC has been reported to portend adverse survival compared to ccRCC.11, 12 Nevertheless, with locally-advanced disease, such as RCC with VTT, there is a paucity of data regarding the interaction of tumor histology and prognosis.13–15
Here, then, we evaluated clinicopathologic outcomes of patients undergoing nephrectomy with tumor thrombectomy found to have non-ccRCC, and compared survival to a matched cohort of patients with ccRCC VTT.
MATERIALS AND METHODS
After obtaining Institutional Review Board approval, we reviewed the Mayo Clinic Nephrectomy Registry to identify 807 patients who underwent nephrectomy with tumor thrombectomy at our institution between 1970 and 2008. Nephrectomy was performed by various surgeons over the time frame of the study using techniques for RCC with VTT previously described.16
One urologic pathologist (JCC) re-reviewed all nephrectomy pathology specimens. Tumor staging followed the 2010 American Joint Committee on Cancer/Union Internationale Contre le Cancer 7th edition TNM classification.17 Tumor thrombus level was categorized according to the classification proposed by Neves and Zincke.4 Histology was assigned according to American Joint Committee on Cancer, Union Internationale Contre le Cancer and Heidelberg guidelines.18 Clinicopathologic variables recorded for analysis here included age, gender, clinical presentation, Eastern Cooperative Oncology Group (ECOG) performance status, pathologic tumor stage, histologic subtype, nuclear grade, coagulative tumor necrosis, sarcomatoid differentiation, tumor thrombus level, lymph node status, and presence of metastatic disease.
The retrospective nature of this study precluded a standardized follow-up protocol in all patients. However, follow-up after nephrectomy with tumor thrombectomy at our institution has generally been recommended semi-annually for the first two years after surgery, including serum electrolyte panel and imaging of the chest, abdomen and pelvis, and then annually thereafter. Local recurrence was defined as tumor recurrence in the ipsilateral kidney, renal fossa or retroperitoneal lymph nodes, while distant metastases included disease in the viscera or bone. Vital status was identified from death certificates or physician correspondence. For patients followed elsewhere, the Mayo Clinic Nephrectomy Registry monitors outcomes annually by correspondence to the patient and treating physician. In addition, national registries including the Social Security Death Index and Accurint® (a healthcare database of approximately 34 billion public records) are utilized to confirm follow-up information on each patient in the registry.
In total, 56 patients (6.9%) were identified with non-ccRCC VTT. These included papillary RCC (n=26), chromophobe RCC (n=11), collecting duct tumors (n=5), and RCC not otherwise specified (n=14). Clinicopathologic demographics for the patients with non-ccRCC were reported and compared to patients with ccRCC VTT (n=751). Given noted differences in pathologic features between the non-ccRCC and ccRCC VTT cohorts, to facilitate an analysis of comparative survival based on histology, we then used a matched cohort design to individually match the 56 patients with non-ccRCC VTT in a 1:2 ratio to patients with ccRCC VTT based on symptoms at presentation (absent versus present), regional lymph node involvement at surgery (pNx/pN0 versus pN1), distant metastases at surgery (M0 versus M1), tumor thrombus level (0 versus I versus II versus III versus IV), nuclear grade (1/2 versus 3/4), and sarcomatoid differentiation (absent versus present). Of the 112 matches identified, 99 were matched exactly on the features listed above and the remaining 13 were allowed to vary on regional lymph node involvement or to be matched exactly after combining tumor thrombus levels I, II, III, and IV (0 versus I/II/III/IV).
Comparison of clinicopathologic features between cases and controls were evaluated using conditional logistic regression. Cancer-specific survival (CSS), local recurrence-free survival, and metastases-free survival were estimated as the time from nephrectomy to the event of interest or last follow up using the Kaplan-Meier method and were compared with the log-rank test. In addition, Cox proportional hazard regression models were used to test the association of various clinicopathological features with outcome.
Statistical analyses were performed using the SAS software package (SAS Institute, Cary, NC). All statistical tests were two-sided, with a p-value <0.05 considered statistically significant.
RESULTS
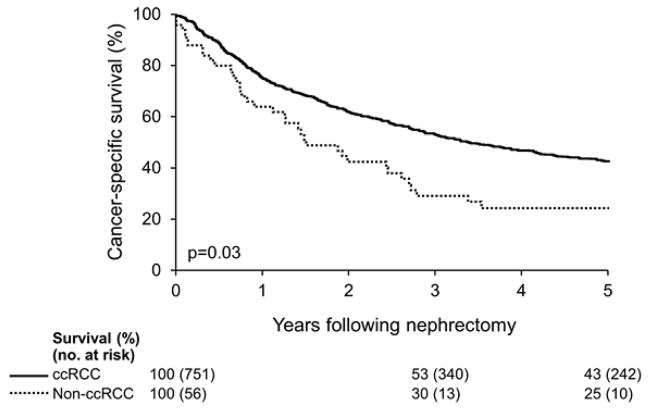
Clinicopathologic demographics for the 56 patients with non-ccRCC VTT, as well as for the 751 unmatched patients with ccRCC VTT, are summarized in Table 1. As can be seen, patients with non-ccRCC were found to have a significantly larger tumor size (median 10.5 cm versus 9.3 cm; p=0.02), greater rate of lymph node invasion (38% versus 13%; p<0.001), high nuclear grade (91% versus 81%; p=0.04) and more frequent sarcomatoid differentiation (25% versus 9%; p<0.001). Not surprisingly, then, patients with non-ccRCC VTT had a significantly worse 5-year CSS (25%) compared to unmatched patients with ccRCC VTT (43%; p=0.03) (Figure 1).
Table 1.
Clinicopathologic features of patients with non-ccRCC versus unmatched patients with ccRCC
| Variable | Non-ccRCC (N=56) | ccRCC (N=751) | p value |
|---|---|---|---|
| Age in years (IQR) | 65 (57,75) | 64(56,72) | 0.42 |
| Tumor size cm (IQR) | 10.5 (8.0,15.0) | 9.3(7.5,12) | 0.02 |
| Gender | |||
| Female | 14 (25%) | 233 (31%) | 0.35 |
| Male | 42 (75%) | 518 (69%) | |
| Local symptoms at presentation | 45 (80%) | 655 (87%) | 0.14 |
| Constitutional symptoms at presentation | 25 (45%) | 358 (48%) | 0.66 |
| ECOG performance status | |||
| 0 | 33 (77%) | 525 (85%) | 0.14 |
| 1 | 6 (14%) | 66 (11%) | |
| 2 | 4 (9%) | 19 (3%) | |
| 3 | 0 (0%) | 10 (2%) | |
| Pathologic tumor stage | |||
| pT3a | 24 (43%) | 466 (62%) | 0.027 |
| pT3b | 23 (41%) | 205 (27%) | |
| pT3c | 4 (7%) | 39 (5%) | |
| pT4 | 5 (9%) | 41 (4%) | |
| pN+ at nephrectomy | 21 (38%) | 99 (13%) | <0.001 |
| M1 at nephrectomy | 16 (29%) | 210 (28%) | 0.92 |
| Tumor thrombus level | |||
| 0 | 28 (50%) | 488 (65%) | 0.27 |
| I | 9 (16%) | 88 (12%) | |
| II | 11 (20%) | 100 (13%) | |
| III | 4 (7%) | 40 (5%) | |
| IV | 4 (7%) | 35 (5%) | |
| Nuclear grade | |||
| 1 | 0 (0%) | 15 (2%) | 0.04 |
| 2 | 5 (9%) | 127 (17%) | |
| 3 | 31 (55%) | 451 (60%) | |
| 4 | 20 (36%) | 158 (21%) | |
| Coagulative tumor necrosis | 38 (68%) | 431 (57%) | 0.13 |
| Sarcomatoid differentiation | 14 (25%) | 68 (9%) | <0.001 |
Figure 1.
Cancer-specific survival following nephrectomy for patients with non-ccRCC VTT versus unmatched patients with ccRCC VTT.
Given the noted difference in clinicopathologic characteristics between patients with non-ccRCC VTT and the unmatched ccRCC cohort, in order to investigate the independent association of tumor histology with survival, the 56 patients with non-ccRCC VTT were then matched 1:2 to 112 patients with ccRCC VTT. Patients were well-matched, with no significant differences noted in the distribution among matching variables. No patients were treated with neoadjuvant chemotherapy, immunotherapy or targeted therapy.
Median follow-up after nephrectomy for those alive at last follow-up was 12 years (range 3–15) for patients with non-ccRCC compared to 6.6 years (range 0–13) for the matched ccRCC cohort. During this time, 20 patients with non-ccRCC who were M0 at nephrectomy developed metastases and a total of 49 patients with non-ccRCC died, with 36 dying of RCC. Among matched ccRCC patients, 55 experienced metastases during follow-up and a total of 100 ccRCC patients died, with 84 dying of RCC. Ten patients with non-ccRCC were treated with systemic therapy at the time of relapse, including 4 who received targeted therapy, while 17 of the matched ccRCC patients received systemic therapy at disease relapse, with 6 receiving targeted agents. Notably, the 5-year metastases-free (41% versus 34%; p=0.24) and local-recurrence free (77% versus 76%; p=0.62) survival were not significantly difference between patients with non-ccRCC VTT and the matched ccRCC VTT cohort. Likewise, 5-year CSS (25% versus 27%; p=0.97) also did not significantly differ between patients with non-ccRCC VTT and matched ccRCC patients.
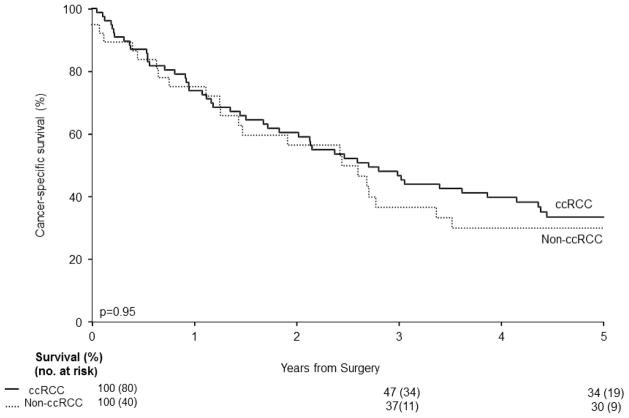
To further investigate the independent association of tumor histology with survival, we then excluded patients with metastatic disease at presentation (n= 226) from analyses. The remaining patients with non-ccRCC (n=40) were then matched to patients with ccRCC (n=80). Again, no significant difference in 5-year CSS was found between cM0 non-ccRCC VTT patients and ccRCC patients (30% versus 34%; p=0.95; Figure 2). Moreover, on multivariate analysis in patients with M0 disease at nephrectomy (Table 2), RCC histologic subtype was not found to be significantly associated with patients’ risk of death from RCC (p=0.43).
Figure 2.
Cancer-specific survival following nephrectomy for cM0 patients with non-ccRCC VTT versus matched patients with ccRCC VTT.
Table 2.
Multivariate Cox proportional hazards regression analysis of factors associated with death from RCC among patients with cM0 RCC and a venous tumor thrombus treated with nephrectomy and tumor thrombectomy
| Variable | HR | 95% CI | p Value |
|---|---|---|---|
| Symptoms at presentation | 1.42 | 0.95, 2.12 | 0.09 |
| Tumor thrombus level (I–IV vs 0) | 1.36 | 1.07, 1.72 | 0.01 |
| Nuclear grade (3–4 vs 1–2) | 1.90 | 1.38, 2.61 | <0.001 |
| Sarcomatoid differentiation | 2.95 | 1.93, 4.50 | <0.001 |
| Positive lymph nodes | 1.69 | 1.22, 2.36 | 0.002 |
| Non-clear cell histology | 1.20 | 0.76, 1.89 | 0.43 |
DISCUSSION
We found here that patients with non-ccRCC VTT frequently present with adverse pathologic features, including large tumor size, high nuclear grade, and more frequent sarcomatoid differentiation and lymph node invasion than patients with ccRCC VTT. However, once these pathologic features were controlled for, tumor histology was not significantly associated with metastasis-free survival or CSS following nephrectomy with tumor thrombectomy.
Among patients with localized RCC, clear cell histology has been largely 7, 8, 19–22 but not universally23 been associated with adverse survival. For example, when comparing outcomes among patients with localized clear cell, papillary, and chromophobe RCC, Leibovich et al. found on multivariate analysis that clear cell RCC was associated with a significantly increased risk of metastasis (HR 2.76; p<0.001) and death from kidney cancer (HR 1.77; p<0.001).7 Meanwhile Teloken et al evaluated 1,863 patients who underwent partial or radical nephrectomy for a renal mass and demonstrated that chromophobe RCC was independently associated with better CSS and metastasis-free survival compared to ccRCC. 8 Likewise, Beck et al. reviewed 1057 RCC patients and demonstrated on multivariate analysis that chromophobe RCC had an improved prognosis, with no difference in outcome noted between ccRCC and papillary RCC.19 Similarly, when evaluating the impact of histologic subtype among 17,605 patients in the SEER database treated with nephrectomy for RCC, Keegan et al. noted improved survival on multivariate analysis among patients with chromophobe tumors compared to those with ccRCC.22 Nevertheless, Patard et al, in a multi-institutional series of 4063 patients undergoing nephrectomy, did not find an independent association between tumor histology and survival.23 Instead similar to the findings in our analysis, these investigators found that after clinicopathologic features were controlled for, histologic subtype was not a significant predictor of survival. 23 One potential contributing factor to the discrepancy in results among these prior series may be the inconsistency of centralized pathologic review.
In the setting of metastatic RCC, meanwhile, prior reports have demonstrated that while the frequency of metastatic disease may be less for non-ccRCC than ccRCC, adverse survival has been associated with metastatic non-ccRCC compared to metastatic ccRCC.11, 12, 24 That is, Romnnen et al. reported a median survival of 8 months in 38 patients with metastatic papillary RCC.24 Moreover, Kassouf et al. compared 94 patients with non-ccRCC undergoing cytoreductive nephrectomy to 514 patients with ccRCC and found that those with non-ccRCC had an increased rate of nodal metastases (77% versus 26%; p<0.0001) and sarcomatoid features (23% versus 13%; p=0.03).12 Additionally, these investigators noted a significantly decreased CSS among patients with non-ccRCC (9.7 versus 20.3 months; p=0.0003).12
An important potential explanation for the reported differences in the association of histology with survival between localized RCC (worse outcomes with ccRCC) and metastatic RCC (worse outcome with non-ccRCC) may be the efficacy of systemic therapies for ccRCC versus non-ccRCC.11, 25 Indeed, recent evidence suggests that tyrosine kinase inhibitors have lower levels of activity in papillary RCC compared to ccRCC.26 Similarly, studies by both Tannir et al. and Choueiri et al. noted a low response rate of non-ccRCC to tyrosine kinase inhibitors.27, 28 However, as only 10 patients in our study received targeted therapies at disease relapse, we cannot assess for a differential response to treatment as a potential cause of survival difference according to histology versus for example disparate disease biology. Further evaluation of systemic therapy in the setting of non-ccRCC will thus be needed to help guide optimal multi-modal treatment in these patients, while the etiology of differences in outcome by histology for patients with localized versus advanced RCC remains to be elucidated.
Limited data have been reported to date regarding the importance of histology among patients with RCC and VTT, despite as noted previously 29 that non ccRCC may be associated with extensive loco-regional involvement. Ciancio et al. reported their experience with 87 patients with RCC and VTT treated by nephrectomy, which included 25 with non-ccRCC, and determined on multivariate analysis that non–clear cell histology was independently associated with adverse survival (HR 2.4; p=0.03).13 Conversely, in a multi-institutional study of patients with RCC and VTT which included 73 patients with non-ccRCC, albeit without pathologic re-review, Wagner et al did not find an association between histology and survival on multivariate analysis.15 Meanwhile, Margulis et al. evaluated the association of pathologic features with survival in 2157 patients with RCC, including 245 patients with papillary RCC, of whom 20 had VTT.14 In an unmatched comparison, they noted that, among patients with a VTT, those with papillary RCC had an adverse 5-year CSS compared to patients with ccRCC VTT (35% versus 66%; p=0.01).14 The relatively low frequency of non-clear cell histology among patients with RCC and VTT noted here (~ 7%) was consistent with the findings from prior studies7, 14, 15 and has likely contributed to the difficulties with establishing the prognostic significance of histology in this setting.
Our study, in a relatively large number of patients with non-ccRCC and VTT, extends these prior series in that: (1) All specimens underwent pathologic re-review to confirm histology, and (2) non-ccRCC patients were matched to ccRCC to control for other adverse pathologic features which might obscure the ability to discern an independent impact of histology with survival following nephrectomy with tumor thrombectomy. Herein, we did not find an association of histology with outcome, suggesting that in the setting of such locally-advanced disease, prognosis is primarily guided by established pathologic predictors such as nodal stage and metastatic status rather than tumor histology. As such, we believe that aggressive surgical resection should remain central to the treatment of patients with RCC and VTT, even for those with non-ccRCC in whom the value of nephrectomy has previously been questioned, likely due to the frequency of other adverse pathologic features in such cases which were controlled for in our study.
We recognize that our study is limited by its retrospective, non-randomized design. Likewise, we acknowledge that the patients evaluated here represent a highly-selected cohort, treated at a single, high-volume academic center. Additionally, given the tertiary referral nature of our practice, many patients receive at least part of their follow-up locally, which introduces heterogeneity into surveillance modalities/frequency. Moreover, given the prolonged time-frame of our analysis, only a minority of patients were treated with targeted therapies at the time of disease relapse, which may differentially impact survival between ccRCC and non-ccRCC patients. Lastly, while our sample size is large given the relative rarity of non-ccRCC with VTT, it remains possible that with larger patient numbers, a significant difference in survival between patients with non-ccRCC VTT and those with ccRCC VTT may be determined.
CONCLUSIONS
Patients with non-ccRCC VTT frequently present with adverse pathologic features. However, when matched to patients with ccRCC and VTT, patients with non-ccRCC VTT did not have increased rates of recurrence or adverse survival. Surgical resection should continue to represent the mainstay of treatment in these cases, while continued efforts to optimize a multimodal management approach to such patients remain necessary.
Footnotes
Financial disclosure: None
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Schefft P, Novick AC, Straffon RA, Stewart BH. Surgery for renal cell carcinoma extending into the inferior vena cava. J Urol. 1978;120(1):28–31. doi: 10.1016/s0022-5347(17)57028-7. [DOI] [PubMed] [Google Scholar]
- 3.Libertino JA, Zinman L, Watkins E., Jr Long-term results of resection of renal cell cancer with extension into inferior vena cava. J Urol. 1987;137(1):21–4. doi: 10.1016/s0022-5347(17)43859-6. [DOI] [PubMed] [Google Scholar]
- 4.Neves RJ, Zincke H. Surgical treatment of renal cancer with vena cava extension. Br J Urol. 1987;59(5):390–5. doi: 10.1111/j.1464-410x.1987.tb04832.x. [DOI] [PubMed] [Google Scholar]
- 5.Tsui KH, Shvarts O, Smith RB, et al. Prognostic indicators for renal cell carcinoma: a multivariate analysis of 643 patients using the revised 1997 TNM staging criteria. J Urol. 2000;163(4):1090–5. doi: 10.1016/s0022-5347(05)67699-9. quiz 295. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka M, Fujimoto K, Okajima E, et al. Prognostic factors of renal cell carcinoma with extension into inferior vena cava. Int J Urol. 2008;15(5):394–8. doi: 10.1111/j.1442-2042.2008.02017.x. [DOI] [PubMed] [Google Scholar]
- 7.Leibovich BC, Lohse CM, Crispen PL, et al. Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. J Urol. 2010;183(4):1309–15. doi: 10.1016/j.juro.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 8.Teloken PE, Thompson RH, Tickoo SK, et al. Prognostic impact of histological subtype on surgically treated localized renal cell carcinoma. J Urol. 2009;182(5):2132–6. doi: 10.1016/j.juro.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amin MB, Tamboli P, Javidan J, et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol. 2002;26(3):281–91. doi: 10.1097/00000478-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Capitanio U, Cloutier V, Zini L, et al. A critical assessment of the prognostic value of clear cell, papillary and chromophobe histological subtypes in renal cell carcinoma: a population-based study. BJU Int. 2009;103(11):1496–500. doi: 10.1111/j.1464-410X.2008.08259.x. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Bacik J, Mariani T, et al. Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol. 2002;20(9):2376–81. doi: 10.1200/JCO.2002.11.123. [DOI] [PubMed] [Google Scholar]
- 12.Kassouf W, Sanchez-Ortiz R, Tamboli P, et al. Cytoreductive nephrectomy for metastatic renal cell carcinoma with nonclear cell histology. J Urol. 2007;178(5):1896–900. doi: 10.1016/j.juro.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 13.Ciancio G, Manoharan M, Katkoori D, et al. Long-term survival in patients undergoing radical nephrectomy and inferior vena cava thrombectomy: single-center experience. Eur Urol. 2010;57(4):667–72. doi: 10.1016/j.eururo.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Margulis V, Tamboli P, Matin SF, et al. Analysis of clinicopathologic predictors of oncologic outcome provides insight into the natural history of surgically managed papillary renal cell carcinoma. Cancer. 2008;112(7):1480–8. doi: 10.1002/cncr.23322. [DOI] [PubMed] [Google Scholar]
- 15.Wagner B, Patard JJ, Mejean A, et al. Prognostic value of renal vein and inferior vena cava involvement in renal cell carcinoma. Eur Urol. 2009;55(2):452–9. doi: 10.1016/j.eururo.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 16.Boorjian SA, Sengupta S, Blute ML. Renal cell carcinoma: vena caval involvement. BJU Int. 2007;99(5 Pt B):1239–44. doi: 10.1111/j.1464-410X.2007.06826.x. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB American Joint Committee on Cancer. Prostate AJCC Cancer Staging Manual. 7. New York: Springer; 2010. pp. 457–68. [Google Scholar]
- 18.Storkel S, Eble JN, Adlakha K, et al. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) Cancer. 1997;80(5):987–9. doi: 10.1002/(sici)1097-0142(19970901)80:5<987::aid-cncr24>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 19.Beck SD, Patel MI, Snyder ME, et al. Effect of papillary and chromophobe cell type on disease-free survival after nephrectomy for renal cell carcinoma. Ann Surg Oncol. 2004;11(1):71–7. doi: 10.1007/BF02524349. [DOI] [PubMed] [Google Scholar]
- 20.Cheville JC, Lohse CM, Zincke H, et al. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27(5):612–24. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Ficarra V, Martignoni G, Galfano A, et al. Prognostic role of the histologic subtypes of renal cell carcinoma after slide revision. Eur Urol. 2006;50(4):786–93. doi: 10.1016/j.eururo.2006.04.009. discussion 93–4. [DOI] [PubMed] [Google Scholar]
- 22.Keegan KA, Schupp CW, Chamie K, et al. Histopathology of surgically treated renal cell carcinoma: survival differences by subtype and stage. J Urol. 2012;188(2):391–7. doi: 10.1016/j.juro.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patard JJ, Leray E, Rioux-Leclercq N, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005;23(12):2763–71. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 24.Ronnen EA, Kondagunta GV, Ishill N, et al. Treatment outcome for metastatic papillary renal cell carcinoma patients. Cancer. 2006;107(11):2617–21. doi: 10.1002/cncr.22340. [DOI] [PubMed] [Google Scholar]
- 25.Upton MP, Parker RA, Youmans A, et al. Histologic predictors of renal cell carcinoma response to interleukin-2-based therapy. J Immunother. 2005;28(5):488–95. doi: 10.1097/01.cji.0000170357.14962.9b. [DOI] [PubMed] [Google Scholar]
- 26.Gordon MS, Hussey M, Nagle RB, et al. Phase II study of erlotinib in patients with locally advanced or metastatic papillary histology renal cell cancer: SWOG S0317. J Clin Oncol. 2009;27(34):5788–93. doi: 10.1200/JCO.2008.18.8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tannir NM, Plimack E, Ng C, et al. A Phase 2 Trial of Sunitinib in Patients with Advanced Non-clear Cell Renal Cell Carcinoma. Eur Urol. 2012;62(6):1013–9. doi: 10.1016/j.eururo.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choueiri TK, Plantade A, Elson P, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol. 2008;26(1):127–31. doi: 10.1200/JCO.2007.13.3223. [DOI] [PubMed] [Google Scholar]
- 29.Mai KT, Landry DC, Robertson SJ, et al. A comparative study of metastatic renal cell carcinoma with correlation to subtype and primary tumor. Pathol Res Pract. 2001;197(10):671–5. doi: 10.1078/0344-0338-00144. [DOI] [PubMed] [Google Scholar]