Abstract
The thymus produces self-tolerant functionally competent T cells. This occurs by the import of multipotent hematopoietic progenitors that are signalled to adopt the T cell fate. Expression of T cell specific genes, including those encoding the T cell receptor (TCR), is followed by positive and negative selection and the eventual export of mature T cells. Significant progress has been made in elucidating the signals that direct progenitor cell trafficking to, within and out of the thymus. These advances are the subject of this Review, with a particular focus on the role of reciprocal cooperative and regulatory interactions between TCR and chemokine receptor-mediated signalling.
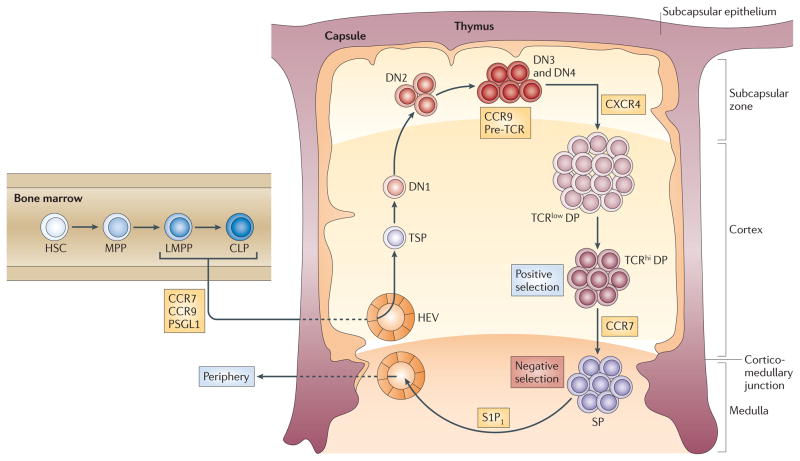
T cells originate from bone marrow-derived progenitors that, in order to complete their maturation, must migrate to, enter and transition through an anatomically distinct organ, namely the thymus. This complex, yet well-orchestrated, movement of cells is controlled by the selective, developmentally regulated expression of G-protein coupled receptors (GPCRs) that respond to chemotactic cytokine (chemokine) gradients in the thymus. Once they reach the thymus, T cell progenitors undergo a series of defined differentiation steps that occur in specific zones of the thymus (Figure 1) 1. Thymocyte maturation is regulated primarily by signal transducing receptors that are distinct from those that control chemotaxis; these include the T cell receptor (TCR) and its precursor, the pre-TCR, each of which controls important checkpoints in T cell development. Interestingly, the signals that drive the differentiation of thymocytes also influence their migration through successive thymic environments and their eventual exit from the thymus 2, 3.
Figure 1.
Overview of regulated migration events during T cell development. Within the bone marrow, hematopoietic stem cells (HSCs) differentiate into multipotential progenitors (MPPs). A subset of MPPs that are FLT3hi initiates transcription of the genes encoding RAG1 and RAG2 and are termed lymphoid-primed multipotential progenitors (LMPPs; also known as ELPs). Subsequent lymphoid-primed bone marrow progenitors include common lymphoid progenitor (CLPs). All these progenitors will make T cells if placed within the thymus. However, the ability of hematopoietic progenitors to migrate to the thymus is regulated, requiring among other molecules the CC-chemokine receptor 9 (CCR9), which is expressed by subsets of LMPPs and CLPs, and CCR7, which is expressed by some LMPPs and highly expressed on CLPs. The identity of in vivo thymus settling progenitors (TSPs) has not been precisely determined, but they likely include LMPPs and CLPs. TSPs enter the thymus near the cortico-medullary junction, where they may spend a prolonged period of time as KIT+ DN1 thymocytes (also known as ETPs) before migrating to the subcapsular zone as double-negative 2 (DN2) and double-negative 3 (DN3) cells. DN3 cells require CXCR4 to efficiently develop into double-positive (DP) thymocytes. These DP thymocytes express high levels of CCR9; however, the function of CCR9 in DP thymocytes remains unclear. DP thymocytes that undertake appropriate interactions with self peptide–MHC complexes on cortical thymic epithelial cells (positive selection), upregulate expression of CCR7 and mature into single-positive (SP) mature T cells that migrate into the thymic medulla. Residence in the medulla (where negative selection occurs) is followed by emigration regulated by S1P1.
In this Review, we summarize what is currently known about the migration of conventional CD4/CD8 lineage thymocytes and their precursors in the adult thymus. Special attention is given to how cross-talk between developmental and chemotactic receptors contributes to T cell maturation and how developmental signals serve to ensure that continued migration and export are linked to and are dependent on proper thymocyte maturation. Discussion of thymus organogenesis and fetal thymus colonization are not included here but are covered in several excellent recent reviews2, 4–6.
T cell progenitors in the bone marrow
The identity of the hematopoietic progenitor cells that colonize the thymus from the blood has not been definitively established. Very low numbers of bone marrow-derived progenitor cells are thought to circulate and populate the thymus, and for this reason, studies of direct thymic colonization by progenitor cells are technically very demanding. Initial efforts to identify T cell progenitors attempted to describe haematopoietic cells expressing specific combinations of cytokine receptors and possessing limited lineage potentials as T cell intermediates7, 8. However, bone marrow-derived progenitors at many stages of differentiation give rise to T cells if directly exposed to the thymic environment1. The difference between physiological progenitors of T cells and other progenitors with T cell potential appears to be the ability to migrate to the thymus. Consequently, a description of physiological T cell progenitors requires a description of expressed thymic homing molecules.
The earliest hematopoietic progenitors in the bone marrow are negative for expression of lineage markers found on differentiated cells, and express the stem cell markers stem cell antigen 1 (SCA1) and KIT; they are therefore known as LSK cells1 (Fig. 1). This LSK cell population is heterogeneous, and has been further characterized based on expression of the cytokine receptor FMS-related tyrosine kinase 3 (FLT3), as well as other markers such as CD150 and CD489. The CD150+ FLT3− subset of LSK progenitors contains long term self-renewing haematopoietic stem cells (HSCs), whereas more mature CD150− FLT3low LSK cells have lost self-renewal capacity but retain the ability to differentiate into all blood cell types, and are therefore designated multipotent progenitors (MPPs)9–12. LSK cells with the highest FLT3 expression express lymphoid-specific genes and are termed lymphoid-primed MPPs (LMPPs) or early lymphoid progenitors (ELPs)11, 13, 14. Common lymphoid progenitors (CLPs), which are not within the LSK pool, were initially defined as devoid of non-lymphoid lineage potentials7 (Figure 1). More recently, the LY6D− subset of CLPs was shown to retain a degree of myeloid and natural killer cell potential in in vitro assays15, 16 (Figure 1). HSCs, MPPs, LMPPs and CLPs will each produce T lineage progeny if directly placed in the thymic environment17, 18.
In addition to these populations, CD4−CD8−CD3−CD44−CD25+ double negative 3 (DN3)-like pre-T cells exist in low numbers in the bone marrow, blood and spleen of normal mice19–24 and expand greatly in the spleen following bone marrow transplantation25–27. However, their role, if any, in normal T cell development requires clarification.
Getting to the thymus
Although T cell potential is broadly distributed across various haematopoietic cell subsets, the ability to home to the thymus is regulated and thymic homing is selective18, 28. Early work established that stem cell activity was not evident within the thymus, suggesting that HSCs were unlikely to efficiently home to and enter the thymus29. More recently, it was shown that whereas HSCs, MPPs, LMPPs and CLPs each possess T cell potential and generate thymocytes when injected intrathymically, only LMPPs and CLPs rapidly generate thymocytes after intravenous transfer18 (Figure 1). These results show that HSCs and most MPPs are not capable of colonizing the thymus from blood and that this ability must instead be attained during later stages of differentiation.
The mechanisms by which progenitors home to the thymus have been suggested to be similar to those by which leukocytes enter lymph nodes and tissues 30, 31. As leukocytes pass through postcapillary venules, they use selectins, chemokine receptors and integrins in a regulated signalling cascade to arrest at the endothelial wall and extravasate. It is likely that progenitor egress from the blood into the thymus mirrors the steps used by other cells in lymph node and tissue homing. Several molecules expressed on progenitor cells have been suggested to play a role in thymic homing. The strongest experimental data exist for P-selectin glycoprotein ligand 1 (PSGL1)32, 33, CC-chemokine receptor 7 (CCR7)28, 34 and CCR918, 35–38 (see below). However, contributions from other molecules including CXC-chemokine receptor 4 (CXCR4) and CCR539, and Ephrin type-B receptor 2 (EPHB2)40 in regulating thymus homing have also been suggested.
CCR7 and CCR9
The ligands for CCR7 (CCL19 and CCL21) and CCR9 (CCL25) are expressed in the thymus. CCL25 is broadly expressed by thymic stromal cells, whereas CCR7 ligands are most densely expressed in the medulla; both sets of ligands are expressed by thymic endothelium32, 36–38, 41–44. In the absence of CCR7 or CCR9, thymic migration and T cell development is delayed in fetal mice36, 41. In adult mice lacking CCR7 and/or CCR9, the thymus is of near-normal size; however, thymic colonization by CCR7−CCR9− progenitors is almost completely blocked under competitive conditions28, 45. LMPPs and CLPs express functional CCR7 and CCR9, whereas HSCs and MPPs do not18, 28, 46. Consistently, efficient thymic colonizing capacity resides within the LIN−KIT+FLT3hi pool of bone marrow progenitors, which includes both LMPPs and CLPs but excludes HSCs and MPPs18, 45, 47. CCR9-expressing LMPPs retain the ability to make lymphoid and myeloid cell lineages, although they do not give rise to erythroid lineages, implying the thymus must be able to prevent incoming progenitors from manifesting significant myeloid and other non-T cell lymphoid fates46, 48–51.
By contrast, both CCR9+ and CCR9− progenitors exhibited similar differentiation potentials and gene expression profiles in the fetal thymus, suggesting that progenitors may access the fetal thymus via distinct homing pathways52. In addition to LMPPs and CLPs, other subsets of T cell progenitors also express CCR7 and/or CCR9, and might also contribute to T cell development (Table 1). The relative contributions of different progenitor populations to T cell development are not known with certainty.
Table 1.
Chemokine receptors expressed by T cell progenitors.
| Progenitor | Definition | GPCRs | Refs |
|---|---|---|---|
| HSC | FLT3− CD150+ LSK | CXCR4, CCR2 | 110, 111 |
| MPP | FLT3+CD150− LSK | CXCR4, CCR2? | 110 |
| LMPP (Overlaps with ELP) | FLT3hi LSK (ELP are LMPP that are GFP+ in RAG–GFP reporter mice) | CXCR4, CCR9 (subset), CCR7 | 18, 28, 34, 46 |
| LY6D− CLP | LIN−SCA1lowFLTt3hiIL-7Rα KITlowLY6D− | CXCR4, CCR7, CCR9? | 18, 28, 34 |
| Ly6D+ CLP | LIN−SCA1lowFLT3hiIL-7Rα+KITlowLY6D+ | CXCR4, CCR9 (subset), CCR7 | 18, 28, 34 |
| DN1 (also known as ETP) | LINlowSCA1+KIT+CD25− in thymus | CXCR4, CCR9, CCR7 | 46, 61, 112 |
| DN2 | LINlowCD25+KIThi | CXCR4, CCR9, CCR7 | 35, 44, 57, 61, 73 |
| DN3 | LINlowCD25+KITlow pre-TCR+ (subset) | CXCR4, CCR9 CCR7 | 35, 44, 57, 61, 73 |
| DN4 | LINlowCD25−KITlow pre-TCR+ | CXCR4, CCR9 (subset) | 35, 44, 57, 61, 73 |
| DP (pre-selection) | CD4+ CD8+ pre-TCR+ | CCR9, CXCR4 | 35, 38, 43, 44, 60, 61, 73, 80 |
| DP (post-selection) | CD4+ CD8+ αβTCR+ | CCR7, CCR9 | 38, 43, 65, 66, 73 |
| CD4 or CD8 SP | CD4+ CD8−, αβTCR+ CD4− CD8+, αβTCR+ | CCR7, CCR9, CXCR4, S1P1 | 35, 38, 43, 44, 66, 73, 80, 84, 85, 113 |
CCR, CC-chemokine receptor; CXCR, CXC-chemokine receptor; CLP, Common lymphoid progenitor; DP, double positive; DN, double negative; ELP, early lymphoid progenitor; ETP, early thymic progenitor; FLT3, FMS-related tyrosine kinase 3; GFP, green fluorescent protein; GPCR, G protein-coupled receptor; HSC, Hematopoietic stem cell; IL, interleukin; LMPP, Lymphoid-primed multipotent progenitor; LSK, LIN−SCA1+KIT+; MPP, Multipotential progenitor; RAG, recombination-activating gene; SCA1, stem-cell antigen 1; S1P1, sphingosine-1-phosphate receptor 1; SP, single positive
PSGL1
PSGL1, a glycoprotein expressed on progenitors, serves as a ligand for P-selectin, which is found on the thymic endothelium32, 33. Expression of functional PSGL1 requires glycosylation, and it is broadly expressed on bone marrow progenitors, whereas expression of its ligand P-selectin has been suggested to be dynamically regulated on thymic endothelial cells32, 33. Expression of P-selectin appears to be periodically upregulated on thymic endothelial cells with CCL25, which may provide a molecular explanation for the observation that the mouse thymus is only periodically receptive to intravenously administered progenitors32, 53.
Migration within the thymus
After entering the thymus, immature progenitors undergo further differentiation and eventually become irreversibly committed to the T cell lineage. The first steps in the T cell developmental process can be defined by four stages of maturation that precede expression of high levels of CD4 and/or CD8 [i.e., double negative (DN) cells] (Table 1). T-lineage commitment occurs at the DN3 stage coincident with the initiation of V-DJ rearrangement of the T cell receptor β-chain (Tcrb), TCR γ-chain (Tcrg) and TCR δ-chain (Tcrd) genes. Productive rearrangement of the Tcrg and Tcrd genes induces commitment to the γδ T cell lineage, whereas productive rearrangement of Tcrb leads to the expression of the pre-TCR, which together with Notch-mediated signalling induces cell proliferation and differentiation of DN3 thymocytes to the DN4 stage (an event referred to as β-selection)54 and then subsequently to the CD4CD8 double-positive (DP) stage.
Migration to the subcapsular zone
In adult mice, T cell progenitors enter the thymus close to the cortico–medullary junction which is highly vascularized. The earliest T cell progenitors (DN1; also known as ETP) may undertake extensive proliferative expansion during a prolonged residence near the CMJ55 before their progeny migrate outward to the subcapsular zone.
As DN1 thymocytes move into the cortex they differentiate to the DN2 and then to the DN3 stage (Figure 1). Roles for CXCR4, CCR7 and CCR9 in regulating the initial migration of early progenitors from the cortico–medullary junction to the cortex have been suggested44, 56, 57; however, none of these GPCRs appears to be critical for this transition. In addition, the expression and localization of ligands for CCR7 (which are predominantly medullary) do not appear to be positioned to induce movement of cells away from the cortico–medullary junction42, 44. It is worth noting that estimates of cortical transit times (~13–15 days) indicate that migration of DN thymocytes from the cortico–medullary junction to the subcapsular zone is unusually slow58 and that movement of cortical DN thymocytes appears to lack obvious directional bias. This could indicate that, rather than migrating via active chemotaxis, cortical migration of DN thymocytes is directed mainly by integrin-mediated cell–cell and cell–matrix interactions that are perhaps facilitated by chemokine signalling in the absence of defined chemokine gradients59. Upon reaching the subcapsular zone, DN3 cells undergo extensive proliferation and then transition to the DN4 stage, a process that depends on expression of and signalling by the pre-TCR. Pre-TCR signalling up-regulates CCR9 on DN3 thymocytes60 and CCR9 has been shown to be important for the proper localization of DN3 thymocytes to the subcapsular zone56. However, in the absence of CCR9 subsequent steps of thymocyte development do not appear to be impacted, suggesting that CCR9-mediated migration of DN thymocytes to the subcapsular zone is not essential for maturation to the DP stage56.
Conditional deletion of CXCR4 also results in accumulation of DN2 and/or DN3 cells in the outer cortex, but unlike deletion of CCR9, differentiation from the DN3 to the DN4 stage is impaired in the absence of CXCR461. This developmental defect appears to be secondary to the loss of CXCR4-mediated survival and proliferation signals rather than to mis-localization of DN thymocytes, indicating that CXCR4 functions as a co-stimulator along with the pre-TCR during β-selection61. Interestingly, the same study demonstrated that CXCR4-mediated chemotaxis is potentiated by pre-TCR signalling, indicating that there is reciprocal cross-talk between the pre-TCR and CXCR4 in DN3 thymocytes and that this is critical for β-selection61.
Migration from the cortex to the medulla
After reaching the subcapsular zone, thymocytes reverse direction and migrate once again back into the cortex (Figure 1). Coincident with their movement into the cortex, DN4 thymocytes begin to express the CD4 and CD8 on their cell surface and transition to the DP stage. Newly formed DP cells express both CXCR4 and CCR9 and can migrate to CXCL12 and CCL25; however, the requirement for these signals for movement of cells out of the subcapsular zone and into the cortex remains unclear35, 44, 57, 60, 62. One hypothesis to explain the “retrograde” migration of DP thymocytes out of the subcapsular zone is that rather than being actively directed by chemokine mediated signals, the proliferative burst that occurs at the DN3 stage results in the passive cortical translocation and inward movement of daughter DN4 and DP cells 59. Indeed, results obtained using two-photon laser scanning microscopy with intact thymus lobe cultures in mice63 and by intravital imaging in fish64 indicate that most immature DP thymocytes move randomly at low motility rates. It has also been proposed that the inward movement of DP thymocytes may be facilitated by interstitial fluid currents that flow directionally from the subcapsular zone to the medulla4. However, as passive or non-directional mechanisms cannot explain both the outward and inward cortical migration of thymocytes, it is clear that additional, as yet undefined factors are involved in regulating the bi-directional movement of thymocytes.
In the cortex, productive rearrangement of TCRαin DP thymocytes results in replacement of the pre-TCR with the clonotypic αβTCR complex. DP cells that express TCRs capable of generating low-intensity activating signals following interaction with self-ligands undergo positive selection in the cortex and they transition into either CD4+CD8− (CD4 single positive (SP)) or CD4−CD8+ (CD8 SP), enter the medulla, and ultimately exit the thymus, populating peripheral lymphoid organs. DP thymocytes that fail to receive activating signals in the cortex are ‘non-selected’ and undergo cell death in the cortex without maturing further. By contrast, cells that express highly self-reactive TCRs that generate strong signals undergo negative selection primarily in the medulla, resulting in death by apoptosis. Positive selection of DP thymocytes results in down-regulation of CXCR465 and concomitant up-regulation of CCR7 and CCR443, 66, 67. Moreover, activated (CD69+) DP thymocytes exhibit enhanced migration to CCR7 and CCR9 ligands38, 67 and directed migration towards the medulla 63, whereas immature DP thymocytes appear unable to migrate on medullary stroma68 suggesting that chemotaxis to the medulla is initiated (or augmented) in DP thymocytes by positively selecting signals transduced by the TCR in the cortex.
Several studies have concluded that entry of immature SP thymocytes into the medulla is dependent on CCR743, 69, 70. Supporting this interpretation, overexpression of CCR7 results in the mislocalization of DP thymocytes in the medulla 69 and negative selection of thymocytes that express TCRs reactive to tissue-restricted antigens is markedly compromised in the absence of CCR770, 71. However, a recent study, although confirming a role for CCR7 in directing migration of cortical CD4 SP thymocytes toward the medulla, suggested that CCR7 is not required for the migration of SP thymocytes on medullary substrates68. In addition, the same study concluded that CCR7 is not required for medullary entry of CD4 SP thymocytes68. However, since these experiments were performed in vitro by seeding of SP thymocytes onto thymus slices, it is unclear if they accurately reflect the in vivo requirements for medullary entry. Another study found a reduction in medullary CD8 SP but not CD4 SP thymocytes in the absence of CCR7, concluding that CCR7 is not essential for entry of CD4 SP thymocytes into the medulla66. In addition, they detected defects in TCR-mediated activation responses in both DP and CD4 SP thymocytes, suggesting that CCR7 augments TCR signalling and that this defect, rather than failure to enter the medulla, may underlie the reduced negative selection efficiency in Ccr7−/− mice66. However, subsequent findings indicate that medullary entry of Ccr7−/− CD4 SP thymocytes is still compromised71. The apparent differential dependence of CD4 SP and CD8 SP thymocytes on CCR7 may be due to the fact that CCR7 is upregulated on a higher percentage of DP thymocytes undergoing positive selection into the CD8 lineage compared with those entering the CD4 lineage 72. Taken together, these data suggest that additional factors may be important for medullary entry, particularly of CD4 SP thymocytes. Interestingly, two studies reported that entry of SP thymocytes into the medulla is blocked completely by the GPCR inhibitor pertussis toxin, indicating that this process is dependent on GPCR-dependent signalling 68, 73.
Export of mature T cells from the thymus
As SP thymocytes migrate within the medulla, they are screened for high affinity TCR-self-peptide interactions capable of triggering negative selection. Expression of tissue specific self-antigens is regulated in thymic epithelial cells (TECs) by autoimmune regulator (AIRE)-dependent transcription 74 and self-peptides are cross presented by medullary dendritic cells (DCs). Medullary thymocytes migrate rapidly and make frequent contacts with DCs; however, their movement becomes markedly slowed if they encounter negatively selecting ligands 68, 75. During their estimated 4–5 day sojourn in the thymus, SP thymocytes undergo terminal differentiation, transitioning from a semi-mature CD69hiCD62LlowCD24hi activated phenotype — during which time they are subjected to negatively selecting stimulation by self antigens and are especially susceptible to pro-apoptotic stimuli — to a mature quiescent CD69lowCD62LhiCD24low phenotype — during which time they become functionally competent, are relatively refractory to apoptosis, and are poised for export76–78. All medullary SP thymocytes express high levels of CCR7 and restricted subsets also express CCR8, CCR9 and CXCR435, 38, 44, 79–81. Although CCR7 and CXCR4 have been shown to have roles in fetal thymocyte egress42, 82, there appears to be no obligatory requirement for any particular chemokine receptor for the export of adult thymocytes37, 43, 83. However, some caution is warranted when interpreting these studies in view of data demonstrating that deletion of these chemokine genes at earlier stages can result in developmental defects 61, 66 and since the effects of SP stage-specific gene deletions have not yet been determined.
In contrast to the relatively mild effects of single chemokine receptor gene deletions, deletion of the GPCR sphingosine-1-phosphate receptor 1 (S1P1) results in marked accumulation of mature SP thymocytes in the medulla due to failure to exit the thymus, identifying S1P1 as a major regulator of thymocyte egress84, 85. S1P1 is preferentially expressed by mature SP thymocytes84, 85 and its ligand, sphingosine-1-phosphate (S1P), is produced by neural crest-derived pericytes in the cortico–medullary junction86 and is highly concentrated in the circulation87 providing a chemotactic gradient for migration of mature SP thymocytes towards the vascularised cortico–medullary junction and then out of the thymus.
A role for TCR signalling in thymocyte export
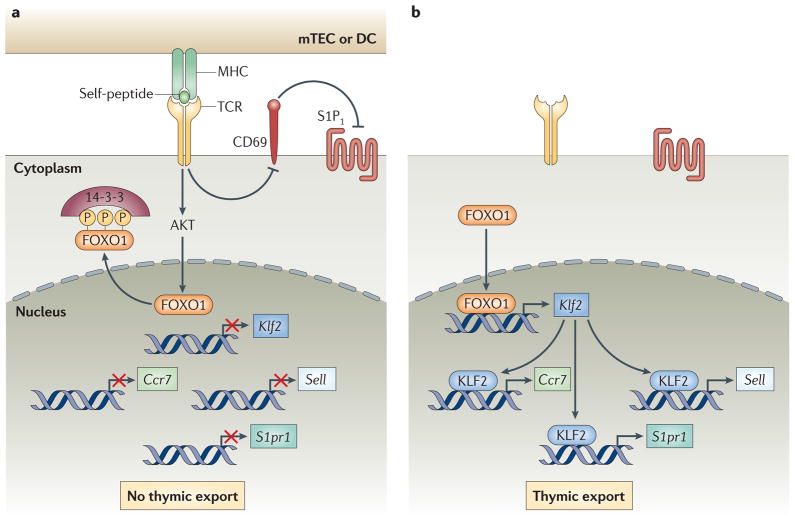
The identification of a pivotal role for S1P1 in controlling thymocyte export provides a clear mechanism for how mature SP T cells are directed to sites of thymic egress. However, an unresolved question is how thymocyte export is appropriately timed such that only functionally mature, post-selection SP cells are permitted to exit the thymus. The importance of this issue is underscored by the consequences of allowing SP thymocytes to exit the thymus prior to completing the process of negative selection, which is exemplified by the severe autoimmune disease in AIRE-deficient humans and mice74. When taken together, the results of several unrelated studies suggest a mechanism by which the timing of thymocyte export is ultimately regulated by TCR signalling (Figure 2). Especially relevant are two recent findings; first, that the transcription factor Kruppel-like factor 2 (KLF2) is required for expression of S1P188, and second, that KLF2 is positively regulated by the transcription factor forkhead box O1 (FOXO1)77, 89. As expected from these results, thymocyte export is also impaired in both Klf2−/− and Foxo1−/− mice90–92.
Figure 2.
Model for regulation of CD4 SP and CD8 SP thymocyte export by TCR signalling. A. “Semi-mature” SP thymocytes receive activating signals through their TCRs by interaction with self-peptide–MHC complexes expressed by medullary TECs (mTECs) and DCs. TCR signalling results in the AKT-mediated phosphorylation of FOXO1 resulting in the transcriptional inactivation and nuclear export of FOXO1. Retention of FOXO1 in the cytoplasm leads to down-regulation of Klf2, which is required for transcription of Ccr7, Sell (encoding CD62L) and S1pr1 (encoding S1P1). In addition, TCR signalling induces expression of CD69, which downregulates the surface expression of S1P1. Loss and/or prevention of S1P1 surface expression prevents premature S1P-mediated thymocyte export from the thymus. B. SP thymocytes that do not undergo or survive negative selection become functionally mature and TCR signalling is terminated. Cessation of TCR signalling terminates AKT-mediated FOXO1 phosphorylation and results in nuclear translocation of transcriptionally active FOXO1. FOXO1 promotes transcription of Klf2, which induces the expression of Ccr7, Sell and S1pr1. Termination of TCR signalling also results in eventual downregulation of CD69, alleviating CD69-directed downregulation of S1P1 surface expression. Increased surface expression of S1P1 renders SP thymocytes responsive to S1P chemotaxis and promotes their migration towards the cortio-medullary junction and their export from the thymus.
FOXO1 protein activity is regulated by its subcellular localization, which in turn is controlled by its post-translational modification93. Notably, phosphorylation of FOXO1 by the serine/threonine protein kinase AKT (also known as PKB) reduces its DNA-binding activity and results in FOXO1 nuclear export by promoting its interaction with the 14-3-3 chaperone proteins 93. TCR engagement is a potent activator of the phosphatidylinositol 3-kinase (PI3K)–AKT pathway 94 providing a mechanism though which TCR stimulation can regulate the expression of S1P1 and therefore control the timing of thymocyte export (Figure 2).
Thus, we propose the following model: in semi-mature CD69+ SP thymocytes that are receiving activating TCR signals through engagement of self-ligands, FOXO1 is phosphorylated by AKT. Downregulation of FOXO1 transcriptional activity leads to reduced transcription of KLF2 and S1P1 resulting in thymic retention (Figure 2). This regulatory mechanism functions to prevent export of SP thymocytes until they have completed their maturation and been screened for self-reactivity by negative selection. Fully mature quiescent CD69− SP thymocytes cease TCR signalling. This results in upregulation of FOXO1 activity and increased KLF2 and S1P1 transcription, thereby priming cells for thymic export (Figure 2). Indirect support for this model is that transgenic expression of constitutively active PI3K95, inactivation of the PI3K inhibitor PTEN (phosphatase and tensin homologue)96 and antigen-mediated activation of mature thymocytes block thymocyte export97. Interestingly, in addition to S1P1, FOXO1 has also been shown to regulate the expression of CD62L and CCR789 perhaps through its regulation of KLF288. Consequently, TCR signalling controls the expression of S1P1, CCR7 and CD62L. Although CCR7 and CD62L do not appear to be required for thymocyte export, both molecules, together with S1P1, are known to have important roles in regulating peripheral transmigration of T cells into secondary lymphoid tissues96, 98.
A second tier of regulatory control of thymocyte export by TCR signalling was recently suggested by the discovery of reciprocal inhibition by CD69 and S1P1.99, 100. CD69 is a transmembrane C-type lectin protein that is rapidly and transiently upregulated following TCR engagement101. An initial clue to a potential regulatory interaction between CD69 and S1P1 was the observation that mature SP thymocytes in S1p1−/− mice fail to downregulate CD6985 and, conversely, that treatment with the S1P1 agonist FTY720 results in downregulation of CD69 on thymocytes102 indicating that S1P1 signalling inhibits CD69 surface expression. Subsequently it was found that CD69 associates with and inhibits the function of S1P1 by inducing its internalization and degradation99, 100. Consistent with these results, constitutive (transgene-mediated) expression of CD69 by SP thymocytes inhibits their export from the thymus103. Thus, TCR-induced expression of CD69 on activated semi-mature SP thymocytes inhibits surface expression of S1P1, thereby preventing premature thymocyte egress (Figure 2). As TCR signalling is terminated and CD69 is downregulated SP thymocytes become responsive to S1P and are capable of being exported from the thymus. For the majority of SP thymocytes which do not express highly self-reactive TCRs and therefore do not undergo negative selection, CD69 downregulation and the acquisition of S1P responsiveness may simply reflect the gradual termination of signals induced by positively selecting interactions. On the other hand, for SP thymocytes that are subjected to and survive negative selection CD69 downregulation may instead result from active “tuning” of TCR signaling through the regulated expression of inhibitory molecules such as CD5104–106.
Outstanding questions and conclusions
The reason why thymic homing is so tightly restricted to specific progenitor populations remains unclear. One possibility is that selective thymic settling serves to exclude inappropriate progenitors such as those retaining efficient myeloid and erythroid lineage potential from the thymus, as the development of myelo-erythroid progenitors into megakaryocytes is not inhibited, but may instead be enhanced by Notch signals65. Regulated expression of CCR7 and CCR9 serves to prevent bona fide long term HSCs from migrating to the thymus, thereby ensuring that only those cells that have committed to differentiation (that is, lost self-renewal potential) are candidates for migration.
The consequences of thymic settling by HSCs may be similar to what is observed in rhombotin-2 (Lmo2)-transgenic mice, where self-renewing progenitors populate the thymus, exclude further seeding, and predispose to tumorigenesis 107. It is further possible that the use of CCR9− and CCR7-expressing progenitors in thymopoiesis may allow the importation of multiple progenitor populations with distinct lineage potentials and possibly distinct functions. Each of these issues represents a potential focus for future work.
At several stages of thymocyte development, multiple chemokine receptors are implicated in regulating migration raising the possibility of functional redundancy. The generation of compound knockout mice (i.e., mice deficient in two or more chemokine receptors), in addition to conditional, stage-specific knockout mice as mentioned above, should help to distinguish whether redundancy exists, or alternatively, if additional mechanisms need to be identified for controlling stage-specific migration in the thymus. These approaches could be particularly informative in helping to elucidate thymocyte migration patterns that are currently poorly understood such as those controlling the bidirectional movement of thymocytes in the cortex. Further investigation into potential cross-talk between chemokine receptor signalling and pre-TCR and/or TCR (or cytokine receptor) signalling should provide clearer insight into the important linkage between thymocyte development and migration. Additional pathways and factors downstream of the TCR are likely to play an important role in regulating thymocyte export. A still largely unexplored area of investigation concerns the molecules that regulate migration of non-T lineage cells to the thymus. These transient cell populations, which include macrophages and DCs, are critical for thymocyte selection, and, similar to T cell progenitors, are derived from bone marrow precursors that home to and enter the thymus prior to undergoing a program of proliferation and differentiation. In this regard, recent data suggest that thymic recruitment of bone marrow-derived DCs is regulated by chemokines and chemokine receptors distinct from those used by thymocytes such as XCR1, a chemokine receptor expressed on thymic DCs and its ligand XCL1 expressed by mTECs 108.
Despite these gaps in our current knowledge, much has already been learned about migration during T cell development. These discoveries are likely to result in significant therapeutic applications. Bone marrow transplantation is a relatively common procedure, and cells of the T cell lineage is the slowest to recover following irradiation and bone marrow transplantation, a delay that can be life-threatening 109. Improvements in understanding the molecular basis of thymic homing may allow acceleration of T cell reconstitution following bone marrow ablative therapy for hematological cancers and genetic disorders.
Acknowledgments
The authors thank Y. Takahama, J.C. Zúñiga-Pflücker, D. A. Zlotoff, S. Zhang, R. Lesourne and K. Pfeifer for critical reading of and helpful suggestions on the manuscript.
Glossary terms
- G-protein-coupled receptor
(GPCR). A receptor that is composed of seven membrane-spanning helical segments. These receptors associate with G-proteins, which are a family of trimeric intracellular-signalling proteins with common β- and γ-chains, and one of several α-chains. The α-chain determines the nature of the signal that is transmitted from a ligand-occupied GPCR to downstream effector systems
- LSK cells
A mouse cell population that is highly enriched in HSCs and defined by the absence of lineage/maturation markers (LIN−), and expression of stem-cell antigen 1 (SCA1) and the receptor for stem-cell factor KIT. This is a heterogeneous population containing multipotent progenitors and true self-renewing stem cells
- double-negative cells
(DN cells). The most immature thymocytes lack expression of the co-receptors CD4 and CD8, and are referred to as DN cells. This compartment can be further subdivided on the basis of CD44 and CD25 expression into four subpopulationsDN1 (CD25−CD44+) DN2 (CD25+CD44+), DN3 (CD25+CD44−) and DN4 (CD25−CD44−)
- pre-TCR
(Pre-T cell receptor). A receptor that is expressed on pre-T cells. It is formed by a TCR β-chain paired with a surrogate TCR α-chain (known as the invariant pre-Tα protein). The receptor complex includes CD3 proteins and transduces signals that allow further T cell development
- Notch
A transmembrane receptor involved in the pathway for direct cell–cell signalling through its association with a transmembrane ligand of the delta or serrate/jagged family on a neighbouring cell
- β-selection
The controlled developmental transition beyond the double-negative 3 (DN3) stage to the double positive (DP) stage that is limited to T cells that have successfully rearranged their cell receptor (TCR) β-chain genes to express a functional cell-surface pre-TCR. The conditional developmental arrest encountered at the DN3 stage is termed the ‘β-selection checkpoint’
- Two-photon laser scanning microscopy
Laser-scanning microscopy that uses pulsed infrared laser light for the excitation of conventional fluorophores or fluorescent proteins. This technique greatly reduces photodamage of living specimens and improves depth of tissue penetration, owing to the low level of light scattering within the tissue
- positive selection
The maturation of immature CD4+CD8+ precursor thymocytes induced by TCR signals that result from binding to self-peptide–MHC ligands on thymic epithelial cells. This process selects thymocytes that express TCRs that are able to interact with self-MHC
- negative selection
T cells that express T cell receptors with high affinity for self-antigens are eliminated from the repertoire by apoptosis after recognition of their target antigen presented by thymic medullary dendritic cells
- Thymic epithelial cells
Medullary thymic epithelial cells (mTECs) and cortical TECs (cTECs) cells are phenotypically and functionally distinctive, providing specialized niches necessary for the stage-specific development of thymocytes migrating through these zones
- 14-3-3
A family of conserved proteins present in all eukaryotic organisms that are involved in such diverse cellular processes as apoptosis and stress, as well as intracellular signalling and cell-cycle regulation. 14-3-3 proteins function as adaptors in protein interactions and can regulate protein localization and enzymatic activity. Approximately 100 binding partners for the 14-3-3 proteins have been reported. Lmo2-transgenic mice
References
- 1.Bhandoola A, von Boehmer H, Petrie HT, Zuniga-Pflucker JC. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: plenty to choose from. Immunity. 2007;26:678–89. doi: 10.1016/j.immuni.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Petrie HT, Zuniga-Pflucker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol. 2007;25:649–79. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- 3.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–35. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 4.Hollander G, et al. Cellular and molecular events during early thymus development. Immunol Rev. 2006;209:28–46. doi: 10.1111/j.0105-2896.2006.00357.x. [DOI] [PubMed] [Google Scholar]
- 5.Boehm T. Thymus development and function. Curr Opin Immunol. 2008;20:178–84. doi: 10.1016/j.coi.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Blackburn CC, Manley NR. Developing a new paradigm for thymus organogenesis. Nat Rev Immunol. 2004;4:278–89. doi: 10.1038/nri1331. [DOI] [PubMed] [Google Scholar]
- 7.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–72. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 8.Karsunky H, Inlay MA, Serwold T, Bhattacharya D, Weissman IL. Flk2+ common lymphoid progenitors possess equivalent differentiation potential for the B and T lineages. Blood. 2008;111:5562–70. doi: 10.1182/blood-2007-11-126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Adolfsson J, et al. Upregulation of Flt3 Expression within the Bone Marrow Lin(−)Sca1(+)c- kit(+) Stem Cell Compartment Is Accompanied by Loss of Self-Renewal Capacity. Immunity. 2001;15:659–69. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 11.Adolfsson J, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci USA. 2001;98:14541–6. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igarashi H, Gregory S, Yokota T, Sakaguchi N, Kincade P. Transcription from the RAG1 Locus Marks the Earliest Lymphocyte Progenitors in Bone Marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 14.Yokota T, et al. Unique properties of fetal lymphoid progenitors identified according to RAG1 gene expression. Immunity. 2003;19:365–75. doi: 10.1016/s1074-7613(03)00231-0. [DOI] [PubMed] [Google Scholar]
- 15.Inlay MA, et al. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes Dev. 2009;23:2376–81. doi: 10.1101/gad.1836009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mansson R, et al. Single cell analysis of the common lymphoid progenitor compartment reveals functional and molecular heterogeneity. Blood. 2009;115:2601–9. doi: 10.1182/blood-2009-08-236398. [DOI] [PubMed] [Google Scholar]
- 17.Allman D, et al. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol. 2003;4:168–74. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz BA, et al. Selective thymus settling regulated by cytokine and chemokine receptors. J Immunol. 2007;178:2008–17. doi: 10.4049/jimmunol.178.4.2008. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjea-Matthes D, et al. Early defect prethymic in bone marrow T cell progenitors in athymic nu/nu mice. J Immunol. 2003;171:1207–15. doi: 10.4049/jimmunol.171.3.1207. [DOI] [PubMed] [Google Scholar]
- 20.Dejbakhsh-Jones S, Garcia-Ojeda ME, Chatterjea-Matthes D, Zeng D, Strober S. Clonable progenitors committed to the T lymphocyte lineage in the mouse bone marrow; use of an extrathymic pathway. Proc Natl Acad Sci U S A. 2001;98:7455–60. doi: 10.1073/pnas.131559798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dejbakhsh-Jones S, Strober S. Identification of an early T cell progenitor for a pathway of T cell maturation in the bone marrow. Proc Natl Acad Sci U S A. 1999;96:14493–8. doi: 10.1073/pnas.96.25.14493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Ojeda ME, et al. Stepwise development of committed progenitors in the bone marrow that generate functional T cells in the absence of the thymus. J Immunol. 2005;175:4363–73. doi: 10.4049/jimmunol.175.7.4363. [DOI] [PubMed] [Google Scholar]
- 23.Krueger A, von Boehmer H. Identification of a T lineage-committed progenitor in adult blood. Immunity. 2007;26:105–16. doi: 10.1016/j.immuni.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodewald HR, Kretzschmar K, Takeda S, Hohl C, Dessing M. Identification of pro-thymocytes in murine fetal blood: T lineage commitment can precede thymus colonization. Embo J. 1994;13:4229–40. doi: 10.1002/j.1460-2075.1994.tb06743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arcangeli ML, et al. Extrathymic hemopoietic progenitors committed to T cell differentiation in the adult mouse. J Immunol. 2005;174:1980–8. doi: 10.4049/jimmunol.174.4.1980. [DOI] [PubMed] [Google Scholar]
- 26.Lancrin C, et al. Major T cell progenitor activity in bone marrow-derived spleen colonies. J Exp Med. 2002;195:919–29. doi: 10.1084/jem.20011475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maillard I, et al. Notch-dependent T-lineage commitment occurs at extrathymic sites following bone marrow transplantation. Blood. 2006;107:3511–9. doi: 10.1182/blood-2005-08-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zlotoff DA, et al. CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus. Blood. 2010;115:1897–905. doi: 10.1182/blood-2009-08-237784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mori S, Shortman K, Wu L. Characterization of thymus-seeding precursor cells from mouse bone marrow. Blood. 2001;98:696–704. doi: 10.1182/blood.v98.3.696. [DOI] [PubMed] [Google Scholar]
- 30.Scimone ML, Aifantis I, Apostolou I, von Boehmer H, von Andrian UH. A multistep adhesion cascade for lymphoid progenitor cell homing to the thymus. Proc Natl Acad Sci U S A. 2006;103:7006–11. doi: 10.1073/pnas.0602024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–34. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 32.Gossens K, et al. Thymic progenitor homing and lymphocyte homeostasis are linked via S1P-controlled expression of thymic P-selectin/CCL25. J Exp Med. 2009;206:761–778. doi: 10.1084/jem.20082502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossi FM, et al. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol. 2005;6:626–34. doi: 10.1038/ni1203. [DOI] [PubMed] [Google Scholar]
- 34.Krueger A, Willenzon S, Lyszkiewicz M, Kremmer E, Forster R. CC chemokine receptor 7 and 9 double-deficient hematopoietic progenitors are severely impaired in seeding the adult thymus. Blood. 2010;115:1906–12. doi: 10.1182/blood-2009-07-235721. [DOI] [PubMed] [Google Scholar]
- 35.Wurbel MA, Malissen B, Campbell JJ. Complex regulation of CCR9 at multiple discrete stages of T cell development. Eur J Immunol. 2006;36:73–81. doi: 10.1002/eji.200535203. [DOI] [PubMed] [Google Scholar]
- 36.Liu C, et al. Coordination between CCR7- and CCR9-mediated chemokine signals in prevascular fetal thymus colonization. Blood. 2006;108:2531–9. doi: 10.1182/blood-2006-05-024190. [DOI] [PubMed] [Google Scholar]
- 37.Uehara S, Grinberg A, Farber JM, Love PE. A role for CCR9 in T lymphocyte development and migration. J Immunol. 2002;168:2811–9. doi: 10.4049/jimmunol.168.6.2811. [DOI] [PubMed] [Google Scholar]
- 38.Uehara S, Song K, Farber JM, Love PE. Characterization of CCR9 expression and CCL25/thymus-expressed chemokine responsiveness during T cell development: CD3(high)CD69+ thymocytes and gammadeltaTCR+ thymocytes preferentially respond to CCL25. J Immunol. 2002;168:134–42. doi: 10.4049/jimmunol.168.1.134. [DOI] [PubMed] [Google Scholar]
- 39.Robertson P, Means TK, Luster AD, Scadden DT. CXCR4 and CCR5 mediate homing of primitive bone marrow-derived hematopoietic cells to the postnatal thymus. Exp Hematol. 2006;34:308–19. doi: 10.1016/j.exphem.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Stimamiglio MA, et al. EphB2-mediated interactions are essential for proper migration of T cell progenitors during fetal thymus colonization. J Leukoc Biol. 2010;88:483–94. doi: 10.1189/jlb.0210079. [DOI] [PubMed] [Google Scholar]
- 41.Liu C, et al. The role of CCL21 in recruitment of T-precursor cells to fetal thymi. Blood. 2005;105:31–9. doi: 10.1182/blood-2004-04-1369. [DOI] [PubMed] [Google Scholar]
- 42.Ueno T, et al. Role for CCR7 ligands in the emigration of newly generated T lymphocytes from the neonatal thymus. Immunity. 2002;16:205–18. doi: 10.1016/s1074-7613(02)00267-4. [DOI] [PubMed] [Google Scholar]
- 43.Ueno T, et al. CCR7 signals are essential for cortex-medulla migration of developing thymocytes. J Exp Med. 2004;200:493–505. doi: 10.1084/jem.20040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Misslitz A, et al. Thymic T cell development and progenitor localization depend on CCR7. J Exp Med. 2004;200:481–91. doi: 10.1084/jem.20040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saran N, et al. Multiple extrathymic precursors contribute to T-cell development with different kinetics. Blood. 2010;115:1137–44. doi: 10.1182/blood-2009-07-230821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benz C, Bleul CC. A multipotent precursor in the thymus maps to the branching point of the T versus B lineage decision. J Exp Med. 2005;202:21–31. doi: 10.1084/jem.20050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serwold T, Ehrlich LI, Weissman IL. Reductive isolation from bone marrow and blood implicates common lymphoid progenitors as the major source of thymopoiesis. Blood. 2009;113:807–15. doi: 10.1182/blood-2008-08-173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bell JJ, Bhandoola A. The earliest thymic progenitors for T cells possess myeloid lineage potential. Nature. 2008;452:764–7. doi: 10.1038/nature06840. [DOI] [PubMed] [Google Scholar]
- 49.Schlenner SM, et al. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity. 2010;32:426–36. doi: 10.1016/j.immuni.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Wada H, et al. Adult T-cell progenitors retain myeloid potential. Nature. 2008;452:768–72. doi: 10.1038/nature06839. [DOI] [PubMed] [Google Scholar]
- 51.Lai AY, Kondo M. Identification of a bone marrow precursor of the earliest thymocytes in adult mouse. Proc Natl Acad Sci U S A. 2007;104:6311–6316. doi: 10.1073/pnas.0609608104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Desanti GE, et al. Clonal Analysis Reveals Uniformity in the Molecular Profile and Lineage Potential of CCR9+ and CCR9− Thymus-Settling Progenitors. J Immunol. 2011 doi: 10.4049/jimmunol.1002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Foss DL, Donskoy E, Goldschneider I. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. J Exp Med. 2001;193:365–74. doi: 10.1084/jem.193.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ciofani M, Knowles GC, Wiest DL, von Boehmer H, Zuniga-Pflucker JC. Stage-specific and differential notch dependency at the alphabeta and gammadelta T lineage bifurcation. Immunity. 2006;25:105–16. doi: 10.1016/j.immuni.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 55.Lind EF, Prockop SE, Porritt HE, Petrie HT. Mapping precursor movement through the postnatal thymus reveals specific microenvironments supporting defined stages of early lymphoid development. J Exp Med. 2001;194:127–34. doi: 10.1084/jem.194.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benz C, Heinzel K, Bleul CC. Homing of immature thymocytes to the subcapsular microenvironment within the thymus is not an absolute requirement for T cell development. Eur J Immunol. 2004;34:3652–63. doi: 10.1002/eji.200425248. [DOI] [PubMed] [Google Scholar]
- 57.Plotkin J, Prockop SE, Lepique A, Petrie HT. Critical role for CXCR4 signaling in progenitor localization and T cell differentiation in the postnatal thymus. J Immunol. 2003;171:4521–7. doi: 10.4049/jimmunol.171.9.4521. [DOI] [PubMed] [Google Scholar]
- 58.Porritt HE, Gordon K, Petrie HT. Kinetics of steady-state differentiation and mapping of intrathymic-signaling environments by stem cell transplantation in nonirradiated mice. J Exp Med. 2003;198:957–62. doi: 10.1084/jem.20030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petrie H. Cell migration and the control of post-natal T-cell lymphopoiesis in the thymus. Nature Reviews Immunology. 2003;3:859–866. doi: 10.1038/nri1223. [DOI] [PubMed] [Google Scholar]
- 60.Norment AM, Bogatzki LY, Gantner BN, Bevan MJ. Murine CCR9, a chemokine receptor for thymus-expressed chemokine that is up-regulated following pre-TCR signaling. J Immunol. 2000;164:639–48. doi: 10.4049/jimmunol.164.2.639. [DOI] [PubMed] [Google Scholar]
- 61.Trampont PC, et al. CXCR4 acts as a costimulator during thymic beta-selection. Nat Immunol. 2010;11:162–70. doi: 10.1038/ni.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim K, Lee CK, Sayers TJ, Muegge K, Durum SK. The trophic action of IL-7 on pro-T cells: inhibition of apoptosis of pro-T1, -T2, and -T3 cells correlates with Bcl-2 and Bax levels and is independent of Fas and p53 pathways. J Immunol. 1998;160:5735–41. [PubMed] [Google Scholar]
- 63.Witt CM, Raychaudhuri S, Schaefer B, Chakraborty AK, Robey EA. Directed migration of positively selected thymocytes visualized in real time. PLoS Biol. 2005;3:e160. doi: 10.1371/journal.pbio.0030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li J, Iwanami N, Hoa VQ, Furutani-Seiki M, Takahama Y. Noninvasive intravital imaging of thymocyte dynamics in medaka. J Immunol. 2007;179:1605–15. doi: 10.4049/jimmunol.179.3.1605. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki G, et al. Loss of SDF-1 receptor expression during positive selection in the thymus. Int Immunol. 1998;10:1049–56. doi: 10.1093/intimm/10.8.1049. [DOI] [PubMed] [Google Scholar]
- 66.Davalos-Misslitz AC, Worbs T, Willenzon S, Bernhardt G, Forster R. Impaired responsiveness to T-cell receptor stimulation and defective negative selection of thymocytes in CCR7-deficient mice. Blood. 2007;110:4351–9. doi: 10.1182/blood-2007-01-070284. [DOI] [PubMed] [Google Scholar]
- 67.Campbell JJ, Pan J, Butcher EC. Cutting edge: developmental switches in chemokine responses during T cell maturation. J Immunol. 1999;163:2353–7. [PubMed] [Google Scholar]
- 68.Ehrlich LI, Oh DY, Weissman IL, Lewis RS. Differential contribution of chemotaxis and substrate restriction to segregation of immature and mature thymocytes. Immunity. 2009;31:986–98. doi: 10.1016/j.immuni.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kwan J, Killeen N. CCR7 directs the migration of thymocytes into the thymic medulla. J Immunol. 2004;172:3999–4007. doi: 10.4049/jimmunol.172.7.3999. [DOI] [PubMed] [Google Scholar]
- 70.Kurobe H, et al. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 2006;24:165–77. doi: 10.1016/j.immuni.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 71.Nitta T, Nitta S, Lei Y, Lipp M, Takahama Y. CCR7-mediated migration of developing thymocytes to the medulla is essential for negative selection to tissue-restricted antigens. Proc Natl Acad Sci U S A. 2009;106:17129–33. doi: 10.1073/pnas.0906956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yin X, et al. CCR7 expression in developing thymocytes is linked to the CD4 versus CD8 lineage decision. J Immunol. 2007;179:7358–64. doi: 10.4049/jimmunol.179.11.7358. [DOI] [PubMed] [Google Scholar]
- 73.Suzuki G, et al. Pertussis toxin-sensitive signal controls the trafficking of thymocytes across the corticomedullary junction in the thymus. J Immunol. 1999;162:5981–5. [PubMed] [Google Scholar]
- 74.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 75.Le Borgne M, et al. The impact of negative selection on thymocyte migration in the medulla. Nat Immunol. 2009;10:823–30. doi: 10.1038/ni.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kishimoto H, Sprent J. Negative selection in the thymus includes semimature T cells. J Exp Med. 1997;185:263–71. doi: 10.1084/jem.185.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fabre S, et al. FOXO1 regulates L-Selectin and a network of human T cell homing molecules downstream of phosphatidylinositol 3-kinase. J Immunol. 2008;181:2980–9. doi: 10.4049/jimmunol.181.5.2980. [DOI] [PubMed] [Google Scholar]
- 78.McCaughtry TM, Wilken MS, Hogquist KA. Thymic emigration revisited. J Exp Med. 2007;204:2513–20. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kremer L, et al. The transient expression of C-C chemokine receptor 8 in thymus identifies a thymocyte subset committed to become CD4+ single-positive T cells. J Immunol. 2001;166:218–25. doi: 10.4049/jimmunol.166.1.218. [DOI] [PubMed] [Google Scholar]
- 80.Schabath R, et al. The murine chemokine receptor CXCR4 is tightly regulated during T cell development and activation. J Leukoc Biol. 1999;66:996–1004. doi: 10.1002/jlb.66.6.996. [DOI] [PubMed] [Google Scholar]
- 81.Kim CH, Pelus LM, White JR, Broxmeyer HE. Differential chemotactic behavior of developing T cells in response to thymic chemokines. Blood. 1998;91:4434–43. [PubMed] [Google Scholar]
- 82.Vianello F, et al. A CXCR4-dependent chemorepellent signal contributes to the emigration of mature single-positive CD4 cells from the fetal thymus. J Immunol. 2005;175:5115–25. doi: 10.4049/jimmunol.175.8.5115. [DOI] [PubMed] [Google Scholar]
- 83.Wurbel MA, et al. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor gammadelta(+) gut intraepithelial lymphocytes. Blood. 2001;98:2626–32. doi: 10.1182/blood.v98.9.2626. [DOI] [PubMed] [Google Scholar]
- 84.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 85.Matloubian M, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–60. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 86.Zachariah MA, Cyster JG. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science. 2010;328:1129–35. doi: 10.1126/science.1188222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pappu R, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–8. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 88.Carlson CM, et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 89.Kerdiles YM, et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10:176–84. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gubbels Bupp MR, et al. T cells require Foxo1 to populate the peripheral lymphoid organs. Eur J Immunol. 2009;39:2991–9. doi: 10.1002/eji.200939427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ouyang W, Beckett O, Flavell RA, Li MO. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity. 2009;30:358–71. doi: 10.1016/j.immuni.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weinreich MA, Hogquist KA. Thymic emigration: when and how T cells leave home. J Immunol. 2008;181:2265–70. doi: 10.4049/jimmunol.181.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–88. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- 94.Cantrell DA. Regulation and function of serine kinase networks in lymphocytes. Curr Opin Immunol. 2003;15:294–8. doi: 10.1016/s0952-7915(03)00052-9. [DOI] [PubMed] [Google Scholar]
- 95.Barbee SD, Alberola-Ila J. Phosphatidylinositol 3-kinase regulates thymic exit. J Immunol. 2005;174:1230–8. doi: 10.4049/jimmunol.174.3.1230. [DOI] [PubMed] [Google Scholar]
- 96.Sinclair LV, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–21. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Uldrich AP, et al. Antigen challenge inhibits thymic emigration. J Immunol. 2006;176:4553–61. doi: 10.4049/jimmunol.176.8.4553. [DOI] [PubMed] [Google Scholar]
- 98.Finlay D, Cantrell D. Phosphoinositide 3-kinase and the mammalian target of rapamycin pathways control T cell migration. Ann N Y Acad Sci. 2010;1183:149–57. doi: 10.1111/j.1749-6632.2009.05134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bankovich AJ, Shiow LR, Cyster JG. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J Biol Chem. 2010;285:22328–37. doi: 10.1074/jbc.M110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shiow LR, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–4. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 101.Sancho D, Gomez M, Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–40. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 102.Rosen H, Alfonso C, Surh CD, McHeyzer-Williams MG. Rapid induction of medullary thymocyte phenotypic maturation and egress inhibition by nanomolar sphingosine 1-phosphate receptor agonist. Proc Natl Acad Sci U S A. 2003;100:10907–12. doi: 10.1073/pnas.1832725100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Feng C, et al. A potential role for CD69 in thymocyte emigration. Int Immunol. 2002;14:535–44. doi: 10.1093/intimm/dxf020. [DOI] [PubMed] [Google Scholar]
- 104.Azzam HS, et al. Fine tuning of TCR signaling by CD5. J Immunol. 2001;166:5464–72. doi: 10.4049/jimmunol.166.9.5464. [DOI] [PubMed] [Google Scholar]
- 105.Grossman Z, Singer A. Tuning of activation thresholds explains flexibility in the selection and development of T cells in the thymus. Proc Natl Acad Sci U S A. 1996;93:14747–52. doi: 10.1073/pnas.93.25.14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wong P, Barton GM, Forbush KA, Rudensky AY. Dynamic tuning of T cell reactivity by self-peptide-major histocompatibility complex ligands. J Exp Med. 2001;193:1179–87. doi: 10.1084/jem.193.10.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McCormack MP, et al. The Lmo2 oncogene initiates leukemia in mice by inducing thymocyte self-renewal. Science. 2010;327:879–83. doi: 10.1126/science.1182378. [DOI] [PubMed] [Google Scholar]
- 108.Lei Y, et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med. 2011;208:383–94. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Storek J, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol. 2008;30:425–37. doi: 10.1007/s00281-008-0132-5. [DOI] [PubMed] [Google Scholar]
- 110.Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195:1145–54. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Si Y, Tsou CL, Croft K, Charo IF. CCR2 mediates hematopoietic stem and progenitor cell trafficking to sites of inflammation in mice. J Clin Invest. 2010;120:1192–203. doi: 10.1172/JCI40310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Benz C, Martins VC, Radtke F, Bleul CC. The stream of precursors that colonizes the thymus proceeds selectively through the early T lineage precursor stage of T cell development. J Exp Med. 2008;205:1187–99. doi: 10.1084/jem.20072168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carramolino L, et al. Expression of CCR9 beta-chemokine receptor is modulated in thymocyte differentiation and is selectively maintained in CD8(+) T cells from secondary lymphoid organs. Blood. 2001;97:850–7. doi: 10.1182/blood.v97.4.850. [DOI] [PubMed] [Google Scholar]