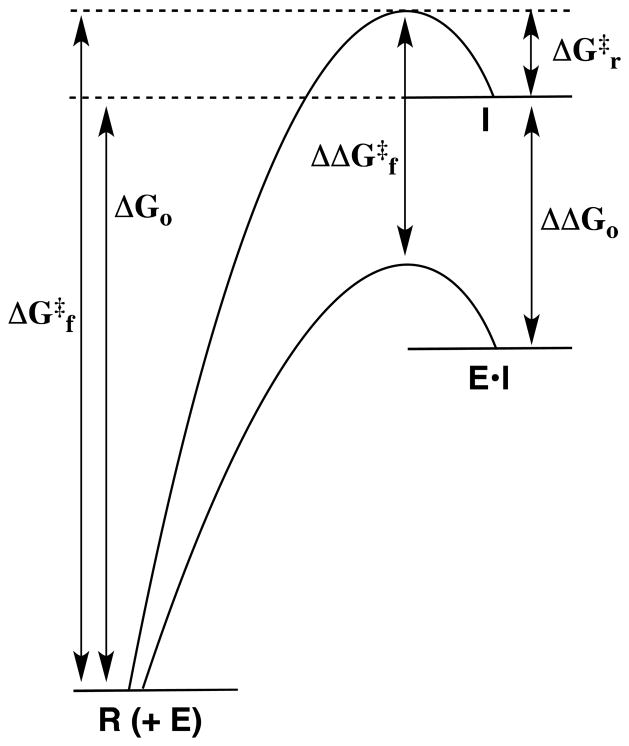
Figure 1.
Reaction coordinate profiles for nonenzymatic (upper profile) and enzymatic (lower profile) heterolytic bond cleavage at a reactant R to give a high energy reactive intermediate I. The activation barrier for formation of the intermediate, ΔG‡f, is equal to the sum of the large thermodynamic barrier to formation of the reactive intermediate, ΔGo, and the small barrier for the reaction in the reverse direction, ΔG‡r. If ΔG‡r is not changed on proceeding to the enzymatic reaction, then the stabilization of the intermediate at the active site of an enzyme catalyst (E•I) by an amount ΔΔGo will result in a corresponding reduction in the activation barrier for formation of the intermediate, such that ΔΔG‡f = ΔΔGo.