Abstract
NADPH oxidase has recently been identified as a promising new therapeutic target in ALS. Genetic deletion of NADPH oxidase (Nox2) in the transgenic SOD1G93A mutant mouse model of ALS was reported to increase survival remarkably by 97 days. Furthermore, apocynin, a widely used inhibitor of NADPH oxidase, was observed to dramatically extend the survival of the SOD1G93A ALS mice even longer to 113 days (Harraz et al. J Clin Invest 118: 474 2008). Diapocynin, the covalent dimer of apocynin, has been reported to be a more potent inhibitor of NADPH oxidase. We compared the protection of diapocynin to apocynin in primary cultures of SOD1G93A-expressing motor neurons against nitric oxide-mediated death. Diapocynin, 10 μM, provided significantly greater protection compared to apocynin, 200 μM, at the lowest statistically significant concentrations. However, administration of diapocynin starting at 21 days of age in the SOD1G93A-ALS mouse model did not extend lifespan. Repeated parallel experiments with apocynin failed to yield protection greater than a 5-day life extension in multiple trials conducted at two separate institutions. The maximum protection observed was an 8-day extension in survival when diapocynin was administered at 100 days of age at disease onset. HPLC with selective ion monitoring by mass spectrometry revealed that both apocynin and diapocynin accumulated in the brain and spinal cord tissue to low micromolar concentrations. Diapocynin was also detected in the CNS of apocynin-treated mice. The failure to achieve significant protection with either apocynin or diapocynin raises questions about the utility for treating ALS patients.
Keywords: Diapocynin, Apocynin, ALS, NADPH Oxidase, G93A SOD1 mice
Introduction
Recently, a dramatic extension has been reported on the lifespan in the high expressing SOD1G93A mouse model of amyotrophic lateral sclerosis (ALS). Disruption of NADPH oxidase (Nox), either as genetic knockouts or through pharmacological inhibition, extended the survival of the ALS mice by about 100 days—the greatest protection ever observed in this animal model (Marden et al., 2007; Harraz et al., 2008). The evidence implicating a role for Nox in the SOD1G93A mouse model is particularly intriguing because Nox catalyzes the formation of superoxide from NADPH and oxygen while SOD1 is the primary cytosolic scavenger of superoxide. The Nox family, which includes seven different known isoforms expressed in various tissue, consists of membrane-associated enzymes that require several cytosolic subunits to bind in order to activate superoxide formation (Bedard and Krause, 2007; Lambeth, 2004). Nox2 (gp91phox) is primarily found in phagocytes and involved in host defense. In the central nervous system (CNS), Nox2 is the predominant isoform found in microglia, astrocytes and neuron cells. Although most highly expressed in the colon, Nox1 is also expressed in the CNS (Sorce and Krause, 2009).
Marden et al. (2007) reported homozygous knockouts of both Nox1 and Nox2 substantially increased the survival of the hybrid B6SJL transgenic SOD1G93A mouse model of ALS with the greatest effect in the Nox2 knockout. Nox2 extended life by 97 days, whereas the Nox1 knockout increased survival by 33 days. The heterozygous Nox2 and Nox1 knockout ALS mice also showed an increased survival of 54 days and 11 days, respectively. Furthermore, a redoxdependent activation of rac1 modulated by SOD1 was identified for Nox activation (Harraz et al., 2008). This interaction was disrupted by ALS-associated mutations in SOD1, which was found to activate Nox to produce superoxide.
However, an earlier investigation into the role of Nox in ALS by Wu et al. (2006) observed Nox2 deletion in an ALS mouse model to be less protective on lifespan. Deletion of Nox2 (gp91phox) in the congenic C57BL/6J transgenic SOD1G93A mouse model of ALS only increased survival by 15 days. The difference in strain background, hybrid B6SJL versus congenic C57BL/6J, of the transgenic SOD1G93A-ALS mice was suggested to partially account for the discrepancy in survival (Marden et al., 2007). Engelhardt's group also observed the occurrence of lethal eye infections only in the Nox2 knockout transgenic SOD1G93A-ALS mice, which might contribute to the discrepancy in survival as well with Przedborski's study (Marden et al., 2007). The eye infections manifested by accumulated secretion that occurred 2-3 weeks before the onset of disease symptoms and resulted in death within 1 week. Cultures of eye secretions tested positive for Staphylococcus aureus and histopathology analysis identified abnormalities in two ocular glands. About 75% of these affected mice were successfully treated with antibiotics, ceftazidime and gentamycin. These two antibiotics were reported to have no effect on the progression or survival of SOD1G93A-ALS mice expressing Nox1 and Nox2 (Marden et al., 2007).
No therapeutic treatment has been found to delay the progression of motor neuron degeneration by more than 2-3 weeks in the high-expressing SOD1G93A-ALS mouse model. However, a subsequent study by the Engelhardt group reported a similar significant protection in survival of the hybrid B6SJL SOD1G93A-ALS mice with apocynin (Harraz et al., 2008), a widely used putative inhibitor of Nox (van den Worm, 2001; Stefanska and Pawliczak, 2008). A high dose of 300 mg/kg/day apocynin administered beginning at 14 days increased survival by a remarkable 113 days over controls and delayed disease onset and progression. Lower dosages of apocynin started at 14 days old, 150 mg/kg/day and 30 mg/kg/day, showed impressive extension of lifespan as well, 81 days and 56 days respectively. Administration of 300 mg/kg/day apocynin at a later age, beginning at 60 and 80 days old, extended lifespan to a lesser extent, 38 and 13 days, respectively. Eye infections were also observed in the apocynin-treated ALS mice prior to the development of disease symptoms.
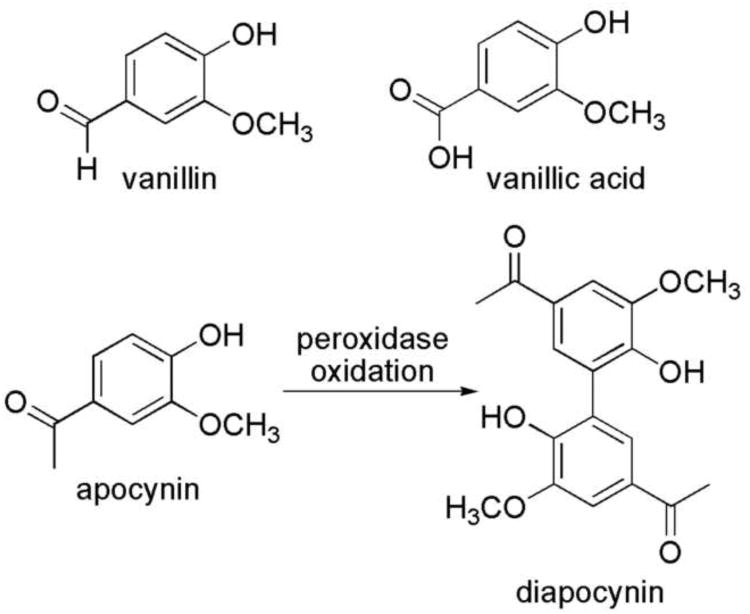
Apocynin, also known as acetovanillone, is a plant-derived ortho-methoxy substituted catechol, which has shown to exhibit anti-inflammatory properties attributed to Nox inhibition (van den Worm, 2001; Stefanska and Pawliczak, 2008). In activated human neutrophils, apocynin is reported to have a 10 μM inhibitory concentration at 50% (IC50) on Nox (Simons et al., 1990). Apocynin is proposed to inhibit the assembly of the activated Nox complex (Stolk, et al., 1994; Ximenes et al., 2007). The mechanism is still unclear but diapocynin, a covalent dimer of apocynin, appears to be the activated metabolite of apocynin produced during neutrophil activation by myeloperoxidase-mediated oxidation (Fig. 1) (Stolk, et al., 1994; Johnson et al., 2002; Steffen et al., 2008).
Figure 1.
Structures of vanillin, vanillic acid, apocynin, and diapocynin -- the covalent dimer of apocynin.
We sought to compare the efficacy of diapocynin to apocynin at extending the survival in the hybrid B6SJL SOD1G93A-ALS mouse model. Administration of diapocynin could potentially offer greater protection than apocynin if it is the active metabolite of apocynin in vivo. Administration of diapocynin would remove the necessity for peroxidase involvement that might limit diapocynin formation in vivo. As a potential therapeutic agent, diapocynin should be more likely to cross the blood-brain barrier due to its greater hydrophobicity and potentially could be efficacious at lower dosages.
Materials and Methods
Chemicals and reagents
Acetovanillone (Apocynin), ferrous sulfate heptahydrate, sodium persulfate, vanillic acid and other reagents were purchased from Sigma-Aldrich in the highest purity available. Reagents and primers for PCR were purchased from Invitrogen. Cell culture media and supplements were from GIBCO (Life Technologies). Glial Cell Line-Derived Neurotrophic Factor (GDNF) was purchased from R&D Systems and NOC-18 from Alexis (Enzo Life Sciences).
Synthesis of diapocynin
Diapocynin was synthesized by oxidative coupling of apocynin and adapted from a literature protocol (Wang et al., 2008). Apocynin, 4 g, was added to deionized water, 400 ml, in an Erlenmeyer flask and heated while stirring until solution was gently boiling. Addition of ferrous sulfate heptahydrate (0.30 g), followed by sodium persulfate (3.2 g) to generate sulfate radical in situ as the oxidizing agent, resulted in the formation of a brown precipitate. The solution was allowed to cool for 10 min and then filtered. The precipitate was dissolved in 3M NH4OH and then reprecipitated with 6M HCl. The diapocynin precipitate was filtered, washed with boiling water, 3 × 150 ml, followed by boiling methanol, 3 × 150 ml. Diapocynin (3.1 g, 77% yield) was dried in vacuo. 1H NMR (Bruker AC 300 MHz, DMSO-d6): 2.49 (CH3), 3.90 (OCH3), 7.45 & 7.46 (aromatic CH), 9.47 (OH) ppm. MS (ESI): [M-H]−m/z = 329.1.
Animal models
Animal procedures and experiments were performed in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and approved by the Oregon State University and the Medical College of Wisconsin Animal Care and Use Committees. Hemizygous transgenic male rats overexpressing human mutant SOD1G93A, NTac:SD-Tg(SOD1G93A)L26H purchased from Taconic (Germantown, NY), were bred with wild-type Sprague-Dawley females, NTac:SD to obtain rat embryos for motor neuron cultures. Male transgenic mice overexpressing human mutant SOD1G93A, B6SJL-Tg(SOD1-G93A)1Gur/J, and the nontransgenic wildtype B6SJLF1/J females were purchased from the Jackson Laboratory (Bar Harbor, ME). The mouse line was maintained by breeding hemizygous SOD1G93A males with wildtype B6SJLF1/J females. When indicated, specific generations of transgenic SOD1G93A male breeder, F2, F6 and F7, were mated with F1 nontransgenic female breeders as well as littermate matched F7 nontransgenic females bred with F7 transgenic SOD1G93A male breeders. Transgenic offspring were identified by PCR with tail DNA using primers specific for human SOD1. This high expressing transgenic ALS mouse line develops disease onset at about 100 days of age and reach clinical death by about 125 days of age (Gurney et al., 1994). Mice were housed in a control environment with free access to food and water. Starting at 60 days of age until the age of clinical death, mice were weighted and motor performance evaluated by paw grip endurance twice a week (Weydt et al., 2003). During end-stage illness, advanced symptomatic mice were individually housed and provided gruel with water soaked chow fines twice a day. Clinical death was determined as the age when the mice could no longer right themselves within 15 seconds of being place on their side and were euthanized.
Primary motor neuron cultures and treatments
Motor neurons were prepared as previously described (Cassina et al., 2002; Henderson et al., 1995). Rat embryos on embryonic day 15 were genotyped by PCR and cultures of SOD1G93A motor neurons and non-transgenic were carried out in parallel. Briefly, the dorsal horns of spinal cords were dissected and incubated in 0.05% trypsin for 15 minutes at 37°C, followed by mechanical dissociation. Motor neurons were then purified by centrifugation on an Optiprep cushion, followed by isolation of p75NTR expressing motor neurons by immunoaffinity selection with the IgG 192 monoclonal antibody. Motor neuron cultures were plated at a density of 300 cells/cm2 in 24 well plates precoated with polyornithine and laminin and maintained in Neurobasal media supplemented with 2% horse serum, 25 μM L-glutamate, 25 μM 2-mercaptoethanol, 500 μM L-glutamine, and 2% B-27 supplement at 37°C in a 5% CO2 humidified atmosphere (Cassina et al., 2002; Henderson et al., 1995). Survival was maintained by the addition of GDNF (1 ng/ml). Two hours after plating apocynin or diapocynin were added from 200X stock solutions prepared in DMSO and half an hour later DETANONOate (20 μM) was added to generate a low steady state concentration of nitric oxide (∼100nM). DETANONOate was resupplied after 24 hours.
Motor neuron survival was assessed after 48 hours of treatment by counting all phase-bright cells with intact neurites longer than 4 body diameters in 2 diameters of the well. Values were expressed as percentage of the number of motor neurons present in control wells maintained with GDNF.
Treatment of ALS mice
Apocynin-treated water was prepared following Engelhardt's protocol, method A (Harraz et al., 2008). Apocynin was added to warm sterile water, about 60°C, at a concentration of 1mg/ml, 2 mg/ml or 5 mg/ml and stirred. Treatment water was allowed to cool to room temperature before administered to mice. When indicated, apocynin was alternately prepared, method B, by its addition to sterile water and heated to 60°C while stirred, then cooled to room temperature. A concentration of 1 mg/ml translates to a dose of about 150 mg/kg/day, 2 mg/ml about 300 mg/kg/day and 5 mg/ml about 750 mg/kg/day based on an average ALS mouse, weighing between 25-30 grams, consumes on average 4 ml water/day (Bachmanov et al., 2002). Treatment was administered at 21 or 100 days of age to littermate matched control ALS mice.
Apocynin-antibiotic treatment was prepared by adding 0.1 mg/ml gentamycin and 0.1 mg/ml ceftazidime to the cooled apocynin treatment water. For this treatment group of mice, animals were switched from regular apocynin water to apocynin-antibiotic water at 100 days of age. The same concentration of antibiotics were added to sterile water and administered at 100 days of age to the littermate matched control mice for this group.
Diapocynin requires dilute buffering to dissolve in water therefore at a concentration of 1 mg/ml diapocynin was added to sterile water and dissolved by increasing to pH ∼10 with 1M NaOH. Once fully dissolved, 1M HCl was added to the solution until pH 7.6-7.8, for a concentration of 10 mM saline in the diapocynin treatment. Control treatment for littermate matched mice was prepared by making a 10 mM saline solution with the same pH as diapocynin treatment. Diapocynin treatment, which translates to a dosage of 150 mg/kg/day, or saline treatment was administered at 21 days or 100 days of age to littermate matched control mice.
Vanillic acid treatment was prepared by adding 0.5 mg/ml or 1 mg/ml to sterile water and heated to 60°C. Once dissolved, it was allowed to cool to room temperature before administered to littermate matched control mice. A concentration of 0.5 mg/ml translates to a dosage of 75 mg/kg/day and 1 mg/ml to 150 mg/kg/day.
Treatment water was replaced weekly. Mice were provided gruel with chow fines soaked in treatment or control water during advanced clinical symptoms.
Tissue preparation and treatment extraction
Mice at the age of clinical death were euthanized by isofluorane intoxication then transcardially perfused with 20 ml of heparinized-saline solution. Brain and spinal cord tissues were removed, flash frozen and stored at −80°C until analysis.
Extraction of treatment compounds was adapted from literature protocol (Wang et al., 2008). Tissue was homogenized in 10 volumes of 17 mM NH4OH, pH 8.0. Ammonium acetate buffer, 1.0 M at pH 5.0, at 0.3 volume, was added to aliquots of tissue homogenates. In trial experiments to hydrolyze potential glycosyl linkages to apocynin or diapocynin, samples were incubated overnight at 37 °C with 10 units/ml β-glucuronidase (Sigma) or without. Samples were centrifuged at 13,000 rpm for 20 min and the supernatant removed. Organic extraction of the supernatant with 3 volumes chloroform-methanol (3:1) followed by centrifugation at 3,000 rpm for 20 min. An aliquot of the upper aqueous layer was removed for HPLC-MS analysis.
HPLC-MS Tissue Analysis
The HPLC-MS data for apocynin and diapocynin was acquired on a Perkin-Elmer Sciex API 365 triple quadrupole mass spectrometer (Applied Biosystems, Foster City, CA) in negative ion mode using turbo ion spray. Chromatographic separations were performed with a Shimadzu Prominence CBM-20 HPLC (Shimadzu Scientific Instruments, Columbia, MD) with auto sampler and equipped with a XTerra MS C8 (2.5 μm, 2.1 × 50 mm) column. Analyses were run in isocratic mode with methanol-acetonitrile (35:65) with 0.1 % formic acid at 0.2 ml/min flow rate for 3 min. Multiple reaction monitoring analysis of two transitions was conducted for apocynin, 165.1-150.0 m/z, and diapocynin, 329.1-313.1 m/z. The corresponding peaks were identified using Q1 full scan comparing retention times and mass spectra of apocynin and diapocynin with those obtained for the standards. Standards were prepared by spiking non-treated control tissue to account for observed matrix suppression at varying concentrations. Minor background noise in non-spiked non-treated control tissue was adjusted for in reported values of treated tissue.
Statistics
Reported values for the motor neuron culture studies are the mean ± SEM of at least 3 independent experiments carried out in triplicates. Statistical analysis was performed by one-way ANOVA, followed by a Fisher's LSD test using KaleidaGraph (Synergy Software). Kaplan-Meier survival curves were generated using SigmaPlot 11 and compared using the long-rank test. Body weight analysis and paw grip endurance test analysis are reported as the mean ± SD using KaleidaGraph and statistical analysis was performed by Student's t-test. A P-value less than 0.05 was considered statistically significant.
Results
Motor Neuron Culture Experiments
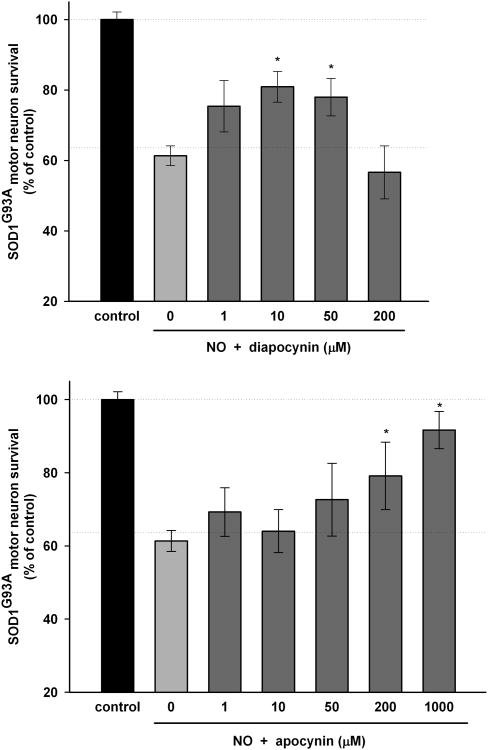
Motor neurons isolated from transgenic SOD1G93A rat models of ALS in primary culture display increased sensitivity to nitric oxide (Sahawneh et al., 2010). About 40% of SOD1G93A motor neurons underwent apoptosis after 48 hours, as previously reported (Sahawneh et al., 2010), when exposed to a steady state concentration of 80 nM nitric oxide (Fig. 2). Both diapocynin and apocynin were effective at preventing motor neuron death after nitric oxide treatment. Diapocynin was protective at lower concentrations, between 10 to 50 μM, but appeared to be toxic to motor neurons at a higher concentration of 200 μM (Fig. 2a). Apocynin was protective at higher concentrations between 200 μM to 1 mM and did not appear to be toxic over the range tested (Fig. 2b).
Figure 2. Diapocynin and apocynin protect SOD1G93A primary motor neurons against nitric oxide mediated death in cell culture.
In transgenic SOD1G93A motor neurons, nitric oxide treatment causes death of about 40% of motor neurons after 48 hours. (A) Survival of motor neurons pretreated with 1, 10, 50 or 200 μM diapocynin before exposure to nitric oxide. (B) Survival of motor neurons pretreated with 1, 10, 50, 200 or 1000 μM apocynin before exposure to nitric oxide. Data are expressed as percentage of control, mean ± SEM from at least 3 independent experiments. *p<0.05, significantly different from motor neurons treated with nitric oxide only.
Diapocynin Administered at Weaning
No significant protective effect was observed at extending survival in either the hybrid B6SJL SOD1G93A male or female ALS mice when 150 mg/kg/day of diapocynin was administered beginning at 21 days of age, Table 1. Weight loss was mildly diminished and paw grip endurance mildly increased in the saline control groups compared to the diapocynin treated male and female groups (Supplementary Figure S1).
Table 1.
Survival analysis, dosage and start age of treatment versus control administered to the SOD1G93A mice.
| Treatment | Dosage (mg/kg/day) | Mean Survival ± SEM (sample number) | Δ Survival | Trial | |
|---|---|---|---|---|---|
| Treated | Control | ||||
| Diapocynin | |||||
| 21 day start | 150 | a1 | |||
| Male | 126 ± 2 (12) | 127 ± 2 (12) | − 1 | ||
| Female | 130 ± 3 (12) | 133 ± 3 (12) | − 3 | ||
|
|
|||||
| Total | 128 ± 2 (24) | 130 ± 2 (24) | − 2 | ||
| Diapocynin | |||||
| 100 day start | 150 | a1 | |||
| Male | 130 ± 2 (12) | 123 ± 2 (12) + | +7 (P=<0.007) | ||
| Female | 139 ± 2 (12) | 129 ± 2 (12) + | +10 (P=<0.003) | ||
|
|
|||||
| Total | 134 ± 2 (24) | 126 ± 2 (24) + | +8 (P=<0.001) | ||
| Apocynin - antibiotics | |||||
| 21 day start | 300 | a | |||
| Male | 129 ± 2 (12) | 127 ± 3 (12) | + 2 | ||
| Female | 130 ± 2 (12) | 131 ± 2 (12) | − 1 | ||
|
|
|||||
| Total | 130 ± 2 (24) | 129 ± 2 (24) | + 1 | ||
| Apocynin + antibiotics | |||||
| 21 day start | 300 | a2 | |||
| + 100 day start | + 15 | ||||
| Male | 131 ± 3 (12) | 126 ± 2 (12) | + 5 | ||
| Female | 131 ± 2 (12) | 132 ± 2 (12) | − 1 | ||
|
|
|||||
| Total | 131 ± 2 (24) | 129 ± 2 (24) | + 2 | ||
| Apocynin dose response | |||||
| 21 day start | |||||
| 150 | 126 ± 2 (12) | 128 ± 2 (13) | − 2 | b3 | |
| 300 | 133 ± 3 (14) | + 5 | |||
| 750 | 135 ± 2 (11) | 135 ± 3 (11) | 0 | b | |
| Apocynin | |||||
| 100 day start | 300 | a | |||
| Male | 130 ± 2 (12) | 127 ± 2 (12) | + 3 | ||
| Female | 134 ± 2 (12) | 131 ± 2 (12) | + 3 | ||
|
|
|||||
| Total | 132 ± 2 (24) | 129 ± 2 (24) | + 3 | ||
| Vanillic Acid | |||||
| 21 day start | b3 | ||||
| 75 | 127 ± 5 (6) | 128 ± 2 (6) | − 1 | ||
| 150 | 133 ± 4 (4) | + 5 | |||
Performed at Oregon State University.
Performed at Medical College of Wisconsin.
Control group was administered saline at the specified age.
Antibiotics started at 100 days of age for apocynin group and control group.
Randomized trial of 2 treatment dosages with 1 control group.
Diapocynin Administered at Disease Onset
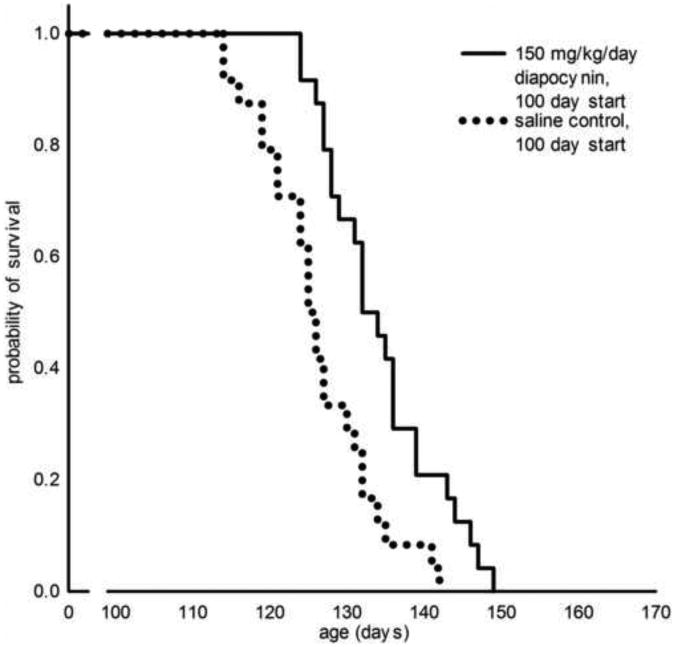
A statistically significant protective effect in mean survival of 8 days was observed in the SOD1G93A total male and female mice combined when 150 mg/kg/day of diapocynin was administered at 100 days of age at disease onset (Fig. 3 and Table 1). The protection was greater in female mice compared to male mice. Weight loss was mildly diminished and paw grip endurance mildly increased after 100 days of age for diapocynin treated male and female mice (Supplementary Figure S2).
Figure 3. Survival analysis of transgenic SOD1G93A mice treated with 150 mg/kg/day diapocynin starting at 100 days of age.
Kaplan-Meier probability of survival analysis shows total male and female mice combined per cohort administered diapocynin (134±2 days, n=24) compared to saline control (126±2, n=24), p<0.001.
Apocynin Plus Antibiotics Administration
In the diapocynin trial starting at 21 days, a parallel trial was conducted using apocynin with and without antibiotics to replicate the Harraz et al. (2008) experiments. Administration of 300 mg/kg/day apocynin started at 21 days of age had no protective effect on survival (Table 1). Weight loss was mildly diminished in apocynin treated males between 70-100 days. No other protection was observed in weight loss or paw grip endurance (Supplementary Figure S3).
No signs of lethal eye infections were observed in the mice prior to disease onset. However, accumulated eye discharge was observed in about 50% of treated and non-treated control ALS mice at the end-stage of disease, about 2-6 days prior to diagnosis of clinical death. In consultation with the veterinary care staff, the cause was attributed to decreased grooming due to compromised forelimb mobility and no evidence was found for eye infections. However, 17 eye culture tests and 3 necropsies were performed on advanced symptomatic male and female mice displaying eye discharge in various treatment or control groups. Necropsy results on two 21 day start apocynin treatment mice and one 100 day start apocynin treatment showed no significant findings and the ocular glands were normal. Eye swab culture tests identified several species from 9 eye swabs: unidentified yeast spp. (Candida albicans or Cryptococcus spp. were excluded), Enterococcus spp., Staphylococcus spp. (coagulase negative), Streptococcus spp. (alpha-hemolytic), Pasteurella spp., and Corynebacterium spp. The other eight eye cultures reported mixed Gram-positive bacteria with no significant bacteria isolated. There were no predominate species in any specific treatment or control group: Enterococcus spp. were isolated from a non-treated control mouse and a 21 day-start apocynin treated mouse; Streptococcus spp. from control and 21 day-start diapocynin mice; Staphylococcus spp. from control and 21 day-start diapocynin mice; Pasteurella spp. from control and 100 day-start apocynin mice.
Several β-lactam antibiotics have been shown to be neuroprotective in ALS mouse models in vitro (Rothstein et al., 2005). One of the antibiotics used in Engelhardt's study, ceftazidime, is a β-lactam antibiotic (Harraz et al., 2008). Although not every apocynin-treated mouse in Engelhardt's study was treated with antibiotics, only about 50% of the mice showed signs of eye infections, perhaps the ceftazidime as part of the antibiotic treatment was contributing some synergistic protective effect with apocynin treatment, since the antibiotic control group did not show any protection. Therefore, we proceeded to administer a group of apocynin-treated and non-treated control ALS mice with the same antibiotic mixture used in the Engelhardt study despite no signs of lethal eye infections.
At 100 days of age, the average age of disease onset, 0.1 mg/ml gentamycin and 0.1 mg/ml ceftazidime antibiotics were administered in the drinking water of both the apocynin-treated mice, 300 mg/kg/day started at 21 days of age, and the littermate control mice on non-treated water. Antibiotics were administered at the average age of onset due to minimal observed hind-limb impairment since Engelhardt's study reported eye infections occurred, therefore antibiotic treatment started, prior to disease development. A 5-day statistically non-significant extension in survival was observed for the male apocynin-antibiotic treatment group whereas no difference was observed in the female group (Table 1). Overall, antibiotic treatment had no significant effect on survival with or without apocynin. Weight loss was mildly diminished in apocynin-antibiotic treated males after 100 days of age until clinical death, while no difference was observed in the female group (Supplementary Figure S4). The male apocynin-antibiotic group exhibited a mild loss in paw grip endurance while the female apocynin-antibiotic group was mildly increased compared to the antibiotic control groups (Supplementary Figure S4).
Apocynin Administered at Disease Onset
A statistically non-significant 3-day extension in life was observed in male and female ALS mice when 300 mg/kg/day apocynin was administered in the drinking water starting at 100 days of age (Table 1). No difference was observed between treatment and control groups in body weight loss and the paw grip endurance test (Supplementary Figure S5).
Apocynin Dose Response
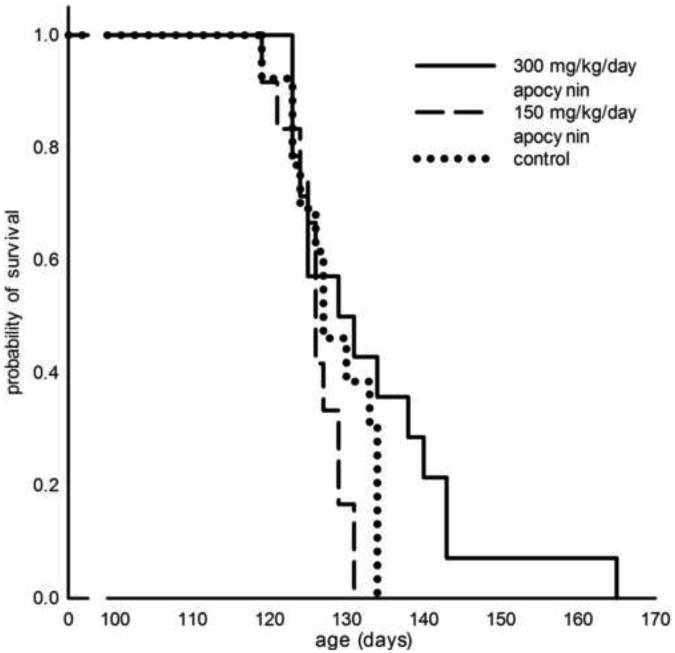
In the second independent study, apocynin was similarly not protective on the lifespan of the hybrid B6SJL SOD1G93A ALS mice when administered three different dosages. Apocynin treatment, 150 mg/kg/day and 300 mg/kg/day started at 21 days, showed no significant protection (Figure 4 and Table 1). An even higher dose of 750 mg/kg/day apocynin did not affect survival in the ALS mice (Table 1).
Figure 4. Survival analysis of apocynin treated SOD1G93A mice started at 21 days of age.
Kaplan-Meier probability of survival analysis shows total male and female mice combined per cohort given dosage of 150 mg/kg/day apocynin (126±2 days, n=12) or 300 mg/kg/day apocynin (133±3 days, n=14) compared to control (128±2 days, n=13). Mean survival of apocynin treated mice on 300 mg/kg/day appears augmented by one mouse that lived to 165 days, which may be due to a spontaneous drop in copy number of the G93A SOD1 transgene (Alexander et al., 2004).
Apocynin treatment in the previous experiments was prepared by the alternate method B (see materials and methods). To account for any difference that might be due to the way apocynin treatment was prepared compared to Engelhardt's method (method A)—even though HPLC-MS analysis of apocynin treatment by either method A or B was found to be identical—mice were treated with 300 mg/kg/day apocynin prepared by either method A or B at 21 days of age. No significant difference in survival was observed between the apocynin preparation groups (Table 2).
Table 2.
Survival analysis of apocynin treated SOD1G93A mice comparing preparation methods of apocynin and parent generations.
| Apocynin Treatment | Dosage (mg/kg/day) | Mean Survival ± SEM (sample number) | Trial |
|---|---|---|---|
| Preparation | 300 | b1 | |
| method A | 130 ± 2 (30) | ||
| method B | 133 ± 3 (14) | ||
| Father Generation | 300 | b2 | |
| F2 males | 133 ± 2 (28) | ||
| F6 males | 138 ± 3 (8) | ||
| Mother Generation | 300 | b3 | |
| F1 females | 132 ± 2 (21) | ||
| littermate females | 129 ± 3 (12) |
Performed at Medical College of Wisconsin.
See material & method for preparation methods.
F generations of hemizygous SOD1G93A male mice mated with F1 wildtype B6SJLF1/J females.
F generation of wildtype B6SJLF1/J females mated with F7 hemizygous SOD1G93A male mice, littermate females were F7 generation.
Varying Genetic Background on Apocynin Treatment
The genetic background in the hybrid B6SJL SOD1G93A mouse model can potentially drift through breeding, which can affect lifespan from transgene copy number drops and littermate clustering (Alexander et al., 2004; Heiman-Patterson et al., 2005; Scott et al., 2008). The hybrid B6SJL SOD1G93A mouse line is generally maintained by breeding a hemizygous transgenic male back to a wild-type B6SJL F1 female. To examine if protection by apocynin may be influenced by variability in genetic background in this mouse model and affect the extent of survival, two trials were conducted varying the generation of the parent male and parent female breeders. Apocynin treated offspring, 300 mg/kg/day at 21 days, of two generations of the transgenic SOD1G93A male breeder either in the F2 generation or F6 generation both mated with F1 wild-type female breeders, were compared. A 5-day statistically non-significant difference in survival was observed between apocynin-treated offspring (Table 2). Furthermore, apocynin treated offspring, 300 mg/kg/day at 21 days, of either wild-type F1 generation females or wild-type F7 generation littermate-matched female breeders were mated with F7 generation transgenic SOD1G93A male breeders. A 3-day statistically non-significant difference in the mean survival was observed between these apocynin treated offspring (Table 2).
Vanillic Acid Therapeutic Study
Vanillic acid is structurally related to apocynin (Fig. 1) and, although reported to be a weaker inhibitor of Nox in neutrophils (van den Worm et al., 2001), has been found to be a more potent inhibitor of Nox in human umbilical vein endothelial cell lysates (Steffen et al., 2008). Therefore, vanillic acid administration to SOD1G93A mice was investigated in a pilot study. No significant protective effect on survival was observed with vanillic acid treatment at two different dosages, 75 mg/kg/day and 150 mg/kg/day, starting at 21 days of age. A statistically non-significant 5 day difference was observed between the control group and 150 mg/kg/day vanillic acid treatment group (Table 1).
HPLC-MS Tissue Analysis of SOD1G93A-ALS Treated Mice
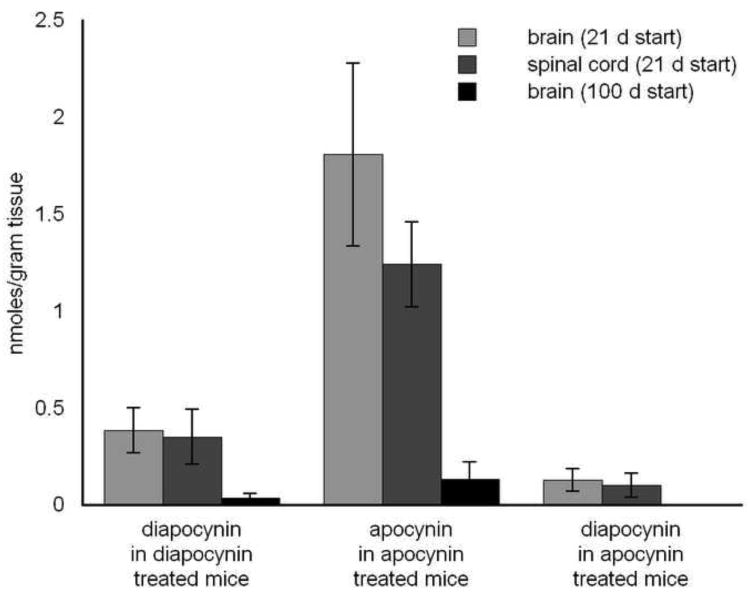
Diapocynin and apocynin were extracted from brain and spinal cord tissue from treated ALS mice and quantified by HPLC-MS analysis (Fig. 5). In treated mice started at 21 days, diapocynin was detected at levels about one-third to one-fifth of apocynin. In the treated mice started at 100 days, diapocynin and apocynin were only detected in brain tissue whereas treatment levels in the spinal cord fell within the margin of error and are not reported (Fig. 5). Diapocynin measured in the 100 day treated brain tissue was about 10 times lower than the 21 day treated brain tissue. Apocynin was about 14 times lower in the 100 day brain tissue compared to the 21 day brain tissue.
Figure 5. CNS tissue analysis by HPLC-MS using selective ion monitoring of mice administered 150 mg/kg/day diapocynin and 300 mg/kg/day apocynin started at 21 and 100 days of age.
Diapocynin and apocynin were measured in brain and spinal cord tissue of treated mice. Diapocynin was detected in tissue of apocynin treated mice started at 21 days of age. Treatment was not detected in spinal cord tissue when administered at 100 days of age. Measurements represent mean ± SD from at least 6 samples and reported in nmoles of treatment per gram of tissue.
Diapocynin was detected in brain and spinal cord tissue from apocynin treated mice started at 21 days old, showing apocynin was converted into diapocynin in vivo and accumulated in the CNS (Fig. 5). The diapocynin concentration measured was about 7-8% of the apocynin concentration in the corresponding tissue. To confirm diapocynin was converted in vivo and not during sample processing, control tissue samples were spiked with apocynin and processed similarly. The detected levels of diapocynin in apocynin spiked tissue fell within the margin of error. Therefore, the detected diapocynin in apocynin treatment was not an artifact formed during the processing of the samples.
Apocynin has been previously reported to form glycoconjugates as the major metabolite in vivo after i.p. administration (Wang et al., 2008). Samples of brain and spinal cord tissue from apocynin and diapocynin treated mice started at 21 days of age were incubated with β-glucuronidase, to hydrolyze the glycosyl linkage if present, and without. No significant difference was observed between the samples therefore this step was not continued in further processing of samples (data not shown).
Discussion
Apocynin has been experimentally used to inhibit superoxide production by Nox in Alzheimer's, Parkinson's, multiple sclerosis, amyotrophic lateral sclerosis, and stroke (Bedard and Krause, 2007; Sorce and Krause, 2009). It was originally isolated from the roots of Apocynum cannabinum in 1883 and found in a Himalayan plant, Picrorhiza kurroa, which has been a thousand year history of human use as part of traditional Indian Ayurvedic medicine. Because of the low toxicity and general lack of side effects, apocynin is an attractive therapeutic candidate for human patients suffering from ALS (van den Worm, 2001; Stefanska and Pawliczak, 2008).
In several different cell-based models, apocynin has been shown to protect motor neurons from mutant SOD1 toxicity. Apparent superoxide production by Nox was reduced along with increased cell viability when apocynin was present in glial cells expressing mutant SOD1 (Harraz et al., 2008). Motor neurons derived from human embryonic stem cells co-cultured with human primary astrocytes expressing mutant SOD1 displayed an increased survival in the presence of apocynin (Marchetto et al., 2008). In SOD1G93A primary motor neurons, we found apocynin to be protective against nitric oxide-mediated death, albeit at concentrations that are far higher than can be achieved in vivo. Diapocynin was protective at lower concentrations that are closer to being achievable in the CNS. These results supported the investigation of diapocynin as a more potent therapeutic agent to treat ALS.
However, we were unable to show any significant extension in survival with diapocynin or apocynin treatment in the SOD1G93A mouse model. The only statistically significant protection was an 8-day increase in lifespan observed when 150 mg/kg/day of diapocynin was administered at disease onset at 100 days of age. The CNS concentrations of diapocynin were barely detectable in this group and less than the treatment groups that showed no extension in lifespan by administering either diapocynin or apocynin at 21 days of age. Possibly, later administration of diapocynin may have another action in muscle or some other alternative target. It is also possible that the slight protection obtained was a statistical fluke arising from the multiple trials conducted.
We were unable to find a basis for our failure to replicate the impressive protective effect by apocynin observed by Engelhardt's group at extending the survival in the ALS mouse model by 113 days (Harraz et al., 2008). Both studies used the same genetic background, the hybrid B6SJL line most commonly used with SOD1G93A mice. The earliest age apocynin treatment was started in our study was at 21 days of age, which was the minimum age for weaning in our experience. The Engelhardt study was started at 14 days old, though it is unclear whether the mice were weaned early. The 7-day difference in administration of apocynin might contribute to the lack in protective we observed. However, Harraz et al. (2008) report protection of 38 days even when the apocynin treatment was begun at 60 days of age.
Antibiotic treatments used to treat lethal eye infections could not account for the absence of protection. Although other groups have found that several β-lactam antibiotics can be neuroprotective in ALS-SOD1 mice, Harraz et al. (2008) found no therapeutic effect of the antibiotic treatment on ALS disease progression. We also found no therapeutic benefit. The β-lactam ceftriaxone delayed the loss of muscle strength and body weight and extended survival by 10 days in the hybrid B6SJL SOD1G93A-ALS mouse model (Rothstein et al., 2005). The related β-lactam, ceftazidime, was used in our trial at a dosage of 15 mg/kg/day, which was about 10 times lower than the dose of ceftriaxone, and started 2 weeks later than the studies showing protection from β-lactam treatment. However, we observed no evidence of eye infections in any of the treated ALS mice in studies conducted at two different institutions. Nevertheless, the same mixture and dosage of antibiotics in Engelhardt's study was not protective when administered to the apocynin-treated and littermate-matched controls.
Similar results for a lack of protection at extending survival with apocynin treatment in the SOD1G93A-ALS mouse model has been reported by the ALS Therapy Development Institute (ALS TDI, http://www.als.net). They used a dosage of 150 mg/kg/day apocynin started at two different ages, 30 and 50 days old. No statistically significant protection was observed in the treated mice. Even at 150 mg/kg/day apocynin (starting at age 14 days), Engelhardt's group reported substantial protection by apocynin by 81 days. A mild protection in the loss of body weight in apocynin treated mice was observed by the ALS TDI.
Only a few studies have examined the metabolites of apocynin in vivo (Daly et al., 1960; Gjertsen et al., 1988; Wang et al., 2008). Daly et al. (1960) and Gjertsen et al. (1988) reported comparable results with the majority of apocynin, about 80%, recovered in the urine as its glucuronide conjugate after i.p. administration of apocynin in rats. Small quantities of other metabolic conversion products of apocynin were also detected, the highest detected in 0.5% as the para-isomer, but diapocynin was not reported as an identified metabolite. These earlier studies were conducted before a conversion mechanism for apocynin to diapocynin in vivo was proposed. The more recent study by Wang et al. (2008) specifically focused on examining the potential formation of diapocynin in vivo after i.p. administration of 5 mg/kg apocynin in rats. Diapocynin was not detected by HPLC-MS in plasma or tissue as a metabolite of apocynin within 30 min to 2 h after administration. The glycoconjugate was found as reported in previous studies (Wang et al., 2008).
We were able to detect diapocynin and apocynin accumulation in low micromolar concentrations in the CNS by HPLC-MS-MS using selective ion monitoring. Diapocynin was also present in apocynin-treated mice at about 7-8% of the apocynin concentration. Thus, diapocynin may be a significant metabolite of apocynin even in diseases without significant neutrophil activation. To the best of our knowledge, this is the first report to measure diapocynin in the CNS in apocynin-treated mice. Diapocynin was only detected in the long term apocynin treated mice which ingested apocynin for about 100 days and may explain the contrasting data from previous studies which analyzed tissue shortly after i.p. injections of apocynin.
In summary, we have not been able to reproduce the previously reported dramatic neuroprotection by apocynin in the ALS mouse model in two separate laboratories. Reasons for this discrepancy are not clear, despite investigating dosage, antibiotics, gender, or the drift in the genetic background from breeding for multiple generations of both female and male breeders. Tissue analysis of brain and spinal cord confirmed both apocynin and diapocynin accumulated in the CNS to micromolar levels in the experimental animals.
Despite the difference in efficacy, apocynin may still be found to be protective in human ALS patients. Results from our study along with supporting evidence from other in vitro studies, stress the importance of testing diapocynin when examining the potential therapeutic properties of apocynin. Although therapeutic protection in ALS animal models has not yet been effectively translated into successful therapeutics in human ALS patients, meta-analysis suggests drugs with anti-inflammatory and antioxidant actions appear to be promising (Benatar, 2007; Barber and Shaw, 2010). Therapeutic drugs that exert these properties have been more effective when administered at disease onset, which is when diapocynin was modestly effective in our study. More studies are clearly needed before embarking on human clinical trials using apocynin supplementation. Our results do not rule out a role of Nox isozymes in the pathogenesis of ALS because apocynin and diapocynin are at best only modest Nox inhibitors. Knockout of NOX has been shown to modestly increase the survival of ALS-mutant SOD transgenic mice (Wu et al., 2006). Hence, the development of more potent Nox inhibitors is worthy of further investigation in ALS.
Supplementary Material
Highlights.
Diapocynin formed from apocynin is a putative inhibitor of NOX
Diapocynin was more protection in primary motor neurons than apocynin.
Neither apocynin nor diapocynin extended life in SOD1G93A-ALS mice.
We could not repeat the reported remarkable protection by apocynin.
Acknowledgments
We thank the Laboratory Animal Resources Center (LARC) staff at Oregon State University for performing mice necropsies and eye culture tests, Kaitlyn Kliman for aid in monitoring mice and Mark Levy for aid with motor neuron cultures. This work was financially supported in part by funding from the National Institute of Health grants NCCAM T32 AT002688 (KAT), NIEHS P30ES000210, NINDS R01NS058628A, and NCCAM P01AT002034, as well as the Amyotrophic Lateral Sclerosis Association (JSB).
Abbreviations
- ALS
amyotrophic lateral sclerosis
- SOD1
copper, zinc superoxide dismutase
- Nox
NADPH oxidase
- CNS
central nervous system
Footnotes
Appendix A. Supplementary data
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GM, Erwin KL, Byers N, Deitch JS, Augelli BJ, Blankenhorn EP, Heiman-Patterson TD. Effects of transgene copy number on survival in the G93A SOD1 transgenic mouse model of ALS. Brain Res Mol Brain Res. 2004;130:7–15. doi: 10.1016/j.molbrainres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32:435–443. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber SC, Shaw PJ. Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med. 2010;48:629–641. doi: 10.1016/j.freeradbiomed.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause K. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Benatar M. Lost in translation: treatment trials in the SOD1 mouse and in human ALS. Neurobiol Dis. 2007;26:1–13. doi: 10.1016/j.nbd.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Cassina P, Peluffo H, Pehar M, Martinez-Palma L, Ressia A, Beckman JS, Estevez AG, Barbeito L. Peroxynitrite triggers a phenotypic transformation in spinal cord astrocytes that induces motor neuron apoptosis. J Neurosci Res. 2002;67:21–29. doi: 10.1002/jnr.10107. [DOI] [PubMed] [Google Scholar]
- Daly JW, Axelrod J, Witkop B. Dynamic aspects of enzymatic O-methylation and - demethylation of catechols in vitro and in vivo. J Biol Chem. 1960;235:1155–1159. [PubMed] [Google Scholar]
- Gjertsen FB, Solheim E, Scheline RR. Metabolism of aromatic plant ketones in rats: acetovanillone and paeonol. Xenobiotica. 1988;18:225–234. doi: 10.3109/00498258809041658. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, Nelson K, Luo M, Paulson H, Schöneich C, Engelhardt JF. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 2008;118:474–478. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman-Patterson TD, Deitch JS, Blankenhorn EP, Erwin KL, Perreault MJ, Alexander BK, Byers N, Toman I, Alexander GM. Background and gender effects on survival in the TgN(SOD1-G93A)1Gur mouse model of ALS. J Neurol Sci. 2005;236:1–7. doi: 10.1016/j.jns.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Bloch-Gallego E, Camu W. Purification and culture of embryonic motor neurons. Oxford: IRL Press; 1995. [Google Scholar]
- Johnson DK, Schillinger KJ, Kwait DM, Hughes CV, McNamara EJ, Ishmael F, O'Donnell RW, Chang MM, Hogg MG, Dordick JS, Santhanam L, Ziegler LM, Holland JA. Inhibition of NADPH oxidase activation in endothelial cells by ortho-methoxy-substituted catechols. Endothelium. 2002;9:191–203. doi: 10.1080/10623320213638. [DOI] [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Marchetto MCN, Muotri AR, Mu Y, Smith AM, Cezar GG, Gage FH. Non-cell-autonomous effect of human SOD1G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3:649–657. doi: 10.1016/j.stem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Marden JJ, Harraz MM, Williams AJ, Nelson K, Luo M, Paulson H, Engelhardt JF. Redox modifier genes in amyotrophic lateral sclerosis in mice. J Clin Invest. 2007;117:2913–2919. doi: 10.1172/JCI31265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Hoberg MD, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su Z, Gupta P, Fisher PB. B-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Sahawneh MA, Ricart KC, Roberts BR, Bomben VC, Basso M, Sahawneh J, Franco MC, Beckman JS, Estévez AG. Cu,Zn-superoxide dismutase increases toxicity of mutant and zinc-deficient superoxide dismutase by enhancing protein stability. J Biol Chem. 2010;285:33885–33897. doi: 10.1074/jbc.M110.118901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S, Kranz JE, Cole J, Lincecum JM, Thompson K, Kelly N, Bostrom A, Theodoss J, AlNakhala BM, Vieira FG, Ramasubbu J, Heywood JA. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph Lateral Scler. 2008;9:4–15. doi: 10.1080/17482960701856300. [DOI] [PubMed] [Google Scholar]
- Simons JM, ‘t Hart BA, Ip Vai Ching TRAM, Dijk HV, Labadie RP. Metabolic activation of natural phenols into selective oxidative burst agonists by activated human neutrophils. Free Radic Biol Med. 1990;8:251–258. doi: 10.1016/0891-5849(90)90070-y. [DOI] [PubMed] [Google Scholar]
- Sorce S, Krause K. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- Stefanska J, Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm. 2008;2008:106507. doi: 10.1155/2008/106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen Y, Gruber C, Schewe T, Sies H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch Biochem Biophys. 2008;469:209–219. doi: 10.1016/j.abb.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- van den Worm E, Beukelman CJ, Van den Berg AJJ, Kroes BH, Labadie RP, Van Dijk H. Effects of methoxylation of apocynin and analogs on the inhibition of reactive oxygen species production by stimulated human neutrophils. Eur J Pharmacol. 2001;433:225–230. doi: 10.1016/s0014-2999(01)01516-3. [DOI] [PubMed] [Google Scholar]
- van den Worm Edwin. Investigation of apocynin, a potent NADPH oxidase inhibitor PhD dissertation. Utrecht University; 2001. [Google Scholar]
- Wang Q, Smith RE, Luchtefeld R, Sun AY, Simonyi A, Luo R, Sun GY. Bioavailability of apocynin through its conversion to glycoconjugate but not to diapocynin. Phytomedicine. 2008;15:496–503. doi: 10.1016/j.phymed.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weydt P, Hong SY, Kliot M, Möller T. Assessing disease onset and progression in the SOD1 mouse model of ALS. Neuroreport. 2003;14:1051–1054. doi: 10.1097/01.wnr.0000073685.00308.89. [DOI] [PubMed] [Google Scholar]
- Wu DC, Ré DB, Nagai M, Ischiropoulos H, Przedborski S. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc Natl Acad Sci USA. 2006;103:12132–12137. doi: 10.1073/pnas.0603670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ximenes VF, Kanegae MP, Rissato SR, Galhiane MS. The oxidation of apocynin catalyzed by myeloperoxidse: proposal for NADPH oxidase inhibition. Arch Biochem Biophys. 2007;457:134–141. doi: 10.1016/j.abb.2006.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.