Abstract
Osteosarcoma (OS) is one of the most common malignant bone tumors in early adolescence. Multi-drug chemotherapy has greatly increased the five year survival rate from 20% to 70%. However, the rate has been staggering for 30 years and the prognosis is particularly poor for patients with recurrence and metastasis. Our study aimed to investigate the role of Wnt-β-catenin, Notch and Hedgehog pathway in OS development because all these pathways are involved in skeletal development, tumorigenesis and chemoresistance. Our results showed that the major components in Wnt-β-catenin pathway, e.g. Wnt3a, β-catenin and Lef1, were consistently upregulated in human osteosarcoma cell line Saos2 cells compared to human fetal osteoblasts (hFOB), whereas the changes in the expression levels of Notch and Hh signaling molecules were not consistent. Knocking down β-catenin increased the Saos2 sensitivity to methotrexate (MTX) induced cell death. Consistently, the expression level of β-catenin protein correlated with the invasiveness of OS, as evidenced by more intensive β-catenin immunoreactivity in higher grade OS samples. Chemical inhibition of the Wnt-β-catenin signaling enhanced MTX mediated death of Saos2 cells. A synergistic effect with MTX was observed when both inhibitors for Wnt-β-catenin and Notch pathways were simultaneously used, while the addition of the Hh inhibitor did not further improve the efficacy. Our findings provide some novel insight to OS pathogenesis and lay a foundation for future application of Wnt-β-catenin and Notch inhibitors together with the currently used chemotherapeutic drugs to improve the outcome of OS treatment.
Keywords: Osteosarcoma, Catenin, Notch, Pathway, Methotrexate, Apoptosis
1. Introduction
Osteosarcoma (OS) is one of the most common malignant bone tumors in childhood and adolescence and the second leading cause of mortality in this age group. Before the advent of multi-agent chemotherapy, 90% of patients died of pulmonary metastasis even after radical surgery such as amputation [1–3]. Due to its life-threatening nature, the relatively low incidence does not fully reflect the real burden of OS on patients and communities. In fact, the disability adjusted life year (DALY) is particularly high in OS patients (17 vs 6.5 years in breast cancer patients), largely due to extensive chemotherapy, destructive surgery and prolonged rehabilitation/follow-ups. Preoperative inductive (neoadjuvant) and postoperative immunogenic (adjuvant) chemotherapy have significantly increased the 5-year survival rate from 20% to 70%. However, the survival rate has remained almost unchanged for three decades since the introduction of multi-drug chemotherapy. It must also be emphasized that chemotherapy alone is not an option, and complete removal of local OS lesion is critical for event-free survival (EFS) [4–7].
A well-established and the most-commonly used chemotherapeutic modality is referred to as MAP, e.g. methotrexate (MTX, M), adriamycin or doxorubicin (A), and platin-derived drugs such as cisplatin (P). Sometimes ifosfamide or other drugs are used with MAP. Recent attempts to further improve survival rate by adding more drugs or increasing the dose of individual drug have been disappointing with the survival rate stagnating around 70%. Increased dose and length of treatment often cause complications and toxicities in various organs, some of which are fatal. The most cumbersome problem in OS treatment is the lack of response to chemotherapy [8–11]. (chemoresistance) in patients with recurrence and metastasis. Not surprisingly, these patients have a very poor prognosis with 5-year survival rates as low as about 20% [12]. Thus, it is imperative to develop novel therapeutic agents that can enhance the efficacy and reduce the toxicity of the currently used drugs. These new agents are expected to work synergistically with the MAP drugs to circumvent the recurrence and metastasis of OS and thus significantly improve the survival rate. Obviously, this goal can only be achieved by a better understanding of the mechanisms underlying its pathogenesis, progression, invasiveness, relapse, metastasis and chemoresistance.
Wnt-β-catenin signaling pathway plays an important role in tumorigenesis, bone development and stem cell biology. Its aberrant activation has been linked to the pathogenesis of various tumors in human. Previous studies have demonstrated that the major molecular components of this pathway are detected in OS cells/samples and abnormal activation of the Wnt signaling plays a role in OS pathogenesis. However, the observations were not always consistent. Indeed, a recent study showed that the Wnt-β-catenin pathway was inactivated in OS samples and that its activation in OS cells inhibited proliferation and induced differentiation. A conclusion was thus reached that the loss of Wnt-β-catenin activity contributed to OS development [13,14]. Besides the Wnt-β-catenin pathway, other pathways may also be involved in OS pathogenesis. Among them, Notch and Hedgehog (Hh) pathways are of particular interests because of their role in cell fate, stemness and carcinogenesis [15,16].
Our study aimed to compare the expression profiles of major molecules in Wnt-β-catenin, Notch and Hh pathways between human OS cell line (Saos2 cells) and human fetal osteoblasts (hFOB). The expression of tumorigenic and angiogenic molecules was also examined in these two cell lines. We then modulated the β-catenin level in Saos2 cells and examined the effect on their apoptosis. After the inhibitors or activators of different signaling pathways were used together with MTX, and the apoptosis and necrosis of tumor cells were investigated. Our results showed that all major molecules in Wnt-β-catenin pathway were upregulated in Saos2 cells. Knocking down β-catenin increased the apoptotic effect of MTX on Saos2 cells. Furthermore, the level of β-catenin was closely related to the invasiveness of OS. Blocking Wnt-β-catenin pathway enhanced MTX effect and a synergistic effect was observed when both Wnt-β-catenin and Notch inhibitors were used together with MTX. Adding Hh inhibitor did not further improve the efficacy.
2. Materials and methods
2.1. Real time quantitative PCR (RT-PCR)
Total RNA was harvested from the cultured cells using a kit from Qiagen (Valencia, CA). Reverse transcription was performed using a kit from Invitrogen (Grand Island, NY). RT-PCR was performed using Sybergreen from Qiagen and relevant primers (Supplementary table).
2.2. Western blotting
The cells were lysed with golden lysis buffer. Western blotting analysis was performed using an Invitrogen system and chemilu-minescence was performed using West Femto Substrate (Pierce, Rockford, IL). The following antibodies were used: total β-catenin (Cell Signaling Technology, Danvers, MA), active β-catenin (Millipore, Billerica, MA), active caspase 3 (Millipore) and β-actin (Sigma, St. Louis, MO).
2.3. Luciferase assay
The following reporter constructs were transfected with Lipofectamine 2000 (Invitrogen) into Saos2 cells: Topflash-Luc for Wnt-β-catenin, RBPjK-Luc for Notch, and Gli2-Luc for Hh pathway. The following reagents were added 24 h after transfection: inhibitors for Wnt-β-catenin CCT036477 (CCT, Enzo, Famingdale, NY), DAPT for Notch pathway (Sigma), GANT61 (GANT, Enzo) for Hh pathway; the Wnt-β-catenin activators BIO (Sigma) and DKK1 neutralizing antibody (DKK1AB, Amgen, Thousand Oaks, CA), and the Hh activator purmorphamine (PUR, Calbiochem, Billerica, MA). Each group was triplicated and Luciferase assay was performed 24 h after treatment.
2.4. Immunohistochemistry (IHC)
Human OS samples (Grade 2: n = 4, Grade 3: n = 4) were collected before the initiation of neoadjuvant chemotherapy after the approval by the Ethic Committee of Nanjing Medical University, China. After antigen retrieval and blocking of non-specific signal, the sections were incubated with an antibody against total β-catenin (Cell Signaling). Color reaction was developed using a kit from Vector. The intensity of the immunoreactivity of total β-catenin was compared between Grade2 and Grade 3 samples.
2.5. Apoptosis and necrosis assay
This assay was performed using a Dead Cell Apoptosis Kit (Invitrogen) containing recombinant Annexin V conjugated to FITC and a ready-to-use solution of the red-fluorescent propidium iodide (PI) nucleic acid binding dye. PI dye is excluded from live and apoptotic cells, but penetrates and stains the dead cells. After treatments, Saos2 cells were harvested and washed with cold PBS. The cells were resuspended in 96-plates with 100 μl binding buffer, and incubated with 5 μl FITC annexin V and 1 μl PI working solution for 15 min at room temperature. The cells were washed with annexin-binding buffer, and fluorescence was observed using appropriate filters. Apoptotic cells exhibited very intensive Annexin V staining. Dead cells showed both membrane staining by Annexin V and strong nuclear PI staining.
3. Results
3.1. Aberrant expression of Wnt-β-catenin, Notch and Hh signaling molecules in Saos2 cells
RT-PCR analysis was performed for the comparison of the expression levels of the Wnt-β-catenin pathway components between hFOB and Saos2 cells. Major molecules of this pathway, including Wnt3 (5.5 folds), β-catenin (5.3 folds) and LEF1 (7.6 folds) were upregulated in Saos2 cells compared to hFOB (Fig. 1A). Western blotting analysis confirmed that the protein levels of both total and active β-catenin were increased in Saos2 cells compared to hFOBs (Fig. 1B). Compared to hFOB, Saos2 cells expressed higher levels of Indian Hh (Ihh, 6.9 folds), Sonic Hh (Shh, 11.4 folds), and Smoothened (Smo, 5.2 folds). However, the expression of transcription factor Gli2 (0.4 fold) was decreased (Fig. 1C). Regarding Notch pathway, the expression of the ligand Jagged1 was slightly increased, but the expression of the surface receptors Notch1 (0.4 fold) and the cleaved Notch receptor intracellular domain (NICD, 0.3 fold) was downregulated in Saos2 cells. In contrast to slight changes in the expression of Notch 2 (0.7 fold) and RBPjK (2.2 folds), nearly 8 fold increase in a Notch target gene HES1 was detected in Saos2 cells (Fig. 1D).
Fig. 1.
The mRNA samples were harvested from cultured Saos2 and hFOB cells and RT-PCR was performed after reverse transcription with the relevant primers. Our results revealed significant upregulation of Wnt3 (5.5 folds), β-catenin (5.3 folds) and LEF1 (7.6 folds) in Saos2 cells compared to hFOB (A). Western blotting analysis confirmed that the proteins of both total and active β-catenin were increased in Saos2 cells compared to hFOBs (B). Saos2 cells showed upregulated expression of Ihh (6.9 folds), Shh (11.4 folds), and Smo (5.2 folds), and downregulated expression of Gli2 (0.4 fold) compared to hFOB (C). With regard to Notch pathway, our results showed a moderate increase of RBPjK mRNA (2.2 folds) expression in Saos2 cells compared to hFOB cells (D). However, the expression level of Notch 1 (0.4 fold) and NICD (0.3 fold) was slightly decreased in Saos2 cells. No significant differences were detected in the expression of Notch 2 and Jagged 1 between Saos2 cells and hFOBs. In contrast, the HES1 expression was significantly increased (near 8 fold) in Saos2 cells (D). β-Cat: β-catenin, T-β-Cat: total β-catenin; A-β-Cat: active β-catenin, Smo: Smoothened, NICD: Notch Intracellular Domain.
3.2. Expression profiles of the relevant molecules in Saos2 and hFOB
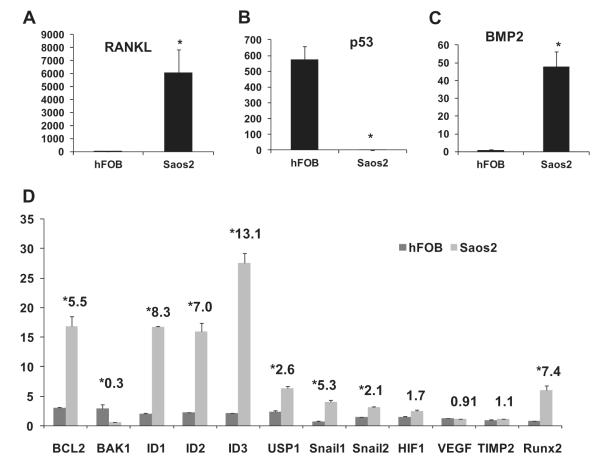
Saos2 cells expressed a very high level of RANKL (over 6000 fold increase vs hFOB), indicating their osteolytic feature (Fig. 2A). Saos2 cells also exhibited strong osteogenic nature as evidenced by a nearly 50 fold increase in BMP-2 (Fig. 2C) and over 7 fold increase in Runx2 expression compared to hFOBs (Fig. 2D). The tumorigenic feature of Saos2 cells was indicated by a very low level of p53 (over 570 fold decrease, Fig. 3B). Consistently, the expression level of anti-apoptotic gene BCL-2 was higher and pro-apoptotic gene BAK-1 lower in Saos2 cells. The products of ID genes (DNA binding protein inhibitors, ID1–ID3) prevent the binding of certain transcription factors to their target genes and therefore negatively regulate cell differentiation. Our results showed that ID1–3 expression levels were higher in Saos2 cells supporting their undifferentiated nature. The ubiquitination and degradation of ID proteins, a commonly seen phenomenon in differentiated tissues, was often lacking in malignant tumors. One of the responsible mechanisms for OS pathogenesis is the deubiquitination of ID proteins by enzyme USP1, which helps maintain the OS cell stemness. Our results showed a 2.6 fold increase in USP-1 expression in Saos2 cells. SNAIL proteins induce the epithelial-mesenchymal transition (EMT) and their expression correlates with OS invasiveness. RT-PCR revealed the increased expression of both Snail1 and Snail2 in Saos2 cells. However, the angiogenesis related genes that are closely involved in carcinogenesis did not show significant changes in Saos2 cells (Fig. 2D).
Fig. 2.
The molecules related to osteogenesis/osteolysis, tumorigenesis, apoptosis, stemness, angiogenesis and invasiveness were analyzed with RT-PCR and compared between Saos2 and hFOB cells. Saos2 exhibited a very high level of RANKL in Saos2 cells (over 6000 fold increase over hFOB cells, (A). Saos2 cells also expressed higher level of an osteogenic molecule BMP-2 and Runx2 over hFOB (C, D). Tumorigenic feature of the Saos2 cells was demonstrated as a very low level of tumor suppressor gene p53 (near 580 folds lower than in hFOBs, (B). Consistently, Saos2 cells expressed a higher level of BCL-2 and lower level of BAK-1 (D). Saos2 cells showed increased expression of ID1–3 and their de-ubiquitination enzyme USP-1. The expression of the Snails (Snail1 and 2), the molecules related to tumor invasiveness, was up regulated in Saos2 cells. However, no significant difference was found between Saos2 and hFOB regarding the angiogenesis related genes such as HIF1, VEGF and TIMP2 (D).
Fig. 3.

Immunohistochemical staining was performed on human OS samples with an antibody against total β-catenin. Representative figures demonstrated much stronger immunoreactivity to total β-catenin in high grade samples with more aggressive nature (G3, n = 4) compared to lower grade OS samples (G2, n = 4, less aggressive) (A). Intracellular β-catenin in Saos2 cells was knocked down using a lentivirus encoding a small hairpin RNA (shRNA) to human β-catenin. Western blot analysis confirmed the efficacy of silencing (B). These cells and cells stabilized with lentiviral LacZ were treated with either MTX (10−4 M) or a vehicle. Knocking down β-catenin not only increased the basal level of active caspase-3 and but also sensitized Saos2 cells to MTX mediated apoptosis (C). β-Cat: β-catenin, MTX: methotrexate, Veh: Vehicle control.
3.3. The expression level of β-catenin correlates with OS invasiveness and silencing β-catenin sensitizes Saos2 cells to chemotherapy
Thus far, our findings suggest Wnt-β-catenin signaling play a critical role in OS pathogenesis. We then performed immunohistochemical (IHC) β-catenin staining on human OS samples collected before the advent of neoadjuvant chemotherapy. The staining intensity was compared between the Grade 2 (n = 4) and Grade 3 (n = 4) samples. Our results revealed a much stronger immunoreactivity in Grade 3 than in Grade 2 samples (Fig. 4A). The effect of silencing of β-catenin in Saos2 cells on the MTX-induced apoptosis was assessed by western blotting with an active caspase-3 antibody, a marker of apoptosis. Saos2 cells were infected with a lentiviral construct encoding a small hairpin RNA (shRNA) to human β-catenin to establish stable cell lines. Western blot analysis showed a satisfactory efficacy with a significant reduction in β-catenin protein level (Fig. 4B). These cells and cells stabilized with lentiviral LacZ were treated with either MTX (10−4 M) or a vehicle. Knocking down β-catenin increased the basal level of active caspase-3 and enhanced the MTX mediated cell death (Fig. 3C).
Fig. 4.
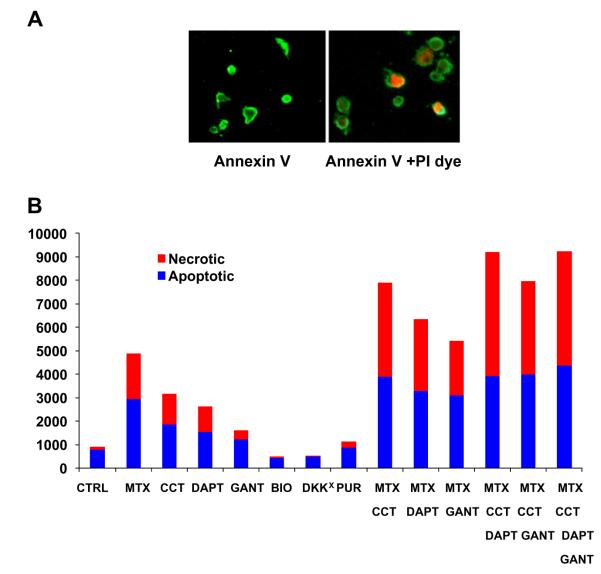
Apoptosis and necrosis of Saos2 cells were examined with the Annexin-V/PI dye kit. While the apoptotic cells were labeled with anti Annexin-V conjugated FITC (green), the necrotic cells were stained with PI dye (red) (A). Saos2 cells were cultured and treated with MTX in conjunction with other reagents/chemicals. The fluorescence from necrotic cells (PI labeling) was measured in a plate reader. MTX treatment resulted in the necrosis of Saos2 cells. The enhanced cell death was also observed when Saos2 cells were treated with the Wnt-β-catenin inhibitor (CCT), Notch inhibitor (DAPT), and Hh inhibitor (GANT). Conversely, Wnt-β-catenin activators BIO and DKK1AB and Hh activator PUR inhibited the necrosis. A synergistic action was detected when MTX was used together with CCT, and such synergy was also noticed in the MTX + DAPT group. The maximal effect was observed when MTX was concomitantly used with both CCT and DAPT. Adding one more agent, e.g. Hh inhibitor GANT, did not further improve the efficacy (B). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Modulation of Wnt-β-catenin, Notch and Hh signaling pathways alters MTX efficacy
The following reagents were used in this experiment: the activators for Wnt-β-catenin BIO and DKK1 neutralizing antibody (DKK1AB), and purmorphamine (PUR) for Hh pathway; the inhibitors for Wnt-β-catenin pathway CCT036477 (CCT), inhibitor for Notch pathway DAPT, inhibitor for Hh pathway GANT61 (GANT). Their efficacies were verified by luciferase assay after transfection with the reporter constructs including Topflash-Luc for Wnt-β-catenin, RBPjK-Luc for Notch and Gli2-Luc for Hh pathway. Wnt activator BIO significantly increased Topflash-Luc activity, while CCT largely abrogated such an effect. Similarly, PUR mediated induction of Gli12-Luc activity was effectively blocked by GANT. DAPT, to a lesser extent, decreased RBPjK-Luc activity (Data not shown). Apoptosis and necrosis of Saos2 cells were examined with the Annexin-V/Propidium iodide (PI) dye kit. While the cells undergoing apoptosis were labeled with Annexin-FITC (green), necrotic cells were stained with PI dye (red). The merged images imply a transition from apoptotic to necrotic process after MTX treatment (Fig. 4A).
Saos2 cells were cultured in 96-well plates and the effect of each treatment was observed in 8 different wells to reduce the errors. The fluorescence was measured by a plate reader at 488 and 530 nm emissions. MTX treatment induced the apoptosis and necrosis of Saos2 cells. Chemical inhibition of Wnt-β-catenin, Notch and Hh pathways also induced cell death and Wnt-β-catenin inhibitor showed the most potent effect. The Wnt-β-catenin pathway activators BIO and DKK1Ab showed an opposite effect. A synergistic effect was observed with MTX + CCT, and such a synergy was also noticed with the MTX + DAPT combination. The maximal effect was observed when MTX was used together with both CCT and DAPT. However, adding more agents such as GANT did not further improve the efficacy (Fig. 4B).
4. Discussion
Our results demonstrate that, compared to hFOB, the major components of the Wnt-β-catenin signaling pathway are upregulated in Saos2 cells. Interestingly, the expression of the hedgehog pathway ligands Ihh and Shh and receptor Smo are higher in Saos2 cells, while the transcription factor Gli2 is downregulated in Sao2 cells. With regards to Notch pathway, the ligand Jagged1 expression is higher but the receptors Notch1 and 2 were lower in Saos2 cells. Surprisingly, NICD, an indicator of the activation of Notch signaling, was downregulated, whereas the transcription factor RBPjK was higher, in Saos2 cells. The most significant change is in the Notch pathway was the increased level of its target gene HES1. Based on these observations, we decided to focus our study mainly on the Wnt-β-catenin pathway although we also tested the effect of the modification of Notch and Hh signaling on the sensitivity of Saos2 cells to MTX. Consistent with the histological features of OS tissue samples, Saos2 simultaneously exhibits both osteogenic and osteolytic potentials. In addition, the tumorigenic features of such cells were reflected by higher BCL2 and lower BAK1 expression. Higher levels of ID proteins indicate an under-differentiated status of OS cells, as such proteins hinder maturation of undifferentiated cells. Snail proteins have been reported to be correlated with the OS malignancy. Our study shows an increased expression of both Snail1 and Snail2.
Aberrant activation of Wnt-β-catenin pathway plays a critical role in OS pathogenesis. LRP5 expression in OS samples is inversely correlated with the EFS. Transfection of dominant negative LRP5 reverses the epithelial-mesenchymal transition (EMT), an initial step for OS development. High β-catenin level in OS samples seems to be positively correlated with lung metastasis. Consistently, the expression of Wnt-β-catenin inhibitors is often suppressed in OS. For example, sFrzB expression was much lower in OS samples than in normal bone [14,17,18]. An extracellular inhibitor of Wnt signaling, Wif1, is epigenetically silenced in human OS, and target disruption of Wif1 accelerates OS formation in mice. In addition, Wif1 deletion enhances radiation-induced OS formation, while increased Wif1 expression downregulates the expression of MMP9 and 14, thereby preventing the invasion and mobility of OS cells. In vitro studies showed that DKK3 treatment reduced the invasion and motility of OS cells [13,19,20]. These findings imply that abnormal activation of the Wnt signaling plays a role in OS formation. Contradictory to these findings, a recent report indicates thatthe Wnt-β-catenin pathway was inactivated in OS and that the loss of Wnt-β-catenin activity induces OS development [21]. Our results support the notion that Wnt-β-catenin signaling is enhanced in OS. Further studies will be performed to provide direct genetic evidence of this pathway in OS pathogenesis.
In addition to Wnt-β-catenin pathway, other pathways, especially Notch and Hh pathways, have also been studied because these signaling events are closely involved in carcinogenesis, cell stemness and bone development. The ligands (Notch1 and 2) and a target gene (HES1) of the Notch pathway were detected in OS samples. Chemical and genetic inhibition of γ-secretase prevented the invasion of human OS cells and reduced tumor growth in mouse xenografts [22–25]. Similarly, some Hh pathway components were aberrantly overexpressed in OS tissue samples. Inhibition of the Hh signaling repressed OS growth, knocking down Gli2 level reduced the proliferation and anchorage independent growth of OS cells, and xenograft tumor growth in mice was retarded when Gli2 level was knocked down [15,26,27]. Our results show that, unlike the Wnt-β-catenin pathway in which all major components are upregulated, the changes in the expression level in Notch and Hh pathways are not always increased. Apoptosis/necrosis assay using Annexin 5-FITC/PI dye kit indicates that the inhibitor for Wnt-β-catenin signaling potentiates the chemotherapeutic effect of MTX. Concomitant use of both Wnt-β-catenin and Notch inhibitors with MTX exhibits a maximal effect with regards to Saos2 cell death. However, adding Hh inhibitor to the above-mentioned treatment does not further enhance the efficacy. Our results lay a foundation for future clinical use of the small molecules/chemicals that are able to suppress Wnt-β-catenin and Notch signaling events in conjunction with the currently used drugs for OS, especially for refractory patients with recurrence and metastasis. Such combined therapy is expected to improve the survival rate of these patients.
Our results demonstrate the increased expression of molecules related to OS invasion, .e.g. Snail1 and Snail2. Furthermore, the protein expression level of β-catenin closely correlates with the OS invasiveness. Our future study will investigate the relationship between these molecules. Histological studies have demonstrated the existence of osteoblastic, chondroblastic and fibroblastic regions in OS samples, thus, it is widely accepted that OS originates from mesenchyme. Genetic evidence implies that OS may origin from the newly committed osteochondral progenitors [28,29]. To date, a link between histological features and biological behaviors has not been established. However, a clinical staging system that emphasizes the margin of spreading and the sites of metastasis has shown a convincing correlation with the prognosis of OS. Cancer stem cells (CSC) plays critical role in the metastasis and recurrence of OS [30]. Our future research will focus on the relationship between Wnt-β-catenin pathway and CSC, with the ultimate objective being able to delineate the mechanism underlying the recurrence, chemoresistance and lung metastasis of OS.
Supplementary Material
Footnotes
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bbrc.2012.12.118.
References
- [1].Messerschmitt PJ, Garcia RM, Abdul-Karim FW, Greenfield EM, Getty PJ. Osteosarcoma. J. Am. Acad. Orthop. Surg. 2009;17:515–527. doi: 10.5435/00124635-200908000-00005. [DOI] [PubMed] [Google Scholar]
- [2].Ritter J, Bielack SS. Osteosarcoma. Ann. Oncol. 2010;21S:320–325. doi: 10.1093/annonc/mdq276. [DOI] [PubMed] [Google Scholar]
- [3].Geller DS, Gorlick R. Osteosarcoma. Cin. Adv. Hematol. Oncol. 2010;8:705–718. [PubMed] [Google Scholar]
- [4].Federman N, Bernthal N, Eilber FC, Tap WD. The multidisciplinary management of osteosarcoma. Options Oncol. Curr. Treat. 2009;10:82–93. doi: 10.1007/s11864-009-0087-3. [DOI] [PubMed] [Google Scholar]
- [5].Niswander LM, Kim SY. Stratifying osteosarcoma: minimizing and maximizing therapy. Curr. Oncol. Rep. 2010;12:266–270. doi: 10.1007/s11912-010-0106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].D’Adamo DR. Appraising the current role of chemotherapy for the treatment of sarcoma. Semin. Oncol. 2011;38S:19–29. doi: 10.1053/j.seminoncol.2011.09.004. [DOI] [PubMed] [Google Scholar]
- [7].Grimer RJ. Surgical options for children with osteosarcoma. Lancet Oncol. 2005;6:85–92. doi: 10.1016/S1470-2045(05)01734-1. [DOI] [PubMed] [Google Scholar]
- [8].Ferrari S, Palmerini E. Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr. Opin. Oncol. 2007;19:341–346. doi: 10.1097/CCO.0b013e328122d73f. [DOI] [PubMed] [Google Scholar]
- [9].Chou AJ, Geller DS, Gorlick R. Therapy for osteosarcoma. Paediatr. Drugs. 2008;10:315–327. doi: 10.2165/00148581-200810050-00005. [DOI] [PubMed] [Google Scholar]
- [10].Jeon DG, Song WS. How can survival be improved in localized osteosarcoma? Expert Rev Anticancer Ther. 2010;10:1313–1325. doi: 10.1586/era.10.79. [DOI] [PubMed] [Google Scholar]
- [11].Janeway KA, Grier HE. Sequelae of osteosarcoma medical therapy. Lancet Oncol. 2010;11:670–678. doi: 10.1016/S1470-2045(10)70062-0. [DOI] [PubMed] [Google Scholar]
- [12].Walters DK, Steinmann P, Langsam B, Schmutz S, Born W, Fuchs B. Identification of potential chemoresistance genes in osteosarcoma. Anticancer Res. 2008;28:673–679. [PubMed] [Google Scholar]
- [13].Kansara M, Tsang M, Kodjabachian L, Sims NA, Trivett MK, Ehrich M, Dobrovic A, Slavin J, Choong PF, Simmons PJ, Dawid IB, Thomas DM. Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. J. Clin. Invest. 2009;119:837–851. doi: 10.1172/JCI37175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].McQueen P, Ghaffar S, Guo Y, Rubin EM, Zi X, Hoang BH. The Wnt signaling pathway: implications for therapy in osteosarcoma. Expert Rev. Anticancer Ther. 2011;11:1223–1232. doi: 10.1586/era.11.94. [DOI] [PubMed] [Google Scholar]
- [15].Nagao H, Ijiri K, Hirotsu M, Ishidou Y, Yamamoto T, Nagano S, Takizawa T, Nakashima K, Komiya S, Setoguchi T. Role of GLI2 in the growth of human osteosarcoma. J. Pathol. 2011;224:169–179. doi: 10.1002/path.2880. [DOI] [PubMed] [Google Scholar]
- [16].Tao J, Chen S, Lee B. Alteration of Notch signaling in skeletal development and disease. Ann. NY. Acad. Sci. 2010;1192:257–268. doi: 10.1111/j.1749-6632.2009.05307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hoang BH, Kubo T, Healey JH, Sowers R, Mazza B, Yang R, Huvos AG, Meyers PA, Gorlick R. Expression of LDL receptor-related protein 5 (LRP5) as a novel marker for disease progression in high-grade osteosarcoma. Int. J. Cancer. 2004;109:106–111. doi: 10.1002/ijc.11677. [DOI] [PubMed] [Google Scholar]
- [18].Iwaya K, Ogawa H, Kuroda M, Izumi M, Ishida T, Mukai K. Cytoplasmic and/or nuclear staining of beta-catenin is associated with lung metastasis. Clin. Exp. Metastasis. 2003;20:525–529. doi: 10.1023/a:1025821229013. [DOI] [PubMed] [Google Scholar]
- [19].Rubin EM, Guo Y, Tu K, Xie J, Zi X, Hoang BH. Wnt inhibitory factor 1 decreases tumorigenesis and metastasis in osteosarcoma. Mol. Cancer Ther. 2010;9:731–741. doi: 10.1158/1535-7163.MCT-09-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hoang BH, Kubo T, Healey JH, Yang R, Nathan SS, Kolb EA, Mazza B, Meyers PA, Gorlick R. Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma cells by modulating the Wnt-beta-catenin pathway. Cancer Res. 2004;64:2734–2739. doi: 10.1158/0008-5472.can-03-1952. [DOI] [PubMed] [Google Scholar]
- [21].Cai Y, Mohseny AB, Karperien M, Hogendoorn PC, Zhou G, Cleton-Jansen AM. Inactive Wnt/beta-catenin pathway in conventional high-grade osteosarcoma. J. Pathol. 2010;220:24–33. doi: 10.1002/path.2628. [DOI] [PubMed] [Google Scholar]
- [22].Zhang P, Yang Y, Zweidler-McKay PA, Hughes DP. Critical role of notch signaling in osteosarcoma invasion and metastasis. Clin. Cancer Res. 2008;14:2962–2969. doi: 10.1158/1078-0432.CCR-07-1992. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [23].Engin F, Bertin T, Ma O, Jiang MM, Wang L, Sutton RE, Donehower LA, Lee B. Notch signaling contributes to the pathogenesis of human osteosarcomas. Hum. Mol. Genet. 2009;18:1464–1470. doi: 10.1093/hmg/ddp057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tanaka M, Setoguchi T, Hirotsu M, Gao H, Sasaki H, Matsunoshita Y, Komiya S. Inhibition of Notch pathway prevents osteosarcoma growth by cell cycle regulation. Br. J. Cancer. 2009;100:1957–1965. doi: 10.1038/sj.bjc.6605060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang P, Yang Y, Nolo R, Zweidler-McKay PA, Hughes DP. Regulation of NOTCH signaling by reciprocal inhibition of HES1 and Deltex 1 and its role in osteosarcoma invasiveness. Oncogene. 2010;29:2916–2926. doi: 10.1038/onc.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Warzecha J, Göttig S, Chow KU, Brüning C, Percic D, Boehrer S, Brude E, Kurth A. Inhibition of osteosarcoma cell proliferation by the Hedgehog-inhibitor cyclopamine. J. Chemother. 2007;19:554–561. doi: 10.1179/joc.2007.19.5.554. [DOI] [PubMed] [Google Scholar]
- [27].Warzecha J, Dinges D, Kaszap B, Henrich D, Marzi I, Seebach C. Effect of the Hedgehog-inhibitor cyclopamine on mice with osteosarcoma pulmonary metastases. Int. J. Mol. Med. 2012;29:423–427. doi: 10.3892/ijmm.2011.851. [DOI] [PubMed] [Google Scholar]
- [28].Lin PP, Pandey MK, Jin F, Raymond AK, Akiyama H, Lozano G. Targeted mutation of p53 and Rb in mesenchymal cells of the limb bud produces sarcomas in mice. Carcinogenesis. 2009;30:1789–1795. doi: 10.1093/carcin/bgp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Walkley CR, Qudsi R, Sankaran VG, Perry JA, Gostissa M, Roth SI, Rodda SJ, Snay E, Dunning P, Fahey FH, Alt FW, McMahon AP, Orkin SH. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev. 2008;22:1662–1676. doi: 10.1101/gad.1656808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Adhikari AS, Agarwal N, Wood BM, Porretta C, Ruiz B, Pochampally RR, Iwakuma T. CD117 and Stro-1 identify osteosarcoma tumor-initiating cells associated with metastasis and drug resistance. Cancer Res. 2010;70:4602–4612. doi: 10.1158/0008-5472.CAN-09-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.