Abstract
BMS-806 and the related compound, #155, are novel inhibitors of human immunodeficiency virus type 1 (HIV-1) entry that bind the gp120 exterior envelope glycoprotein. BMS-806 and #155 block conformational changes in the HIV-1 envelope glycoproteins that are induced by binding to the host cell receptor, CD4. We tested a panel of HIV-1 envelope glycoprotein mutants and identified several that were resistant to the antiviral effects of BMS-806 and #155. In the CD4-bound conformation of gp120, the amino acid residues implicated in BMS-806 and #155 resistance line the “phenylalanine 43 cavity” and a water-filled channel that extends from this cavity to the inner domain. Structural considerations suggest a model in which BMS-806 and #155 bind gp120 prior to receptor binding and, upon CD4 binding, are accommodated in the Phe-43 cavity and adjacent channel. The integrity of the nearby V1/V2 variable loops and N-linked carbohydrates on the V1/V2 stem indirectly influences sensitivity to the drugs. A putative binding site for BMS-806 and #155 between the gp120 receptor-binding regions and the inner domain, which is thought to interact with the gp41 transmembrane envelope glycoprotein, helps to explain the mode of action of these drugs.
The global epidemic of infection by human immunodeficiency virus type 1 (HIV-1), the cause of AIDS (1, 12), has created an urgent need for new classes of antiretroviral agents. The entry of HIV-1 into host cells consists of several steps, each of which is a potential target for intervention. The HIV-1 envelope glycoprotein complex is a trimer consisting of three gp120 exterior envelope glycoproteins and three gp41 transmembrane glycoproteins (6, 34, 43, 48). Most of the surface-exposed elements of the trimeric envelope glycoprotein complex are contained on the gp120 glycoprotein (26, 31). When the gp120 glycoproteins from different HIV-1 strains or from different strains of the related HIV-2 and simian immunodeficiency viruses (SIVs) are compared, five conserved regions (C1 to C5) and five variable regions (V1 to V5) can be identified (24, 33). Intramolecular disulfide bonds in the gp120 glycoprotein result in the incorporation of the first four variable regions (V1 to V4) into large, surface-exposed loops (21, 25). The conserved gp120 regions fold into a core, which contains elements of gp120 important for binding the receptors on the target cell, CD4 and chemokine receptors.
HIV-1 infection is initiated by gp120 binding to CD4 on the target cell surface (8, 14). The conserved core of HIV-1 gp120 is conformationally changed by CD4 binding, as evidenced by unusually large changes in gp120 entropy documented by isothermal titration calorimetry (27). These studies suggest that both full-length gp120 and the gp120 core are flexible proteins that are rigidified by CD4 binding (27). X-ray crystallographic studies have revealed the structure of the HIV-1 gp120 core in the CD4-bound conformation (19, 20, 46). The HIV-1 gp120 core consists of an inner domain, which is thought to interact with the gp41 ectodomain, and an outer domain, which is heavily glycosylated and thought to be exposed on the trimer surface. Connecting these two domains is the bridging sheet, a four-stranded antiparallel β-sheet. The V1 and V2 variable loops project from two of the bridging sheet strands, which serve as a conserved “stem” for the V1/V2 stem-loop on gp120. CD4 contacts all three gp120 core domains and is thought to bring the inner and outer domains into proximity and to structure the conformationally labile bridging sheet. Many of the important contacts between gp120 and CD4 occur at the interface of the three gp120 core domains. The binding of gp120 and CD4 creates a roughly spherical 152 Å3 cavity at this location. This cavity extends deep into the interior of gp120 and is bounded by amino acid residues from each of the gp120 core domains. These cavity-lining gp120 residues are highly conserved among HIV-1 strains. Phe 43 of CD4, which alone accounts for 23% of the total contacts with gp120, is the only CD4 residue that bounds this cavity. Hence, the cavity has been designated the Phe 43 cavity. In the available crystal structures of gp120 core-CD4 complexes (19, 20), isopropanol, a component of the crystallization medium, occupies the Phe 43 cavity. The X-ray crystal structures also reveal a water-filled channel that connects the Phe 43 cavity to the gp120 surface and is flanked by the inner domain and bridging sheet. The Phe 43 cavity was suggested to be a potential target for small-molecule binding and inhibition of HIV-1 entry (19, 20, 46).
The conformational changes induced in the HIV-1 envelope glycoproteins by CD4 binding allow gp120 to interact efficiently with one of the chemokine receptors, CCR5 and CXCR4, that serve as obligate coreceptors for HIV-1 (7, 9, 10, 41, 44). In addition, CD4 binding can promote conformational rearrangements in the gp41 glycoprotein that are thought to drive the fusion of the viral and target cell membranes (11, 13, 17, 32a).
Inhibitors of HIV-1 entry have been identified that target the viral envelope glycoproteins and the chemokine receptors. Most gp120-directed inhibitors are large (soluble CD4 derivatives, peptides, and antibodies) and therefore are not orally bioavailable; others bind gp120 less specifically due to electrostatic or lectin-carbohydrate interactions (sulfated polymers, cyanovirin). These properties create challenges for practical implementation of these molecules as drugs. BMS-378806, here called BMS-806, and related compounds are low-molecular-weight inhibitors of HIV-1 entry that were recently identified by using a viral-infection-based screen (2, 22, 42). BMS-806 was shown to be specific for HIV-1, with no activity against HIV-2 or SIV. BMS-806 is active against HIV-1 isolates irrespective of chemokine receptor preference (22). BMS-806 has been shown to bind the gp120 glycoprotein (12a, 22). We identify here several gp120 amino acid residues that, when altered, result in the generation of viruses that are much less sensitive to BMS-806 and the related compound, #155, than the wild-type viruses. The location of these amino acid changes provides insight into drug-gp120 binding and the mechanism of action of this class of HIV-1 entry inhibitors.
MATERIALS AND METHODS
Compounds.
Compounds were synthesized as previously described (2, 22), dissolved in dimethyl sulfoxide, and stored at 5 mM concentrations at 4°C. Just prior to use, the compounds were diluted in phosphate-buffered saline to create 1 mM solutions. The 1H nuclear magnetic resonance, 13C nuclear magnetic resonance, infrared, and mass spectra, as well as the specific rotation and melting point of the synthesized BMS-806, match those reported by Wang et al. (42).
Cell lines.
Human 293T embryonic kidney and canine Cf2Th thymocytes (ATCC) were grown at 37°C and 5% CO2 in Dulbecco modified Eagle medium (Invitrogen) containing 10% fetal bovine serum (Sigma) and 100 μg of penicillin-streptomycin (Meditech, Inc.)/ml. Cf2Th cells stably expressing human CD4 and either CCR5 or CXCR4 (23) were grown in medium supplemented with 0.4 mg of G418 (Invitrogen)/ml and 0.15 mg of hygromycin B (Roche Diagnostics)/ml.
Plasmids expressing HIV-1 envelope glycoproteins.
The wild-type and mutant HIV-1 envelope glycoproteins were expressed from the pSVIIIenv vector. The glycoprotein mutants were created by site-directed mutagenesis, as previously described (18, 28). The residue numbering is based upon that of the prototypic HIV-1HXBc2 envelope glycoproteins, according to current convention (16). The env genes of the mutated plasmids were sequenced to verify the presence of the desired mutation and the absence of unwanted changes. The mutants are designated by the following nomenclature: wild-type amino acid in single-letter code, residue number, and amino acid to which the residue has been changed.
The ΔV1/V2 envelope glycoproteins have residues 128 to 194 replaced by a Gly-Ala-Gly linker and have been previously described (45, 47).
Generation of recombinant HIV-1 expressing luciferase.
Recombinant viruses capable of expressing firefly luciferase were created by transfection of human 293T embryonic kidney cells with the pCMVΔP1ΔenvpA HIV-1 packaging plasmid, with the HIV-1 vector encoding luciferase (pHIV-1Luc), and with pSVIIIenv plasmids expressing the HIV-1 envelope glycoprotein variants at a DNA ratio of 1:3:1 by using the Effectene transfection reagent (Qiagen). For the production of viruses pseudotyped with the vesicular stomatitis virus (VSV) G glycoprotein, the pHCMV-G plasmid was used instead of pSVIIIenv. The virus-containing supernatants were harvested 24 to 30 h after transfection, filtered (0.45-μm pore size), divided into aliquots, and stored at −80°C until further use. The reverse transcriptase activities of all viruses were measured as described previously (30).
Assay of virus infectivity and drug sensitivity.
Cf2Th/CD4-CCR5 or Cf2Th/CD4-CXCR4 target cells were seeded at a density of 6 × 103 cells/well in 96-well luminometer-compatible tissue culture plates (Dynex) 24 h before infection. On the day of infection, BMS-806 or compound #155 (1 to 300 nM) was added to recombinant viruses (10,000 reverse transcriptase units) in a final volume of 50 μl and incubated at 37°C for 30 min. The medium was removed from the target cells, which were then incubated with the virus-drug mixture for 48 h at 37°C. The medium was removed from each well, and the cells were lysed with 30 μl of passive lysis buffer (Promega) and by three freeze-thaw cycles. An EG&G Berthold Microplate Luminometer LB 96V was used to measure luciferase activity in each well after the addition of 100 μl of luciferin buffer (15 mM MgSO4, 15 mM KPO4 [pH 7.8], 1 mM ATP, 1 mM dithiothreitol) and 50 μl of 1 mM d-luciferin potassium salt (BD Pharmingen).
Syncytium inhibition assay.
Approximately 5 × 105 293T cells were seeded in a 60-mm tissue culture plate. After 24 h, the cells were transfected by using Polyfect transfection reagent (Qiagen) with 2 μg of the pSVIIIenv plasmid expressing the HIV-1 YU2 envelope glycoproteins. After 24 h, the cells were lifted from the plates by using 5 mM EDTA. Then, 104 cells were incubated for 15 min at 37°C with various concentrations of BMS-806 or #155. After incubation, the 293T cells were added to 4 × 104 Cf2Th/CD4-CCR5 cells that had been seeded in a 96-well tissue culture plate the day before. The cells were cocultured at 37°C overnight. Syncytia were counted by using a Nikon TE300 inverted microscope.
Modeling BMS-806 binding to HIV-1 gp120.
The d1d2 CD4-gp120 core-17b Fab crystal structure (pdb code, 1G9 M) from the HXBc2 strain (19) was used in modeling calculations. The Flo software program (22a) was used to conduct Monte Carlo-based docking studies of the binding of inhibitors to the gp120 core containing polar hydrogen atoms. Crystallographic waters and asparagine-linked glucosamine molecules were included in the gp120 core structure for docking calculations. Water molecules were deleted from either the water-filled channel or the Phe 43 cavity of gp120 on an individual basis prior to docking. The inhibitor molecules were built by using coordinates of similar small-molecule crystal structures. Docking of the inhibitors was conducted with flexible protein residues in the location of either the Phe 43 cavity or water-filled channel.
RESULTS
Specific inhibition of viruses with HIV-1 envelope glycoproteins by compounds.
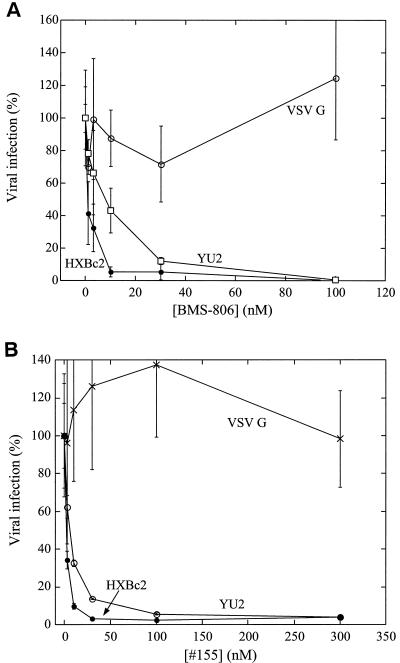
BMS-806 and related molecules have been reported to inhibit HIV-1 specifically (2, 12a, 22). We synthesized BMS-806 and the related compound #155 and examined their abilities to inhibit the single-round infection of recombinant HIV-1 encoding firefly luciferase. The recombinant viruses were pseudotyped with HIV-1 envelope glycoproteins derived from either the CXCR4-using (X4), laboratory-adapted HXBc2 isolate or the CCR5-using (R5) primary YU2 isolate. As a control for specificity, viruses were also pseudotyped with the VSV G glycoprotein. Both BMS-806 and #155 inhibited infection by the viruses with the HXBc2 and YU2 HIV-1 envelope glycoproteins, with 50% inhibitory concentrations (IC50) ranging from 1 to 10 nM (Fig. 1). Neither compound inhibited infection by viruses with the VSV G glycoprotein. We conclude that BMS-806 and #155 potently and specifically inhibit the infection of viruses with HIV-1 envelope glycoproteins regardless of the chemokine receptor used.
FIG. 1.
Effects of BMS-806 and #155 on HIV-1 infection. The infection of Cf2Th-CD4 target cells expressing the appropriate chemokine receptor by recombinant HIV-1 expressing firefly luciferase was measured in the presence of the indicated concentrations of BMS-806 (A) or #155 (B). Cf2Th/CD4-CXCR4 target cells were used for infection by viruses with the HIV-1 HXBc2 or VSV G envelope glycoproteins. Cf2Th/CD4-CCR5 target cells were used for infection by viruses with the HIV-1 YU2 envelope glycoproteins. The values are represented as a percentage of the level of target cell luciferase observed for each virus in the absence of drug. The means and standard deviations of the results obtained in triplicate samples are shown. The results are representative of those obtained in five independent experiments.
The observed requirement for the HIV-1 envelope glycoproteins for the antiviral effects of BMS-806 and #155 is consistent with the proposal that these drugs exert their inhibitory effects by blocking HIV-1 envelope glycoprotein function. To examine this in a context different from that of virus infection, the ability of the compounds to inhibit syncytium formation mediated by the HIV-1 envelope glycoproteins was examined. BMS-806 and #155 inhibited fusion between 293T cells expressing the HIV-1 YU2 envelope glycoproteins and Cf2Th cells expressing CD4 and CCR5, with IC50 values of 5 to 10 μM (data not shown). Blocking cell-cell fusion mediated by HIV-1 envelope glycoproteins generally requires higher concentrations of Env-directed inhibitors than blocking virus entry (32). These results indicate that BMS-806 and #155 inhibit the function of the HIV-1 envelope glycoproteins.
Effects of changes in the gp120 V1/V2 stem-loop on drug sensitivity.
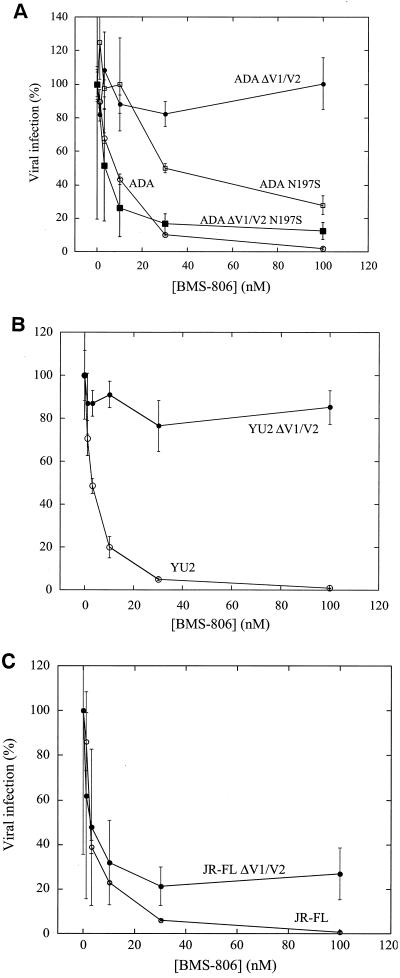
BMS-806 has been shown to bind the HIV-1 gp120 envelope glycoprotein (12a, 22, 32a). We studied the sensitivity to BMS-806 and #155 of viruses with changes in the gp120 glycoprotein. The V1/V2 variable loops of gp120 are dispensable for HIV-1 replication in tissue-cultured cells (4, 45, 47). The drug sensitivity of recombinant viruses with either wild-type or V1/V2 loop-deleted (ΔV1/V2) envelope glycoproteins was examined. The envelope glycoproteins were derived from three primary R5 HIV-1 isolates: ADA, YU2, and JR-FL. Recombinant viruses with the wild-type ADA, YU2, and JR-FL envelope glycoproteins were inhibited by BMS-806 and #155 with IC50 values of <7 nM (Fig. 2 and Table 1). Compared to the viruses bearing the wild-type ADA and YU2 envelope glycoproteins, viruses with the ΔV1/V2 envelope glycoproteins from these two HIV-1 strains were very resistant to BMS-806 and #155 (Fig. 2 and Table 1). In contrast, viruses with the V1/V2 loop-deleted JR-FL envelope glycoproteins were inhibited by BMS-806 and #155, although not as efficiently as viruses with the wild-type JR-FL envelope glycoproteins (Fig. 2C and Table 1). Thus, deletion of the V1/V2 variable loops of the gp120 glycoprotein dramatically alters the sensitivity of some HIV-1 strains, but not others, to BMS-806 and #155.
FIG. 2.
Effects of removal of V1/V2 loops and V1/V2 stem carbohydrate on BMS-806 sensitivity. The infection of Cf2Th/CD4-CCR5 cells by viruses with wild-type or the indicated mutant envelope glycoproteins from the ADA (A), YU2 (B), or JR-FL (C) strains of HIV-1 was measured in the presence of increasing concentrations of BMS-806. The values are represented as a percentage of the level of target cell luciferase observed for each virus in the absence of drug. The means and standard deviations of the results obtained in triplicate samples are shown. The results are representative of those obtained in five independent experiments.
TABLE 1.
Sensitivity of HIV-1 with alterations in the V1/V2 stem-loops to drugsa
| Inhibitor and envelope glycoprotein | IC50 (nM)
|
|||||
|---|---|---|---|---|---|---|
| Wild type | ΔV1/V2 | ΔV2 | N197S | ΔV1/V2 N197S | ΔV1/V2 N197Q | |
| BMS-806 | ||||||
| ADA | 1.9 ± 0.5 | >100 | 4.4 ± 0.7 | 17.2 ± 4.7 | 8.2 ± 2.3 | 1.1 ± 0.1 |
| YU2 | 3.7 ± 1.6 | >100 | ND | ND | ND | ND |
| JR-FL | 5.5 ± 1.5 | 9.4 ± 0.20 | >100 | ND | ND | ND |
| #155 | ||||||
| ADA | 5.9 ± 0.4 | >300 | 2.2 ± 0.0 | 51.1 ± 13.0 | ND | ND |
| YU2 | 6.3 ± 1.0 | >300 | ND | ND | ND | ND |
| JR-FL | 4.8 ± 1.0 | 22.8 ± 1.6 | >300 | ND | ND | ND |
The IC50 values (nM) for BMS-806 and #155 for viruses with the indicated envelope glycoprotein variants derived from the ADA, YU2, or JR-FL HIV-1 strains are reported. The IC50 values represent the means and standard deviations of triplicate samples from a representative experiment of three to five independent experiments. In the absence of drugs, the infection efficiencies (luciferase units) for viruses with the various envelope glycoprotein were as follows: ADA, 1.3 × 107; ADA ΔV1/V2, 5.1 × 104; ADA ΔV2, 4.0 × 103; ADA N197S, 4.2 × 106; ADA ΔV1/V2 N197S, 3.0 × 103; ADA ΔV1/V2 N197Q, 5 × 103; YU2, 2.2 × 106; YU2 ΔV1/V2, 1.7 × 104; JR-FL, 4.8 × 106; JF-FL ΔV1/V2, 1.8 × 104; and JR-FL ΔV2, 1.3 × 105. ND, not determined.
When only the V2 variable loop was deleted from the ADA and JR-FL envelope glycoproteins, the sensitivity of the resulting viruses to BMS-806 and #155 exhibited a pattern opposite to that seen with the complete deletion of the V1/V2 loops (Table 1). Thus, depending on the particular variable loop deleted and the HIV-1 strain of origin of the envelope glycoproteins, viruses with deletions of the V1/V2 variable loops may exhibit dramatic differences in sensitivity to these compounds.
Given the ability of BMS-806 and related compounds to inhibit infection by diverse HIV-1 strains (22), the effects of alteration of variable gp120 structures on the efficacy of these drugs was unexpected. We considered the possibility that the effects of V1/V2 loop deletion on drug sensitivity might be due to conformational effects on the adjacent, highly conserved V1/V2 stem. Sequence comparisons revealed that the ADA and YU2 envelope glycoproteins have a site of N-linked glycosylation at Asn 197 in the V1/V2 stem, whereas the JR-FL envelope glycoproteins lack this site. The presence or absence of the asparagine 197 glycan has been previously shown to influence the position of the V1/V2 variable loops (15). We hypothesized that the presence of this carbohydrate moiety influences the resistance of viruses with the ADA ΔV1/V2 envelope glycoproteins to BMS-806. Asn 197 was altered in the wild-type ADA and the ADA ΔV1/V2 envelope glycoproteins, and recombinant viruses with these envelope glycoproteins were examined for BMS-806 sensitivity. Viruses with the ADA N197S envelope glycoproteins exhibited a modest degree of resistance (IC50 = 17 nM) to BMS-806 compared to viruses with the wild-type ADA envelope glycoproteins (Fig. 2A and Table 1). In contrast, viruses with the ΔV1/V2 N197S and ΔV1/V2 N197Q envelope glycoproteins, which lack the glycan at Asn 197, were much more sensitive to BMS-806 compared to viruses with the ADA ΔV1/V2 envelope glycoproteins (Fig. 2A and Table 1). These results indicate that the gp120 V1/V2 loops are not absolutely required for sensitivity to BMS-806. Also, the resistance of V1/V2 loop-deleted viruses to BMS-806 and #155 is dependent upon the presence of an N-linked glycan at Asn 197 in the V1/V2 stem of gp120.
Effects of single HIV-1 gp120 amino acid changes that fill the Phe 43 cavity on drug sensitivity.
The apparently indirect effects of the changes described above in the V1/V2 stem-loop on HIV-1 sensitivity to BMS-806 and #155 prompted an examination of the contribution of adjacent, more conserved envelope glycoprotein structures to these phenotypes. The V1/V2 stem, which is part of the gp120 bridging sheet, is proximal to the gp120 inner domain and to the interfacial regions of gp120 involved in receptor binding (19, 20, 46). We tested a panel of HIV-1 envelope glycoprotein mutants, most of which contain single-amino-acid changes in these gp120 regions, for susceptibility to inhibition by BMS-806 and #155. This panel of mutants has been extensively characterized for recognition by conformation-dependent gp120 ligands, including soluble CD4 and multiple monoclonal antibodies (28, 35-38, 40). All of the mutant envelope glycoproteins included in the panel are able to bind at least one conformation-dependent ligand efficiently. Moreover, the mutants all support HIV-1 entry, albeit with different efficiencies (Table 2). Previous studies with these mutant envelope glycoproteins indicate that the sensitivity of HIV-1 infection to entry inhibitors is independent of the basal level of virus infectivity (28, 32, 35-38, 40).
TABLE 2.
Phenotypes of the HIV-1 HXCBc2 envelope glycoprotein mutants
| HXBc2 Env | Infection efficiencya (luciferase units) | IC50 (nM)b
|
|
|---|---|---|---|
| BMS-806 | #155 | ||
| Wild type | 7.0 × 105 | 2.2 ± 0.2 | 2.2 ± 0.6 |
| W112A | 4.0 × 103 | >100 | >300 |
| D113A | 7.5 × 104 | 188.0 ± 43.9 | 164.4 ± 47.6 |
| K117W | 3.0 × 104 | 3.5 ± 0.6 | 3.7 ± 2.6 |
| V120L/K121E | 1.0 × 103 | 4.4 ± 2.0 | >300 |
| T123A | 9.2 × 103 | 1.4 ± 0.4 | 5.9 ± 1.3 |
| L125G | 1.7 × 105 | 36.9 ± 9.7 | >300 |
| Q203A | 4.9 × 104 | 2.3 ± 1.6 | 2.6 ± 0.7 |
| T257R | 4.0 × 104 | >100 | >300 |
| T257G | 4.0 × 105 | 40.5 ± 6.0 | 94.7 ± 19.0 |
| T257A | 3.4 × 105 | 15.4 ± 4.5 | 9.3 ± 0.4 |
| D368E | 8.1 × 103 | 2.7 ± 0.9 | 2.6 ± 0.4 |
| E370K | 2.0 × 105 | 6.3 ± 0.7 | 2.2 ± 0.6 |
| E370D | 1.8 × 105 | 2.7 ± 0.6 | 17.5 ± 4.4 |
| S375W | 2.0 × 103 | >100 | >300 |
| S375A | 6.4 × 103 | 2.6 ± 0.4 | 2.2 ± 0.0 |
| N377L | 1.3 × 104 | 16.0 ± 6.2 | 8.2 ± 4.9 |
| F382L | 6.0 × 103 | >100 | >300 |
| N425A | 2.4 × 104 | 0.6 ± 0.2 | 1.9 ± 0.4 |
| M426L | 9.7 × 104 | >300 | >300 |
| W427F | 2.4 × 105 | 1.2 ± 0.1 | 2.6 ± 0.4 |
| Q428A | 1.5 × 105 | 3.2 ± 0.9 | 4.8 ± 1.0 |
| K429L | 1.9 × 105 | 14.3 ± 3.3 | 47.8 ± 12.5 |
| V430S | 1.2 × 103 | ND | >300 |
| K432A | 1.7 × 105 | 5.0 ± 0.6 | 3.7 ± 0.4 |
| A433L | 3.0 × 104 | 41.2 ± 13.2 | >300 |
| M475S | 3.7 × 105 | 18.4 ± 6.2 | 69.6 ± 3.9 |
| D477V | 1.9 × 104 | 9.6 ± 3.8 | 9.3 ± 3.2 |
| A582T | 2.0 × 105 | 4.9 ± 2.0 | 1.9 ± 0.4 |
| W596M | 1.0 × 104 | 3.2 ± 1.7 | 5.6 ± 1.1 |
Recombinant, luciferase-expressing HIV-1 with the indicated HXBc2 envelope glycoprotein mutants were incubated with Cf2Th/CD4-CXCR4 target cells, and luciferase activity in the target cells was measured after 48 h.
The IC50 values were determined as described in Materials and Methods. The values represent the means and standard deviations of triplicate samples from a representative experiment of three independent experiments. ND, not determined.
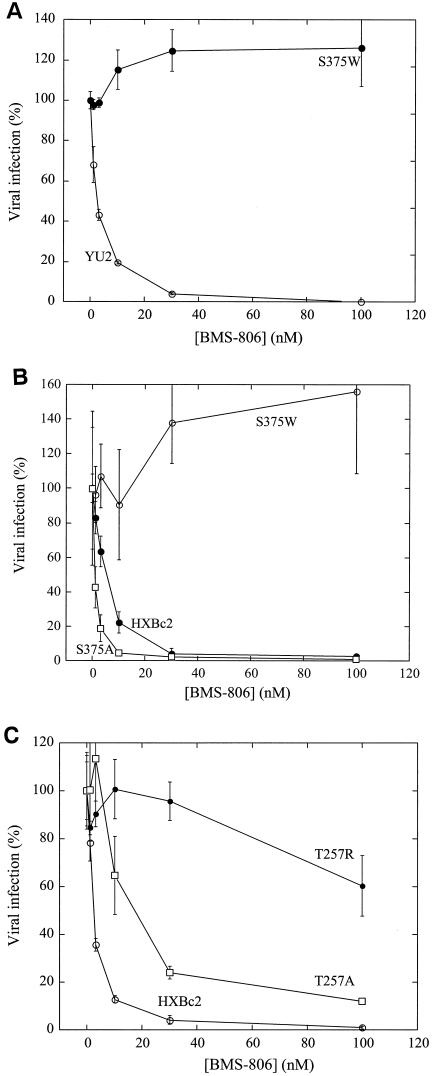
The Phe 43 cavity represents a potentially favorable binding site for low-molecular-weight inhibitors of HIV-1 gp120 function (19, 20). We explored the potential role of the Phe 43 cavity in HIV-1 susceptibility to the inhibitory effects of BMS-806 and #155. Previous studies have demonstrated that a tryptophan substitution for serine 375, which contacts the Phe 43 cavity, fills the cavity and predisposes gp120 to assume a CD4-bound conformation (49). In viruses with either HXBc2 or YU2 HIV-1 envelope glycoproteins, the S375W change resulted in dramatic levels of resistance to BMS-806 and #155 (Fig. 3A and B and Table 2). In contrast, when serine 375 in the HXBc2 envelope glycoproteins was replaced by alanine (S375A), the resulting viruses were as sensitive as the wild-type viruses to these drugs. Thus, an amino acid change that fills the Phe 43 cavity results in significant levels of resistance to both BMS-806 and #155.
FIG. 3.
Effects on BMS-806 sensitivity of gp120 changes that fill the Phe 43 cavity. The infection of Cf2Th/CD4-CCR5 cells (A) or Cf2Th/CD4-CXCR4 cells (B and C) by recombinant HIV-1 expressing luciferase was measured in the presence of the indicated concentrations of BMS-806. The viruses contained the wild-type YU2 (A) or HXBc2 (B and C) envelope glycoproteins or the indicated mutants. The values are represented as a percentage of the level of target cell luciferase observed for each virus in the absence of drug. The means and standard deviations of the results obtained in triplicate samples are shown. The results are representative of those obtained in three independent experiments.
Similarly, substitution of Thr 257, which resides near Ser 375 on the outer domain wall of the Phe 43 cavity, resulted in various degrees of resistance to BMS-806 and #155 in proportion to the size of the substituted side chain. The large arginine side chain in the T257R mutant envelope glycoproteins was associated with high levels of resistance to both BMS-806 and #155 (Fig. 3C and Table 2). Viruses with the T257A and T257G mutant envelope glycoproteins, with smaller side chains at residue 257, were less resistant to these drugs. These results are consistent with a requirement for the Phe 43 cavity for optimal BMS-806 and #155 antiviral activity.
Effects of other HIV-1 gp120 amino acid changes on drug sensitivity.
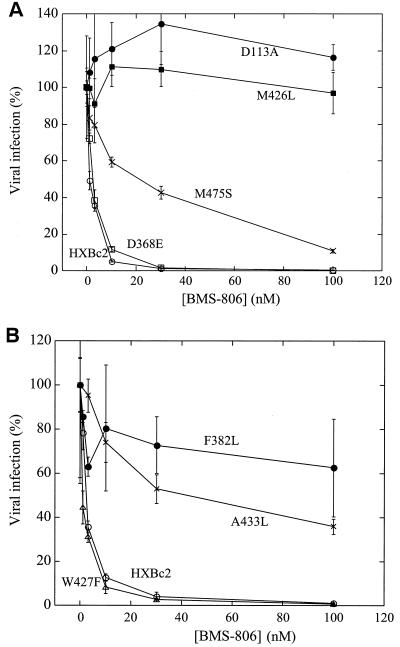
The effects of changes in additional amino acid residues in the HIV-1 HXBc2 envelope glycoproteins on sensitivity to BMS-806 and #155 were investigated. Most of the amino acid residues studied are located within the gp120 bridging sheet, inner domain and/or receptor-binding regions. Changes in four gp120 residues (Trp 112, Asp 113, Phe 382, and Met 426) resulted in viruses that exhibited at least 40-fold increases in the IC50 values for BMS-806 and #155 (Fig. 4 and Table 2). Changes in Leu 125, Lys 429, Ala 433, and Met 475 were associated with moderate to high levels of resistance to #155 and intermediate levels of resistance to BMS-806. Viruses with the V120L/K121E envelope glycoproteins were efficiently inhibited by BMS-806 (IC50 = 7 nM) but were resistant to the highest concentrations of #155 tested (IC50 > 300 nM) (Table 2). These results indicate that changes in particular gp120 residues confer degrees of resistance to BMS-806 and #155. Most of the studied amino acid changes affected sensitivity to both drugs, although some changes resulted in specific resistance to #155.
FIG. 4.
Effects of single gp120 amino acid changes on BMS-806 sensitivity. (A and B) The infection of Cf2Th/CD4-CXCR4 cells by recombinant HIV-1 expressing luciferase was measured in the presence of the indicated concentrations of BMS-806. The viruses contained wild-type HXBc2 envelope glycoproteins or the indicated mutants. The values are represented as a percentage of the level of target cell luciferase observed for each virus in the absence of drug. The means and standard deviations of the results obtained in triplicate samples are shown. The results are representative of those obtained in three independent experiments.
We also tested the effects of alteration of two amino acid residues in the gp41 exterior domain on BMS-806 and #155 sensitivity. These gp41 amino acids have been previously associated either with resistance to gp120-directed antibodies or with gp120-gp41 interactions that contribute to Env-mediated syncytium formation (3, 5, 29, 39). Viruses with the A582T and W596M envelope glycoproteins exhibited levels of sensitivity to BMS-806 and #155 near those of wild-type viruses.
DISCUSSION
We identified several gp120 amino acid residues, changes in which resulted in viruses with marked decreases in sensitivity to the novel HIV-1 entry inhibitors, BMS-806 and #155. Consistent with their related chemical structures, resistance to BMS-806 and #155 demonstrated similar but not identical patterns. The sensitivity of HIV-1 to BMS-806 and #155 was affected by the alteration of variable and conserved gp120 structures. Removal of the V1/V2 variable loops decreased the efficacy of these drugs in a manner influenced by the viral strain from which the envelope glycoproteins were derived. The V1/V2 variable loop-deleted ADA and YU2 viruses were very resistant to BMS-806 and #155, whereas the equivalent construct in the JR-FL strain remained sensitive to the drugs. The opposite pattern of sensitivity was seen when only the V2 loop was removed. The strain-dependent presence of an N-linked carbohydrate on the stem of the V1/V2 loops at asparagine 197 was shown to be a critical determinant of the strain-dependent differences in the drug sensitivity of viruses lacking the V1/V2 variable loops. Our results suggest that neither the V1/V2 variable loops nor this N-linked glycan is absolutely required for the ability of gp120 to bind BMS-806 and #155. However, both of these structures may stabilize the conformation of the V1/V2 stem, which is a bridging sheet component and likely contributes to the drug-binding sites (see below). The loss of either variable loops or the glycan leads to some level of drug resistance, depending upon the presence of the remaining element(s). These observations suggest a model in which the absence of some of the large structural elements on the V1/V2 stem-loop leads to indirect conformational effects on drug binding or efficacy mediated by the remaining untethered loops or glycans.
In keeping with the ability of BMS-806 and #155 to inhibit diverse HIV-1 strains, conserved gp120 structures adjacent to the V1/V2 variable regions were identified to be important for drug sensitivity. Changes in several single gp120 amino acid residues allowed significant levels of resistance to BMS-806 and/or #155. In most cases, the envelope glycoproteins with these changes exhibited lower ability to support HIV-1 entry than the corresponding wild-type envelope glycoproteins. Thus, some of the available evolutionary pathways to BMS-806 or #155 escape incur a penalty with respect to viral fitness, at least in tissue-cultured cells. This is consistent with the invariant or highly conserved nature of gp120 residues 112, 113, 125, 257, 375, 382, 426, 433, and 475 in diverse HIV-1 isolates from different phylogenetic clades (16). Some variability, however, is tolerated in gp120 residues such as lysine 429 and valine 430, changes in which resulted in decreases in sensitivity to BMS-806 and/or #155. Moreover, the M426L mutant exhibited a high level of resistance to BMS-806 and #155 but was quite efficient in supporting HIV-1 infection. Thus, the generation of HIV-1 variants resistant to BMS-806 and #155 appears to be possible, at least in tissue culture. Indeed, escape from BMS-806 inhibition has been observed after tissue culture passage of HIV-1 in the presence of the drug (12a, 22). One of the virus variants selected by this approach exhibited changes in Met 475 of gp120, which in our study conferred modest reductions in BMS-806 and #155 sensitivity but allowed efficient virus replication.
The location of the HIV-1 gp120 residues important for BMS-806 and #155 sensitivity is noteworthy. X-ray crystallographic studies of gp120 complexed with CD4 identified the Phe 43 cavity of gp120 and predicted it to be an attractive binding site for low-molecular-weight inhibitors of HIV-1 entry (19, 20). Our results suggest that BMS-806 and #155 binding and/or activity can be affected by alteration of gp120 structures surrounding the Phe 43 cavity. Changes in five gp120 residues (Trp 112, Thr 257, Ser 375, Phe 382, and Met 426) that contact the Phe 43 cavity resulted in escape from BMS-806 and #155 inhibition. Two of these residues, Ser 375 and Thr 257, are situated on the wall of the Phe 43 cavity formed by the outer domain (Fig. 5). Previous studies indicated that replacing Ser 375 with tryptophan fills the Phe 43 cavity and predisposes gp120 to assume a CD4-bound conformation even in the absence of ligands (49). Likewise, substitution of Thr 257 with a larger amino acid residue such as arginine is expected to diminish the size of the Phe 43 cavity. These cavity-filling substitutions dramatically diminished sensitivity to BMS-806 and #155. However, alteration of Ser 375 to alanine resulted in a virus with wild-type levels of sensitivity to BMS-806 and #155. Replacing Thr 257 with amino acid residues containing smaller side chains resulted in viruses more sensitive to these drugs than the virus in which Thr 257 was replaced by arginine. Thus, the major effect of changes in Ser 375 and Thr 257 on BMS-806/#155 sensitivity appears to derive less from the loss of direct contacts between the drugs and the side chains of these gp120 residues than from filling the Phe 43 cavity. In contrast, the changes introduced in Trp 112, Phe 382, and Met 426 do not significantly increase the size of the side chain and therefore potentially disrupt drug-gp120 contacts. Interestingly, although well conserved in HIV-1 isolates, four of the five Phe 43 cavity-lining residues identified in the present study are altered in HIV-2 and SIV (16), accounting for the lack of effect of BMS-806 and related compounds on these viruses (22).
FIG. 5.
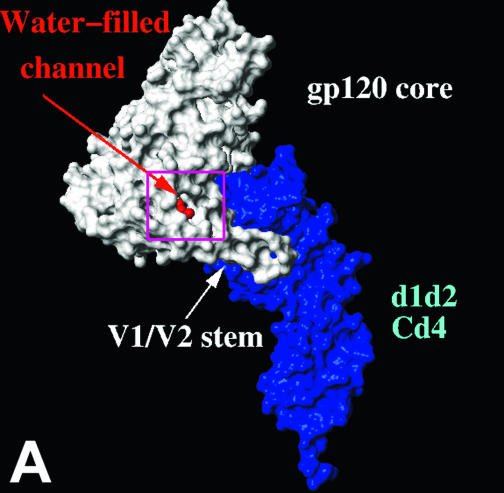
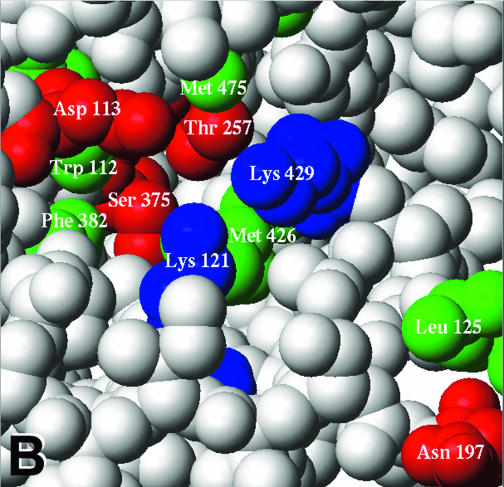
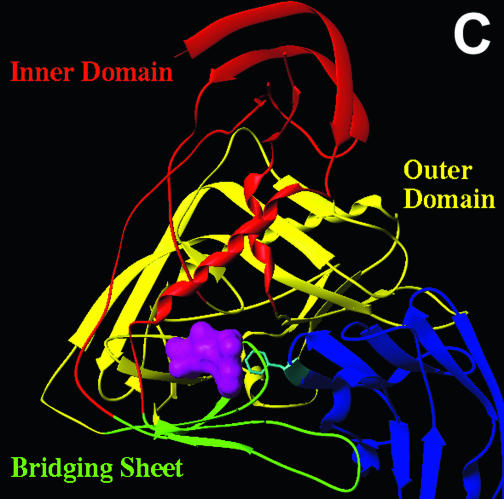
Location of gp120 changes associated with resistance to BMS-806 and #155. (A) The molecular surfaces of the HXBc2 gp120 core (white) complexed with two-domain CD4 (d1d2 CD4) (blue) (19) are shown. The gp120 V1/V2 stem is labeled. A chain of water molecules (red) extends from the solvent interface of the V1/V2 stem to the Phe 43 cavity. The area boxed in magenta is shown in detail in panel B. (B) A CPK model of the gp120 region highlighted in panel A is shown. CD4 and two gp120 core residues (Trp 427 and Glu 428) have been removed to allow the gp120 residues on the distal wall of the Phe 43 cavity to be visualized. The colored gp120 residues are associated with >10-fold increases in the IC50 values for either BMS-806 or #155 or both drugs. Hydrophobic, acidic-polar, and basic residues are colored green, red, and blue, respectively. Ala 433, changes in which are associated with resistance to both BMS-806 and #155, is obscured by Lys 121 in this orientation. (C) Ribbon diagrams of the gp120 core and two-domain CD4 are shown, in the same orientation as in panel A. The gp120 inner domain is red, the outer domain is yellow, and the bridging sheet is green. CD4 is blue, with Phe 43 colored cyan. The binding of BMS-806 may occur in the water-filled channel of gp120 (as shown), the Phe 43 cavity (not shown), or with portions of the drug occupying both sites (not shown). The molecular surface of the modeled BMS-806 is shown in magenta.
Other gp120 changes associated with BMS-806 and/or #155 escape involved Asp 113, Val 120, Lys 121, Lys 429, and Ala 433. In the CD4-bound conformation of gp120, these residues, along with Phe 382, Trp 112, and Met 426, line a water-filled channel that extends from the Phe 43 cavity to the gp120 surface (Fig. 5A and B). This channel exists at the interface of the bridging sheet and the inner domain of gp120 (Fig. 5C). Of note, #155 seems to be more sensitive to changes in residues (Val 120, Lys 121, Lys 429, and Ala 433) near the inner domain surface than is BMS-806.
Escape from inhibition of BMS-806 and #155 could hypothetically involve decreases in the affinity of drug binding or a diminution in the efficacy of bound drug. The discrete location of the amino acid residues implicated in BMS-806 and #155 resistance is consistent with a potential drug-binding site. Precise predictions of binding modes for these drugs are made difficult by the absence of structural information on drug-gp120 complexes or on gp120 free of bound ligands. Docking studies of these drugs with the available gp120 structures, all of which represent the CD4-bound state (19, 20), have limitations in light of the significant conformational changes that occur in gp120 upon CD4 binding (27). Nonetheless, both BMS-806 and #155 can be accommodated without steric clashes in the CD4-bound conformation of gp120, either in the Phe 43 cavity, or in the contiguous water-filled channel, or with part of the drug occupying each of these gp120 elements (Fig. 5C). In all of these models, the terminal aromatic rings of BMS-806 and #155 form aromatic stacking interactions with key gp120 residues, Trp 112 and Phe 382, implicated in drug resistance. The Phe 43 cavity of gp120 has been suggested as a potential target for drug discovery efforts (19, 20). Our mutational data and modeling studies suggest that the water-filled channel also plays a prominent role in the binding of BMS-806 and #155. This preliminary model should be useful in guiding future attempts to define the gp120/BMS-806 interaction in greater detail.
Our data on BMS-806 and #155 resistance are consistent with recent studies on the mechanism of the antiviral effect of this class of inhibitors (32a). At concentrations of BMS-806 near those required for inhibition of HIV-1 infection, we have not observed inhibition of gp120 binding to either CD4 or chemokine receptors. Instead, BMS-806 and related compounds potently interfere with a CD4-induced conformational change in the HIV-1 envelope glycoproteins. This conformational change results in the exposure of the helical heptad repeat region (HR1) on the gp41 envelope glycoprotein (32a). This may activate the gp41 glycoprotein to undergo subsequent conformational changes that form a six-helix bundle and drive the fusion of the viral and target cell membranes. The location of gp120 changes associated with BMS-806 and #155 resistance is compatible with a binding model in which these drugs make no contact with the bound CD4 (Fig. 5C). Furthermore, the compatibility of BMS-806 binding with the CD4-bound conformation of gp120 is consistent with the lack of BMS-806 interference with CD4:gp120 binding. Apparently, BMS-806 does not prevent CD4 from structuring the unusually flexible gp120 molecule and locking gp120 into the CD4-bound conformation. Rather, the presence of BMS-806 wedged into the bridging sheet-inner domain interface may disrupt intermolecular gp120-gp41 signaling triggered by CD4. This model is consistent with the predicted involvement of the gp120 inner domain in interactions with the gp41 ectodomain (19, 20, 46). This model also helps to explain the emergence of changes in gp41 and the gp120 inner domain in some BMS-806-resistant HIV-1 variants generated in tissue culture (12a, 22).
In functional HIV-1 envelope glycoprotein trimers, the presence of the gp41 ectodomain may limit the access routes of BMS-806 and related compounds to the potential binding sites identified in the present study. In this context, access of the drugs would need to be achieved via the entrance to the Phe 43 cavity. Thus, antiviral efficacy would require that BMS-806 and related molecules bind gp120 prior to CD4 binding. Residue changes such as S375W that fill the Phe 43 cavity and predispose the gp120 glycoprotein to assume a CD4-bound conformation (49) would also be expected to limit the ability of these drugs to access their binding sites on the virus.
In addition to their potential clinical role, BMS-806 and related compounds are interesting tools to study events in HIV-1 entry that occur after receptor binding. Insights into the envelope glycoprotein structures involved in receptor-induced activation of membrane fusion may facilitate the improvement of drugs targeting this process.
.
Acknowledgments
We thank Yvette McLaughlin and Sheri Farnum for manuscript preparation.
This study was supported by grants from the National Institutes of Health (AI24755, AI39420, and PO1-GM56550), the Bristol-Myers Squibb Foundation, the International AIDS Vaccine Initiative, and the late William F. McCarty-Cooper. N.M. was supported by an NRSA postdoctoral fellowship (F32 NS43260 M) from the National Institutes of Health. J.M.C. was supported by an American Cancer Society postdoctoral fellowship (PF-03-005-01-CDD) with contributions by the National Fisheries Institute.
REFERENCES
- 1.Barre-Sinoussi, F., J. C. Chermann, F. Rey, M. T. Nugeyre, S. Chamaret, J. Gruest, C. Dauguet, C. Axler-Blin, F. Vezinet-Brun, C. Rouzioux, W. Rozenbaum, and L. Montagnier. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220:868-871. [DOI] [PubMed] [Google Scholar]
- 2.Blair, W. S., M. Deshpande, H. Fang, P.-F. Lin, T. P. Spicer, O. B. Wallace, T. Wang, Z. Zhang, and K.-S. Yeung. 21 December 2000. Antiviral indoleoxoacetyl piperazine derivatives. International Publication Number WO 00/76521 A1. International application patent number PCT/US00/14359.
- 3.Cao, J., L. Bergeron, E. Helseth, M. Thali, H. Repke, and J. Sodroski. 1993. Effects of amino acid changes in the extracellular domain of the human immunodeficiency virus type 1 gp41 envelope glycoprotein. J. Virol. 67:2747-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao, J., N. Sullivan, E. Desjardin, C. Parolin, J. Robinson, R. Wyatt, and J. Sodroski. 1997. Replication and neutralization of human immunodeficiency virus type 1 lacking the V1 and V2 variable loops of the gp120 envelope glycoprotein. J. Virol. 71:9808-9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao, J., B. Vasir, and J. G. Sodroski. 1994. Changes in the cytopathic effects of human immunodeficiency virus type 1 associated with a single amino acid alteration in the ectodomain of the gp41 transmembrane glycoprotein. J. Virol. 68:4662-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. [DOI] [PubMed] [Google Scholar]
- 7.Choe, H., M. Farzan, Y. Sun, N. Sullivan, B. Rollins, P. D. Ponath, L. Wu, C. R. Mackay, G. LaRosa, W. Newman, N. Gerard, C. Gerard, and J. Sodroski. 1996. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85:1135-1148. [DOI] [PubMed] [Google Scholar]
- 8.Dalgleish, A. G., P. C. Beverley, P. R. Clapham, D. H. Crawford, M. F. Greaves, and R. A. Weiss. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:763-767. [DOI] [PubMed] [Google Scholar]
- 9.Dragic, T., V. Litwin, G. P. Allaway, S. R. Martin, Y. Huang, K. A. Nagashima, C. Cayanan, P. J. Maddon, R. A. Koup, J. P. Moore, and W. A. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC- CKR-5. Nature 381:667-673. [DOI] [PubMed] [Google Scholar]
- 10.Feng, Y., C. C. Broder, P. E. Kennedy, and E. A. Berger. 1996. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272:872-877. [DOI] [PubMed] [Google Scholar]
- 11.Furuta, R. A., C. T. Wild, Y. Weng, and C. D. Weiss. 1998. Capture of an early fusion-active conformation of HIV-1 gp41. Nat. Struct. Biol. 5:276-279. [DOI] [PubMed] [Google Scholar]
- 12.Gallo, R. C., S. Z. Salahuddin, M. Popovic, G. M. Shearer, M. Kaplan, B. F. Haynes, T. J. Palker, R. Redfield, J. Oleske, B. Safai, et al. 1984. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science 224:500-503. [DOI] [PubMed] [Google Scholar]
- 12a.Guo, Q., H.-T. Ho, I. Dicker, L. Fan, N. Zhou, J. Friborg, T. Wang, B. V. McAuliffe, H.-G. H. Wang, R. E. Rose, H. Fang, H. T. Scarnati, D. R. Langely, N. A. Meanwell, R. Abraham, R. J. Colonno, and P.-F. Lin. 2003. Biochemical and genetic characterizations of a novel human immunodeficiency virus type 1 inhibitor that blocks gp120-CD4 interactions. J. Virol. 77:10528-10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He, Y., R. Vassell, M. Zaitseva, N. Nguyen, Z. Yang, Y. Weng, and C. D. Weiss. 2003. Peptides trap the human immunodeficiency virus type 1 envelope glycoprotein fusion intermediate at two sites. J. Virol. 77:1666-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klatzmann, D., E. Champagne, S. Chamaret, J. Gruest, D. Guetard, T. Hercend, J. C. Gluckman, and L. Montagnier. 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312:767-768. [DOI] [PubMed] [Google Scholar]
- 15.Kolchinsky, P., E. Kiprilov, P. Bartley, R. Rubinstein, and J. Sodroski. 2001. Loss of a single N-linked glycan allows CD4-independent human immunodeficiency virus type 1 infection by altering the position of the gp120 V1/V2 variable loops. J. Virol. 75:3435-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korber, B., B. Foley, C. Kuiken, S. Pillai, and J. Sodroski. 1998. Numbering positions in HIV relative to HXBc2: human retroviruses and AIDS 1998. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 17.Koshiba, T., and D. C. Chan. 2003. The prefusogenic intermediate of HIV-1 gp41 contains exposed C-peptide regions. J. Biol. Chem. 278:7573-7579. [DOI] [PubMed] [Google Scholar]
- 18.Kunkel, T. A., J. D. Roberts, and R. A. Zakour. 1987. Rapid and efficient site-directed mutagenesis without phenotypic selection. Methods Enzymol. 154:367-382. [DOI] [PubMed] [Google Scholar]
- 19.Kwong, P. D., R. Wyatt, S. Majeed, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 2000. Structures of HIV-1 gp120 envelope glycoproteins from laboratory-adapted and primary isolates. Struct. Fold Des. 8:1329-1339. [DOI] [PubMed] [Google Scholar]
- 20.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonard, C. K., M. W. Spellman, L. Riddle, R. J. Harris, J. N. Thomas, and T. J. Gregory. 1990. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J. Biol. Chem. 265:10373-10382. [PubMed] [Google Scholar]
- 22.Lin, P. F., W. Blair, T. Wang, T. Spicer, Q. Guo, N. Zhou, Y. F. Gong, H. G. Wang, R. Rose, G. Yamanaka, B. Robinson, C. B. Li, R. Fridell, C. Deminie, G. Demers, Z. Yang, L. Zadjura, N. Meanwell, and R. Colonno. 2003. A small molecule HIV-1 inhibitor that targets the HIV-1 envelope and inhibits CD4 receptor binding. Proc. Natl. Acad. Sci. USA 100:11013-11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.McMartin, C., and R. S. Bohacek. 1997. QXP: powerful, rapid computer algorithms for structure-based design. J. Comput. Aided Mol. Des. 11:333-344. [DOI] [PubMed] [Google Scholar]
- 23.Mirzabekov, T., N. Bannert, M. Farzan, W. Hofmann, P. Kolchinsky, L. Wu, R. Wyatt, and J. Sodroski. 1999. Enhanced expression, native purification, and characterization of CCR5, a principal HIV-1 coreceptor. J. Biol. Chem. 274:28745-52870. [DOI] [PubMed] [Google Scholar]
- 24.Modrow, S., B. H. Hans, G. M. Shaw, F. W.-S. R. C. Gallo, and H. Wolf. 1987. Computer assisted analysis of envelope protein sequences of seven human immunodeficiency virus isolates: prediction of antigenic epitopes in conserved and variable regions. J. Virol. 61:570-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore, J. P., Q. J. Sattentau, R. Wyatt, and J. Sodroski. 1994. Probing the structure of the human immunodeficiency virus surface glycoprotein gp120 with a panel of monoclonal antibodies. J. Virol. 68:469-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore, J. P., and J. Sodroski. 1996. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J. Virol. 70:1863-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myszka, D. G., R. W. Sweet, P. Hensley, M. Brigham-Burke, P. D. Kwong, W. A. Hendrickson, R. Wyatt, J. Sodroski, and M. L. Doyle. 2000. Energetics of the HIV gp120-CD4 binding reaction. Proc. Natl. Acad. Sci. USA 97:9026-9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olshevsky, U., E. Helseth, C. Furman, J. Li, W. Haseltine, and J. Sodroski. 1990. Identification of individual human immunodeficiency virus type 1 gp120 amino acids important for CD4 receptor binding. J. Virol. 64:5701-5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reitz, M. S., Jr., C. Wilson, C. Naugle, R. C. Gallo, and M. Robert-Guroff. 1988. Generation of a neutralization-resistant variant of HIV-1 is due to selection for a point mutation in the envelope gene. Cell 54:57-63. [DOI] [PubMed] [Google Scholar]
- 30.Rho, H. M., B. Poiesz, F. W. Ruscetti, and R. C. Gallo. 1981. Characterization of the reverse transcriptase from a new retrovirus (HTLV) produced by a human cutaneous T-cell lymphoma cell line. Virology 112:355-360. [DOI] [PubMed] [Google Scholar]
- 31.Sattentau, Q. J., and J. P. Moore. 1995. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J. Exp. Med. 182:185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Si, Z., M. Cayabyab, and J. Sodroski. 2001. Envelope glycoprotein determinants of neutralization resistance in a simian-human immunodeficiency virus (SHIV-HXBc2P 3.2) derived by passage in monkeys. J. Virol. 75:4208-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Si, Z., N. Madani, J. M. Cox, J. J. Chruma, J. C. Klein, A. Schorn, N. Phan, L. Wang, A. C. Biorn, S. Cocklin, I. Chaiken, E. Freire, A. B. Smith III, and J. G. Sodroski. Small-molecule inhibitors of HIV-1 entry block receptor-induced conformational changes in the viral envelope glycoproteins. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 33.Starcich, B. R., B. H. Hahn, G. M. Shaw, P. D. McNeely, S. Modrow, and H. Wolf. 1986. Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell 45:637-648. [DOI] [PubMed] [Google Scholar]
- 34.Tan, K., J. Liu, J. Wang, S. Shen, and M. Lu. 1997. Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc. Natl. Acad. Sci. USA 94:12303-12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thali, M., U. Olshevsky, C. Furman, D. Gabuzda, M. Posner, and J. Sodroski. 1991. Characterization of a discontinuous human immunodeficiency virus type 1 gp120 epitope recognized by a broadly reactive neutralizing human monoclonal antibody. J. Virol. 65:6188-6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thali, M., U. Olshevsky, C. Furman, D. Gabuzda, J. Li, and J. Sodroski. 1991. Effects of changes in gp120-CD4 binding affinity on HIV-1 envelope glycoprotein function and soluble CD4 sensitivity. J. Virol. 65:5007-5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thali, M., C. Furman, D. Ho, J. Robinson, S. Tilley, A. Pinter, and J. Sodroski. 1992. Discontinuous, conserved neutralization epitopes overlapping the CD4 binding region of the HIV-1 gp120 envelope glycoprotein. J. Virol. 66:5635-5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thali, M., J. P. Moore, C. Furman, M. Charles, D. D. Ho, J. Robinson, and J. Sodroski. 1993. Characterization of conserved human immunodeficiency virus type 1 (HIV-1) gp120 neutralization epitopes exposed upon gp120-CD4 binding. J. Virol. 67:3978-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thali, M., M. Charles, C. Furman, L. Cavacini, M. Posner, J. Robinson, and J. Sodroski. 1994. Resistance to neutralization by broadly reactive antibodies to the human immunodeficiency virus type 1 gp120 glycoprotein conferred by a gp41 amino acid change. J. Virol. 68:674-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trkola, A., T. Dragic, J. Arthos, J. M. Binley, W. C. Olson, G. P. Allaway, C. Cheng-Mayer, J. Robinson, P. J. Maddon, and J. P. Moore. 1996. CD4-dependent, antibody-sensitive interactions between HIV-1 and its coreceptor CCR-5. Nature 384:184-187. [DOI] [PubMed] [Google Scholar]
- 42.Wang, T., Z. Zhang, O. B. Wallace, M. Deshpande, H. Fang, Z. Yang, L. M. Zadjura, D. L. Tweedie, S. Huang, FR. Zhao, S. Ranadive, B. S. Robinson, Y.-F. Gong, K. Ricarddi, T. P. Spicer, C. Deminie, R. Rose, H.-G. H. Wang, W. S. Blair, P.-Y. Shi, P.-F. Lin, R. J. Colonno, and N. A. Meanwell. 2003. Discovery of 4-benzoyl-1-[(4-methoxy-1H-pyrrolo[2,3-b]pyridin-3-yl)oxoacetyl]-2-(R)-methylpiperazine (BMS-378806): a novel HIV-1 attachment inhibitor that interferes with CD4-gp120 interactions. J. Med. Chem. 46:4236-4239. [DOI] [PubMed] [Google Scholar]
- 43.Weissenhorn, W., A. Dessen, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1997. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387:426-430. [DOI] [PubMed] [Google Scholar]
- 44.Wu, L., N. P. Gerard, R. Wyatt, H. Choe, C. Parolin, N. Ruffing, A. Borsetti, A. A. Cardoso, E. Desjardin, W. Newman, C. Gerard, and J. Sodroski. 1996. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384:179-183. [DOI] [PubMed] [Google Scholar]
- 45.Wyatt, R., N. Sullivan, M. Thali, H. Repke, D. Ho, J. Robinson, M. Posner, and J. Sodroski. 1993. Functional and immunologic characterization of human immunodeficiency virus type 1 envelope glycoproteins containing deletions of the major variable regions. J. Virol. 67:4557-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wyatt, R., P. D. Kwong, E. Desjardins, R. W. Sweet, J. Robinson, W. A. Hendrickson, and J. G. Sodroski. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705-711. [DOI] [PubMed] [Google Scholar]
- 47.Wyatt, R., J. Moore, M. Accola, E. Desjardin, J. Robinson, and J. Sodroski. 1995. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J. Virol. 69:5723-5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884-1888. [DOI] [PubMed] [Google Scholar]
- 49.Xiang, S. H., P. D. Kwong, R. Gupta, C. D. Rizzuto, D. J. Casper, R. Wyatt, L. Wang, W. A. Hendrickson, M. L. Doyle, and J. Sodroski. 2002. Mutagenic stabilization and/or disruption of a CD4-bound state reveals distinct conformations of the human immunodeficiency virus type 1 gp120 envelope glycoprotein. J. Virol. 76:9888-9899. [DOI] [PMC free article] [PubMed] [Google Scholar]