Abstract
Strong experimental evidence suggests the involvement of photo-oxidative stress mediated by reactive oxygen species as a crucial mechanism of solar damage relevant to human skin photoaging and photocarcinogenesis. Based on the established role of antioxidant response element (ARE)-mediated gene expression in cancer chemoprevention, we tested the hypothesis that small molecule Nrf2-activators may serve a photo-chemopreventive role by targeting skin cell photo-oxidative stress. A luciferase-based reporter gene assay was used as a primary screen for the identification of novel agents that modulate the Nrf2-Keap1 signaling pathway. A series of cinnamoyl-based electrophilic Michael acceptors including cinnamic aldehyde and methyl-1-cinnamoyl-5-oxo-2-pyrrolidine-carboxylate was identified as potent Nrf2-activators. Hit confirmation was performed in a secondary screen, based on immunodetection of Nrf2 protein upregulation in human Hs27 skin fibroblasts, HaCaT keratinocytes, and primary skin keratinocytes. Bioefficacy profiling of positive test compounds in skin cells demonstrated compound-induced upregulation of hemeoxygenase I and NAD(P)H-quinone oxidoreductase, two Nrf2 target genes involved in the cellular antioxidant response. Pretreatment with cinnamoyl-based Nrf2-activators suppressed intracellular oxidative stress and protected against photo-oxidative induction of apoptosis in skin cells exposed to high doses of singlet oxygen. Our pilot studies suggest feasibility of developing cinnamoyl-based Nrf2-activators as novel photo-chemopreventive agents targeting skin cell photo-oxidative stress.
Keywords: Nrf2, skin cancer, photo-oxidative stress, photo-chemoprevention, Michael acceptor, cinnamic aldehyde, singlet oxygen
Introduction
Solar radiation is a potent environmental human carcinogen as summarized in the Eleventh Report on Carcinogens of the National Institute of Environmental Health Sciences (NIEHS, 2005, available at ntp.niehs.nih.gov) [1-4]. Nonmelanoma skin cancers (NMSC) constitute more than one third of all human cancers in the U.S. and the rate of increase in melanoma occurrence now surpasses that of any other human cancer [5-7]. The mechanisms by which solar UV-irradiation causes skin photodamage are wavelength dependent [3, 8, 9]. UVB (290-320 nm) is thought to cause direct structural damage to DNA in the form of epidermal cyclobutane pyrimidine dimers (CPD) and other photoproducts. Most of the solar UV energy incident on human skin derives from the deeply penetrating UVA region (> 95%, 320-400 nm) that is not directly absorbed by DNA. Accumulative evidence suggests that UVA-induced photodamage and carcinogenesis of human skin occur by photo-oxidative mechanisms mediated by reactive oxygen species (ROS) [9-14]. Recent research indicates that light-driven redoxcycling of non-DNA skin chromophores (including porphyrins, riboflavin, and collagen crosslinks) acting as endogenous photosensitizers is a major source of ROS in UVA-exposed human skin [2, 4, 12, 14-18, 47]. The molecular consequences downstream of light-driven ROS production on skin structural integrity, signal transduction, gene expression and ultimately tumorigenic initiation and progression are well established [6]. Targeting ROS is therefore an important strategy for skin photoprotection and photo-chemoprevention [4, 19, 20]. Moderate skin photoprotection by topical application of antioxidants has been demonstrated in many experiments on animal and human skin, and inhibition of mouse skin photocarcinogenesis by these agents has been observed as reviewed recently [2]. However, the therapeutic efficacy of topical antioxidants is limited by their sacrificial depletion, potential photodegradation, and spontaneous UV-enhanced redox chemistry which can exert prooxidant effects and interfere with physiological redox signaling [4, 21]. Due to these limitations, topical antioxidants can only play an adjuvant role in skin protection against photo-oxidative damage. Another strategy that aims at enhancing skin resistance to environmental oxidative insult is based on recent research that reveals the role of the transcription factor Nrf2 (NF-E2-related factor 2) in coordinating the cellular antioxidant response [22-31]. Nrf2 binds to cis-acting elements in the promoters of target genes, called antioxidant response elements (ARE) or electrophilic response elements. Nrf2 binding induces expression of major phase II detoxification and antioxidant defense enzymes including NAD(P)H:quinone oxidoreductase (NQO1), several glutathione S-transferases, γ-glutamylcysteine synthase heavy and light subunit, heme oxygenase 1 (HO-1), ferritin, and peroxiredoxin 1 [32, 33]. Remarkably, heterogeneous electrophilic pharmacophores contained in many chemopreventive prototype agents such as the β-dicarbonyl compound curcumin, the isothiocyanate sulforaphane, and the dithiolethione oltipraz, activate Nrf2-dependent gene expression [25, 27, 30, 34, 35]. These thiol-reactive, electrophilic pharmacophores activate Nrf2 through inhibition of Keap1-mediated degradation, followed by nuclear translocation of Nrf2 and induction of ARE-dependent gene expression [22-27, 30, 36]. Thus, oxidative modification of critical cysteine residues of Keap1 (including C151, C273, C288) by heterogeneous electrophilic agents and ROS is now recognized as the critical trigger that initiates the upregulation of the cellular antioxidant response and is therefore an attractive molecular target for the development of novel chemopreventive agents. Indeed, germ line Nrf2-null mice are highly susceptible to electrophilic and oxidative stress [35]. Moreover, recent studies strongly suggest a role of Nrf2-mediated gene expression in the suppression of acute photo-oxidative damage in skin cells and also in the prevention of epidermal chemical (TPA/DMBA-induced) and UV-induced carcinogenesis [37-39]. In the skin of Nrf2 knockout mice, UVB-irradiation induced a more pronounced sunburn reaction and oxidative DNA damage as compared to wildtype mice, but no alterations in UVB-induced carcinogenesis were observed [40]. However, pharmacological induction of Nrf2 by the chemopreventive activator sulforaphane has been demonstrated to protect retinal pigment epithelial cells against photo-oxidative damage by upregulating NQO1 expression and elevating cellular glutathione content [39]. The same study demonstrated increased photosensitivity of fibroblasts from Nrf2 knockout mouse embryonic fibroblasts. Other studies report sulforaphane-inhibition of UV-induced AP-1 activation in human keratinocytes and protection against UV-induced skin carcinogenesis in SKH-1 high-risk mice by sulforaphane-containing broccoli sprout extracts [41]. Most recently, human skin photoprotection as assessed by reduced susceptibility to UVB-induced erythema was achieved by topical application of sulforaphane-rich broccoli extracts that induced up-regulation of phase 2 enzymes [29].
Based on the hypothesis that topical Nrf2-activators could serve a chemopreventive role by suppressing photo-oxidative pathways involved in skin photocarcinogenesis and photoaging, we have established a rapid screening method that identifies Nrf2-activators and examined their potential photo-chemopreventive activity in cell-based assays. Here, we report the identification of cinnamoyl-based Nrf2-activators that protect cultured human skin keratinocytes and fibroblasts against photo-oxidative stress.
Material and Methods
Chemicals
Most chemicals were from Sigma Chemical Co, St. Louis, MO, including R715360 (methyl-1-cinnamoyl-5-oxo-2-pyrrolidine-carboxylate) taken from the Sigma-Aldrich Rare Chemical Library (SA-RCL).
Chemical Synthesis
N-(1’-Oxo-3’-phenylprop-2’-enyl)pyrrolidine-2-carboxylic Acid ( N-cinnamoyl-L-proline, ProCA)
Synthesis of ProCA was completed following a published procedure [42]. Briefly, to a stirred solution of L-proline (5.7 g) in 1N NaOH (50 mL) prepared and maintained at 0 °C was added dropwise phenylpropanoyl chloride (16.7 g, 2 M in 1N NaOH) with stirring. Sufficient 2N NaOH was added to maintain the solution at alkaline pH using phenolphthalein as indicator. Stirring was continued for an additional hour before acidifying the solution using 2N HCl and Congo red as indicator. After stirring for an additional 30 min, the precipitate was collected and washed with ice-cold water to yield the crude product (3.88 g, 15.8 mmol, 32%). 500 mg of crude product was recrystallized from ethanol (270 mg, 1.10 mmol, 54%) and characterized by mass spectrometry {(ESI+): m/z calculated for C14H15O3N: 245.2; observed 246.0 [M+H]+} and 1H-NMR {(400 MHz, D6-DMSO): δ 1.90 (2H, m), 2.20 (2H, m), 3.60 (2H, m, H2O overlap), 3.80 (1H, t), 4.85 (1H, d), 7.05 (1H, d), 7.40 (3H, m), 7.75 (2H, d), 12.55 (1H, br s).
General cell culture
Human MDA-MB-231 breast carcinoma cells from ATCC (Manassas, VA, USA) were cultured in MEM supplemented with 10% fetal bovine serum, 25 mM HEPES, 26 mM sodium bicarbonate, 5000 units/mL penicillin G, 5000 ug/mL streptomycin, and 6 ng/mL bovine insulin (Sigma, St. Louis, MO). Dermal neonatal foreskin Hs27 fibroblasts from ATCC and human immortalized HaCaT keratinocytes were cultured in DMEM containing 10% fetal bovine serum. Cells were maintained at 37 °C in 5% CO2, 95% air in a humidified incubator. Primary human epidermal keratinocytes (neonatal HEKn-APF, from Cascade Biologics, Portland, OR) were cultured using Epilife medium supplemented with EDGS growth supplement and passaged using recombinant trypsin/EDTA and defined trypsin inhibitor.
Determination of comparative Michael acceptor reactivities of test compounds
Thiol adduction of CA, proCA, and oxoProCA was followed by UV-spectroscopy following a recently published standard procedure with minor modifications [43]. In brief, in a 1.5 ml UV-cuvette containing 500 μl acetonitrile, 500 μl of a 100 mM stock Tris-HCl buffer (pH 8.8), and 1 μl of a 10 mM stock of test compound in DMSO, the model thiol β-mercaptoethanol (40 μl, 100 mM stock in PBS) was added. The time course of loss of UV-absorbance at the wavelength of maximal enone-pharmacophore absorbance [λmax (CA): 290 nm; λmax (ProCA): 280 nm; λmax (oxoProCA): 300 nm] was determined over 120 min after initiation of reaction and expressed for every time point (tx) as percent of initial absorbance Aλmax(t0) using the formula:
In order to compare relative potencies as Michael acceptors, the relative initial reaction rates of CA-, ProCA-, and oxoProCA-adduction by β-mercaptoethanol were calculated by determining the ratio between the slopes of the linear graphs obtained during the first 120 min of thiol-adduction of test compounds.
Nrf2 reporter gene assay
Regulation of Nrf2-dependent transcriptional activity by test compounds was examined as published recently [24, 30, 44]. Briefly, human MDA-MB-231 breast carcinoma cells were transfected using Lipofectamine Plus (Gibco BRL) according to the manufacturer's instructions. DNA amounts in each transfection were kept constant by addition of empty pcDNA3 plasmid. Cells were cotransfected with the NQO1-ARE TATA-Inr firefly luciferase reporter plasmid pARE-Luc (Fig. 1A) together with expression plasmids for Nrf2, Keap1, and an additional plasmid encoding renilla luciferase driven by the herpes simplex virus thymidine kinase promoter to normalize for transfection efficiency. Cells were treated with the test compounds for 16 hours prior to cell lysis for analysis of reporter gene activity. Reporter assays were performed using the Promega Dual-luciferase reporter gene assay system. All samples were run in duplicate for each experiment and the data represent the means of three independent experiments.
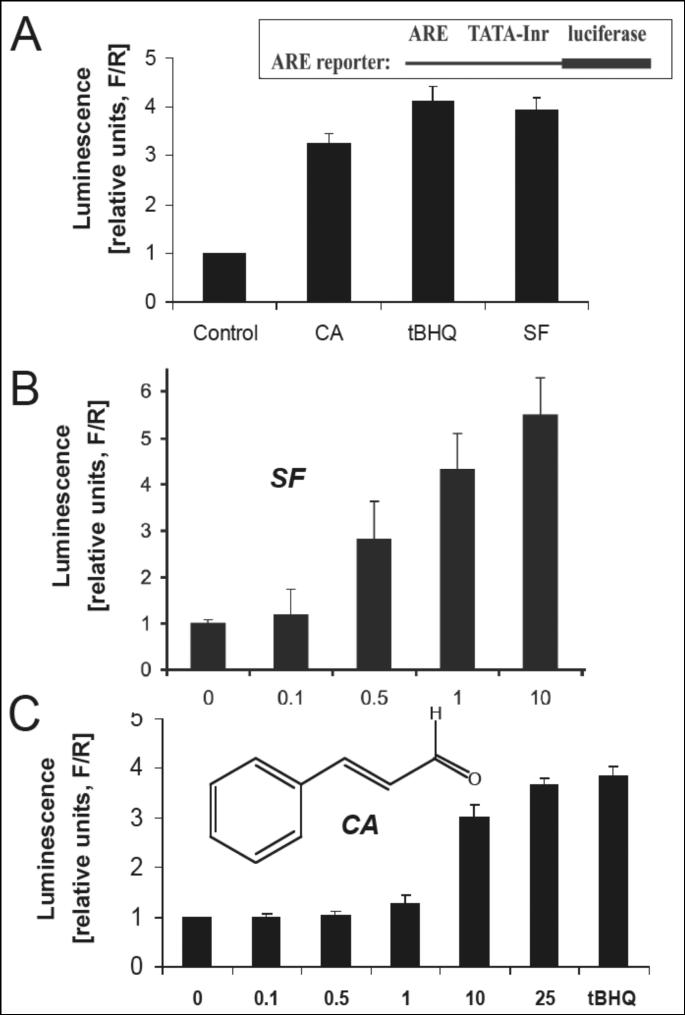
Figure 1. Nrf2 transcriptional activation by established Nrf2-inducers and cinnamic aldehyde.
(A) MDA-MB-231 cells were cotransfected with plasmids containing an ARE-dependent firefly luciferase (F) reporter gene (construct as shown) and expression plasmids for the Nrf2 and Keap1 proteins. A plasmid encoding renilla luciferase (R), driven by the herpes simplex virus thymidine kinase promoter, was included in all transfections to normalize transfection efficiency. Cells were treated with tBHQ (50 μM), SF (10 μM), and CA (10 μM) for 16 h. (B) Dose response of Nrf2 transcriptional activation by SF. (C) Dose response of Nrf2 transcriptional activation by CA. Potency of fold-induction is expressed as relative luminescence units (R/F) versus untreated control transfectants (mean ± SD, n=3).
HO-1 immunoblot analysis
One day before treatment, 2×106 cells were seeded in T-75 flasks. Cell growth medium was replaced 24 h after seeding, followed by addition of test compounds 60 min after medium change. Cells were incubated for 24 h (37 °C, 5% CO2), then washed with PBS, lysed in 1x SDS-PAGE sample buffer (200 μl, 0.375 M Tris HCl pH 6.8, 50% glycerol, 10% SDS, 5% β-mercaptoethanol, 0.25% bromophenol blue), and heated for 3 min at 95 °C. Samples (10 μL, containing approximately 45 μg total protein as determined by the BCA assay) were separated by 15% SDS-PAGE followed by immediate transfer to nitrocellulose membranes (Optitran, Whatman, Bedford, MA). The membrane was blocked with 5% milk in 0.1% PBST for 1 h. Rabbit anti-HO-1 polyclonal antibody (Stressgen Bioreagents, Ann Arbor, MI) was used 1:5000 in 5% milk-PBST overnight at 4° C. The membrane was washed three times for 10 min in 0.1% PBST before adding HRP-conjugated goat anti-rabbit antibody (Jackson Immunological Research, West Grove, PA) at 1:10,000 dilution. Protein detection was accomplished using enhanced chemiluminescence detection reagents. Equal protein loading was examined by actin-detection using a mouse anti-actin monoclonal antibody (Sigma, Saint Louis, MO) at 1:1500 for 2 h. After three times 10 min wash in 0.5% PBST, incubation with secondary antibody and chemiluminescence detection were performed as above.
Nrf2 Immunoblot analysis
For detection of compound-induced modulation of Nrf2 protein levels, cultured human skin cells were treated with the test compound for 4 hours prior to lysis in sample buffer (50 mM Tris-HCl [pH 6.8], 2% SDS, 10% Glycerol, 100 mM DTT, 0.1% bromophenol blue). After boiling the samples, the proteins were separated by 7.5% SDS-PAGE, and subjected to immunoblot analysis with anti-Nrf2 (1:2000 dilution, H-300, rabbit polyclonal antibody) and anti-tubulin antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) as published recently [44]. Subcellular fractionation was performed using the NXTRACT NuCLEAR™ Extraction Kit from Sigma and immunodetection of lamin A was performed as loading control for the nuclear fraction. Nrf2 immunodetection on 10% SDS-polyacrylamide gels revealed a single Nrf2 band, whereas analysis on 7.5% gels revealed a double band as observed earlier [44].
NQO1, HO-1, CAT, and MT2A expression analysis by real time RT-PCR
Total cellular RNA (3×106 cells) was prepared according to a standard procedure using the RNEasy kit from Qiagen. Reverse transcription was performed using TaqMan Reverse Transcription Reagents (Roche Molecular Systems, Branchburg, NJ) and 200 ng of total RNA in a 50-μl reaction. Reverse transcription was primed with random hexamers and incubated at 25 °C for 10 minutes followed by 48 °C for 30 minutes, 95°C for 5 minutes, and a chill at 4 °C. Each PCR reaction consisted of 3.75 μl of cDNA added to 12.5 μl of TaqMan Universal PCR Master Mix (Roche Molecular Systems), 1.25 μl of gene-specific primer/probe mix (Assays-by-Design; Applied Biosystems, Foster City, CA) and 7.5 μl of PCR water. PCR conditions were: 95 °C for 10 minutes, followed by 40 cycles of 95 °C for 15 seconds, alternating with 60 °C for 1 minute using an Applied Biosystems 7000 SDS and Applied Biosystems’ Assays On Demand primers specific to NQO1 (assay ID Hs00168547_m1), HO-1 (assay ID Hs00157965_m1), CAT (catalase, assay ID Hs00156308_m1), MT2A (metallothionein 2A, assay ID Hs02379661_g1), and GAPDH (assay ID Hs99999905_m1). Gene-specific product was normalized to GAPDH and quantified using the comparative (ΔΔCt) Ct method as described in the ABI Prism 7000 sequence detection system user guide. Expression values were averaged across two independent experiments and standard error of the mean was calculated for graphing.
Measurement of NQO1-specific enzymatic activity
Determination of NQO1 specific activity was performed according to a published standard procedure [46]. In brief, cells (2 × 106) were harvested by trysinization and resuspended in ice-cold TE (20 mM Tris-HCl with 2 mM EDTA, pH 7.4). Cells were disrupted in three cycles of freeze/thawing using liquid nitrogen and a 37°C waterbath, followed by centrifugation (12,000 g, 5 min). Protein concentration in the supernatant was determined using the BCA assay (Pierce). For determination of NQO1 specific activity, the reaction mixture (1 mL final volume) contained: 25 mM Tris-HCl (pH 7.4), 180 μM NADPH, BSA (0.2 mg/mL), Tween 20 [0.01 % (v/v)], and cell lysate (5 μl). The reaction was started by the addition of 2 μL 2,6-dichlorophenolindophenol (DCPIP, 20 mM stock in DMSO). Reduction of DCPIP was measured at room temperature for 1 min at 600 nm (ε = 21 × 103 M-1cm-1) with or without 20 μM dicoumarol. The dicoumarol-inhibitable part of DCPIP reduction was used to calculate NQO1 activity expressed as nmol DCPIP/mg protein/min. A minimum of triplicate cultures were assayed.
Assay for human skin cell protection against photo-oxidative induction of apoptosis
Cells (100,000 per 35 mm dish) were pretreated (24 h) with various concentrations of Nrf2 activator test compound and then exposed to photo-oxidative stress using a validated singlet oxygen (1O2)-generating dye sensitization system as published earlier [47]. In brief, toluidine blue O (TB) photosensitization was achieved using a ‘Sylvania 15 W Cool White’ light tube delivering visible light at an irradiance of 4.29 mW/cm2. The irradiance in the visible region (400-700 nm) was determined using a spectroradiometer, Model 754 from Optronic Laboratories (Orlando, FL). Cells received visible radiation at a distance of 50 mm from the source through the polystyrene lids of cell culture dishes. For 1O2 exposure, cells pretreated with test compounds were washed with PBS and immediately exposed to the combined action of visible light (1.8 J/cm2) and toluidine blue O [TB, 3.3 μM in PBS] as sensitizer dye. Following 5 min incubation in the dark after irradiation, the cells were washed with PBS, fresh culture medium was added, and cells were returned to the incubator. Induction of cell death (early and late apoptosis/necrosis) was then examined 24 h after exposure by annexin-V-FITC/propidium iodide (PI) dual staining followed by flow cytometric analysis using an apoptosis detection kit (APO-AF, Sigma, St. Louis, MO) as published previously [16, 48].
Detection of intracellular oxidative stress by flow cytometric analysis
Photodynamic induction of intracellular oxidative stress and its suppression by Nrf2-activators was analyzed by flow cytometry using 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) as a sensitive non-fluorescent precursor dye according to a published standard procedure [45, 49]. Human Hs27 skin fibroblasts were pretreated with test compounds for 24 h and then exposed to singlet oxygen generated by dye-sensitization as described above (visible light dose 0.3 J/cm2), followed by DCFH-DA loading. Cells were incubated for 60 min in the dark (37°C, 5% CO2) with culture medium containing DCFH-DA (5 μg/mL final concentration). Cells were then washed with PBS, harvested by trypsinization, resuspended in 300 μl PBS and immediately analyzed by flow cytometry.
Statistical Analysis
Unless indicated differently, the results are presented as means ± S.D. of at least three independent experiments.
Results
Identification of cinnamoyl-based Michael acceptors as Nrf2-activators using a luciferase reporter assay-based screen
The causative role of oxidative stress in skin cell photodamage led us to explore the feasibility of using novel Nrf2-activators as potential photo-chemopreventive modulators of the skin cell antioxidant response.
First, a cell-based screening assay for the rapid identification of small molecule Nrf2-activators was established as published earlier [24, 30, 44]. A dual-luciferase reporter gene assay allowed detection of Nrf2 transcriptional activation in MDA-MB-231 cells cotransfected with plasmids containing an ARE-dependent firefly luciferase reporter gene and expression plasmids for the Nrf2 and Keap1 proteins as indicated in Fig. 1A. Based on the known activity of electrophiles containing an α,β-unsaturated carbonyl pharmacophore (‘Michael acceptors’) as potent inducers of the Nrf2 target gene NQO1, we examined various dietary unsaturated aldehydes and related compounds including dibenzoylmethane (from licorice), (R)- and (S)-carvone (from cumin), vanillin (from vanilla), and trans-cinnamic aldehyde (CA, from cinnamon) for activity as Nrf2 transcriptional activators. Among these test compounds, only CA exhibited activity as potent Nrf2 transcriptional activator (Fig. 1A and C). Remarkably, CA induced Nrf2 transcriptional activation at low micromolar levels (10 μM) being slightly less potent than the established Nrf2-inducer sulforaphane (Fig. 1B). The established Nrf2-inducer tert.-butylhydroquinone (tBHQ) required dosing at higher concentrations (50 μM) to achieve comparable levels of induction obtained with CA.
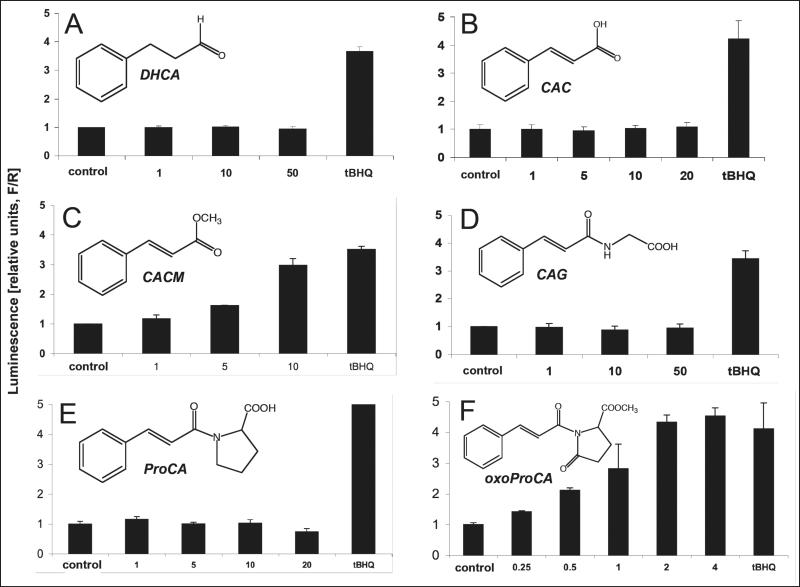
Next, the structure activity relationship of Nrf2 transcriptional activation by CA was explored in detail and more potent structural derivatives were identified as shown in Fig. 2. Consistent with the Michael acceptor pharmacophore serving as a structural prerequisite for Nrf2 activation by CA, the Michael-inactive dihydrocinnamic aldehyde (DHCA, 3-phenylpropionaldehyde), devoid of the α,β-unsaturated carbonyl pharmacophore, did not induce Nrf2 transcriptional activation even at high concentrations (50 μM, Fig. 2A). Equally, cinnamic acid (CAC), again devoid of Michael acceptor activity due to exchange of the aldehyde for a carboxylic acid group, was inactive over a broad dose range (Fig. 2B). In contrast, esterification of the carboxylic acid group as in cinnamic acid methylester (CACM) is expected to restore the Michael acceptor pharmacophore as published previously [43], and consequently, Nrf2 transcriptional activation was observed when MDA-MB-231 cells were treated with micromolar concentrations of CACM (Fig. 2C). In contrast, as observed with CAC, the cinnamic acid amide-derivative N-cinnamoylglycine (CAG), devoid of an electrophilic Michael pharmacophore due to carbonyl inactivation by amide formation, was Nrf2-inactive (Fig. 2D).
Figure 2. Nrf2 transcriptional activation by cinnamoyl-based Michael acceptors and Michael-inactive analogs.
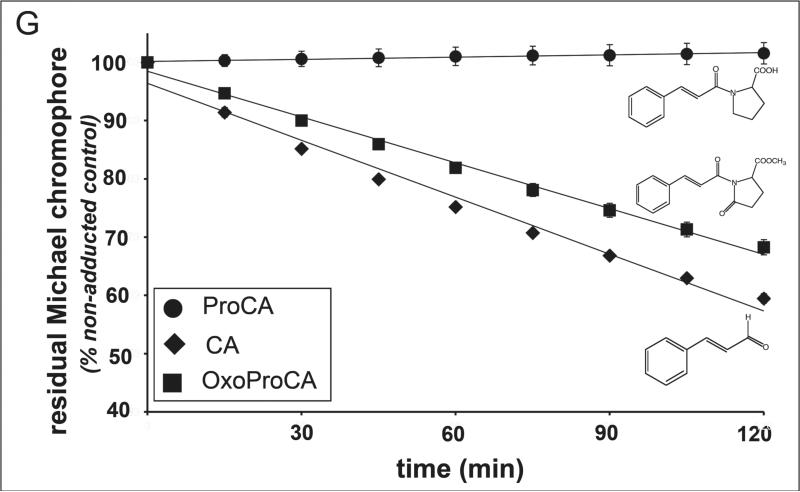
Dose response of Nrf2 transcriptional activation by Michael-active and -inactive cinnamoyl-derivatives: (A) dihydrocinnamic aldehyde (DHCA); (B) cinnamic acid (CAC); (C) cinnamic acid methylester (CACM); (D) N-cinnamoylglycine (CAG); (E) N-cinnamoyl-L-proline (ProCA); (F) N-cinnamoyl-5-oxo-L-proline methylester (oxoProCA); y-axis: potency of induction is expressed as relative luminescence units (R/F) versus untreated control transfectants (mean ± SD, n=3) as detailed above. (G) Comparative Michael acceptor reactivities of test compounds. Spontaneous adduction of CA (λmax: 290 nm), proCA (λmax: 280 nm), and oxoProCA (λmax: 300 nm), by the model thiol compound β-mercaptoethanol was followed by UV-spectroscopy over 120 min after initiation of the reaction as detailed in Materials and Methods. Time course of loss of UV-absorbance at the wavelength of maximal enone-pharmacophore absorbance was expressed for every time point as percent of initial absorbance (mean ± SEM, n=2).
In an attempt to explore the potential use of modified cinnamoyl-amides as Nrf2-activators we then synthesized the secondary amide N-cinnamoyl-L-proline (ProCA) that again was Nrf2-inactive, consistent with the peptide character of the secondary amide bond that would inactivate the Michael acceptor pharmacophore (Fig. 2E). Next, we tested methyl-1-cinnamoyl-5-oxo-2-pyrrolidine-carboxylate (N-cinnamoyl-5-oxo-L-proline methylester, oxoProCA), a close derivative of ProCA designed to exhibit considerable Michael acceptor reactivity due to introduction of an additional carbonyl group that forms an amide bond within the pyrrolidine ring system, thereby reconstituting the electrophilicity of the α,β-unsaturated carbonyl-pharmacophore of the N-cinnamoyl substituent (Fig. 2F). Remarkably, oxoProCA induced Nrf2 transcriptional activation in the submicromolar concentration range. Indeed, when the relative Michael acceptor reactivities of CA, proCA, and oxoProCA were determined by following thiol adduction of their unsaturated carbonyl chromophores using UV-spectroscopy, proCA was completely inert, but CA and oxoProCA were rapidly adducted by the model thiol β-mercaptoethanol (Fig. 2G). The relative initial rate of CA thiol-adduction, as extrapolated from the time course of Michael acceptor chromophore destruction, exceeded that of oxoProCA by approximately 1.3 fold, suggesting a slightly attenuated reactivity of oxoProCA as an electrophilic Michael acceptor when compared to CA. Comparative adduction kinetics and structure elucidation of reaction products between model thiols and a range of cinnamoyl-based Michael acceptors will be published elsewhere (Cabello and Wondrak, manuscript in preparation). Remarkably, in the reporter assay for Nrf2-transcriptional activity, oxoProCA was active at concentrations significantly lower than those required for Nrf2-activation by other positive test compound (tBHQ, CA, CACM) suggesting that in addition to chemical Michael acceptor potency additional parameters such as lipophilicity, membrane permeability, and metabolic deactivation may influence cell-based activity of test compounds.
Taken together, these findings demonstrate (I) feasibility of using our cell-based screen for the rapid identification of potent small molecule Nrf2 transcriptional activators and (II) feasibility of using the dietary natural product CA and synthetic derivatives including oxoProCA and CACM to achieve pharmacological upregulation of Nrf2 transcriptional activity.
Upregulation of Nrf2 protein levels in cultured human skin fibroblasts and keratinocytes exposed to cinnamoyl-based Michael acceptors
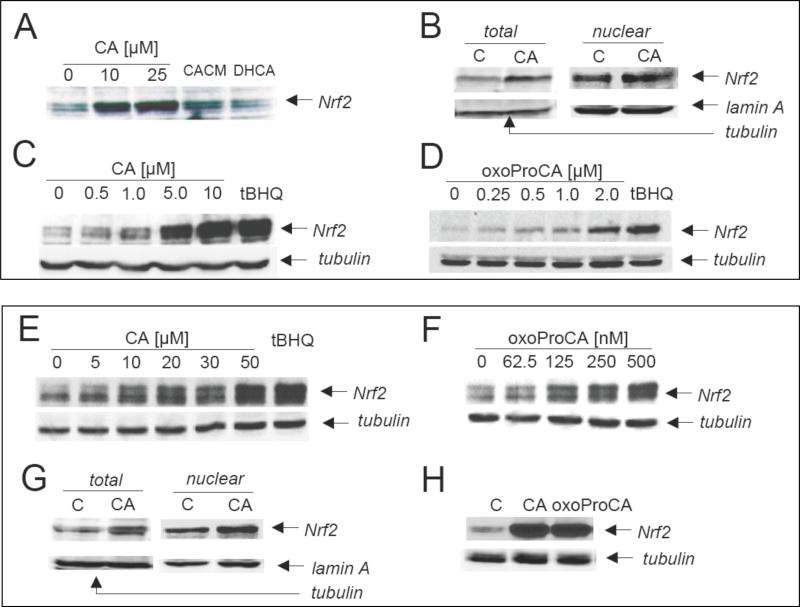
Nrf2-transcriptional activation by small molecule modulators generally occurs by interference with the Keap1-mediated proteasomal degradation of Nrf2 leading to upregulation of cellular Nrf2-protein levels [30, 36]. Therefore, positive hits identified in the luciferase-based reporter gene assay were confirmed by examining upregulation of Nrf2-protein levels in cultured skin cells. Consistent with transcriptional activation as observed above (Figs. 1 and 2), immunoblot analysis of Nrf2 levels indicated its accumulation in human dermal Hs27 fibroblasts exposed to CA and CACM, but not the Michael-inactive cinnamoyl-derivative DHCA (Fig. 3A). Moreover, the dose response of Nrf2 accumulation indicated that low micromolar concentrations of CA (Fig. 3C) and oxoproCA (Fig. 3D) are sufficient to induce Nrf2 protein upregulation in Hs27 fibroblasts. In addition, CA and oxoProCA treatment also upregulated Nrf2 protein levels in immortalized human skin HaCaT keratinocytes (Fig. 3E and F) and primary human skin keratinocytes (Fig. 3H). After subcellular fractionation, cinnamoyl-induced nuclear Nrf2 accumulation was observed in Hs27 and HaCaT keratinocytes (Fig. 3B and G).
Figure 3. Nrf2 protein upregulation by cinnamoyl-based Michael acceptors in cultured human skin cells.
Cultured human Hs27 skin fibroblasts (panels A-D), HaCaT keratinocytes (panels E-G), and primary skin keratinocytes (panel H) were exposed to the following agents: tBHQ (100 μM), DHCA (10 μM), CACM (10 μM), CA (dose range as indicated), and oxoProCA (dose range as indicated) for 4 h, and cellular Nrf2 protein levels were then examined in total cellular protein extracts by SDS-PAGE followed by immunoblot detection using enhanced chemiluminescence as detailed in Materials and Methods. Total and nuclear protein extracts were examined for Nrf2 levels in Hs27 fibroblasts (panel B) and HaCaT keratinocytes (panel G) exposed to CA (10 μM, 4 h) using subcellular fractionation as detailed in Materials and Methods.
Based on these data, it was concluded that cinnamoyl-induced Nrf2-activation (I) depends on upregulation of cellular Nrf2-protein levels, (II) is detectable in both fibroblasts and keratinocytes, and (III) is most pronounced in HaCaT keratinocytes in response to oxo-ProCA.
Transcriptional activation and expression of Nrf2 antioxidant target genes by cinnamoyl-based Michael acceptors in cultured human skin cells
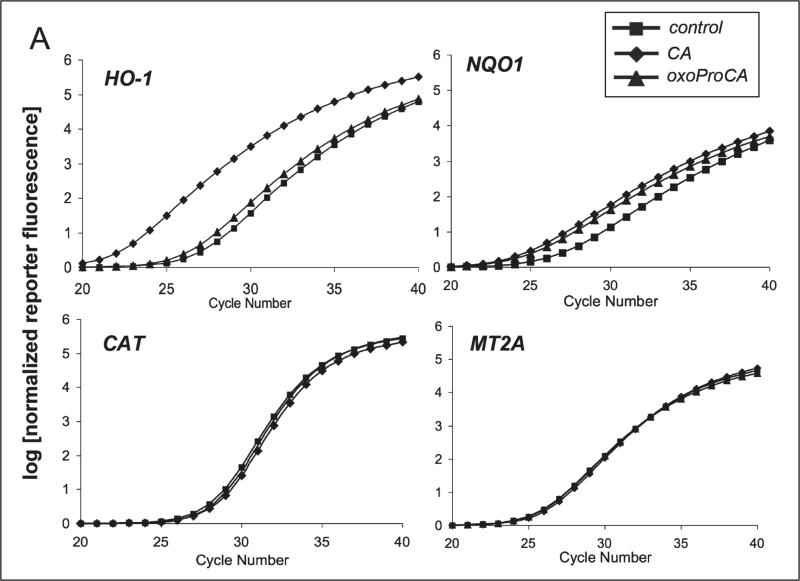
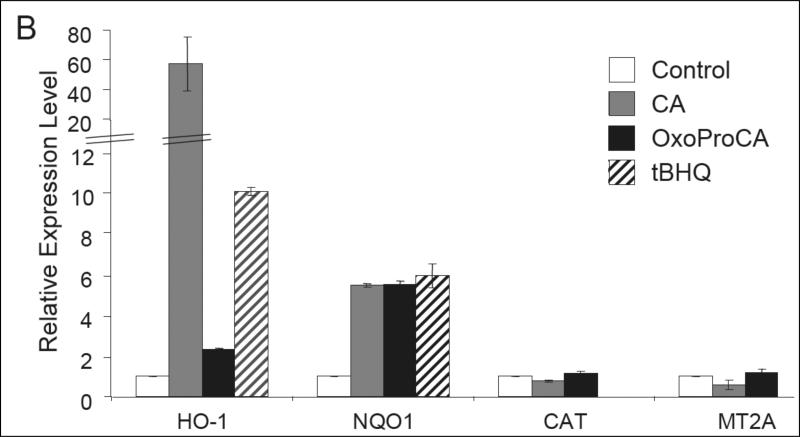
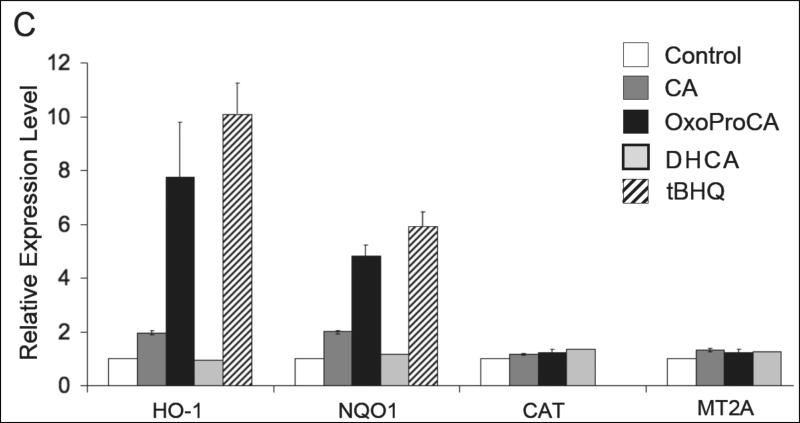
Nrf2-modulation of the cellular antioxidant response occurs by transcriptional activation of antioxidant target genes including NQO1 and HO-1 [22-28, 32]. After demonstrating Nrf2 protein upregulation in Hs27 human skin fibroblasts and HaCaT keratinocytes exposed to cinnamoyl-based Michael acceptors, modulation of antioxidant gene expression downstream of Nrf2 was examined. Using real time RT-PCR, transcriptional activation of selected Nrf2-target genes (NQO1 and HO-1) and other Nrf2-independent antioxidant response encoding genes [catalase (Cat) and metallothionein 2A (MT2A)] was examined in cultured human skin cells exposed to Nrf2-active and -inactive cinnamoyl-derivatives. Indeed, after 24 h exposure to CA or oxoProCA, NQO1 and HO-1 transcription was upregulated in human Hs27 fibroblasts and HaCaT keratinocytes (Fig. 4). In Hs27, CA treatment upregulated HO-1 transcript levels by more than 50 fold, whereas oxoProCA achieved only a moderate twofold induction (Fig. 4A-B). In contrast, HaCaT keratinocytes showed a reversed pattern of HO-1 induction, with oxoProCA and CA achieving eightfold and twofold upregulation, respectively (Fig. 4C). NQO1-mRNA levels were increased approximately fivefold in CA- or oxoProCA-treated Hs27 fibroblasts and oxoProCA-treated HaCaT keratinocytes, whereas a moderate twofold upregulation of NQO1 expression was observed in response to CA treatment in HaCaT keratinocytes. This suggests that in HaCaT keratinocytes, but not in Hs27 fibroblasts, oxoProCA is a more potent Nrf2-inducer than CA. In contrast, mRNA levels of other genes encoding the antioxidant proteins Cat and MT2A and the housekeeping enzyme GAPDH were unaffected by treatment with cinnamoyl-based Michael acceptors. Importantly, the Michael-inactive CA-derivatives DHCA and ProCa, incapable of Nrf2 transcriptional activation (Fig. 2) or Nrf2 protein upregulation (Fig. 3A), did not modulate Nrf2 target gene expression as shown for DHCA-treated HaCaT keratinocytes (Fig. 4C).
Figure 4. Induction of Nrf2-target gene expression by cinnamoyl-based Michael acceptors.
Human skin Hs27 fibroblasts and HaCaT keratinocytes were cultured for 24h in the presence of the test compounds tBHQ (50 μM) and CA, DHCA, and oxoProCA (10 μM, each). Total RNA was prepared and Nrf2 target gene expression was then quantitatively examined using real time RT-PCR analysis with normalization for GAPDH expression levels. (A) Representative amplification curves for NQO1, HO-1, CAT, and MT2A obtained in Hs27 fibroblasts; (B and C) relative expression levels in response to 24 h mock treatment (control) and exposure to test compounds obtained in fibroblasts (B) and keratinocytes (C). For every treatment group, the experiment was performed independently two times in each cell line (n=2, mean ± SEM).
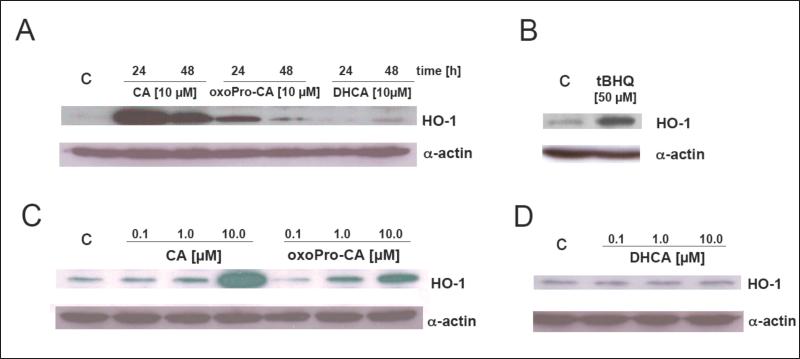
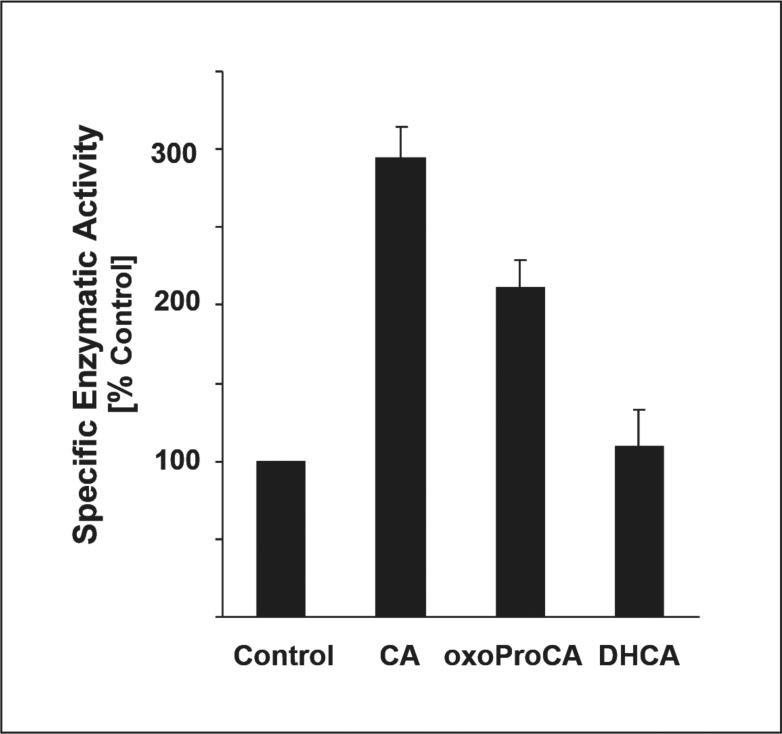
Next, upregulation of HO-1 and NQO1 by CA and oxoProCA was examined in Hs27 fibroblasts by assessing modulation of cellular protein level and specific enzymatic activity, respectively. HO-1 protein levels as assessed by immunoblot analysis were elevated after CA-exposure and more moderately upregulated in response to oxoProCA exposure (Fig. 5A and C). HO-1 upregulation was also observed in response to tBHQ treatment (Fig. 5B), but remained unchanged upon exposure to Michael-inactive DHCA (Fig. 5D). Prolonged upregulation of HO-1 protein levels was detected at 24 h of treatment with cinnamoyl-based Michael acceptors and was diminished at 48 h of exposure (Fig. 5A). Next, upregulation of cellular NQO1 specific enzymatic activity, a cellular marker widely used for screening of electrophilic chemopreventive agents [50], by cinnamoyl-based Michael acceptors was examined. Indeed, transcriptional activation of NQO1 in Hs27 fibroblasts correlated with upregulation of NQO1 specific enzymatic activity as determined using the DCPIP reduction assay (Fig. 6). Interestingly, upregulation of NQO1 specific enzymatic activity was more pronounced in response to 24 h exposure to CA than to oxoProCA, even though equal expression was detected at the mRNA level (Fig. 4A). In contrast, no upregulation of NQO1 specific enzymatic activity was observed as a result of exposure to DHCA, suggesting that NQO-1 upregulation in Hs27 fibroblasts requires an active Michael acceptor pharmacophore as contained in CA and oxoProCA.
Figure 5. Sustained upregulation of cellular HO-1 protein levels in human Hs27 skin fibroblasts exposed to cinnamoyl-based Nrf2-activators.
Cultured human Hs27 skin fibroblasts were exposed to a dose range of DHCA (panels A and D), CA (panels A and C), oxoProCA (panels A and C) for 24 (panels A-D) and 48 h (panel A), and cellular HO-1 protein levels were then examined in total cellular protein extracts by 15% SDS-PAGE followed by immunoblot detection using enhanced chemiluminescence. Exposure to tBHQ (50 μM, 24 h) served as a positive control (panel B).
Figure 6. Induction of NQO1 specific enzymatic activity in human Hs27 skin fibroblasts exposed to cinnamoyl-based Nrf2-activators.
NQO1 specific enzymatic activity of cytosolic protein preparations from cells exposed to various test compounds (CA, oxoProCA, DHCA, 10 μM each, 24 h) was determined using the DCPIP reduction assay as specified in Materials and Methods. The results are presented as means ± S.D. of three independent experiments.
Taken together, these findings demonstrate that the cinnamoyl-based Michael acceptors CA and oxoProCA significantly upregulate Nrf2 antioxidant target gene expression in human fibroblasts and keratinocytes. This suggests feasibility of upregulating the antioxidant response of human skin cells using these compounds as pharmacological agents.
Targeting
1O2-induced skin cell photo-oxidative stress using cinnamoyl-based Nrf2-activators
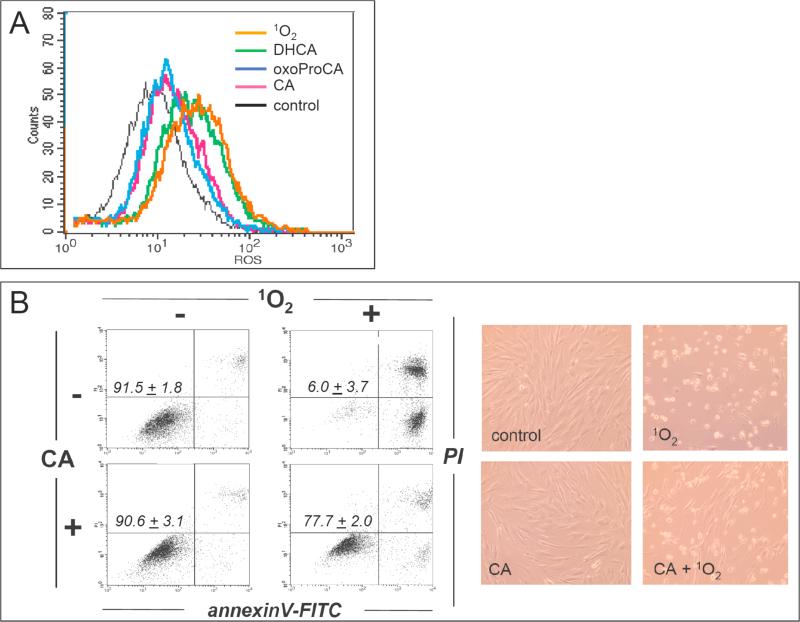
In order to examine feasibility of achieving cellular protection against photo-oxidative damage by cinnamoyl-based Nrf2-activators, human Hs27 skin fibroblasts and HaCaT keratinocytes were cultured for 24 h in the presence or absence of test compounds and then exposed to 1O2, an established key mediator of skin cell photo-oxidative stress [47]. Cellular photo-oxidative stress, resulting from exposure to 1O2 formed in situ by dye-sensitization [combined action of 0.3 J/cm2 visible light and 3.3 μM sensitizer dye toluidine blue O (TB) in PBS], was then monitored by flow cytometric analysis. Mock-pretreated fibroblasts exposed to the combined action of visible light and the sensitizer dye TB and then incubated in the presence of 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) displayed an approximately fourfold increase in green fluorescence intensity over unirradiated control cells exposed to DCFH-DA only, indicative of cellular photo-oxidative stress resulting from ROS formation during dye-sensitization as published earlier (Fig. 7A) [47]. The exact molecular nature of the oxidizing species responsible for DCFH-DA oxidation in dye-sensitized fibroblasts was not examined at this point, but formation of singlet oxygen and peroxides as a result of toluidine blue-sensitization is well documented and likely involved in the oxidative transformation of the nonfluorescent precursor dye [47,51]. Importantly, no suppression of photo-oxidative stress was observed when fibroblasts were pretreated with DHCA and then exposed to dye-sensitization (Fig. 7A). In contrast, pretreatment with CA or oxo-ProCA significantly reduced cellular green fluorescence intensity resulting from 1O2 exposure [approximately 70% reduction] suggesting that pretreatment with cinnamoyl-based Nrf2-activators antagonizes photo-oxidative stress.
Figure 7. Chemoprevention of skin cell photo-oxidative stress using cinnamoyl-based Nrf2-activators.
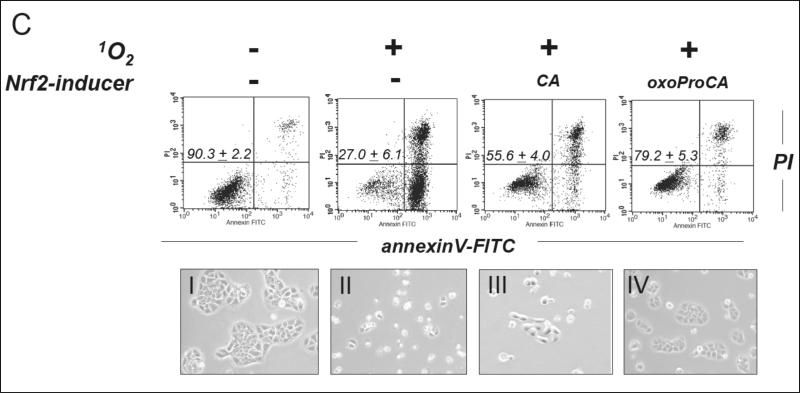
(A) Suppression of intracellular photo-oxidative stress in human Hs27 fibroblasts by pretreatment with CA and oxoProCA. Cells were pre-incubated with DHCA, CA, and oxoProCA (10 μM each, 24 h) and then exposed to photo-oxidative stress using dye-sensitization with generation of 1O2 (visible light dose 0.3 J/cm2). After exposure, intracellular oxidative stress was assessed by 2’,7’-dichloro-dihydrofluorescein diacetate staining followed by flow cytometric analysis. One representative experiment of three similar repeats is shown. (B) Protection of Hs27 fibroblasts by pretreatment with CA (24 h, 10 μM) against photodynamic-induction of cell death (visible light dose 1.8 J/cm2) was examined 24 h after exposure to 1O2 by light microscopy and flow cytometric analysis of annexinV-FITC/propidium iodide-stained cells. (C) Protection of HaCaT keratinocytes by pretreatment with CA and oxo-ProCA (24 h, 10 μM each) against photodynamic induction of cell death was analyzed as described above (panels I-IV: light microscopy of treatment groups shown before flow cytometric analysis). The numbers indicate viable cells (AV-, PI-, lower left quadrant) in percent of total gated cells (mean ± SD, n=3).
Pronounced induction of cell death was observed in Hs27 fibroblasts 24 h after exposure to apoptogenic doses of 1O2. To this end, a six-fold higher photon dose was used over photooxidative stress conditions [combined action of 1.8 J/cm2 visible light and 3.3 μM TB in PBS] (Fig. 7B). Consistent with photodynamic induction of apoptosis/necrosis, photosensitized fibroblasts exhibited pronounced morphological changes characterized by cell rounding, membrane blebbing, and cytoplasmic condensation. Induction of cellular apoptosis was confirmed by annexinV-FITC/PI staining followed by flow cytometric analysis performed 24 h after cell photosensitization (Fig. 7 B). Cells in early apoptosis [annexinV+/PI-] and late apoptosis/necrosis [annexinV+/PI+] were identified starting 6 h after treatment (data not shown) and 24 h later only 6% of cells remained viable. As published before, these changes were not observed with administration of photosensitizer in the dark or in the presence of light alone, and photodynamic cell kill was completely suppressed when dye sensitization was performed in the presence of 1O2-quenchers including NaN3 [data not shown] [47]. In contrast, significant cell protection (almost 80% viable cells) was observed both by light microscopy and flow cytometry, when fibroblasts received 24 h pretreatment with CA before exposure to 1O2 (Fig 7B). Similar protection was achieved after preincubation with oxoProCA (data not shown). In contrast, chemopreventive suppression of photo-oxidative damage in cultured human HaCaT keratinocytes was more pronounced after oxoProCA (over 80% surviving cells) than after CA (approximately 55% surviving cells) pretreatment as shown in Fig. 7C. This may result from more pronounced upregulation of cellular Nrf2 levels that occurs even at submicromolar levels of oxoproCA as shown in Fig. 3F. Importantly, cellular protection against photooxidative stress and induction of apoptosis was not a result of direct absorption of photons by cinnamoyl-based test compounds since photo-activation and 1O2 formation by the sensitizer dye TB depends on excitation in the visible wavelength range [47], whereas photon absorption by CA and oxoProCA is confined to the UVB region (data not shown). Moreover, CA and oxo-ProCA were inactive when used in photoprotection studies without pre-incubation period, consistent with Nrf2 upregulation and subsequent gene expression mediating the protective effects of these cinnamoyl-derivatives (data not shown).
These findings demonstrate that the cinnamoyl-based Nrf2-activators CA and oxoProCA are potent antagonists of 1O2-induced photo-oxidative stress and photodynamic induction of apoptosis in cultured human skin fibroblasts and keratinocytes.
Discussion
The rising incidence of nonmelanoma and melanoma skin cancers and the established role of solar UV-radiation as a major environmental carcinogen create an urgent need for better agents for skin photoprotection [4]. Strong experimental evidence suggests the involvement of photo-oxidative stress mediated by reactive oxygen species as a crucial mechanism of solar damage relevant to human skin photoaging and photocarcinogenesis [2, 9, 12, 19]. Previous research demonstrates the feasibility of targeting photo-oxidative mechanisms for skin photoprotection and cancer chemoprevention [2, 4], and the mechanisms by which small molecule modulation of the Nrf2-Keap1 signaling pathway induces antioxidant gene expression and cell protection are established [22-29]. Based on the hypothesis that activation of ARE-mediated gene expression can suppress photo-oxidative pathways of skin cell photo-damage, we established a cell-based screening methodology and report here the identification and efficacy of cinnamoyl-based Nrf2-activators as potential photo-chemopreventive agents.
First, using a luciferase reporter assay-based screen in human MDA-MB-231 cells the dietary Michael acceptor CA was identified as potent lead Nrf2 activator. A series of closely related cinnamoyl-based Michael acceptors and Michael-inactive derivatives (DHCA, CAC, CACM, CAG, ProCA, oxoProCA) was tested in order to further explore the structure activity relationship of Nrf2-activation by these agents. Apart from CA and CACM, a N-cinnamoyl-substituted secondary amide-based derivative (oxoProCA) was identified as potent Nrf2 activator, consistent with a Michael acceptor reactivity of this compound as assessed using a simple UV-spectrophotometric thiol-adduction assay (Fig. 2G). In contrast, Michael-inactive N-cinnamoyl-substituted primary (CAG) and secondary (ProCA) amide-derivatives did not induce Nrf2 transcriptional activity. To the best of our knowledge, Nrf2 activation by the α,β-unsaturated N-acyl-5-oxo-pyrrolidine Michael acceptor pharmacophore as contained in the CA-derivative oxoProCA has not been described before. Therefore, the secondary amide oxoProCA may represent a novel class of non-aldehyde Nrf2-activators, where amide formation with 5-oxopyrrolidine reconstitutes the electrophilic Michael acceptor reactivity of Michael-inactive α,β-unsaturated carboxylic acids, a situation similar to the known Michael acceptor reactivity of cinnamoyl-alkylesters [43].
Next, hit confirmation was performed in a secondary screen. To this end, upregulation of Nrf2 protein levels was assessed by cinnamoyl-based Michael acceptors using immunoblot analysis performed in cultured human skin Hs27 fibroblasts, immortalized HaCaT keratinocytes, and primary skin keratinocytes. Treatment with CA- and oxo-ProCA induced a pronounced elevation of cellular Nrf2 protein levels, whereas no change was observed in skin cells exposed to Michael-inactive test agents including DHCA (Fig. 3). Subsequently, the ability of cinnamoyl-based Michael acceptors to upregulate skin cell expression of HO-I and NQO1 was examined using real time RT-PCR analysis (Fig. 4). Results were confirmed by HO-1 immunoblotting (Fig. 5) and determination of NQO1 specific enzymatic activity (Fig. 6), respectively.
Finally, feasibility of targeting 1O2-induced skin cell photo-oxidative stress using cinnamoyl-based Nrf2-activators as experimental photo-chemopreventive agents was demonstrated (Fig. 7). Cellular photo-oxidative stress resulting from exposure to 1O2 was reduced significantly in Hs27 fibroblasts pretreated with CA and oxoProCA as assessed by flow cytometric analysis of cellular peroxide levels using DCFH-DA. Photodynamic induction of fibroblast and keratinocyte apoptosis, an established assay designed to assess antioxidant protection of skin cells exposed to 1O2 [47], was strongly antagonized by pretreatment with cinnamoyl-based Nrf2-activators. In contrast, no suppression of photo-oxidative stress was observed upon pretreatment with the Michael-inactive cinnamoyl-derivative DHCA.
It is now well established that the cellular oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2 and that diverse thiol-reactive electrophilic pharmacophores activate Nrf2 through inhibition of Keap1-mediated degradation, followed by nuclear translocation of Nrf2 and induction of ARE-dependent gene expression [23-26]. Earlier work has elucidated the structure-activity relationship of phase II enzyme induction by chemopreventive small molecule electrophiles, and α,β-unsaturated carbonyl compounds (enone-type Michael acceptors) have emerged as a potent class of NQO1-inducers [25, 34, 50]. Importantly, potency of phase II enzyme induction by test compounds assessed in cell based assays paralleled reactivity as Michael addition acceptor, and cinnamic acid methylester has been identified as a phase II enzyme inducer [43]. Our study demonstrates that cinnamoyl-based Michael acceptors are potent Nrf2-activators that protect cultured human skin cells against photo-oxidative stress induced by 1O2. The N-cinnamoyl-5-oxopyrrolidine based Michael acceptor oxoProCA was identified as a promising Nrf2 activator that displayed increased potency over the initial lead compound CA. Indeed, upregulation of Nrf2 target gene expression in keratinocytes was more pronounced in response to oxoProCA than to CA, and protection against 1O2-induced photo-oxidative stress achieved in keratinocytes was superior in response to oxoProCA pretreatment. In contrast to the other cinnamoyl-based Nrf2-activators CA or CACM, the amide-based Michael acceptor oxoProCA is distinguished by the absence of a reactive aldehyde group that may cause unwanted adductive reactivity towards skin proteins and may also display increased stability against skin esterase-mediated cleavage and deactivation that could limit efficacy of topical delivery of ester-based Michael acceptors including CACM, a hypothesis to be tested by future experiments. Interestingly, 5-oxo-L-proline, the secondary amine building block contained in oxo-ProCA, is an important endogenous component of human skin stratum corneum involved in osmoregulation and barrier function [52].
In summary, our findings demonstrate that cinnamoyl-based Nrf2-activators may serve as potent antagonists of 1O2-induced photo-oxidative stress in cultured human skin cells suggesting a potential use of these prototype agents as skin photo-chemopreventive factors [29, 39-41, 47]. Future studies must evaluate the photoprotective performance of cinnamoyl-based and other Nrf2-activators as a function of solar spectral range (UVB, UVA, visible) in acute (sunburn) and chronic models of skin photodamage in appropriate animal models in order to define their potential use as combinatorial photoprotective ingredients [29]. Lead development will then aim at agents optimized for targeted topical delivery, photostability, long-term safety, and compatibility with other photoprotective agents.
Acknowledgements
Supported in part by grants from the National Institutes of Health [R01CA122484, SWEHSC pilot research grant (ES06694), and GI Cancer Pilot Grant (SPORE, CA95060)], from the Arizona Biomedical Research Commission (ABRC 0721, to GTW), American Cancer Society (RSG-07-154-01-CNE, to DDZ), and NSF (DGE-0114420 to CMC). Preliminary data from this research were part of an oral presentation at the 14th Annual Meeting of the Society for Free Radical Biology and Medicine, November 15, 2007, in Washington, DC.
Abbreviations
- ARE
antioxidant response element
- AV
annexinV
- CA
trans-cinnamic aldehyde
- CAC
cinnamic acid
- CACM
cinnamic acid methylester
- CAG
N-cinnamoylglycine
- DC
dicoumarol
- DCFH-DA
2’,7’-dichlorodihydrofluorescein diacetate
- DCPIP
2,6-dichlorophenolindophenol
- DHCA
dihydrocinnamic aldehyde
- GSH
glutathione
- HO-1
heme oxygenase 1
- Nrf2
NF-E2-related factor 2
- NQO1
NAD(P)H:quinone oxidoreductase
- 1O2
singlet oxygen
- oxoProCA
N-cinnamoyl-5-oxo-L-proline methylester
- PI
propidium iodide
- ProCA
N-cinnamoyl-L-proline
- ROS
reactive oxygen species
- SDS-PAGE
Sodium Dodecylsulfate Polyacrylamide Gel Electrophoresis
- TB
toluidine blue O
- tBHQ
tert.-butylhydroquinone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gasparro FP. Sunscreens, skin photobiology, and skin cancer: the need for UVA protection and evaluation of efficacy. Environ Health Perspect. 2000;108(Suppl 1):71–8. doi: 10.1289/ehp.00108s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wondrak GT, Jacobson MK, Jacobson EL. Endogenous UVA-photosensitizers: mediators of skin photodamage and novel targets for skin photoprotection. Photochem Photobiol Sci. 2006;5:215–37. doi: 10.1039/b504573h. [DOI] [PubMed] [Google Scholar]
- 3.de Gruijl FR. Photocarcinogenesis: UVA vs. UVB radiation. Skin Pharmacol Appl Skin Physiol. 2002;15:316–20. doi: 10.1159/000064535. [DOI] [PubMed] [Google Scholar]
- 4.Wondrak GT. Let the sun shine in: mechanisms and potential for therapeutics in skin photodamage. Curr Opin Investig Drugs. 2007;8:390–400. [PubMed] [Google Scholar]
- 5.Dennis LK. Analysis of the melanoma epidemic, both apparent and real: data from the 1973 through 1994 surveillance, epidemiology, and end results program registry. Arch Dermatol. 1999;135:275–80. doi: 10.1001/archderm.135.3.275. [DOI] [PubMed] [Google Scholar]
- 6.Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat Rev Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 7.Markovic SN, Erickson LA, Rao RD, Weenig RH, Pockaj BA, Bardia A, et al. Malignant melanoma in the 21st century, part 1: epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin Proc. 2007;82:364–80. doi: 10.4065/82.3.364. [DOI] [PubMed] [Google Scholar]
- 8.Kielbassa C, Roza L, Epe B. Wavelength dependence of oxidative DNA damage induced by UV and visible light. Carcinogenesis. 1997;18:811–6. doi: 10.1093/carcin/18.4.811. [DOI] [PubMed] [Google Scholar]
- 9.Agar NS, Halliday GM, Barnetson RS, Ananthaswamy HN, Wheller M, Jones AM. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: A role for UVA in human skin carcinogenesis. PNAS. 2004;101:4954–9. doi: 10.1073/pnas.0401141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kvam E, Tyrrell RM. Induction of oxidative DNA base damage in human skin cells by UV and near visible radiation. Carcinogenesis. 1997;18:2379–84. doi: 10.1093/carcin/18.12.2379. [DOI] [PubMed] [Google Scholar]
- 11.Tyrrell RM. Ultraviolet radiation and free radical damage to skin. Biochem Soc Symp. 1995;61:47–53. doi: 10.1042/bss0610047. [DOI] [PubMed] [Google Scholar]
- 12.Scharffetter-Kochanek K, Wlaschek M, Brenneisen P, Schauen M, Blaudschun R, Wenk J. UV-induced reactive oxygen species in photocarcinogenesis and photoaging. Biol Chem. 1997;378:1247–57. [PubMed] [Google Scholar]
- 13.Tyrrell RM. Solar ultraviolet A radiation: an oxidizing skin carcinogen that activates heme oxygenase-1. Antioxid Redox Signal. 2004;6:835–40. doi: 10.1089/ars.2004.6.835. [DOI] [PubMed] [Google Scholar]
- 14.Hiraku Y, Ito K, Hirakawa K, Kawanishi S. Photosensitized DNA damage and its protection via a novel mechanism. Photochem Photobiol. 2007;83:205–12. doi: 10.1562/2006-03-09-IR-840. [DOI] [PubMed] [Google Scholar]
- 15.Wondrak GT, Roberts MJ, Jacobson MK, Jacobson EL. Photosensitized growth inhibition of cultured human skin cells: mechanism and suppression of oxidative stress from solar irradiation of glycated proteins. J Invest Dermatol. 2002;119:489–98. doi: 10.1046/j.1523-1747.2002.01788.x. [DOI] [PubMed] [Google Scholar]
- 16.Wondrak GT, Roberts MJ, Jacobson MK, Jacobson EL. 3-hydroxypyridine chromophores are endogenous sensitizers of photooxidative stress in human skin cells. J Biol Chem. 2004;279:30009–20. doi: 10.1074/jbc.M404379200. [DOI] [PubMed] [Google Scholar]
- 17.Wondrak GT, Roberts MJ, Cervantes-Laurean D, Jacobson MK, Jacobson EL. Proteins of the Extracellular Matrix Are Sensitizers of Photo-oxidative Stress in Human Skin Cells. J Invest Dermatol. 2003;121:578–86. doi: 10.1046/j.1523-1747.2003.12414.x. [DOI] [PubMed] [Google Scholar]
- 18.Kipp C, Young AR. The soluble eumelanin precursor 5,6-dihydroxyindole-2-carboxylic acid enhances oxidative damage in human keratinocyte DNA after UVA irradiation. Photochem Photobiol. 1999;70:191–8. [PubMed] [Google Scholar]
- 19.Wlaschek M, Tantcheva-Poor I, Naderi L, Ma W, Schneider LA, Razi-Wolf Z, et al. Solar UV irradiation and dermal photoaging. J Photochem Photobiol B. 2001;63:41–51. doi: 10.1016/s1011-1344(01)00201-9. [DOI] [PubMed] [Google Scholar]
- 20.Einspahr JG, Stratton SP, Bowden GT, Alberts DS. Chemoprevention of human skin cancer. Crit Rev Oncol Hematol. 2002;41:269–85. doi: 10.1016/s1040-8428(01)00185-8. [DOI] [PubMed] [Google Scholar]
- 21.Meves A, Stock SN, Beyerle A, Pittelkow MR, Peus D. Vitamin C derivative ascorbyl palmitate promotes ultraviolet-B-induced lipid peroxidation and cytotoxicity in keratinocytes. J Invest Dermatol. 2002;119:1103–8. doi: 10.1046/j.1523-1747.2002.19521.x. [DOI] [PubMed] [Google Scholar]
- 22.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–22. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 23.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99:11908–13. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23:8137–51. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinkova-Kostova AT, Holtzclaw WD, Kensler TW. The role of Keap1 in cellular protective responses. Chem Res Toxicol. 2005;18:1779–91. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K, et al. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26:221–9. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:1–21. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 28.Dinkova-Kostova AT, Fahey JW, Wade KL, Jenkins SN, Shapiro TA, Fuchs EJ, et al. Induction of the phase 2 response in mouse and human skin by sulforaphane-containing broccoli sprout extracts. Cancer Epidemiol Biomarkers Prev. 2007;16:847–51. doi: 10.1158/1055-9965.EPI-06-0934. [DOI] [PubMed] [Google Scholar]
- 29.Talalay P, Fahey J, Healy Z, Wehage S, Benedict A, Min C, et al. Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc Natl Acad Sci U S A. 2007;104:17500–5. doi: 10.1073/pnas.0708710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–53. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang XJ, Sun Z, Chen W, Eblin KE, Gandolfi JA, Zhang DD. Nrf2 protects human bladder urothelial cells from arsenite and monomethylarsonous acid toxicity. Toxicol Appl Pharmacol. 2007 doi: 10.1016/j.taap.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–203. [PubMed] [Google Scholar]
- 33.Kwak MK, Egner PA, Dolan PM, Ramos-Gomez M, Groopman JD, Itoh K, et al. Role of phase 2 enzyme induction in chemoprotection by dithiolethiones. Mutat Res. 2001;480-481:305–15. doi: 10.1016/s0027-5107(01)00190-7. [DOI] [PubMed] [Google Scholar]
- 34.Dinkova-Kostova AT, Abeygunawardana C, Talalay P. Chemoprotective properties of phenylpropenoids, bis(benzylidene)cycloalkanones, and related Michael reaction acceptors: correlation of potencies as phase 2 enzyme inducers and radical scavengers. J Med Chem. 1998;41:5287–96. doi: 10.1021/jm980424s. [DOI] [PubMed] [Google Scholar]
- 35.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98:3410–5. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24:7130–9. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.auf dem Keller U, Huber M, Beyer TA, Kumin A, Siemes C, Braun S, et al. Nrf transcription factors in keratinocytes are essential for skin tumor prevention but not for wound healing. Mol Cell Biol. 2006;26:3773–84. doi: 10.1128/MCB.26.10.3773-3784.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Durchdewald M, Beyer TA, Johnson DA, Johnson JA, Werner S, Auf dem Keller U. Electrophilic Chemicals but not UV Irradiation or Reactive Oxygen Species Activate Nrf2 in Keratinocytes In Vitro and In Vivo. J Invest Dermatol. 2006;127:646–653. doi: 10.1038/sj.jid.5700585. [DOI] [PubMed] [Google Scholar]
- 39.Gao X, Talalay P. Induction of phase 2 genes by sulforaphane protects retinal pigment epithelial cells against photooxidative damage. Proc Natl Acad Sci U S A. 2004;101:10446–51. doi: 10.1073/pnas.0403886101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawachi Y, Xu X, Taguchi S, Sakurai H, Nakamura Y, Ishii Y, et al. Attenuation of UVB-induced sunburn reaction and oxidative DNA damage with no alterations in UVB-Induced skin carcinogenesis in Nrf2 gene-deficient mice. J Investig Dermatol. 2008 Jan 17; doi: 10.1038/sj.jid.5701245. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Dinkova-Kostova AT, Jenkins SN, Fahey JW, Ye L, Wehage SL, Liby KT, et al. Protection against UV-light-induced skin carcinogenesis in SKH-1 high-risk mice by sulforaphane-containing broccoli sprout extracts. Cancer Lett. 2006;240:243–52. doi: 10.1016/j.canlet.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Babidge PJ, Massy-Westropp RA, Pyne SG, Shiengthong D, Ungphakorn A, Veerchat G. The Synthesis and Stereochemistry of Odorine. Aust J Chem. 1980;33:1841–5. [Google Scholar]
- 43.Dinkova-Kostova AT, Massiah MA, Bozak RE, Hicks RJ, Talalay P. Potency of Michael reaction acceptors as inducers of enzymes that protect against carcinogenesis depends on their reactivity with sulfhydryl groups. Proc Natl Acad Sci U S A. 2001;98:3404–9. doi: 10.1073/pnas.051632198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Z, Zhang S, Chan JY, Zhang DD. Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol Cell Biol. 2007;27:6334–49. doi: 10.1128/MCB.00630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pani G, Colavitti R, Bedogni B, Anzevino R, Borrello S, Galeotti T. Determination of intracellular reactive oxygen species as function of cell density. Methods Enzymol. 2002;352:91–100. doi: 10.1016/s0076-6879(02)52010-3. [DOI] [PubMed] [Google Scholar]
- 46.De Haan LH, Boerboom AM, Rietjens IM, van Capelle D, De Ruijter AJ, Jaiswal AK, et al. A physiological threshold for protection against menadione toxicity by human NAD(P)H:quinone oxidoreductase (NQO1) in Chinese hamster ovary (CHO) cells. Biochem Pharmacol. 2002;64:1597–603. doi: 10.1016/s0006-2952(02)01383-7. [DOI] [PubMed] [Google Scholar]
- 47.Wondrak GT, Jacobson MK, Jacobson EL. Identification of quenchers of photoexcited states as novel agents for skin photoprotection. J Pharmacol Exp Ther. 2005;312:482–91. doi: 10.1124/jpet.104.075101. [DOI] [PubMed] [Google Scholar]
- 48.Wondrak GT, Jacobson MK, Jacobson EL. Antimelanoma activity of apoptogenic carbonyl scavengers. J Pharmacol Exp Ther. 2006;316:805–14. doi: 10.1124/jpet.105.094953. [DOI] [PubMed] [Google Scholar]
- 49.Wondrak GT. NQO1-activated phenothiazinium redox cyclers for the targeted bioreductive induction of cancer cell apoptosis. Free Radic Biol Med. 2007;43:178–90. doi: 10.1016/j.freeradbiomed.2007.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dinkova-Kostova AT, Fahey JW, Talalay P. Chemical Structures of Inducers of Nicotinamide Quinone Oxidoreductase 1 (NQO1). Methods Enzymol. 2004;382:423–49. doi: 10.1016/S0076-6879(04)82023-8. [DOI] [PubMed] [Google Scholar]
- 51.Hempel SL, Buettner GR, O'Malley YQ, Wessels DA, Flaherty DM. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2',7'-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2',7'-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic Biol Med. 1999;27:146–59. doi: 10.1016/s0891-5849(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 52.Caspers PJ, Lucassen GW, Carter EA, Bruining HA, Puppels GJ. In vivo confocal Raman microspectroscopy of the skin: noninvasive determination of molecular concentration profiles. J Invest Dermatol. 2001;116:434–42. doi: 10.1046/j.1523-1747.2001.01258.x. [DOI] [PubMed] [Google Scholar]