Summary
Although estrogens are widely considered circulating ‘sex steroid hormones’ typically associated with female reproduction, recent evidence suggests that estrogens can act as local modulators of brain circuits in both males and females. Functional implications of this newly-characterized estrogen signaling system have begun to emerge. This essay summarizes evidence in support of the hypothesis that the rapid production of estrogens in brain circuits can drive acute changes in both the production and perception of acoustic communication behaviors. These studies reveal two fundamental neurobiological concepts: 1) estrogens can be produced locally in brain circuits independent of levels in nearby circuits and in the circulation, and 2) estrogens can have very rapid effects within these brain circuits to modulate social vocalizations, acoustic processing, and sensorimotor integration. This research relies on a vertebrate-wide span of investigations, including vocalizing fishes, amphibians and birds, emphasizing the importance of comparative model systems in understanding principles of neurobiology.
Keywords: Nongenomic, Estrogen, Signaling, Behavior, Cortex
Introduction
Like most sensorimotor behaviors, the neural pathways for both vocalization and audition (here grouped as ‘acoustic communication behaviors’) ultimately pass through the hindbrain/spinal cord. Specifically, despite widely different mechanisms of sound production across the vertebrate radiation (larynx, syrinx, swimbladder, etc.) the production of social vocalizations depends upon hindbrain-spinal circuits that are organized according to a conserved developmental/molecular ‘blueprint’ across all major vertebrate lineages [1-3]. Similarly, ascending auditory pathways that project from peripheral hair cells through brainstem, thalamic and telencephalic structures rely on a conserved basic functional architecture in frogs, fishes, birds, as well as both eutherian and marsupial mammals [4-7]. Therefore the evolution of acoustic communication in birds, mammals, fishes, and other vertebrates appears to have relied on similar toolkits for molecular and neural connectivity [1].
How can the conserved neural wiring diagram for the production and perception of acoustic signals account for the wide variation in vertebrate acoustic behaviors seen in nature? One mechanism for both species- and individual-level behavioral variation is the actions of neuromodulators, which can alter the moment-by-moment functional connectivity within neural circuits. For example, the nonapeptides (e.g,. arginine vasotocin and vasopressin) regulate the neural and behavioral patterning of vocalizations in birds, mammals and teleost fishes [8-10]. Here, I summarize recent research that has uncovered a parallel neuromodulatory system – local signaling by estrogens within brain circuits – that can also rapidly modulate both the production and perception of acoustic communication signals in vertebrates. Collectively, this evidence shows there is strong support for acute estrogen-dependent modulation of acoustic communication circuits and behavior. As this signaling system comes into focus, these findings can guide investigations regarding how estrogens exert rapid and local actions on neural circuits more broadly (i.e., pathways not involved in acoustic communication per se). Following the evaluation of the hypothesis below, I then present several key pieces of the puzzle that currently await more detailed investigation before this estrogen-dependent neuromodulatory system can be fully understood.
A working hypothesis linking brain estrogen signaling and behavior
The canonical mode of the actions of estrogens is via intracellular receptors that mediate long-term changes in gene expression. These changes typically manifest within hours to in some cases weeks after initiation. It is now clear that estrogens can also act extremely rapidly (i.e., within seconds to minutes) on cellular functions throughout the body, including the central nervous system [11,12]. These nonclassical actions are of particular interest to neurobiologists and neuroendocrinologists because this rapid timescale is consistent with that of momentary changes in neural activity and behavior. A parallel line of evidence demonstrates that the brain itself can produce its' own local supply of estrogens, on demand and locally at the synaptic connections between neurons [13-15]. The working hypothesis presented here evaluates whether these two recent observations are fundamentally linked in certain brain circuits, and whether this synthesis provides mechanistic explanations for rapid changes in behavior.
The historical backdrop to the central hypothesis of this essay consists of four main themes, drawn from a literature focused broadly on neuroendocrinology/neurobiology (and not communication circuits per se). First was the identification of the rapid actions of steroids (including estrogens) on the excitability of neurons using both in vitro and in vivo preparations [16-19]. Second was the observation that rapid changes in the social environment (including stimuli such as acoustic communication signals and social challenges) were accompanied by rapid changes in the levels of circulating steroid hormones, such as plasma testosterone [20-25]. Third was the discovery that plasma androgens could be converted into estrogens via the enzyme aromatase within specific brain regions [26,27]. Last was the observation that relatively fast changes in brain aromatase activity were coupled to changes in sociosexual behaviors [28-30]. A forthcoming volume published by Oxford University Press on brain aromatase provides further exploration and context of the above foundational concepts that cannot be addressed in depth in this essay [31].
Because acoustic communication behaviors are largely accessible and quantifiable in the laboratory, combining the study of rapid estrogen signaling with acoustic communication behaviors became an attractive area of study. The above foundational observations led to the hypothesis that the rapid production and action of estrogens in brain circuits drives acute, minute-by-minute changes in acoustic signal production and perception. Below, I present the evidence in favor of this hypothesis, first for the rapid actions of estrogens on acoustic signal production, followed then by evidence for the rapid actions of estrogens on acoustic perception and sensorimotor integration. I then describe recent studies characterizing a precise control mechanism for estrogen synthesis in brain circuits that could provide the necessary spatial and temporal resolution for this newly-identified mode of estrogen signaling. This essay relies mainly on studies in amphibians, birds and teleost fishes, and therefore emphasizes the continued importance of a comparative approach to neurobiology and neuroendocrinology.
Estrogens rapidly influence vocal motor output
Estrogen receptors and the estrogen-synthesis enzyme aromatase are expressed in the neural pathways involved in acoustic signal production and perception in a wide range of vertebrates, including teleost fishes [32,33], frogs [34,35], and birds [36-38]. Indeed, estrogens can alter the activity of neurons in communication circuits via long-term mechanisms [39], and estrogens can change vocalizations in rodents [40,41] and human beings [42,43]. However, only recently have we come to understand that rapid estrogen signaling can occur within acoustic communication circuits to guide moment-by-moment changes in sensorimotor behaviors.
The neuromuscular junction of amphibians has historically provided a wealth of knowledge about how neuronal synapses operate and are modulated [e.g., 44]. Studies focused on the larynx of Xenopus demonstrated that estradiol can have acute effects on synaptic strength at the neuromuscular junction governing vocalizations [45]. Using laryngeal stimulation to evoke neuromuscular potentials, the authors of this study showed that the transmission of vocal motor commands was altered by estradiol within 60 min, consistent with a relatively rapid action of this steroid on vocalizations. To date, this is the only demonstration of an acute effect of any steroid on the peripheral control of acoustic communication behaviors.
Experiments over the past decade have uncovered evidence that estrogens can have rapid, neuromodulatory effects on the patterning of vocalizations by acting within the central nervous system itself. These studies focused on the plainfin midshipman, a teleost fish that uses vocalizations for mate attraction and territoriality [6,46]. A neural circuit in the midshipman hindbrain patterns both the frequency and duration of midshipman vocalizations [47], and this circuit is enriched with the expression of aromatase enzyme [48,49]. In vivo electrophysiology recordings of the activity of this hindbrain circuit showed that estrogens can modulate the duration of vocal motor output signals within 5 min of injection [50]. These fast effects of estradiol on vocal patterning were transient and were observed in the hindbrain of both males and females [51], consistent with the local and high levels of aromatase and estrogen-receptor expression surrounding the hindbrain circuit [48,49]. Therefore, these studies showed that estrogens can have acute actions on neural circuits that pattern vertebrate vocalizations.
Because the midshipman brain contains widespread aromatase expression, one hypothesis consistent with these findings is that estrogens could be produced locally within brain circuits, and that these events could occur within a very fast timescale in support of rapid changes in communication behaviors. Therefore, the studies above led to a new direction of investigations into whether neural circuits involved in acoustic communication could drive estrogen-dependent modulation by synthesizing estrogens themselves, locally and acutely. This idea has since received experimental support from multiple labs that have examined local estrogen production and action in the brain of songbirds.
Measurement of acute estrogen fluctuations in avian brain
In theory, brain circuits can generate their own supply of estrogens – rapidly, on-demand, precisely targeted, and independent of the circulation – to guide rapid changes in behavior. Support for this idea has come from studies of birds, in which behavioral stimuli such as copulation [52] and social challenges [53] have been associated with rapid changes in steroidogenic enzyme activity in brain tissues. Thus, enzymatic proteins that synthesize steroids in the brain exhibit rapid changes in their activity during changes in the social environment.
As a complement to enzyme activity assays, brain estrogen content itself has been examined using combined liquid- and solid-phase extraction procedures. In the zebra finch, brain estrogen levels are elevated relative to levels in the circulation [54,55], supporting the view that the brain provides the predominant source of circulating estrogens in male zebra finches [56]. The zebra finch therefore presents a model system in which the role of steroid ‘microenvironments’ could be measured and manipulated in the laboratory to understand how estrogens locally and acutely regulate brain function and behavior. Until recently, existing methods have not enabled the spatial or temporal resolution to follow changing levels of estrogens in brain nuclei. To address this question a method for measuring fluctuating estrogens in discrete brain circuits – in vivo steroid microdialysis coupled to highly-sensitive assays – was recently developed [57]. This technology has since provided evidence that estrogens fluctuate in the brain on acute time scales in awake, behaving animals, as described below.
Initial in vivo microdialysis experiments [57] were focused on the caudomedial nidopallium (NCM) of the zebra finch, which is enriched in expression of aromatase [58,59]. These methods were first validated through in vitro studies and via independent confirmation using unequivocal detection with gas chromatography coupled to mass spectrometry. Subsequent in vivo microdialysis experiments were conducted with probes directed at the NCM of awake males and samples were collected in 30-min time bins. These experiments showed that local levels of estradiol increased when males were interacting with females and hearing experimental playback of songs [57]. Similar observations were made in females, emphasizing a shared, sex-independent auditory role for rapid elevations in NCM estrogens, since females do not sing in this species [60]. In both males and females, circulating levels or levels within an adjacent auditory region were unchanged during similar social manipulations, collectively emphasizing the region-specificity of the events in NCM. Taken together, these experiments indicated that brain circuits can respond to social and/or auditory stimuli with acute elevations in local concentrations of estrogens. The consequences of rapid changes in estrogens within brain circuits for the activity of nearby neurons as well as the control of sensorimotor behaviors then became a primary research focus.
Estrogens can rapidly influence auditory processing
The NCM of songbirds has been identified as an auditory processing region analogous to mammalian secondary auditory cortex based on experiments using electrophysiological and immediate-early gene approaches [61-66]. Therefore, the observation that NCM estradiol levels were elevated in response to auditory stimuli in awake, behaving animals led to the hypothesis that acute estrogen signaling could regulate either the firing properties of NCM neurons, auditory-dependent behaviors, or both. One approach coupled reverse-microdialysis (‘retrodialysis’) of estrogens or the estrogen-synthesis inhibitor fadrozole (FAD) with extracellular recordings of neuronal activity in the NCM of males, in vivo [67]. These experiments revealed that rapid elevations in local estrogens caused rapid increases in the auditory-evoked activity of NCM neurons, and caused the firing activity of NCM neurons to switch to a mode of burst-firing (i.e., less tonic and greater phasic spiking activity). Conversely, inhibiting estrogen production via retrodialysis of FAD caused a rapid suppression of burst firing in NCM [67]. Largely convergent findings (published prior to as well as concurrent with the above-mentioned study) were generated by an independent laboratory using awake-restrained extracellular recordings in the zebra finch NCM. These observations showed that estrogens and estrogen blockers each exerted acute effects on NCM auditory encoding in zebra finches [68,69]. This same research group further showed that estrogens can rapidly suppress inhibitory synaptic currents in NCM neurons using whole-cell patch recordings, providing a candidate mechanism for the rapid actions of estrogens on auditory processing in NCM [68]. In sum, independently-replicated findings together strongly indicate that estrogens can exert rapid actions on the auditory-response patterning of neurons in NCM.
The functional consequences for rapid estrogen signaling in NCM were recently evaluated in two independent studies. Each of these studies in awake, behaving animals took advantage of an established literature on the reliable preferences that zebra finches express for familiar vs. unfamiliar songs in laboratory playback experiments [70,71]. In one study, FAD was retrodialyzed for 30 min into the left NCM of adult male zebra finches, and the behavioral expression of song preferences (ordinarily ∼75% biased in favor of familiar songs) was reduced to 50:50 chance [67]. Furthermore, when FAD was washed out with normal aCSF over 30 min, the song preference of individuals for a familiar song was restored to control levels (∼75%). Similar findings for song preference were observed in an independent lab and published at approximately the same time [69]. This study showed that song preferences were similarly disrupted when estrogen receptors or aromatase blockers were infused via intracranial injections into NCM [69]. These authors also showed that blocking estrogen signaling disrupted innate behavioral preferences that were specific for complex song stimuli, while preferences for simple calls were unaffected. In summary, convergent studies from independent research labs have shown that acute estrogen signaling within the NCM can enhance auditory processing as well as behavioral song preferences in adult zebra finches.
Estrogens can rapidly influence sensorimotor integration
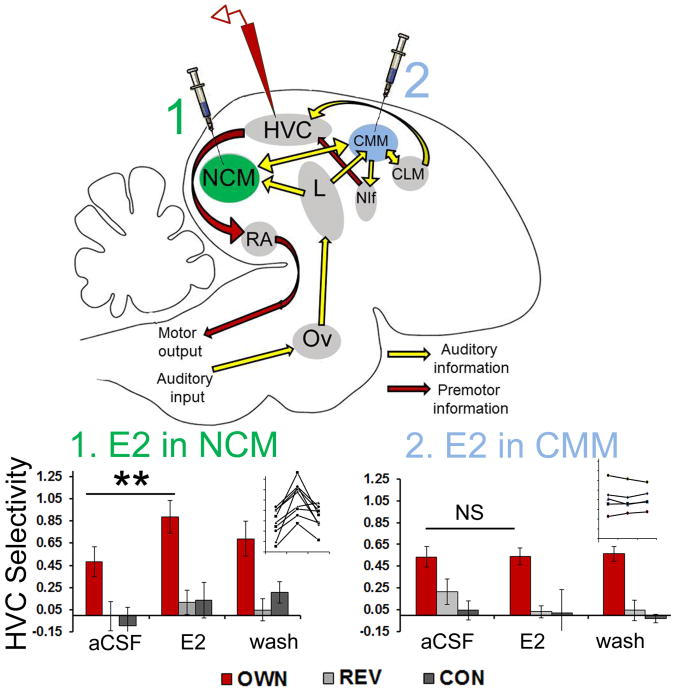
The discovery of rapid actions of estrogens on neural activity and behavioral responses in the NCM of zebra finches raised the question whether these actions were entirely local within NCM (e.g., to enhance the efficiency of NCM song coding and neural discrimination [e.g., 69]) or also further disseminated through the interconnected network of forebrain nuclei (Fig. 1). Recent evidence demonstrates that estrogen signaling events in the auditory NCM are in fact communicated to downstream sensorimotor regions [72]. A downstream nucleus that receives indirect auditory input from NCM (see Fig. 1) is the sensorimotor HVC. The critical role of HVC in sensorimotor integration has been identified through lesion and recording experiments, in which HVC neurons have been found essential for both the perception and the production of complex vocalizations [73-75]. In particular, neurons within HVC are active both when a male hears his own song as well as when he is singing. Individual auditory-motor ‘mirror’ neurons were identified in HVC that are activated in both auditory and motor contexts with exquisite syllable-level precision [76]. The inherent neuronal ‘selectivity’ of enhanced responses to the birds' own vocalizations in HVC (see Fig. 1) therefore likely relates to its central role in sensorimotor integration.
Figure 1.
Rapid actions of estradiol (E2) in NCM on auditory processing and stimulus selectivity in a downstream nucleus HVC. Top, a schematic of the zebra finch brain in the sagittal plane, showing the primary auditory network (light yellow arrows) and the song motor pathway (dark red arrows). The caudomedial nidopallium (NCM) is particularly enriched with expression of aromatase, the protein that catalyzes the local synthesis of estrogens. Ov, ovoidalis; L, primary thalamorecipient Field L; HVC (proper name); CMM, caudomedial mesopallium; CLM, caudolateral mesopallium; NIf, nucleus interface; RA, robust nucleus of the arcopallium. Bottom, a selective neural representation in HVC for playback of the bird's own vocalizations (‘OWN’) is enhanced by rapid delivery of estrogens (E2) to NCM, but not to nearby CMM. Bar graphs show mean ± s.e. for HVC responses to stimulation with OWN (bird's own song), REV (reverse OWN), or CON (conspecific song), each standardized as a ratio of responses to white noise (a measure of stimulus selectivity). The HVC selectivity for OWN is significantly elevated during E2 delivery to NCM (**p < 0.01 for paired Wilcoxon test vs. aCSF) but not to CMM (NS = nonsignificant). Insets show individual data for HVC OWN selectivity during aCSF, E2, and wash manipulations in NCM (n = 9) and CMM (n = 5). Adapted from Remage-Healey and Joshi, In press, J Neurosci, (ref 72).
As shown in Figure 1, when estrogens are delivered to NCM via retrodialysis, the responses of single neurons in HVC to the bird's own vocalizations are enhanced. This is in contrast to the responses of these same single HVC neurons to other vocal stimuli (such as conspecific vocalizations (CON)), which are not altered by estradiol retrodialysis into NCM. Therefore, estrogens acting in NCM cause the selectivity of neurons in downstream HVC to be even further enhanced above baseline for sensorimotor-relevant stimuli. These transsynaptic effects in NCM are region-specific, since there is no effect of identical rapid estrogen delivery into the adjacent CMM (right panel of Fig. 1). Importantly, when local aromatase activity is blocked via retrodialysis of the inhibitor fadrozole into NCM, the HVC selectivity for the bird's own vocalizations are rapidly suppressed, confirming the endogenous nature of rapid estrogen signaling in NCM. These findings therefore show that rapid estrogen signaling events are not locally restricted to one brain region and can be transmitted to downstream brain circuits. Specifically, the neural representation of incoming auditory stimuli is rapidly altered by estrogen signaling in NCM and this information is transmitted indirectly into the sensorimotor HVC [72]. A likely scenario is that this auditory-sensorimotor transformation by estrogens enhances either the perception or production of complex behaviors, or both, occurring in HVC and its downstream targets. It is also likely that this modulation depends upon established mechanisms for shifting HVC response properties and selectivity, and chiefly among these is catecholamine input to HVC afferents [77]. Regardless of mechanism, it is now evident that rapid estrogen signaling can lead to enhanced neural representations of complex stimuli that are distributed across a neural network, with potential impacts for cognitive function.
These findings resonate with studies in canaries, in which the HVC selectivity for the bird's own vocalizations were enhanced during the long-day (breeding) season relative to short-days [78]. Though not explicitly measured in that study, their finding suggests a role for seasonal changes in plasma- or brain-derived steroids such as estrogens in modulating HVC neuronal selectivity in a more protracted, seasonal timescale. In a similar fashion, seasonal changes in estrogen availability at the level of HVC can also transsynaptically regulate downstream motor targets of HVC, specifically causing elevated firing rates in the premotor RA [79]. Taken together, this recent body of work has shown that estrogens can act transsynaptically at multiple timescales to alter auditory processing, sensorimotor integration, and premotor firing patterns in the songbird forebrain. These actions at multiple neural loci can provide proximate clues for the way that estrogens alter behavioral song preferences as well as song motor output. They also lead to the prediction that rapid estrogen signaling can modulate ongoing song motor output(s), such as song stereotypy [for similar seasonal effects on stereotypy, see: 79]. Together, these recent findings give insight into how and why estrogen signaling events within the CNS are transmitted within and between brain circuits in support of behaviors (such as song) that depend on distributed neural representations.
A proposed mechanism for rapid estrogen signaling in neurons
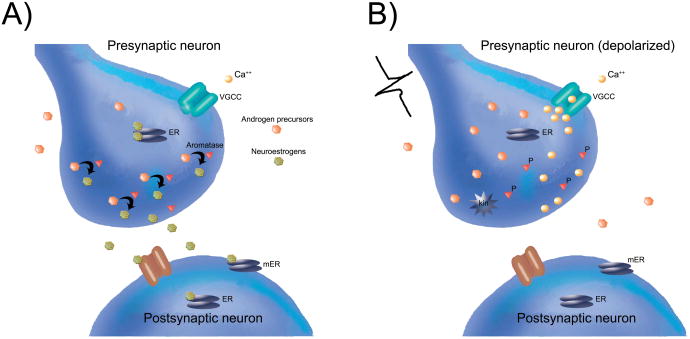
An important consideration regarding rapid estrogen signaling within neural circuits (including but not limited to acoustic communication circuits) is the extent to which the bioavailability of estrogens within these circuits fluctuates with sufficient spatiotemporal precision to enable rapid neuro-behavioral actions to occur. Aromatase expression in the brain is most commonly localized to neuronal cell bodies [80], however, in some vertebrate brain regions the aromatase enzyme is also expressed along axonal processes and is even found at presynaptic terminals [81-83]. In principle, this alternative ‘synaptocrine’ source of aromatase activity provides a means for highly localized and rapid delivery of estrogens to precise synaptic targets, much in the way of classically-defined neuromodulators [14,15].
As a model system for ‘synaptocrine’ signaling, the zebra finch brain contains a substantial fraction of aromatase expressed [58,81] and active [82,84] in presynaptic terminals. A recent series of studies identified a key control mechanism for the rapid estradiol changes at the presynaptic terminal in zebra finches. First, local estradiol levels were shown to be rapidly regulated by excitatory events in the NCM, using a combined in vivo retrodialysis/microdialysis approach [57,85]. In particular, increased local excitation within NCM (either with retrodialysis of glutamate or elevated K+ concentrations) each induced an acute downregulation of estradiol concentrations within NCM in vivo, which then recovered during washout conditions. These actions were somewhat specific to glutamatergic inputs, because similar treatments with NMDA or GABA produced no significant changes in local estradiol concentrations. Second, a similar approach was used to deliver the specific presynaptic voltage-gated calcium channel blocker omega conotoxin to NCM and simultaneously measure local changes in estradiol levels. In the presence of omega conotoxin, the rapid excitatory-induced downregulation of NCM estradiol levels were blocked. The authors of this study concluded that a primary component of rapid estrogen fluctuations occurs directly at the level of presynaptic terminals, which were dependent upon calcium channel opening [85]. More recently, the biochemical activity of the aromatase enzyme was shown to be specifically regulated within presynaptic terminals (synaptosomes) by acute calcium-dependent phosphorylation events [86].
Together, these studies indicate that momentary neuronal excitation within the songbird forebrain drives depolarization-sensitive phosphorylation of the aromatase protein within presynaptic boutons. This mechanism can therefore theoretically provide acute and highly localized fluctuations in estradiol levels at precise synaptic targets. This model is summarized schematically in Figure 2. The extent to which this precise control mechanism accounts for the rapid regulation of auditory processing or vocalizations (as presented above) remains to be determined. This model also opens up several unexplored avenues for further research [see also 14,15]. First, the nature of calcium-dependent phosphorylation of the aromatase enzyme is unclear at the level of synaptic terminals. In quail and human preparations, phosphorylating conditions induce rapid downregulation of aromatase activity, but the search for critical amino acid residues that enable this process has proven difficult [87]. Second, the extent to which the aromatase protein can be controlled at presynaptic terminals independently from the regulation of the abundant aromatase in cell bodies is unclear. In theory, alternative splice variants of the aromatase protein [e.g., 88,89] could be localized to somal vs. synaptic cellular compartments and may confer differential sensitivity to rapid calcium-dependent phosphorylation. Perhaps most importantly, studies to date consistently show that excitatory-induced, calcium-dependent phosphorylation of aromatase in synaptic terminals accounts for a rapid downregulation of aromatase/estradiol levels, whereas the behavioral studies above rely on an as yet undefined control mechanism for rapid increases in estradiol levels within acoustic circuits. The molecular control of aromatase to enable rapid increases in local estradiol levels, especially within synaptic terminals, is now an active area of research.
Figure 2.
Synaptocrine Signalling:Testosterone acts as a precursor (orange hexagons) diffusing from the extracellular space to be available as a substrate for the enzyme aromatase (red triangles) located within the presynaptic bouton. Estrogens such as 17-beta-estradiol (green hexagons) synthesized within the presynaptic bouton are then available to bind presynaptic estrogen receptors (ERs; grey ovals) and modulate presynaptic physiology. Synaptocrine estrogens may also diffuse across the synaptic cleft to interact with postsynaptic ion channel receptors (brown channel), and/or postsynaptic ERs (grey ovals, cytosolic) or membrane ERs (mER; grey ovals, membrane-bound). Synaptocrine estrogens could therefore modulate postsynaptic neurotransmitter receptors, cell signaling pathways, and/or have genomic effects on the postsynaptic cell. Because the activity of the aromatase enzyme is highest in a dephosphorylated state, the synaptocrine actions of estradiol are most likely to occur when the cell membrane is at rest (i.e., not depolarized) and when voltage-gated calcium channels (VGCCs) are closed. In contrast, (right panel), depolarization of the presynaptic neuron leads to calcium influx into the presynaptic bouton. This can activate a calcium-dependent kinase (kin) to phosphorylate presynaptic aromatase (P) and thereby cause a rapid reduction in the synthesis of synaptocrine estrogens, while simultaneously potentially increasing the local concentration of androgens. Adapted from Remage-Healey et al., 2011, Frontiers in Endocrinology, (ref 14).
Conclusions and outlook
This essay presents evidence supporting the hypothesis that estrogens produced in the brain modulate vocal patterning, auditory processing, and sensorimotor integration via local and acute actions in identified brain circuits. These initial conclusions are drawn mainly from studies of vocalizing fish and birds, but may be broadly applicable to the wide variety of vertebrates that use vocalizations for social communication purposes. Interestingly, the elevated expression of aromatase and estrogen receptors in the song learning/auditory processing regions of the songbird brain have not been found in the equivalent brain regions in bird species that do not learn vocalizations, such as quail, pigeons, etc. [e.g., 37,90,91]. Moreover, aromatase expression is elevated in human temporal cortex [13,92,93], which is the primary locus for speech sensorimotor processing. Therefore, the evolution of complex vocal signaling and perhaps even vocal learning itself may be intimately linked to the innovative use of estrogen signaling in the cortex.
These initial observations open new research directions regarding acute estrogen production and action in brain circuits for acoustic communication. First, the specific receptor mechanisms and downstream cell signaling pathways are poorly understood for the modulation of acoustic communication circuits, although the availability of specific receptor ligands has led to a wealth of new information in other neural systems regarding the rapid actions of estrogens in recent years [94-98]. Second, in addition to emphasizing the central actions of estrogens on acoutic communication within brain circuits, it will be important to characterize how estrogens may coordinate peripheral mechanisms for both perception and production of acoustic communication signals. The expression of estrogen receptors in both vocal organs [99-102] and auditory end-organs [38,48,103,104] appears to be a conserved feature among vertebrates and can guide these investigations. It is intriguging to consider that the evolutionary innovation that enabled brain circuits to locally synthesize estrogens could have been linked to the common ancestral origins of acoustic communication circuits among vertebrates [3]. Continuing to explore these questions from a comparative perspective will provide a rich understanding of neuromodulatory mechanisms that regulate vertebrate communication behavior.
Acknowledgments
The author thanks Ben Pawlisch and two anonymous reviewers for comments on the manuscript, and Andrew Bass, Elizabeth Adkins-Regan, Barney Schlinger and Art Arnold for guidance and thoughtful discussion about the topic. Research support was provided by NINDS R00NS066179.
References
- 1.Bass AH, Gilland EH, Baker R. Evolutionary origins for social vocalization in a vertebrate hindbrain-spinal compartment. Science. 2008;321(5887):417–421. doi: 10.1126/science.1157632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodson JL, Bass AH. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: Convergence with terrestrial vertebrates reveals conserved traits. J Comp Neurol. 2002;448(3):298–322. doi: 10.1002/cne.10258. [DOI] [PubMed] [Google Scholar]
- 3.Bass AH, Chagnaud BP. Shared developmental and evolutionary origins for neural basis of vocal-acoustic and pectoral-gestural signaling. Proc Natl Acad Sci U S A. 2012;109(1):10677–84. doi: 10.1073/pnas.1201886109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aitkin L. The auditory neurobiology of marsupials: a review. Hear Res. 1995;82(2):257–66. doi: 10.1016/0378-5955(94)00182-p. [DOI] [PubMed] [Google Scholar]
- 5.Hoke KL, Ryan MJ, Wilczynski W. Integration of sensory and motor processing underlying social behaviour in tungara frogs. Proc Biol Sci. 2007;274(1610):641–9. doi: 10.1098/rspb.2006.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bass A, McKibben JR. Neural mechanisms and behaviors for acoustic communication in teleost fish. Prog Neurobiol. 2003;69(1):1–26. doi: 10.1016/s0301-0082(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Brzozowska-Prechtl A, Karten HJ. Laminar and columnar auditory cortex in avian brain. Proc Natl Acad Sci U S A. 2010;107(28):12676–81. doi: 10.1073/pnas.1006645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodson JL, Bass AH. Forebrain peptides modulate sexually polymorphic vocal circuitry. Nature. 2000;403(6771):769–772. doi: 10.1038/35001581. [DOI] [PubMed] [Google Scholar]
- 9.Winslow JT, Insel TR. Effects of central vasopressin administration to infant rats. Eur J Pharmacol. 1993;233(1):101–7. doi: 10.1016/0014-2999(93)90354-k. [DOI] [PubMed] [Google Scholar]
- 10.Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Horm Behav. 1998;34(1):67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava DP, Waters EM, Mermelstein PG, Kramar EA, Shors TJ, et al. Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry. J Neurosci. 2011;31(45):16056–63. doi: 10.1523/JNEUROSCI.4097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 13.Azcoitia I, Yague JG, Garcia-Segura LM. Estradiol synthesis within the human brain. Neuroscience. 2011;191:139–47. doi: 10.1016/j.neuroscience.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Remage-Healey L, Saldanha CJ, Schlinger BA. Estradiol synthesis and action at the synapse: Evidence for ‘synaptocrine’ signaling. Frontiers in Endocrinology. 2011;2(28):1–13. doi: 10.3389/fendo.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine Signaling: Steroid Synthesis and Action at the Synapse. Endocr Rev. 2011 doi: 10.1210/er.2011-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruf K, Steiner FA. Steroid-Sensitive Single Neurons in Rat Hypothalamus and Midbrain - Indentification by Microelectrophoresis. Science. 1967;156(3775):667–669. doi: 10.1126/science.156.3775.667. [DOI] [PubMed] [Google Scholar]
- 17.Wong M, Moss RL. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J Neurosci. 1992;12(8):3217–25. doi: 10.1523/JNEUROSCI.12-08-03217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose JD, Marrs GS, Moore FL. Rapid, corticosterone-induced disruption of medullary sensorimotor integration related to suppression of amplectic clasping in behaving roughskin newts (Taricha granulosa) Horm Behav. 1998;34(3):268–282. doi: 10.1006/hbeh.1998.1483. [DOI] [PubMed] [Google Scholar]
- 19.Kelly MJ, Ronnekleiv OK, Eskay RL. Identification of estrogen-responsive LHRH neurons in the guinea pig hypothalamus. Brain Res Bull. 1984;12(4):399–407. doi: 10.1016/0361-9230(84)90112-6. [DOI] [PubMed] [Google Scholar]
- 20.Leshner AI. The hormonal responses to competition and their behavioral significance. In: Svare B, editor. Hormones and Aggressive Behavior. New York: Plenum Press; 1983. pp. 393–404. [Google Scholar]
- 21.Batty J. Acute Changes in Plasma Testosterone Levels and Their Relation to Measures of Sexual-Behavior in Male House Mouse (Mus-Musculus) Anim Behav. 1978;26(MAY):349–357. doi: 10.1016/0003-3472(78)90053-2. [DOI] [PubMed] [Google Scholar]
- 22.Harding CF, Follett BK. Hormone changes triggered by aggression in a natural population of blackbirds. Science. 1979;203(4383):918–20. doi: 10.1126/science.570304. [DOI] [PubMed] [Google Scholar]
- 23.Wingfield JC. Short-Term Changes in Plasma-Levels of Hormones During Establishment and Defense of a Breeding Territory in Male Song Sparrows, Melospiza-Melodia. Horm Behav. 1985;19(2):174–187. doi: 10.1016/0018-506x(85)90017-0. [DOI] [PubMed] [Google Scholar]
- 24.Wingfield JC, Hegner RE, Dufty AM, Ball GF. The Challenge Hypothesis - Theoretical Implications for Patterns of Testosterone Secretion, Mating Systems, and Breeding Strategies. Am Nat. 1990;136(6):829–846. [Google Scholar]
- 25.Hirschenhauser K, Oliveira RF. Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim Behav. 2006;71:265–277. [Google Scholar]
- 26.Naftolin F, MacLusky NJ. Aromatization Hypothesis Revisited. 1. Vol. 87. Serono Symposia publications from Raven Press; 1984. pp. 79–92. [Google Scholar]
- 27.Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, et al. The formation of estrogens by central neuroendocrine tissues. Recent Prog Horm Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- 28.Steimer T, Hutchison JB. Androgen Increases Formation of Behaviorally Effective Estrogen in Dove Brain. Nature. 1981;292(5821):345–347. doi: 10.1038/292345a0. [DOI] [PubMed] [Google Scholar]
- 29.Balthazart J, Surlemont C. Androgen and estrogen action in the preoptic area and activation of copulatory behavior in quail. Physiol Behav. 1990;48(5):599–609. doi: 10.1016/0031-9384(90)90198-d. [DOI] [PubMed] [Google Scholar]
- 30.Cornil CA, Taziaux M, Baillien M, Ball GF, Balthazart J. Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Horm Behav. 2006;49(1):45–67. doi: 10.1016/j.yhbeh.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balthazart J, Ball GF. Brain Aromatase, Estrogens, and Behavior. In: Ball GF, Balthazart J, Nelson RJ, editors. Oxford Series in Behavioral Neuroendocrinology. Oxford: Oxford University Press; 2012. [Google Scholar]
- 32.Forlano PM, Deitcher DL, Bass AH. Distribution of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. The Journal of Comparative Neurology. 2005;483(1):91–113. doi: 10.1002/cne.20397. [DOI] [PubMed] [Google Scholar]
- 33.Sisneros JA. Steroid-Dependent Auditory Plasticity Leads to Adaptive Coupling of Sender and Receiver. Science. 2004;305(5682):404–407. doi: 10.1126/science.1097218. [DOI] [PubMed] [Google Scholar]
- 34.Kelley DB. Locations of androgen-concentrating cells in the brain of Xenopus laevis: autoradiography with 3H-dihydrotestosterone. J Comp Neurol. 1981;199(2):221–31. doi: 10.1002/cne.901990206. [DOI] [PubMed] [Google Scholar]
- 35.Wu KH, Tobias ML, Thornton JW, Kelley DB. Estrogen receptors in Xenopus: duplicate genes, splice variants, and tissue-specific expression. Gen Comp Endocrinol. 2003;133(1):38–49. doi: 10.1016/s0016-6480(03)00148-5. [DOI] [PubMed] [Google Scholar]
- 36.Caras ML, Brenowitz E, Rubel EW. Peripheral auditory processing changes seasonally in Gambel's white-crowned sparrow. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010;196(8):581–99. doi: 10.1007/s00359-010-0545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metzdorf R, Gahr M, Fusani L. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J Comp Neurol. 1999;407(1):115–129. [PubMed] [Google Scholar]
- 38.Noirot IC, Adler HJ, Cornil CA, Harada N, Dooling RJ, et al. Presence of aromatase and estrogen receptor alpha in the inner ear of zebra finches. Hear Res. 2009;252(1-2):49–55. doi: 10.1016/j.heares.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Schaefer J, Zakon HH. Opposing actions of androgen and estrogen on in vitro firing frequency of neuronal oscillators in the electromotor system. J Neurosci. 1996;16(8):2860–8. doi: 10.1523/JNEUROSCI.16-08-02860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nyby J, Matochik JA, Barfield RJ. Intracranial androgenic and estrogenic stimulation of male-typical behaviors in house mice (Mus domesticus) Horm Behav. 1992;26(1):24–45. doi: 10.1016/0018-506x(92)90029-u. [DOI] [PubMed] [Google Scholar]
- 41.Holman SD, Hutchison RE, Hutchison JB. Microimplants of Estradiol in the Sexually Dimorphic Area of the Hypothalamus Activate Ultrasonic Vocal Behavior in Male Mongolian Gerbils. Horm Behav. 1991;25(4):531–548. doi: 10.1016/0018-506x(91)90019-e. [DOI] [PubMed] [Google Scholar]
- 42.Amir O, Biron-Shental T, Muchnik C, Kishon-Rabin L. Do oral contraceptives improve vocal quality? Limited trial on low-dose formulations. Obstet Gynecol. 2003;101(4):773–7. doi: 10.1016/s0029-7844(02)03126-5. [DOI] [PubMed] [Google Scholar]
- 43.Firat Y, Engin-Ustun Y, Kizilay A, Ustun Y, Akarcay M, et al. Effect of intranasal estrogen on vocal quality. J Voice. 2009;23(6):716–20. doi: 10.1016/j.jvoice.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952;117(1):109–28. [PMC free article] [PubMed] [Google Scholar]
- 45.Wu KH, Tobias ML, Kelley DB. Estrogen and laryngeal synaptic strength in Xenopus laevis: opposite effects of acute and chronic exposure. Neuroendocrinology. 2001;74(1):22–32. doi: 10.1159/000054667. [DOI] [PubMed] [Google Scholar]
- 46.Brantley RK, Bass AH. Alternative Male Spawning Tactics and Acoustic-Signals in the Plainfin Midshipman Fish Porichthys-Notatus Girard (Teleostei, Batrachoididae) Ethology. 1994;96(3):213–232. [Google Scholar]
- 47.Bass AH, Baker R. Sexual Dimorphisms in the Vocal Control-System of a Teleost Fish - Morphology of Physiologically Identified Neurons. J Neurobiol. 1990;21(8):1155–1168. doi: 10.1002/neu.480210802. [DOI] [PubMed] [Google Scholar]
- 48.Forlano PM, Deitcher DL, Bass ARH. Distribution of estrogen receptor alpha mRNA in the brain and inner ear of a vocal fish with comparisons to sites of aromatase expression. J Comp Neurol. 2005;483(1):91–113. doi: 10.1002/cne.20397. [DOI] [PubMed] [Google Scholar]
- 49.Forlano PM, Deitcher DL, Myers DA, Bass AH. Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: Aromatase enzyme and mRNA expression identify glia as source. J Neurosci. 2001;21(22):8943–8955. doi: 10.1523/JNEUROSCI.21-22-08943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. J Neurosci. 2004;24(26):5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Remage-Healey L, Bass AH. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J Neurosci. 2007;27(5):1114–1122. doi: 10.1523/JNEUROSCI.4282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, et al. Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior. Endocrinology. 2005;146(9):3809–3820. doi: 10.1210/en.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charlier TD, Newman AE, Heimovics SA, Po KW, Saldanha CJ, et al. Rapid effects of aggressive interactions on aromatase activity and oestradiol in discrete brain regions of wild male white-crowned sparrows. J Neuroendocrinol. 2011;23(8):742–53. doi: 10.1111/j.1365-2826.2011.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chao A, Schlinger BA, Remage-Healey L. Combined liquid and solid-phase extraction improves quantification of brain estrogen content. Front Neuroanat. 2011;5:57. doi: 10.3389/fnana.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Charlier TD, Po KW, Newman AE, Shah AH, Saldanha CJ, et al. 17beta-Estradiol levels in male zebra finch brain: combining Palkovits punch and an ultrasensitive radioimmunoassay. Gen Comp Endocrinol. 2010;167(1):18–26. doi: 10.1016/j.ygcen.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schlinger BA, Arnold AP. Circulating Estrogens in a Male Songbird Originate in the Brain. Proc Natl Acad Sci U S A. 1992;89(16):7650–7653. doi: 10.1073/pnas.89.16.7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11(11):1327–34. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, et al. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423(4):619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 59.Jeong JK, Burrows K, Tremere LA, Pinaud R. Neurochemical organization and experience-dependent activation of estrogen-associated circuits in the songbird auditory forebrain. Eur J Neurosci. 2011;34(2):283–91. doi: 10.1111/j.1460-9568.2011.07743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. J Neurophysiol. 2012;107(6):1621–31. doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci U S A. 1992;89(15):6818–22. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chew SJ, Vicario DS, Nottebohm F. A large-capacity memory system that recognizes the calls and songs of individual birds. Proc Natl Acad Sci U S A. 1996;93(5):1950–1955. doi: 10.1073/pnas.93.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc Natl Acad Sci U S A. 2006;103(4):1088–1093. doi: 10.1073/pnas.0510136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nature Reviews Neuroscience. 2006;7(5):347–357. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- 65.Woolley SC, Doupe AJ. Social context-induced song variation affects female behavior and gene expression. PLoS Biol. 2008;6(3):e62. doi: 10.1371/journal.pbio.0060062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nature Neuroscience. 2008;11(5):579–586. doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A. 2010;107(8):3852–7. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tremere LA, Jeong JK, Pinaud R. Estradiol shapes auditory processing in the adult brain by regulating inhibitory transmission and plasticity-associated gene expression. J Neurosci. 2009;29(18):5949–63. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tremere LA, Pinaud R. Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. J Neurosci. 2011;31(9):3271–89. doi: 10.1523/JNEUROSCI.4355-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holveck MJ, Riebel K. Preferred songs predict preferred males: consistency and repeatability of zebra finch females across three test contexts. Anim Behav. 2007;74:297–309. [Google Scholar]
- 71.Adret P. Operant-Conditioning, Song Learning and Imprinting to Taped Song in the Zebra Finch. Anim Behav. 1993;46(1):149–159. [Google Scholar]
- 72.Remage-Healey L, Joshi NR. Changing Neuroestrogens Within the Auditory Forebrain Rapidly Transform Stimulus Selectivity in a Downstream Sensorimotor Nucleus. J Neurosci. 2012;32(24):8231–41. doi: 10.1523/JNEUROSCI.1114-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brenowitz EA. Altered Perception of Species-Specific Song by Female Birds after Lesions of a Forebrain Nucleus. Science. 1991;251(4991):303–305. doi: 10.1126/science.1987645. [DOI] [PubMed] [Google Scholar]
- 74.Gentner TQ, Hulse SH, Bentley GE, Ball GF. Individual vocal recognition and the effect of partial lesions to HVc on discrimination, learning, and categorization of conspecific song in adult songbirds. J Neurobiol. 2000;42(1):117–33. doi: 10.1002/(sici)1097-4695(200001)42:1<117::aid-neu11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 75.Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature. 2008;456(7219):189–194. doi: 10.1038/nature07448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory–vocal mirroring in neurons for learned vocal communication. Nature. 2008;451(7176):305–310. doi: 10.1038/nature06492. [DOI] [PubMed] [Google Scholar]
- 77.Cardin JA, Schmidt MF. Noradrenergic Inputs Mediate State Dependence of Auditory Responses in the Avian Song System. J Neurosci. 2004;24(35):7745–7753. doi: 10.1523/JNEUROSCI.1951-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Del Negro C, Lehongre K, Edeline JM. Selectivity of canary HVC neurons for the bird's own song: modulation by photoperiodic conditions. J Neurosci. 2005;25(20):4952–63. doi: 10.1523/JNEUROSCI.4847-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meitzen J, Moore IT, Lent K, Brenowitz EA, Perkel DJ. Steroid hormones act transsynaptically within the forebrain to regulate neuronal phenotype and song stereotypy. J Neurosci. 2007;27(44):12045–12057. doi: 10.1523/JNEUROSCI.3289-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Canick JA, Vaccaro DE, Livingston EM, Leeman SE, Ryan KJ, et al. Localization of aromatase and 5 alpha-reductase to neuronal and non-neuronal cells in the fetal rat hypothalamus. Brain Res. 1986;372(2):277–82. doi: 10.1016/0006-8993(86)91135-2. [DOI] [PubMed] [Google Scholar]
- 81.Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proceedings of the Royal Society B-Biological Sciences. 2005;272(1576):2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rohmann KN, Schlinger BA, Saldanha CJ. Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. Dev Neurobiol. 2007;67(1):1–9. doi: 10.1002/dneu.20303. [DOI] [PubMed] [Google Scholar]
- 83.Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, et al. Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology. 1996;63(2):149–55. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- 84.Remage-Healey L, Oyama RK, Schlinger BA. Elevated aromatase activity in forebrain synaptic terminals during song. J Neuroendocrinol. 2009;21(3):191–9. doi: 10.1111/j.1365-2826.2009.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Remage-Healey L, Dong S, Maidment NT, Schlinger BA. Presynaptic control of rapid estrogen fluctuations in the songbird auditory forebrain. J Neurosci. 2011;31(27):10034–8. doi: 10.1523/JNEUROSCI.0566-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cornil CA, Leung CH, Pletcher ER, Naranjo KC, Blauman SJ, et al. Acute and specific modulation of presynaptic aromatization in the vertebrate brain. Endocrinology. 2012;153(6):2562–7. doi: 10.1210/en.2011-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Charlier TD, Harada N, Balthazart J, Cornil CA. Human and Quail Aromatase Activity Is Rapidly and Reversibly Inhibited by Phosphorylating Conditions. Endocrinology. 2011 doi: 10.1210/en.2011-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ramachandran B, Schlinger BA, Arnold AP, Campagnoni AT. Zebra finch aromatase gene expression is regulated in the brain through an alternate promoter. Gene. 1999;240(1):209–216. doi: 10.1016/s0378-1119(99)00399-6. [DOI] [PubMed] [Google Scholar]
- 89.Toffolo V, Belvedere P, Colombo L, Valle LD. Tissue-specific transcriptional initiation of the CYP19 genes in rainbow trout, with analysis of splicing patterns and promoter sequences. Gen Comp Endocrinol. 2007;153(1-3):311–9. doi: 10.1016/j.ygcen.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 90.Yoder KM, Vicario DS. To modulate and be modulated: estrogenic influences on auditory processing of communication signals within a socio-neuro-endocrine framework. Behav Neurosci. 2012;126(1):17–28. doi: 10.1037/a0026673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Silverin B, Baillien M, Foidart A, Balthazart J. Distribution of aromatase activity in the brain and peripheral tissues of passerine and nonpasserine avian species. Gen Comp Endocrinol. 2000;117(1):34–53. doi: 10.1006/gcen.1999.7383. [DOI] [PubMed] [Google Scholar]
- 92.Yague JG, Munoz A, De Monasterio-Schrader P, DeFelipe J, Garcia-Segura LM, et al. Aromatase expression in the human temporal cortex. Neuroscience. 2006;138(2):389–401. doi: 10.1016/j.neuroscience.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 93.Stoffel-Wagner B, Watzka M, Schramm J, Bidlingmaier F, Klingmuller D. Expression of CYP19 (aromatase) mRNA in different areas of the human brain. J Steroid Biochem. 1999;70(4-6):237–241. doi: 10.1016/s0960-0760(99)00114-4. [DOI] [PubMed] [Google Scholar]
- 94.Bryant DN, Sheldahl LC, Marriott LK, Shapiro RA, Dorsa DM. Multiple pathways transmit neuroprotective effects of gonadal steroids. Endocrine. 2006;29(2):199–207. doi: 10.1385/ENDO:29:2:199. [DOI] [PubMed] [Google Scholar]
- 95.Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid effects of estrogen receptor alpha and beta selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152(4):1492–502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
- 96.Frick KM. Building a better hormone therapy? How understanding the rapid effects of sex steroid hormones could lead to new therapeutics for age-related memory decline. Behav Neurosci. 2012;126(1):29–53. doi: 10.1037/a0026660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol. 2009;23(3):349–59. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane Estrogen Receptors Stimulate Intracellular Calcium Release and Progesterone Synthesis in Hypothalamic Astrocytes. J Neurosci. 2010;30(39):12950–12957. doi: 10.1523/JNEUROSCI.1158-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Veney SL, Wade J. Post-hatching syrinx development in the zebra finch: an analysis of androgen receptor, aromatase, estrogen receptor alpha and estrogen receptor beta mRNAs. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191(2):97–104. doi: 10.1007/s00359-004-0577-5. [DOI] [PubMed] [Google Scholar]
- 100.Narbaitz R, Stumpf WE, Sar M. Estrogen target cells in the larynx: autoradiographic studies with 3H-diethylstilbestrol in fetal mice. Horm Res. 1980;12(2):113–7. doi: 10.1159/000179111. [DOI] [PubMed] [Google Scholar]
- 101.Wu KH, Tobias ML, Kelley DB. Estrogen receptor expression in laryngeal muscle in relation to estrogen-dependent increases in synapse strength. Neuroendocrinology. 2003;78(2):72–80. doi: 10.1159/000071962. [DOI] [PubMed] [Google Scholar]
- 102.Holt GR, Aufdemorte TB, Sheridan PJ. Estrogen receptor in the larynx of the aged baboon (Papio cynocephalus) Ann Otol Rhinol Laryngol. 1986;95(6 Pt 1):608–17. doi: 10.1177/000348948609500614. [DOI] [PubMed] [Google Scholar]
- 103.Sisneros JA. Adaptive hearing in the vocal plainfin midshipman fish: getting in tune for the breeding season and implications for acoustic communication. Integr Zool. 2009;4(1):33–42. doi: 10.1111/j.1749-4877.2008.00133.x. [DOI] [PubMed] [Google Scholar]
- 104.Hultcrantz M, Simonoska R, Stenberg AE. Estrogen and hearing: a summary of recent investigations. Acta Oto-Laryngol. 2006;126(1):10–14. doi: 10.1080/00016480510038617. [DOI] [PubMed] [Google Scholar]