Abstract
The present studies were designed to evaluate the adjuvant activity of polyanhydride microparticles prepared in the absence of additional stabilizers, excipients, or immune modulators. Microparticles composed of varying ratios of either 1,6-bis(p-carboxyphenoxy)hexane (CPH) and sebacic acid (SA) or 1,8-bis(p-carboxyphenoxy)-3,6-dioxaoctane (CPTEG) and CPH were added to in vitro cultures of bone marrow-derived dendritic cells (DCs). Microparticles were efficiently and rapidly phagocytosed by DCs in the absence of opsonization and without centrifugation or agitation. Within 2 h, internalized particles were rapidly localized to an acidic, phagolysosomal compartment. By 48 h, only a minor reduction in microparticle size was observed in the phagolysosomal compartment, indicating minimal particle erosion consistent with being localized within an intracellular microenvironment favoring particle stability. Polyanhydride microparticles increased DC surface expression of MHC II, the co-stimulatory molecules CD86 and CD40, and the C-type lectin CIRE (murine DC-SIGN; CD209). In addition, microparticle stimulation of DCs also enhanced secretion of the cytokines IL-12p40 and IL-6, a phenomenon found to be dependent on polymer chemistry. DCs cultured with polyanhydride microparticles and ovalbumin induced polymer chemistry-dependent antigen-specific proliferation of both CD4+ OT-II and CD8+ OT-I T cells. These data indicate that polyanhydride particles can be tailored to take advantage of the potential plasticity of the immune response, resulting in the ability to induce immune protection against many types of pathogens.
Keywords: Polyanhydrides, Dendritic cells, Adjuvants, Vaccine delivery, Microparticles
Introduction
The World Health Organization (WHO) estimated that in 2002, 2.1 million deaths occurred worldwide that resulted from diseases that could have been prevented by routine vaccination [1]. In an effort to minimize the mortality associated with infectious diseases, the WHO, United Nations Children’s Fund (UNICEF) and several other partnering organizations developed a Global Immunization Vision and Strategy (GIVS). The primary mission of GIVS is the introduction of new and efficacious vaccines and delivery technologies to combat diseases for which no treatment currently exists [2]. The rapid development of protein-based biopharmaceuticals suggests that many future vaccines will involve the delivery of peptide or protein subunits. Currently, such vaccines lack a suitable carrier, and, thus, there is an urgent need to develop new adjuvants for delivery of efficacious vaccines [3].
An adjuvant is a substance which when incorporated into a vaccine will enhance the immune response to the antigen [4]. Classically, adjuvants fulfill one of three roles: 1) act as a depot, preventing rapid clearance of the antigen; 2) direct the antigen to antigen presenting cells (APCs) for phagocytosis, processing, and presentation; and 3) induce co-stimulatory signals on APCs necessary for activation of naïve T cells [5,6]. Currently, the common adjuvants employed in human vaccines are aluminum-based salts (e.g., alum) and often require multiple doses to achieve protective immunity. The resulting immune response is primarily antibody-mediated with limited engagement of an effector T cell population [7]. Use of aluminum-based salts is often associated with the induction of adverse reactions at the site of injection. Alternatively, we and others have extensively studied polyanhydrides, a novel class of biodegradable polymers, as protein carriers and/or vaccine delivery systems with inherent adjuvant properties [8-20]. Currently approved by the FDA for use in humans, polyanhydrides are tissue compatible and degrade into non-mutagenic and non-cytotoxic products [8,21-25]. Another advantage of these polymers is their degradation by a surface erosion mechanism resulting in controlled release of the antigen with predictable degradation profiles that can vary from days to months depending on the polymer chemistry [8,9,16]. Moreover, this controlled release of antigen enhances the induction of an immune response and modulates the ensuing immune response with respect to Th1-Th2 bias, and may potentially improve patient compliance by requiring only a single injection [20,26]. Investigations have also shown that polyanhydrides may have intrinsic adjuvant properties, distinct from any antigen payload [20,27,28].
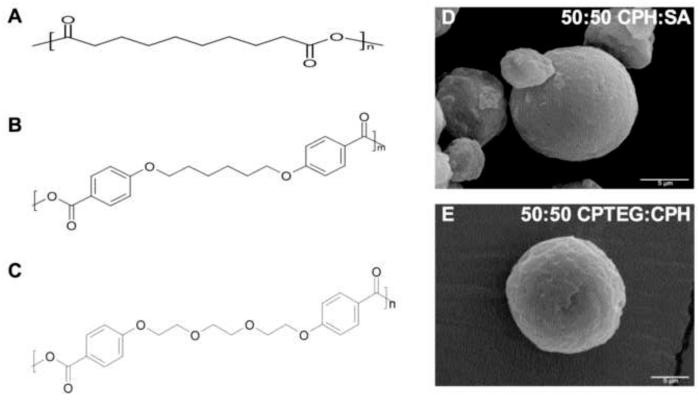
In this study, we have further investigated the intrinsic adjuvanticity of two polyanhydride chemistries based on the aliphatic sebacic anhydride (SA), the aromatic 1,6-bis(p-carboxyphenoxy)hexane (CPH), and the amphiphilic 1,8-bis(p-carboxyphenoxy)-3,6-dioxaoctane (CPTEG) (Fig. 1A). In particular, 50:50 CPH:SA and 50:50 CPTEG:CPH copolymers were chosen based on previous studies indicating the ability of these materials to serve as vaccine adjuvants and protein carriers [19,20]. As adjuvants are thought to elicit many of their effects via directing antigen to and activation of APCs, mouse bone marrow-derived dendritic cells (DCs), the most potent APC involved in the induction of an immune response, were selected for use in these experiments. It was found that polyanhydride microparticles were efficiently phagocytosed by DCs and localized to phagolysosomal compartments. Dendritic cell surface expression of MHC II, CD86, CD40, and CIRE was significantly increased after exposure to microparticles, as was secretion of IL-12p40 and IL-6. Lastly, DCs incubated in the presence of polyanhydride microparticles and ovalbumin induced antigen-specific proliferation of both CD4+ OT-II and CD8+ OT-I T cells. The results of these studies demonstrate that polyanhydride microparticles are capable of activating DCs as well as inducing, in a chemistry-dependent manner, antigen-specific T cell responses in vitro.
Figure 1.
(A) Chemical structures of (i) poly(SA), (ii) poly(CPH), and (iii) poly CPTEG. Here, n and m represent degree of polymerization. (B) Scanning electron photomicrographs of 50:50 CPH:SA and 50:50 CPTEG:CPH microparticles. Scale bar: 5 μm.
Materials and Methods
Materials
Chemicals used for synthesis of CPH and CPTEG monomers included: 4-p-hydroxybenzoic acid, 1,6-dibromohexane, 1-methyl-2-pyrrolidinone, and tri-ethylene glycol. All these chemicals and sebacic acid (99%) were purchased from Sigma Aldrich (St Louis, MO); 4-p-fluorobenzonitrile was obtained from Apollo Scientific (Cheshire, UK); potassium carbonate, dimethyl formamide, toluene, sulfuric acid, acetic acid, acetonitrile, acetic anhydride, methylene chloride, and petroleum ether were purchased from Fisher Scientific (Fairlawn, NJ).
Polymer synthesis and characterization
CPH:SA and CPTEG:CPH copolymers were synthesized by melt polycondensation as described previously [15,17]. The purity and degree of polymerization of the polymers was analyzed using 1H NMR spectra obtained from a Varian VXR-300 MHz NMR spectrometer. Detailed analysis of 1H NMR characteristic spectra of polyanhydrides can be found elsewhere [15,16]. The molecular weight and molecular weight distribution of the copolymers was analyzed by gel permeation chromatography (GPC) as previously reported [17]. The number average molecular weight of 50:50 CPTEG:CPH was 8,000 Da (PDI = 1.7) and that of 50:50 CPH:SA was 17,000 Da (PDI = 2.1).
Microparticle fabrication by cryogenic atomization
To prevent microbial contamination, all glassware and equipment was soaked in 70% ethanol prior to fabricating microparticles. The procedure used to fabricate microparticles was modified from previously reported studies [29,30]. Briefly, polymer dissolved in methylene chloride was pumped through an 8700-1200 MS ultrasonic atomizing nozzle (Sono Tek Corporation, Milton, NY) into 200 mL of ultra-cold ethanol overlaid with ~100 mL of liquid nitrogen. This procedure was performed at 4°C for 50:50 CPH:SA and CPTEG:CPH microparticles in order to maintain the temperature below the glass transition temperature of the polymers during microparticle preparation. After atomization, microparticles were stored at −80 °C for three days to allow the methylene chloride to be extracted into the ethanol. Microparticles were then collected by filtration and dried under vacuum overnight. The microparticle morphology was characterized by scanning electron microscopy (SEM). Particle size distribution was obtained from SEM images (150-250x) by using an image analysis software package (analySIS®, Soft Imaging System Corp, Lakewood, CO). An average of 800 particles per image was analyzed.
Endotoxin assay
To ensure that the DC activation observed was due to the polymers and not endotoxin contamination, endotoxin levels of polyanhydride microparticles were tested with the Limulus Amebocyte Lysate (LAL) QCL-1000 test kit (Cambrex, Walkersville, MD). Solutions of CPTEG:CPH and CPH:SA microparticles (5 mg/mL) fabricated as described above were prepared using endotoxin-free, sterile water and incubated overnight at 37°C while shaking. After centrifugation of microparticles, the LAL test was performed on the supernatant according to manufacturer’s procedure. All the polyanhydride microparticles exhibited an endotoxin content of less than 0.1 EU/mL, which is five times lower than the maximum level permitted by the United States FDA for new drugs tested by the LAL test [31]. Solutions of ovalbumin were prepared in endotoxin-free water and also tested for endotoxin content according to manufacturer’s instructions. Ovalbumin, as purchased, was found to contain high levels of endotoxin, which was removed using AffinityPak Detoxi-Gel endotoxin removing gel columns (Thermo Scientific, Rockford, IL) according to manufacturer’s instructions. The resulting ovalbumin contained less than 10 EU/mg, which is equivalent to ~1 ng of endotoxin per mg of protein. Endotoxin-free ovalbumin was lyophilized and stored at −20°C until use.
Mice
C3H/HeNHsd and C57BL/6 mice were purchased from Harlan Sprague Dawley. Male and female C57BL/6 Tg(TcraTrab)1100Mjb/J (OT-I) and C57BL/6 Tg(TcraTrab)425Cbn/J (OT-II) TCR transgenic mice were purchased from Jackson Laboratory (Bar Harbor, Maine). All mice were housed under specific pathogen-free conditions and all bedding, caging, water, and feed were sterilized prior to use. Animal procedures were conducted with the approval of the Iowa State University Institutional Animal Care and Use Committee.
Isolation and culture of DCs
Bone marrow-derived dendritic cells (DCs) were isolated from the femurs and tibia of C3H/HeNHsd or C57BL/6 mice and cultured in vitro in the presence of 10 ng/mL murine GM-CSF (PeproTech, Rocky Hill, NJ) according to a previously published protocol with minor modifications [32]. At day 6 of culture, ~90% of DCs were positive for the DC marker CD11c and veiled projections were visible on the cells. Six-day-old DCs were harvested and transferred to 24-well plates (2.5 × 106 cells/well).
Microparticle internalization by DCs using fluorescence microscopy
To visualize cellular interactions with microparticles, the particles (250 μg/mL) were incubated with DCs for 30 min and then washed briefly to remove extracellular and non-adherent particles. Cultures continued to incubate with fresh medium for either 2 or 48 h. Dendritic cell monolayers incubated with microparticles were fixed at either 2 h or 48 h post-internalization with 4% paraformaldehyde (PFA) for 10 min at room temperature. Acidic vesicles and lipid rafts were labeled by incubating cells for 20 min prior to fixation with either Lysotracker at 1:2000 dilution (DND-99) (acidic vesicles) or Alexa555 conjugated cholera toxin B-subunit (CTx) at 1:150 dilution (lipid rafts) (Molecular Probes-Invitrogen, Carlsbad, CA) [27]. Intracellular structures were visualized by immunofluorescence microscopy by incubating fixed cover slips with primary and secondary antibodies in PBS containing albumin and 0.1% saponin (BSP) [33]. Stained cover slips were washed and mounted on glass slides (Pro-Long with Dapi; Molecular Probes-Invitrogen). Wide field, epifluorescence images were captured using a DP-70 CCD camera, with a 40x LWDPlanFluor objective mounted on an IX-61 inverted microscope (Olympus, USA). Detailed high resolution imaging in X, Y, and Z dimensions was subsequently performed with a Leica NTS laser scanning confocal microscopy (LSCM) with Ar, Kr and HeNe laser lines. The step size for Z-stack image data was maintained at 0.24 μm while total stack volume varied to match cell thickness for each field. Epifluorescence and confocal image data sets were exported to ImageJ v1.36b for cell and particle analysis (epifluorescence) and constructing Z-projection images (confocal) [34].
DC stimulation using flow cytometry
For flow cytometric analysis of surface molecule expression, bone marrow-derived dendritic cells (DCs) were isolated and cultured as described above. Polyanhydride microparticles were suspended in complete culture medium, sonicated briefly, and added to the DC cultures at day nine at a concentration 250 μg/mL, corresponding to a microparticle:DC ratio of 12:1. Unstimulated DCs and DCs stimulated with LPS (200 ng/mL) were used as negative and positive controls, respectively. Cultures were incubated for 48 h (37 °C, 5% CO2). DCs were harvested after 48 h of stimulation and washed in fluorescence-activated cell sorting buffer (FACS, 0.1% sodium azide and 0.1% bovine serum albumin in phosphate buffer saline). Fc receptors were blocked with 10 μg/mL purified rat anti-mouse CD16/CD32 antibody (eBioscience, San Diego, CA), 0.5% homologous mouse serum and 50 μg/mL rat IgG (Sigma) for 1 h at 4°C to prevent nonspecific binding. DCs were then incubated with the appropriate antibody or isotype control for 1 h on ice. Antibodies used included Alexa Fluor® 700 anti-mouse CD11c (clone N418), FITC conjugated anti-mouse/rat MHC Class II (I-Ek, clone 14-4-4S), PE/Cy7 anti-mouse CD86 (clone GL-1), allophycocyanin (APC) anti-mouse CD40 (clone 1C10) and PE conjugated anti-mouse CIRE (DC-SIGN CD209; clone 5H10) antibodies. Isotype-specific control antibodies included Alexa Fluor® 700 conjugated Armenian hamster IgG (clone eBio299Arm), FITC IgG2a κ (clone eBM2a), PE/Cy7 conjugated rat IgG2b (clone KLH/G2b-1-2), APC rat IgG2a κ (clone eBR2a) and PE-conjugated rat IgG2a (clone eBR2a). Anti-CD86 was purchased from BD Biosciences (San Jose, CA) and all other antibodies and isotype controls were purchased from eBioscience (San Diego, CA). Propidium iodide (PI) was used to establish the live/dead cell gate. Following staining, cells were washed in FACS buffer and fixed in 1% paraformaldehyde and stored at 4°C until analysis. Analysis was performed on a BD FACScanto flow cytometer (Becton Dickinson, San Jose, CA), and data analyzed using FlowJo software (Tree Star, Inc., Ashland, OR). No significant differences in surface marker expression were observed between DCs co-cultured with polyanhydride particles plus free ovalbumin as compared to particles alone or between DCs co-cultured with free ovalbumin as compared to unstimulated DCs (data not shown).
Cytokine analysis
Polyanhydride microparticles were suspended in complete culture medium, sonicated briefly, and added to the DC cultures at day nine at a concentration of 250 μg/mL, corresponding to a microparticle:DC ratio of 12:1. Unstimulated DCs and DCs stimulated with LPS (200 ng/mL) were used as negative and positive controls, respectively. Cell-free supernatants were collected from DCs cultured for 48 h in the presence of microparticles and stored at −20°C until analysis. Cytokines (TNF-α, IL-4, IL-6, IL-10, and IL-12p40) were measured using a multiplex bead assay and a Luminex® 100 system (Austin, TX). Multiple experiments were analyzed individually.
OT-I and OT-II T cell stimulation
Dendritic cells were cultured and harvested as described above and incubated with 250 μg/mL microparticles and 100 μg/mL ovalbumin for 12 h. Lymph nodes were removed from either OT-I or OT-II mice and single cell suspensions were prepared. Lymph node cells were washed by centrifugation and resuspended in complete culture medium. To assess in vitro immune stimulation, 2.5 × 105 lymphocytes from OT-I or OT-II mice were combined with 0.5 × 105 DCs in 96-well round bottom culture plates. Plates were incubated at 37 °C in humidified 5% CO2. After 72 h of co-culture, 0.5 μCi of methyl-[3H]-thymidine (specific activity of 6.7 Ci mmole−1, Amersham Life Science, Arlington Heights, IL) was added to each well and cells were incubated for additional 18 h. Well contents were harvested onto glass fiber filters and the incorporated radioactivity was measured using a liquid scintillation counter. Assays were performed in triplicate and data are presented as mean counts per minute (cpm) of triplicate wells.
Statistical analysis
Differences among group means were tested by one-way analysis of variance (ANOVA) F-test. If the F-test was significant, post-hoc Tukey’s t-tests were performed for pairwise comparisons of group means. Significance was defined as p < 0.05.
Results
Polymer synthesis and microparticle fabrication
The chemical structures of SA, CPH, and CPTEG are shown in Fig. 1A. NMR spectroscopy was used to confirm that the desired compositions of the copolymers (i.e., 50:50 CPH:SA and 50:50 CPTEG:CPH) were synthesized. Next, microparticles were fabricated by cryogenic atomization and typical yields for both chemistries were ~60%, which is consistent with previous work [29]. The morphology of the microparticles was analyzed with SEM and microparticles of both chemistries exhibited a relatively smooth surface (Fig. 1B), which is consistent with previously published observations [19,35,42]. An analysis of particle size distribution revealed that more than 50% of the polyanhydride microparticles used in this study were below 10 μm in diameter (data not shown). This particle size has been reported to be readily phagocytosed by APCs [36]. More specifically, the mean diameter of the 50:50 CPH:SA microparticles was 10 ± 5 μm and that of the 50:50 CPTEG:CPH microparticles was 13 ± 8 μm.
Efficient uptake and intracellular delivery of microparticles to DC lysosomes
After ensuring that polyanhydride microparticles were not contaminated with endotoxin, initial experiments were performed to determine the best microparticle-DC incubation conditions to accurately visualize intracellular microparticles. These studies revealed that incubating DCs with microparticles for 30 min at 37 6 C without agitation, centrifugation, or opsonization of particles introduced the fewest staining artifacts and allowed for detailed microscopic analysis of particle uptake and intracellular localization. Non-adherent and extracellular microparticles were effectively removed by PBS washes at 30 min to establish the time zero for “post-internalization” analysis of the microparticles. At 2 h, large phagosomes positive for the lysosomal membrane protein Lamp1 contained individual microparticles of 50:50 CPH:SA or 50:50 CPTEG:CPH chemistries (Fig. 2A). Staining of replicate samples revealed that the Lamp1+ vesicles harboring the particles were also acidic (data not shown). At 2 h, approximately 25% of DCs had engulfed microparticles for each of the chemistries (data not shown). Although no difference in the percentage of DCs positive for microparticles was observed between the two chemistries, the number of microparticles internalized was significantly different. Specifically, the number of 50:50 CPTEG:CPH microparticles internalized was higher than that for 50:50 CPH:SA microparticles. This result is consistent with a previous study from our laboratories in which we demonstrated that the hydrophobicity of particles influenced their uptake by monocytes [27].
Figure 2.
Efficient uptake and intracellular delivery of microparticles to DC lysosomes. Laser scanning confocal microscopy of DCs (blue nuclei) at (A) 2 and (B) 48 h post-internalization demonstrated that 50:50 CPH:SA and 50:50 CPTEG:CPH microparticles (green) are localized within phagosomes positive for the lysosomal membrane protein Lamp1 (red). Representative wide field views of monolayers are shown in the top panels to allow for relative comparisons of cell health and morphology and changes observed over time (scale bar = 20 μm). Confocal image data sets were collected to construct 0 6 (top-down view) and 90 6 Z-plane projections (side-view) for each sample (scale bar = 5 μm). A 2 μm wide slice of the image data set (yellow box) was used to generate the 90 6 Z-plane projections. Both wide field and Z-plane images shown are representative of five fields of view analyzed for each chemistry and consistently observed over three independent experiments. ROI = region of interest.
Intracellular microparticles were also observed at 48 h and the majority of the microparticles remained inside a Lamp1+ phagolysosomal membrane (Fig. 2B). DCs appeared healthy and shared a similar morphology with cells that had not phagocytosed microparticles and with cells not cultured in the presence of microparticles (data not shown). Within 48 h, the shape of phagocytosed microparticles changed in a chemistry-dependent manner without appreciable loss of particle fluorescence. Initially observed by epifluorescence and confirmed by confocal microscopy, the surface-eroding 50:50 CPH:SA microparticles retained uniform shapes while 50:50 CPTEG:CPH microparticles appeared more irregular in shape, while remaining sufficiently intact to be readily visible as a microparticle within cells (Fig. 2B). These results are consistent with the degradation profiles of both polymers; our previous work has shown that 50:50 CPH:SA copolymers were largely intact after 48 h [16], whereas 50:50 CPTEG:CPH copolymers exhibited a significant molecular weight loss in aqueous media for over the same incubation period [15]. During confocal imaging, the central portion of microparticles frequently exhibited either partial or complete absence of FITC fluorescence, despite the fact that the surfaces of all of these particles were highly fluorescent (Fig. 2). This phenomenon was observed for both chemistries and observed at both 2 and 48 h post-internalization. Based on confocal images, surface fluorescence typically extended from 0.5 to 1 μm into the intact microparticle. In separate experiments, these results were also seen with microparticles not incubated with cells (data not shown).
Microparticles enhance DC expression of MHC II and co-stimulatory molecules
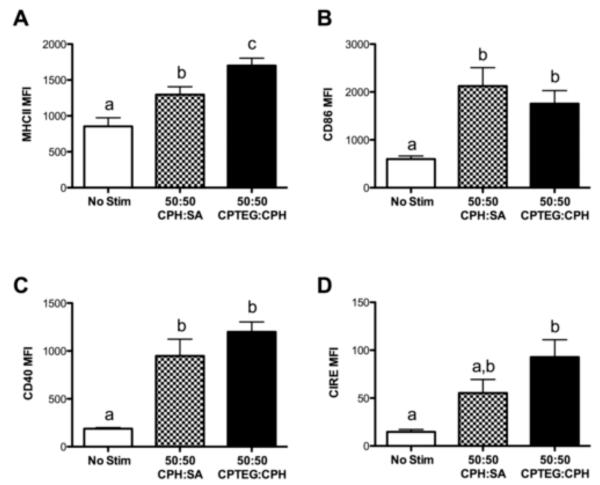
To assess activation of CD11c+ DCs by 50:50 CPH:SA or 50:50 CPTEG:CPH endotoxin-free microparticles, flow cytometry was used to measure levels of cell surface expression of the major histocompatibility complex molecule class II (MHC II), the co-stimulatory molecules CD86 and CD40, and the C-type lectin CIRE (the murine homologue of human DC-SIGN). A significant upregulation was observed in the MHC II mean fluorescence intensity (MFI) on DCs cultured with microparticles as compared to unstimulated DCs (Fig. 3A). Moreover, DCs cultured with 50:50 CPTEG:CPH microparticles expressed significantly greater levels of MHC II than those stimulated with 50:50 CPH:SA microparticles. Both polymer chemistries equally enhanced the surface expression of the co-stimulatory molecules CD86 and CD40 (Fig. 3B and 3C). In contrast, surface expression of CIRE, a molecule important for pathogen uptake and DC migration, was only significantly increased over the unstimulated cultures by the less hydrophobic 50:50 CPTEG:CPH microparticles. Taken together, these results indicate that both 50:50 CPH:SA and 50:50 CPTEG:CPH microparticles were able to significantly enhance the expression of key surface markers that are involved in DC maturation and antigen presentation.
Figure 3.
Microparticles enhance DC expression of MHC II and co-stimulatory molecules. DCs were stimulated with either 50:50 CPH:SA or 50:50 CPTEG:CPH microparticles or LPS or left unstimulated for 48 h. DCs were harvested and analyzed via FACS for surface expression of (A) MHC II, (B) CD86, (C) CD40, or (D) CIRE. Data are expressed as the mean ± the SEM of three independent experiments. Treatments with different letters are significantly different from one another at p < 0.05. MFI = mean fluorescence intensity. Mean MFI for DCs stimulated with LPS (200 ng/mL): MHC II = 2,009 ± 303; CD86 = 4,120 ± 1,235; CD40 = 6,739 ± 1,552; CIRE = 27 ± 10.
Polyanhydride microparticles induce DC cytokine secretion
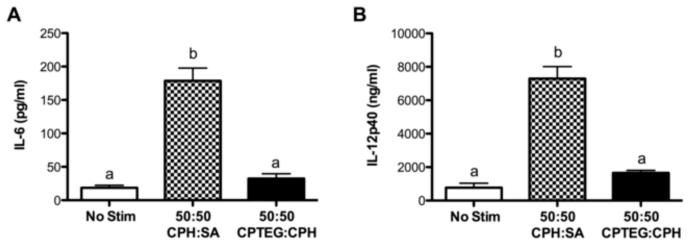
In addition to antigen presentation and T cell activation, DCs also modulate immune responses via the cytokines they secrete. After a 48 h culture with microparticles, only 50:50 CPH:SA microparticles enhanced DC secretion of IL-12p40 and IL-6 in comparison to the unstimulated DCs (Fig. 4). The presence of the cytokines TNF-α, IL-10, and IL-4 in culture supernatants were below the limits of detection when DCs were stimulated with either formulation of polyanhydride microparticles, but were detectable when DCs were stimulated with LPS, lipotecholic acid, or monophosphoryl lipid A (data not shown) as previously reported in other studies [37]. In contrast to the data obtained for surface expression of MHC II and DC co-stimulatory molecules, hydrophobicity of the microparticle formulations significantly influenced DC cytokine production.
Figure 4.
Polyanhydride microparticles induce DC cytokine secretion. DCs were stimulated with either 50:50 CPH:SA or 50:50 CPTEG:CPH microparticles or LPS or left unstimulated for 48 h. Supernatants were harvested and assayed for (A) IL-6 and (B) IL-12p40. Data are expressed as the mean ± the SEM of four independent experiments. Treatments with different letters are significantly different from one another at p < 0.05. Mean cytokine production for DCs stimulated with LPS (200 ng/mL): IL-6 = 14,387 ± 4,928 pg/ml; IL-12p40 = 33,321 ± 8,851 pg/ml.
Polyanhydride chemistries differentially impact T cell proliferation
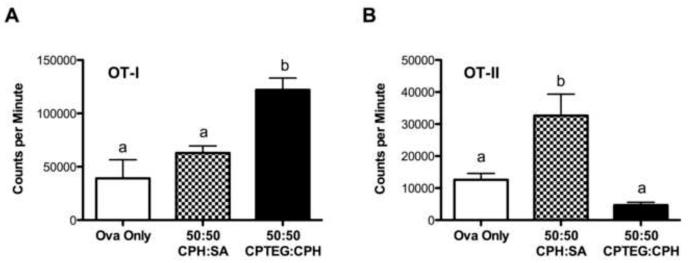
To assess the effect of polymer chemistry on the ability of DCs to prime naïve lymphocytes, unfractionated lymphocytes from OT-I and OT-II transgenic mice were co-cultured with ovalbumin-pulsed DCs either stimulated with microparticles and ovalbumin or with ovalbumin only. Approximately 90% of the CD8+ T cells from OT-I mice express a clonotypic T cell receptor that recognizes residues 257-264 of ovalbumin while approximately 90% of the CD4+ T cells from OT-II mice recognize residues 323-339 of ovalbumin [38,39]. A significantly enhanced antigen-specific proliferative response was observed when OT-I lymphocytes (CD8+ T cells) were incubated with DCs stimulated with 50:50 CPTEG:CPH microparticles plus ovalbumin as compared to ovalbumin alone or 50:50 CPH:SA microparticles plus ovalbumin (Fig. 5A). In contrast, antigen-specific OT-II lymphocyte (CD4+ T cell) proliferation was significantly increased in the presence of DCs stimulated with 50:50 CPH:SA microparticles plus ovalbumin but not 50:50 CPTEG:CPH microparticles plus ovalbumin (Fig. 5B). These data clearly demonstrate a role for particle chemistry in enhancing both antigen processing and presentation in the context of antigen-specific T cell responses.
Figure 5.
Polyanhydride chemistries differentially impact antigen-specific T cell proliferation. Unfractionated lymph node cells were isolated from either (A) OT-I (CD4+ T cells) or (B) OT-II (CD8+ T cells) mice and co-cultured with DCs either stimulated with 50:50 CPH:SA or 50:50 CPTEG:CPH microparticles plus ovalbumin or with ovalbumin (Ova) only for 72 h. Proliferation was assessed via 3H-thymidine incorporation. Data from one representative experiment of three independent experiments is shown and is expressed as the mean ± the SEM of replicate wells. Treatments with different letters are significantly different from one another at p < 0.05.
Discussion
Adjuvants provide much-needed immunostimulatory properties to vaccine preparations by either acting as a depot to prevent rapid antigen clearance, by delivering the vaccine antigen to APCs for uptake, processing, and presentation, and/or by inducing APC expression of the co-stimulatory molecules necessary for T cell activation [4-6]. The data presented herein demonstrate that polyanhydride microparticles were efficiently phagocytosed by DCs, localized within the phagolysosomal compartment, and subsequently activated DCs in vitro. The combination of a rapid and efficient intracellular delivery vehicle along with strong DC activation potential supports the concept that these materials have intrinsic adjuvant properties and excellent potential for vaccine delivery.
The microparticles were efficiently phagocytosed by immature DCs within 30 min after addition to the culture without the addition of opsonins, culture centrifugation, or agitation to enhance uptake (Fig. 2A). DC internalization of microparticles is clearly a phagocytic event. We observed large amounts of polymerized actin at the surface of the cell, immediately juxtaposed to the microparticle contacting the plasma membrane (data not shown). The exact mechanism of internalization is the subject of ongoing research. The microparticles were observed to persist intracellularly for at least 48 h within a phagolysosomal compartment. During this time, erosion of the particles was evident by the shift in average particle size from 2 to 48 h. Related to particle erosion was the apparent caging/uncaging phenomenon of FITC fluorescence observed with both the chemistries. It was frequently noted using confocal microscopy that the center portion of FITC positive microparticles did not emit fluorescence. While FITC-dextran was uniformly dispersed throughout the particle regardless of chemistry, the CPH:SA microparticles appeared hollow when observed under fluorescence microscopy (Fig. 2). Microparticles composed of both chemistries produced images showing that there was brighter fluorescence at the surface of the particle than its core. The enhanced surface fluorescence of FITC suggests that the surface of the particle presents a unique microenvironment that changes the fluorescence spectra of FITC to one characterized by either ablation of excitation or emission of photons of light. The phenomenon of spatial variation of fluorescence characteristics in microparticles is mostly likely related to “molecular caging.”
Polyanhydride microparticles may also function as adjuvants by enhancing APC expression of key molecules involved in antigen presentation and T cell co-stimulation. Indeed, these particles enhanced DC surface expression of MHC II (Fig. 3A), a molecule critical for presentation of antigen to and the activation of naïve CD4 helper T cells. Both polymer chemistries studied similarly enhanced DC expression of the T cell co-stimulatory molecules CD86 and CD40 (Figs 3B and 3C). CD86, a member of the B7 family of co-stimulatory molecules, is upregulated following activation of DCs by microbial stimuli and promotes T cell survival and proliferation [40]. The co-stimulatory molecule CD40 binds to CD154 expressed predominantly on T cells and promotes bidirectional signaling that amplifies the activation status of both the antigen presenting cell and the lymphocyte [41]. In addition, polyanhydride microparticles enhanced the T cell priming capacity of DCs, as evidenced by increased antigen-specific CD4+ and CD8+ T cell proliferation (Fig. 5).
Differential effects of particle chemistry were observed throughout these studies. Only stimulation with 50:50 CPH:SA microparticles enhanced DC secretion of the cytokines IL-12p40 and IL-6 (Figs. 3 and 4). Particle hydrophobicity also influenced T cell responses. Amphiphilic 50:50 CPTEG:CPH microparticles induced a greater antigen-specific CD8+ T cell proliferative response while the more hydrophobic 50:50 CPH:SA microparticles enhanced antigen-specific CD4+ T cell proliferation in vitro. With these observations, it is tempting to speculate that 50:50 CPH:SA and 50:50 CPTEG:CPH are recognized by different DC receptors and/or trigger unique signaling pathways resulting in differential DC phenotypes that preferentially present antigenic peptides in the context of MHC I or MHC II. This leads to the hypothesis that polyanhydride microparticles are internalized by immature DCs after being recognized by distinct pathogen-recognition receptors on their surface. Taken together, these results indicate that polyanhydride particles possess intrinsic adjuvant properties, and that immune modulation can be achieved by altering the chemistry of the polymer adjuvants.
Other work from our laboratory has shown that polyanhydride chemistry also affects the release kinetics and stability of encapsulated immunogens [42-44]. In those studies, amphiphilic particles rich in CPTEG provided an environment that favored enhanced antigen stability. This observation is consistent with previous reports in which carriers containing both hydrophobic and hydrophilic domains provided superior conditions for protein stabilization [45,46]. The amphiphilic nature of these CPTEG-containing materials results in the presence of a combination of surface (as seen with the 50:50 CPH:SA copolymers) and bulk (like that of PLGA) erosion.
Relative to previously published studies, an important advantage of biodegradable polyanhydride microparticles over other microparticle adjuvants (e.g., poly(lactic-co-glycolic acid) or PLGA) lies in the lower amount of copolymer required to significantly enhance DC activation [47-49]. Specifically, the ratio of polyanhydride microparticles to DCs (1:12) used in our studies was markedly lower (by as much as 50X) compared to the amounts of polyester microparticles used in other published studies to elicit DC activation [48]. In other studies, the addition of the adjuvant MPLA (a known TLR4 ligand) was required in order for PLGA microparticles to facilitate the activation of immune cells [47]. The ability to use a low dose of polyanhydride microparticles without the addition of stabilizers, excipients, or immune modulators is particularly favorable when considering its use in vaccine design, as the induction of more robust immune responses while using a minimum amount of adjuvant would be desirable.
The data presented herein strongly support the concept that polyanhydrides particles are potential vaccine adjuvants. Only a small number of activated DCs are sufficient to induce strong immune responses [50]. Small amounts of materials derived from either pathogens or damaged cells, are capable of triggering the activation of innate immune cells through Toll-like receptors, C-type lectin receptors and other pattern recognition receptors [51]. Matzinger and colleagues have proposed that hydrophobic molecules may also trigger signal transduction pathways via interactions with pattern recognition receptors [51]. Polyanhydride particles are relatively hydrophobic, especially when compared to sugars and lipids, and it is likely that DCs were activated by the hydrophobic characteristics of the polymers. Moreover, this study demonstrated that the extent of activation is further influenced by the individual chemistries. We suggest that an optimal vaccine formulation might consist of a cocktail of microparticles of different compositions to balance antigen release kinetics [19,20], antigen stability [9,29,42,44], and DC activation phenotype in order to facilitate the development of a robust immune response tailored to a specific immunogen, pathogen, or disease.
Conclusions
Currently, aluminum salts and monophosphoryl lipid A are two of the adjuvants approved for use in human vaccines and provide only a one-size-fits-all approach to enhancing immunogenicity. These compounds are not tunable and, as many pathogens have evolved to evade the host immune response, using such approaches may not induce adequate protective immunity. The next generation of efficacious vaccine adjuvants must possess the ability to be tailored to generate the optimal type of immune response to and provide protection against a particular pathogen. The results of this study demonstrate that polyanhydride microparticles activate DCs, supporting the concept that these materials have intrinsic adjuvant properties. Moreover, DC cytokine secretion and the priming of specific T cell subsets were further influenced by individual microparticle chemistries. These data indicate that polyanhydride particles can be tailored to take advantage of the potential plasticity of the immune response, resulting in the ability to induce immune protection against many types of pathogens.
Acknowledgments
The authors would like to thank Shawn Rigby for his expertise in flow cytometry, Bradley VanDeWoestyne and Anne-Marie Overstreet for assistance with statistical analysis and Andrea Dorn, Mary Byron, and Gretchen Anderson for technical assistance. B.N. and M.J.W. would like to acknowledge ONR-MURI (NN00014-06-1-1176) for financial support. M.P.T. acknowledges support of the NSF-Iowa AGEP Fellowship and the Ruth L. Kirschstein National Service Award (5F31CA126533-02) from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weekly epidemiological record. World Health Organization; Geneva: 2006. pp. 189–196. Report nr 19. [Google Scholar]
- 2.GIVS-Global Immunization Vision and Strategy 2006-2015. 2005.
- 3.Hanes J, Cleland JL, Langer R. New advances in microsphere-based single-dose vaccines. Adv Drug Del Rev. 1997;28(1):97–119. doi: 10.1016/s0169-409x(97)00053-7. [DOI] [PubMed] [Google Scholar]
- 4.Wilson-Welder J, Torres MP, Kipper MJ, Mallapragada SK, Wannemuehler MJ, Narasimhan B. Vaccine adjuvants: current challenges and future approaches. J Pharm Sci. 2009;98(4):1278–1316. doi: 10.1002/jps.21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schijns VE. Antigen delivery systems and immunostimulation. Vet Immunol Immunopathol. 2002;87(3-4):195–8. doi: 10.1016/s0165-2427(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 6.Woodland DL, Blackman MA. Vaccine development: baring the ‘dirty little secret’. Nat Med. 2005;11(7):715–6. doi: 10.1038/nm0705-715. [DOI] [PubMed] [Google Scholar]
- 7.O’Hagan DT, Singh M, Gupta RK. Poly(lactide-co-glycolide) microparticles for the development of single-dose controlled-release vaccines. Adv Drug Del Rev. 1998;32(3):225–246. [PubMed] [Google Scholar]
- 8.Tamada J, Langer R. The development of polyanhydrides for drug delivery applications. J Biomater Sci Polym Ed. 1992;3(4):315–353. doi: 10.1163/156856292x00402. [DOI] [PubMed] [Google Scholar]
- 9.Determan AS, Trewyn BG, Lin VS-Y, Nilsen-Hamilton M, Narasimhan B. Encapsulation, stabilization, and release of BSA-FITC from polyanhydride microspheres. J Contr Rel. 2004;100(1):97–109. doi: 10.1016/j.jconrel.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Tabata Y, Gutta S, Langer R. Controlled delivery systems for proteins using polyanhydride microspheres. Pharm Res. 1993;10(4):487–96. doi: 10.1023/a:1018929531410. [DOI] [PubMed] [Google Scholar]
- 11.Fu J, Fiegel J, Krauland E, Hanes J. New polymeric carriers for controlled drug delivery following inhalation or injection. Biomaterials. 2002;23(22):4425–4433. doi: 10.1016/s0142-9612(02)00182-5. [DOI] [PubMed] [Google Scholar]
- 12.Hanes J, Chiba M, Langer R. Degradation of porous poly(anhydride-co-imide) microspheres and implications for controlled macromolecule delivery. Biomaterials. 1998;19(1-3):163–172. doi: 10.1016/s0142-9612(97)00221-4. [DOI] [PubMed] [Google Scholar]
- 13.Ron E, Turek T, Mathiowitz E, Chasin M, Hageman M, Langer R. Controlled release of polypeptides from polyanhydrides. Proc Natl Acad Sci USA. 1993;90:4176–80. doi: 10.1073/pnas.90.9.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallapragada SK, Narasimhan B. Immunomodulatory biomaterials. Int J Pharm. 2008;364:265–271. doi: 10.1016/j.ijpharm.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 15.Torres MP, Vogel BM, Narasimhan B, Mallapragada SK. Synthesis and characterization of novel polyanhydrides with tailored erosion mechanisms. J Biomed Mater Res A. 2006;76:102–110. doi: 10.1002/jbm.a.30510. [DOI] [PubMed] [Google Scholar]
- 16.Shen EE, Kipper MJ, Dziadul B, Lim MK, Narasimhan B. Mechanistic relationships between polymer microstructure and release kinetics in bioerodible polyanhydrides. J Contr Rel. 2002;82:115–125. doi: 10.1016/s0168-3659(02)00125-6. [DOI] [PubMed] [Google Scholar]
- 17.Shen E, Pizsczek R, Dziadul B, Narasimhan B. Microphase separation in bioerodible copolymers for drug delivery. Biomaterials. 2001;22(3):201–210. doi: 10.1016/s0142-9612(00)00175-7. [DOI] [PubMed] [Google Scholar]
- 18.Petersen LK, Xue L, Rajan K, Wannemuehler MJ, Narasimhan B. The simultaneous effect of polymer chemistry and geometry on the in vitro activation of murine dendritic cells. Biomaterials. 2009;30(28):5131–5142. doi: 10.1016/j.biomaterials.2009.05.069. [DOI] [PubMed] [Google Scholar]
- 19.Torres MP, Determan AS, Anderson GL, Mallapragada SK, Narasimhan B. Amphiphilic polyanhydrides for protein stabilization and release. Biomaterials. 2007;28(1):108–116. doi: 10.1016/j.biomaterials.2006.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kipper MJ, Wilson JH, Wannemuehler MJ, Narasimhan B. Single dose vaccine based on biodegradable polyanhydride microspheres can modulate immune response mechanism. J Biomed Mater Res A. 2006;76:798–810. doi: 10.1002/jbm.a.30545. [DOI] [PubMed] [Google Scholar]
- 21.Leong KW, Amore PD, Marletta M, Langer R. Bioerodible polyanhydrides as drug-carrier matrices. 2. Biocompatibility and chemical reactivity. J Biomed Mater Res. 1986;20:51–64. doi: 10.1002/jbm.820200106. [DOI] [PubMed] [Google Scholar]
- 22.Jiang HL, Tang GP, Weng LH, Zhu KJ. In vivo degradation and biocompatibility of a new class of alternate poly(ester-anhydrides) based on aliphatic and aromatic diacids. J Biomater Sci Polymer Ed. 2001;12(12):1281–1292. doi: 10.1163/156856202753419222. [DOI] [PubMed] [Google Scholar]
- 23.Katti DS, Lakshmi S, Langer R, Laurencin CT. Toxicity, biodegradation and elimination of polyanhydrides. Adv Drug Del Rev. 2002;54:933–961. doi: 10.1016/s0169-409x(02)00052-2. [DOI] [PubMed] [Google Scholar]
- 24.Adler AF, Petersen LK, Wilson JH, Torres MP, Thorstenson JB, Gardner SW, Mallapragada SK, Wannemuehler MJ, Narasimhan B. High throughput cell-based screening of biodegradable polyanhydride libraries. Comb Chem High Through Screen. 2009;12(7):634–645. doi: 10.2174/138620709788923764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poshusta AK, Burdick JA, Mortisen DJ, Padera RF, Ruehlman D, Yaszemski MI, Anseth KS. Histocompatibility of photocrosslinked polyanhydrides: A novel in situ forming orthopaedic biomaterial. J Biomed Mater Res A. 2002;64:62–69. doi: 10.1002/jbm.a.10274. [DOI] [PubMed] [Google Scholar]
- 26.Cleland JL. Single-administration vaccines: controlled-release technology to mimic repeated immunizations. Trends Biotechnol. 1999;17(1):25–9. doi: 10.1016/s0167-7799(98)01272-4. [DOI] [PubMed] [Google Scholar]
- 27.Ulery BD, Phanse Y, Sinha A, Wannemuehler MJ, Narasimhan B, Bellaire BH. Polymer chemistry influences uptake of nanospheres by immune cells. Pharm Res. 2009;26(3):683–690. doi: 10.1007/s11095-008-9760-7. [DOI] [PubMed] [Google Scholar]
- 28.Tamayo I, Irache JM, Mansilla C, Ochoa-Reparaz J, Lasarte JJ, Gamazo C. Poly(anhydride) nanoparticles act as active Th1 adjuvants through Toll-like receptor exploitation. Clin Vaccine Immunol. 2010;17(9):1356–1362. doi: 10.1128/CVI.00164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Determan A, Graham J, Pfeiffer K, Narasimhan B. The role of microsphere fabrication methods on the stability and release kinetics of ovalbumin encapsulated in polyanhydride microspheres. J Microencap. 2006;23(8):832–843. doi: 10.1080/02652040601033841. [DOI] [PubMed] [Google Scholar]
- 30.Lam XM, Duenas ET, Daugherty AL, Levin N, Cleland JL. Sustained release of recombinant insulin-like growth factor-I for treatment of diabetes. J Contr Rel. 2000;67:281–292. doi: 10.1016/s0168-3659(00)00224-8. [DOI] [PubMed] [Google Scholar]
- 31.(FDA) FaDA, editor. Guideline on validation of the Limulus amebocyte test as an end-product endotoxin test for human and animal parenteral drugs, biological products, and medical devices. Center for Drug Evaluation and Research; 1987. pp. 1–10. [Google Scholar]
- 32.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Meth. 1999;223(1):77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 33.Bellaire BH, Roop RM, III, Cardelli JA. Opsonized virulent Brucella abortus replicates within nonacidic, endoplasmic reticulum-negative, LAMP-1-positive phagosomes in human monocytes. Infect Immun. 2005;73(6):3702–3713. doi: 10.1128/IAI.73.6.3702-3713.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ImageJ [cited August 3, 2009];Image Processing and Analysis in Java. Available from http://rsb.info.nih.gov/ij/
- 35.Kipper MJ, Shen E, Determan A, Narasimhan B. Design of an injectable system based on bioerodible polyanhydride microspheres for sustained drug delivery. Biomaterials. 2002;23(22):4405–4412. doi: 10.1016/s0142-9612(02)00181-3. [DOI] [PubMed] [Google Scholar]
- 36.Tabata Y, Ikada Y. Effect of the size and surface charge of polymer microspheres on their phagocytosis by macrophage. Biomaterials. 1988;9:356–362. doi: 10.1016/0142-9612(88)90033-6. [DOI] [PubMed] [Google Scholar]
- 37.Barr TA, Brown S, Ryan G, Zhao J, Gray D. TLR-mediated stimulation of APC: Distinct cytokine responses of B cells and dendritic cells. Eur J Immunol. 2007;37(11):3040–53. doi: 10.1002/eji.200636483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76(1):17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 39.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76(1):34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 40.Smith KM, Garside P, McNeil RC, Brewer JM. Analysis of costimulatory molecule expression on antigen-specific T and B cells during the induction of adjuvant-induced Th1 and Th2 type responses. Vaccine. 2006;24(15):3035–43. doi: 10.1016/j.vaccine.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 41.O’Sullivan B, Thomas R. CD40 and dendritic cell function. Crit Rev Immunol. 2003;23(1-2):83–107. doi: 10.1615/critrevimmunol.v23.i12.50. [DOI] [PubMed] [Google Scholar]
- 42.Lopac SK, Torres MP, Wilson-Welder JH, Wannemuehler MJ, Narasimhan B. Effect of polymer chemistry and fabrication method on protein release and stability from polyanhydride microspheres. J Biomed Mater Res B. 2009;91(2):938–47. doi: 10.1002/jbm.b.31478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Determan AS, Wilson JH, Kipper MJ, Wannemuehler MJ, Narasimhan B. Protein stability in the presence of polymer degradation products: consequences for controlled release formulations. Biomaterials. 2006;27(17):3312–20. doi: 10.1016/j.biomaterials.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 44.Petersen LK, Sackett CK, Narasimhan B. High throughput analysis of protein stability in polyanhydride nanoparticles. Acta Biomater. 2010;6(10):3873–81. doi: 10.1016/j.actbio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Kissel T, Li YX, Volland C, Gorich S, Koneberg R. Parenteral protein delivery systems using biodegradable polyesters of ABA block structure, containing hydrophobic poly(lactide-co-glycolide) A blocks and hydrophilic poly(ethylene oxide) B blocks. J Contr Rel. 1996;39:315–326. [Google Scholar]
- 46.Ronneberger B, Kao WJ, Anderson JM, Kissel T. In vivo biocompatibility study of ABA triblock copolymers consisting of poly(L-lactic-co-glycolic acid) A blocks attached to central poly(oxyethylene) B blocks. J Biomed Mater Res. 1996;30:31–40. doi: 10.1002/(SICI)1097-4636(199601)30:1<31::AID-JBM5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 47.Elamanchili P, Diwan M, Cao M, Samuel J. Characterization of poly(D,L-lactic-co-glycolic acid) based nanoparticulate system for enhanced delivery of antigens to dendritic cells. Vaccine. 2004;22(19):2406–12. doi: 10.1016/j.vaccine.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida M, Mata J, Babensee JE. Effect of poly(lactic-co-glycolic acid) contact on maturation of murine bone marrow-derived dendritic cells. J Biomed Mater Res A. 2007;80(1):7–12. doi: 10.1002/jbm.a.30832. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida M, Babensee JE. Poly(lactic-co-glycolic acid) enhances maturation of human monocyte-derived dendritic cells. J Biomed Mater Res A. 2004;71(1):45–54. doi: 10.1002/jbm.a.30131. [DOI] [PubMed] [Google Scholar]
- 50.Steinman RM. DC-SIGN: a guide to some mysteries of dendritic cells. Cell. 2000;100(5):491–4. doi: 10.1016/s0092-8674(00)80684-4. [DOI] [PubMed] [Google Scholar]
- 51.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4(6):469–78. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]