Abstract
Neural networks in the spinal cord known as central pattern generators produce the sequential activation of muscles needed for locomotion. The overall locomotor network architectures in limbed vertebrates have been much debated, and no consensus exists as to how they are structured. Here, we use optogenetics to dissect the excitatory and inhibitory neuronal populations and probe the organization of the mammalian central pattern generator. We find that locomotor-like rhythmic bursting can be induced unilaterally or independently in flexor or extensor networks. Furthermore, we show that individual flexor motor neuron pools can be recruited into bursting without any activity in other nearby flexor motor neuron pools. Our experiments differentiate among several proposed models for rhythm generation in the vertebrates and show that the basic structure underlying the locomotor network has a distributed organization with many intrinsically rhythmogenic modules.
Keywords: channelrhodopsin-2, halorhodopsin, motor neurons, interneurons
Spinal cord networks that drive and coordinate walking are, to a large extent, innate. Even in species that do not walk at birth, such as rodents and humans, the networks that generate walking are already present (1, 2). The early development of functional locomotor networks has been exploited and studied in the neonatal rodent spinal cord in vitro preparation in which locomotor-like activity can be induced pharmacologically or by electrical activation of descending or afferent fibers (3–6). The mammalian locomotor networks have also been studied by using the adult cat where locomotor-like activity similarly can be induced pharmacologically or electrically (7–9). A general notion from these studies is that forelimb and hindlimb locomotion are controlled by independent limb-controlling circuits (10) and that rhythm-generating excitatory neurons, and pattern-generating neurons, interact to produce the coordinated motor output (11–14). The network layout within these limb-controlling circuits has, however, been debated. A number of conceptual models have been advanced. The classical half-center model asserts that flexor and extensor bursting is generated by two reciprocally coupled half-centers driving all flexors and extensors (8, 15). The flexor burst generator model is asymmetric and consists of a flexor burst generator that provides active excitation of flexor motor neurons and inhibition of extensor motor neurons that are otherwise tonically active (14, 16, 17). In response to evidence that the central pattern generator (CPG) could produce a more complex motor output than just a mere flexor-extensor alternation (9), the unit burst generator (UBG) model was proposed (18). According to this theory, separate modules can generate a rhythm in close muscle synergies and are distributed around each joint (18, 19), or in the swimming network in each hemisegment (20). The UBGs therefore generate a local rhythmic activity that during locomotion will be recruited so that they form an interconnected network of rhythm generators. The conceptual models for the overall locomotor network architecture have been deduced from microlesion studies (21–24), pharmacological activation of restricted areas of the cord (25, 26), afferent perturbation studies (27–29), and deletion studies (14, 16, 30). More recently, genetic ablation studies have also added to the understanding of details of the organizational structure of the rodent locomotor network (31).
In this study, we use an optogenetic approach in which spinal excitatory and inhibitory interneurons are genetically targeted for selective activation or inhibition to probe the overall organization of the spinal hindlimb locomotor network in mice. We first show that spinal glutamatergic neurons are both sufficient and necessary for generating rhythmic activity. Activating smaller populations of glutamatergic neurons in the cord limits the rhythmic output to restricted flexor-related or extensor-related motor neurons. Our experiments demonstrate a modular organization of the hindlimb locomotor network where multiple rhythmogenic flexor and extensor modules are combined to produce an integrated motor output.
Results
Glutamatergic Neurons Are Sufficient and Necessary for Generating Locomotor-Like Activity.
We have shown earlier that tonic light-induced activation of a ventral population of excitatory interneurons in the rostral lumbar spinal cord can elicit locomotor-like activity in the hindlimb motor neuron pools (32). To further assess the role of the spinal interneurons in generating the locomotor rhythm, we used a conditional approach that allowed us to activate or inhibit spatially segregated populations of excitatory or inhibitory neurons in the spinal cord.
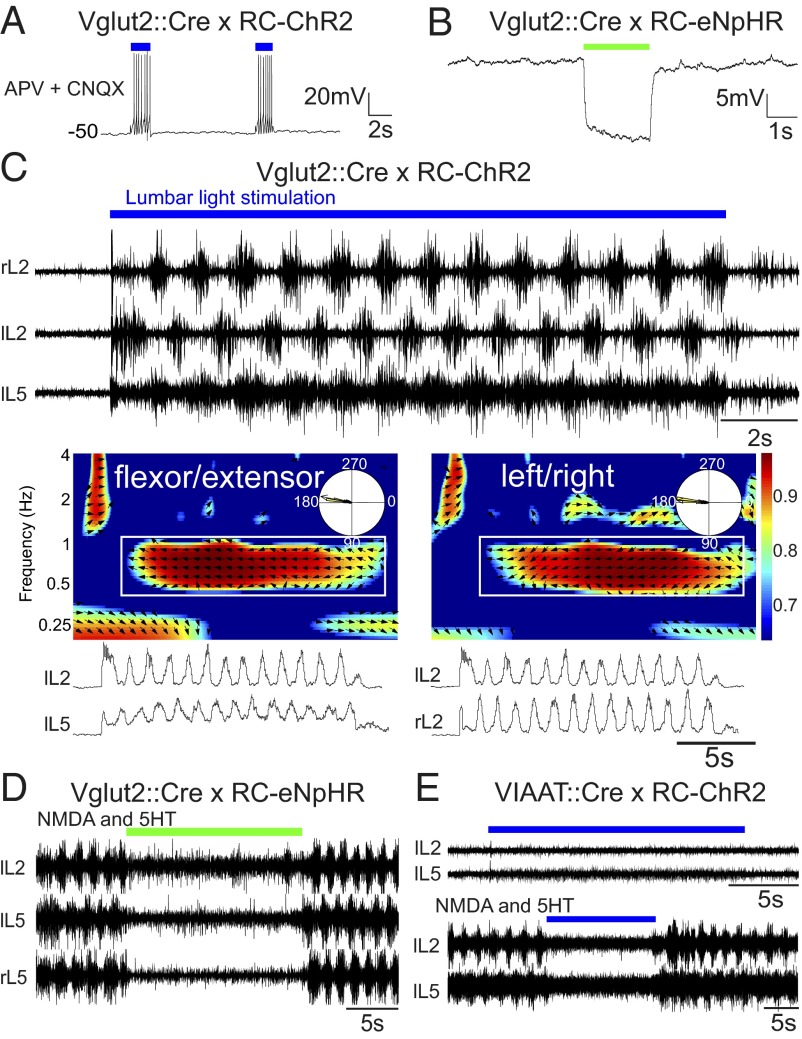
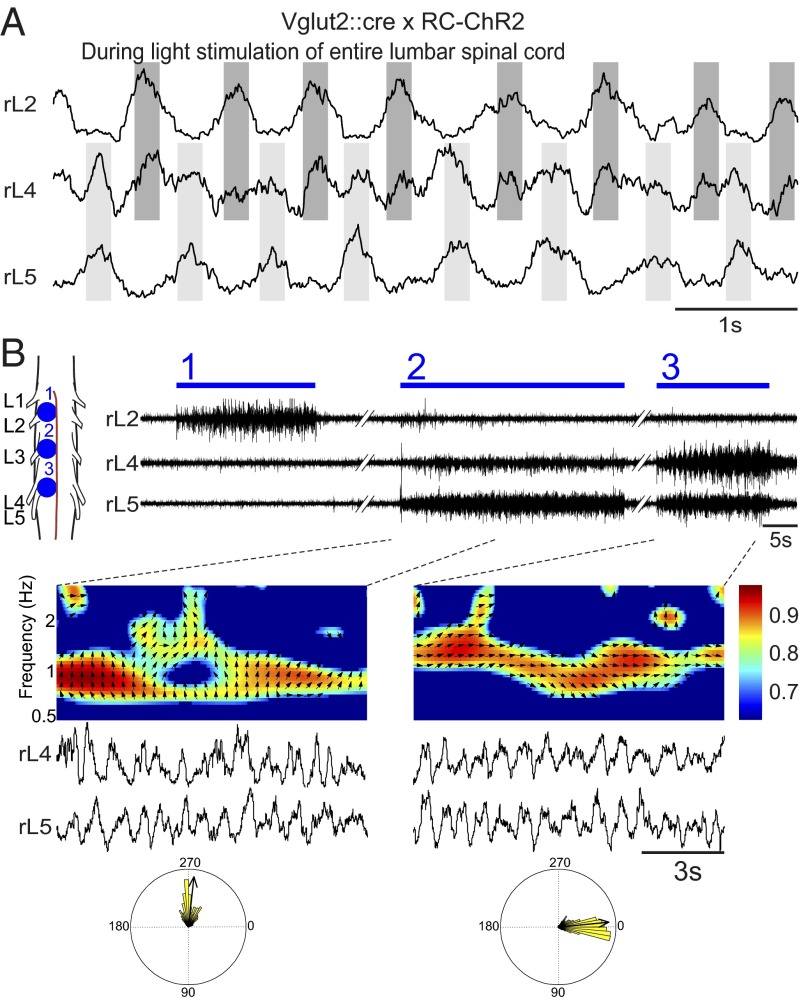
To activate the excitatory population of neurons in the spinal cord, we crossed Vesicular glutamate transporter 2 (Vglut2)::causes recombination (Cre) with Rosa26-cytomegalovirus early enhancer element/chicken beta-actin promoter (CAG)-loxP–Stop codons–3x SV40 polyA–loxP (LSL)-channelrhodopsin2 (ChR2)-enhanced yellow fluorescent protein (EYFP)-woodchick hepatitis virus posttranscriptional enhancer (WPRE) (RC-ChR2) mice (33). Vglut2 is the dominant glutamatergic transporter in interneurons in the spinal cord, and Vglut2::Cre mice have expression specifically restricted to all glutamatergic cells (34). YFP-positive neurons recorded in these mice show strong responses to light with persistent spiking when all excitatory fast synaptic transmission is blocked by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and D-(-)-2-amino-5-phosphonopentanoic acid (AP5) (Fig. 1A), demonstrating that these neurons respond directly to the light stimulus. To monitor locomotor activity, the output of ventral roots from lumbar 2 (L2) and lumbar 5 (L5) segments on the left (lL2, lL5) and right sides (rL2, rL5) were recorded. When blue light was shined onto the ventral surface of the lumbar cord (L1–L6), locomotor-like activity was evoked, characterized by an alternation in bursts from ipsilateral flexor-dominant L2 and extensor-dominant L5 ventral roots and ipsilateral and contralateral L2 (or L5) roots, respectively (Fig. 1C). Instantaneously light-evoked locomotor-like activity was seen in all preparations, in absence of any other locomotor-stimulating activity (n = 14, average frequency = 1.06 Hz, range 0.40–2.70 Hz, flexor-extensor coordination r value = 0.90, left-right r value = 0.83). In contrast to what was seen in previous experiments using the Vglut2-ChR2 mouse (32), the locomotor activity persisted for a longer time, essentially as long as the light stimulus was on. The patterning between flexor-extensor and left-right activity was finely tuned, as seen in the time-frequency and circular plot from the locomotor period (Fig. 1C). The color coding denotes the coherence of the two signals and the vector field shows the phase relationship between flexor-extensor and left-right signals, respectively. These experiments demonstrate the sufficiency of Vglut2-positive cells for generating hindlimb locomotion.
Fig. 1.
Glutamatergic neurons are both sufficient and necessary for evoking locomotor-like activity, whereas inhibitory neurons can only abolish ongoing locomotor activity. Light depolarizes Vglut2::Cre; RC-ChR2-expressing neurons despite blockade of glutamatergic neurotransmission (AP5 and CNQX) (A) and hyperpolarizes Vglut2::Cre; RC-eNpHR-expressing neurons (B). (C) Stimulating the ventral surface of the lumbar spinal cord of Vglut2::Cre; RC-ChR2 mice evokes locomotor-like activity with direct onset and well-tuned flexor-extensor and left-right coordination. The time-frequency plot shows the coordination between flexor-extensor (Middle Left) and left-right (Middle Right) activity. The color coding denotes the coherence between the two signals and the vector field shows phase delay (also indicated in the circular plots, right = 0°, down = 90°) (Bottom). (D) Inhibiting glutamatergic neurons in the Vglut2::Cre; RC-eNpHR mouse turns off ongoing drug-evoked (5-HT/NMDA: 7 µM/8 µM) activity. Activating inhibitory interneurons does not elicit any rhythmic activity (E) but turns off ongoing drug-evoked (5-HT/NMDA: 7 µM/8 µM) activity.
To probe the necessity of glutamatergic neurons for locomotion, we inhibited Vglut2-positive neurons during ongoing locomotor-like activity induced by the locomotor-evoking drugs serotonin (5-HT)/NMDA, brainstem stimulation (BS), or dorsal root stimulation. For these experiments, we used Vglut2::Cre crossed with Rosa26-CAG-LSL-enhanced natronomonas pharaonis halorhoposin (eNpHR)-EYFP-WPRE (RC-eNpHR) mice. Intracellular recordings of excitatory cells show that shining light onto them caused a persistent and strong hyperpolarization (Fig. 1B). When light was shined onto the en bloc spinal cord during locomotor-like activity induced by descending (n = 9), dorsal root (n = 3), or pharmacological activation [N-methyl-D-aspartate (NMDA) and 5-hydroxytryptamine (5-HT), n = 4] (Fig. 1D), a complete inhibition of the ongoing rhythm was seen.
As a counterpart to manipulating the glutamatergic population of cells, we performed light stimulation of inhibitory neurons in the spinal cord, identified by their expression of the vesicular inhibitory amino acid transporter (VIAAT) (35). To target these cells, we used a transgenic VIAAT::Cre mouse (Fig. S1). This mouse strain expresses Cre exclusively in GABAergic and glycinergic cells in the spinal cord (98.7% recombination in glycine and GABA-positive cells; SI Methods). The VIAAT::Cre mice were crossed with RC-ChR2 mice. Light stimulation of the spinal cord of VIAAT::Cre; RC-ChR2 mice does not evoke any kind of rhythmic activity (n = 3) (Fig. 1E, Upper). Conversely, during drug-evoked (5-HT/NMDA) locomotor-like activity, tonic light stimulation of the entire lumbar spinal cord in VIAAT::Cre; RC-ChR2 (Fig. 1E, Lower) mice caused a strong inhibition of ventral root output.
Together, these experiments show that Vglut2-positive cells are both sufficient and necessary for rhythm-generation and locomotion, whereas activation of VIAAT-positive cells cannot initiate locomotion but can inhibit ongoing bursting.
A Locomotor-Like Rhythm Can Be Initiated from both Rostral and Caudal Parts of the Spinal Cord.
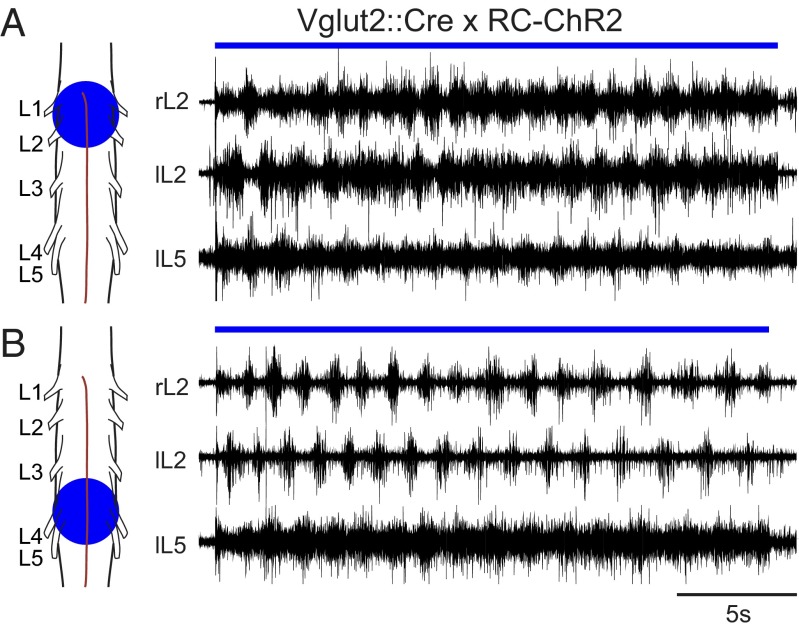
To probe the distribution of the locomotor network, we light-stimulated different areas of the lumbar spinal cord in Vglut2::Cre; RC-ChR2 mice. A rhythmic output with left-right and flexor-extensor alternation could be evoked when the rostral lumbar spinal cord (L1–L3) was stimulated with a light spot centered at the L2 segment covering an area of 0.4–2.5 mm2 (Fig. 2A). A similar result was seen when the caudal lumbar (L4–L6) spinal cord was stimulated (Fig. 2B). The light threshold for evoking coordinated locomotor-like output was variable across different animals, but always lower in the rostal lumbar spinal cord compared with the caudal lumbar spinal cord. Moreover the frequency of the evoked locomotor-like activity was lower when evoked by stimulating the caudal compared with the rostral lumbar spinal cord (0.73 Hz and 1.14 Hz, respectively; P = 0.014, n = 9). This rostrocaudal gradient in rhythmogenic potential corresponds to what has been observed when the locomotor network was activated with neuroactive substances (23, 25, 36)
Fig. 2.
Locomotor-like activity can be elicited from rostral or caudal regions. Smaller stimulation areas can evoke locomotor-like activity. Spots ranging from 0.4–2.5 mm2 could successfully evoke locomotor-like activity when centered over rostral (A) or caudal (B) lumbar spinal cord.
Locomotor Activity Can Be Unilaterally Induced.
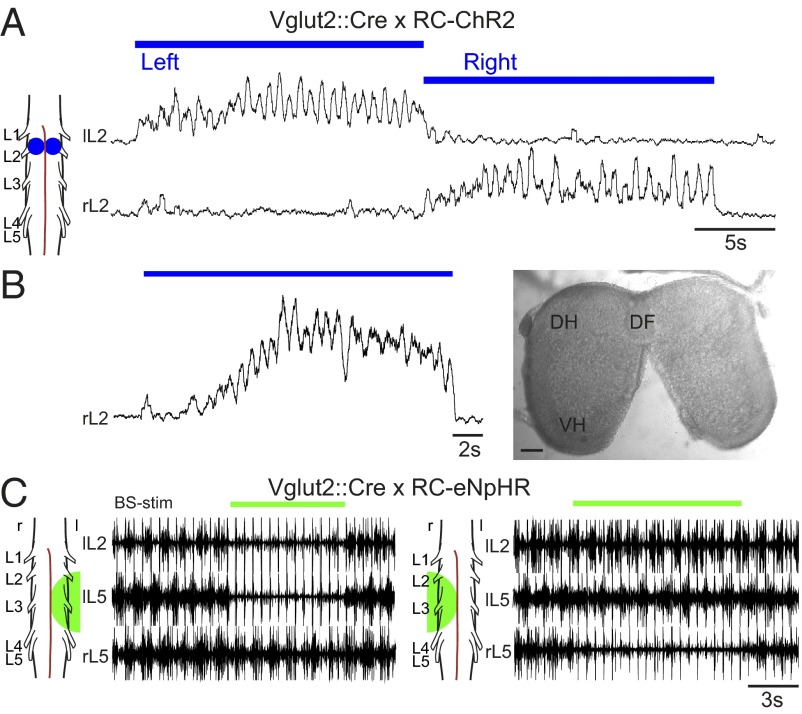
Having established that the locomotor-like activity can be induced by stimulating large areas of the cord in Vglut2::Cre; RC-ChR2 mice, we aimed to activate more restricted areas to test whether rhythmic activity could be induced in isolation. Unilateral activity could be induced by stimulating one side of the cord (n = 6, mean frequency = 1.00 Hz, range 0.69–1.49 Hz). An example is shown in Fig. 3A where an area of interest was stimulated consecutively on first the left, then the right, side of the cord in L2. Persistent rhythmic activity was initiated on the left side with no rhythmic activity on the right when the left side was stimulated and vice versa. The change of rhythmic activity was instantaneous when the stimulation side was switched. The rhythmogenic capability on one side did not depend on bilateral shared networks (37), because rhythmic activity could also be induced on one side of the cord when the ventral commissure was cut from the upper thoracic to the sacral spinal cord (n = 3) (Fig. 3B).
Fig. 3.
Locomotor-like activity can be initiated and eliminated unilaterally. Stimulating a unilateral spot at L2 in Vglut2::Cre; RC-ChR2 mice elicits unilateral locomotor-like activity in left and right sides consecutively (A), and this activity can be produced when the two sides are separated by cutting the ventral commissure (B; DF, dorsal funiculus; DH, dorsal horn; VH, ventral horn). (Scale bar: 100 µm.) Hyperpolarizing glutamatergic neurons unilaterally, using Vglut2::Cre; RC-eNpHR mice can abolish brainstem-evoked (BS-stim: 2 Hz, 0.5 mA) locomotor-like activity on one side of the cord, whereas activity is maintained on the side contralateral to the light (C; l and r, left and right sides, respectively).
These experiments support the notion that a hemicord can generate a rhythmic motor output, which has also been demonstrated pharmacologically (38, 39). This concept was further supported by experiments in the Vglut2::Cre; RC-eNpHR2 mice where light applied to one hemicord could shut down brainstem-evoked (BS) locomotor-like activity on one side of the cord with activity persisting on other side of the cord without any clear change in frequency (paired t test P = 0.38, n = 7) on the unstimulated side (Fig. 3C).
Rhythmic Bursting Can Be Induced or Persist Independently in Flexor and Extensor-Related Motor Neuron Pools.
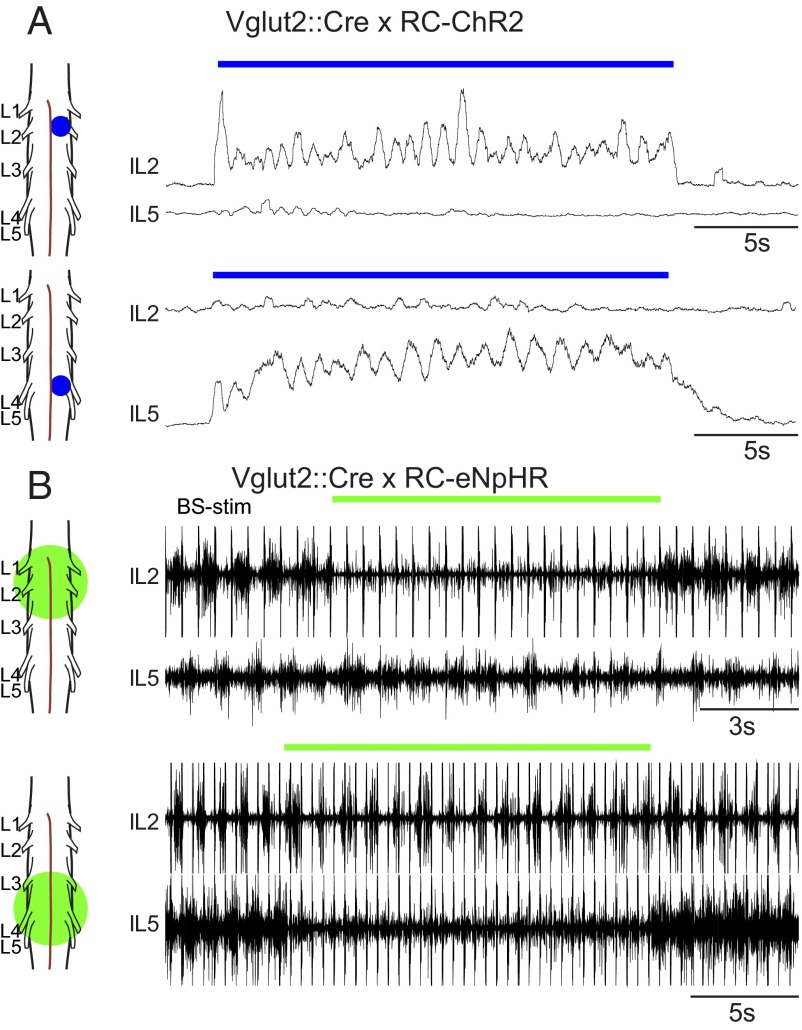
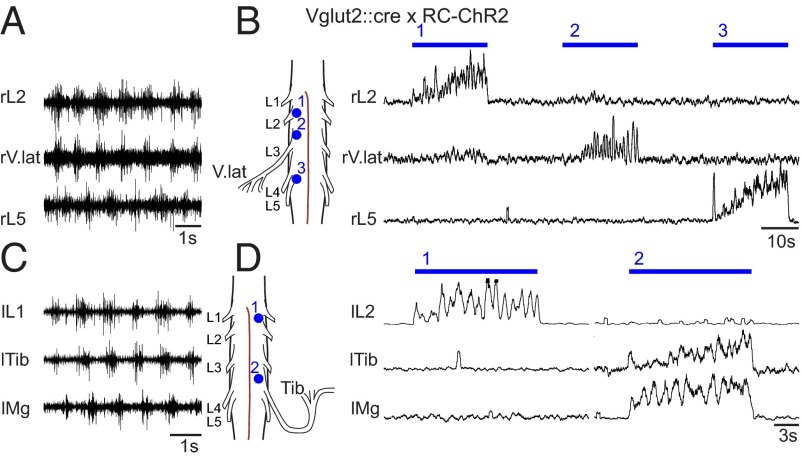
The possibility to stimulate restricted areas with light allowed us to ask two additional questions about the rhythm-generating capability: (i) can flexor and extensor bursting exist independently and (ii) if so, can flexor- or extensor-related motor neuron pools burst independently of other flexor- or extensor-related motor neuron pools? To answer the first question, we used the Vglut2::Cre; RC-ChR2 mice and shined small laser spots onto different parts of the cord. When a small spot (320 µm2 in diameter) was placed at the L2 segment, it was possible to evoke rhythmic flexor bursting in the L2 ventral root without bursting in extensor-related L5 ventral root (n = 9, mean frequency = 0.93 Hz; Fig. 4A, Upper). Similarly, it was possible to evoke extensor bursting in L5 without bursting in L2 (n = 7, mean frequency = 0.94 Hz; Fig. 4A, Lower). Furthermore, it was possible to completely suppress either flexor or extensor bursting during brainstem-evoked locomotor-like activity in Vglut2::Cre; RC-eNpHR mice by inhibiting glutamatergic cells locally (Fig. 4B). Exposing the rostral lumbar cord to light (through a 10× or 50× objective) abolished L2 bursting, but L5 bursting continued; however, the frequency was reduced by 34% (0.13 Hz, n = 4, P = 0.0012; Fig. 4B, Upper). When the caudal lumbar cord was exposed to light in a similar manner, bursting in L5 was blocked and L2 bursting frequency was reduced by 17% (0.9 Hz, n = 2, P = 0.0194; Fig. 4B, Lower). Together, these experiments demonstrate that flexor and extensor bursting can exist independently, without the need for the reciprocity suggested by the half-center and asymmetric flexor-burst models.
Fig. 4.
Flexor and extensor networks can be independently bursting. (A) Activating glutamatergic neurons in a restricted area at the rostral lumbar cord can evoke locomotor-like activity exclusively in the flexor network (Upper), whereas similar activation at more caudal levels evokes locomotor-like activity exclusively in the extensor network (Lower). (B) Inhibiting glutamatergic neurons in the rostral lumbar spinal cord during brainstem evoked locomotor-like activity can turn off the flexor output from the L2 root while the L5 is still rhythmically active (Upper), albeit lower in frequency. Similar inhibition of glutamatergic neurons in the caudal lumbar spinal cord abolishes extensor output from the L5 root and causes a slight reduction in the frequency of the L2 locomotor bursts (Lower).
To answer the second question, we took advantage of the fact that the L4 ventral root projects to both flexor and extensor related muscles (40). A flexor-related burst (in phase with L2) and an extensor-related burst (in phase with L5) is seen as a double bursting pattern in the L4 ventral root when light is shined over the entire lumbar cord in Vglut2::Cre; RC-ChR2 mice (Fig. 5A). With this stimulus paradigm, flexor-related bursting could be individually induced in the L2 or the L4 root. An example is shown in Fig. 5 B where L2 bursting was first evoked without bursting in L4 and L5 (n = 2, mean frequency = 0.98 Hz; Fig. 5 B, 1), then flexor bursting was evoked in L4 with extensor bursting in L5 demonstrated by their out-of-phase bursting (Fig. 5 B, 2; circular plot and time/frequency plot), and then finally extensor bursting in L4 in synchrony with extensor-related L5 bursting (Fig. 5 B, 3; circular plot and time/frequency plot). Moreover, it was possible to evoke bursting in L4 without bursting in L2 or L5 (Fig. S2). In this case, it is impossible to know whether L4 bursting is flexor- or extensor-related, but it clearly indicates that it is not necessary to have bursting in all flexors or all extensors at the same time.
Fig. 5.
The mixed flexor and extensor motor output of L4 can be segregated by site-specific focal light stimulation. The L4 root carries a similar amount of motor output to extensor muscles as to the flexor muscles evident as a double burst pattern seen during locomotor-like activity (by whole preparation Vglut2::Cre x RC-ChR2 stimulation in A). The flexor activity in L2 (B, 1) and L4 (B, 2) can be independently activated depending on the site of focal light stimulation. The L4 output in B2 is flexor-related because it has a phase lag to L5 output (left circle plot). The extensor activity in L4 is also demonstrated by yet another stimulation spot (B, 3 and right circle plot).
The definitive confirmation of independent driven rhythmicity of motor neuron pools would be to get independent rhythmicity in two closely related muscles that fire in the same phase and reside close to each other in the spinal cord. We therefore recorded the activity in the nerves of a pure flexor related muscle (tibialis anterior) and a muscle that shows both flexor and extensor activity (vastus lateralis) (41, 42). The nerves to either the tibialis anterior or the vastus lateralis were dissected, and the nerve endings were cut right next to their respective muscle. Suction electrode recordings of these nerves during light stimulation of the entire lumbar cord showed that they were in phase with the L2-related flexor activity (Fig. 6 A and C). Small spot stimulation was able to elicit flexor activity in L2 separate from the flexor activity in vastus lateralis (coming from L3 ventral root, n = 2), or the tibialis anterior (coming from the L4 ventral root, n = 5) (Fig. 6 B and D). These experiments demonstrate that flexor-related motor neuron pools can burst independently of other close by flexor-related motor neuron pools.
Fig. 6.
Tibialis anterior or vastus lateralis nerve output can be independently rhythmic without activity in L2. Light simulation of the whole lumbar cord in the Vglut2::Cre; RC-ChR2 mice elicits flexor-related activity in the nerve that projects to vastus lateralis (A) and tibialis anterior (C) muscles. Small spot stimulation shows that these motor neuron pools can be rhythmically active in flexor phase, independent from the rhythmic flexor activity in the L1 and L2 roots (B and D).
Discussion
We have shown before (32) that activation of excitatory interneurons in the lumbar spinal cord is sufficient to evoke hindlimb locomotor-like activity. The present study demonstrates that activation of glutamatergic neurons is both sufficient and necessary for generating locomotor-like activity. In contrast, selective light-induced activation of inhibitory neurons will not elicit locomotion, but can instantaneously abolish any ongoing activity. Using this genetically driven functional dichotomy, we demonstrate that the mammalian hindlimb locomotor network can be segregated into multiple intrinsically rhythmogenic modules, each module composed of a presynaptic network driving a motor neuron pool, not unlike the unit burst model. Our experiments provide mechanistic insights into the functional organization of spinal rhythm-generating networks in the rodent and differentiate among several proposed models for rhythm-generation in the vertebrates.
Light-Induced Locomotor-Like Activity Is an Important Model for Locomotion.
We have used intersectional mouse genetics to robustly express ChR2 and eNpHR in glutamatergic or GABAergic/glycinergic neurons combined with light stimulation of both global and restricted areas of the isolated spinal cord, using nerve activity as a proxy for locomotor-like activity. The cell-targeted, instantaneous, and spatially specific stimulation allows for a selective probing of the macroscopic architecture of the hindlimb locomotor network without having to induce lesions or use other irreversible manipulations. Using the optogenetic approach, we firmly establish that excitatory neurons are both sufficient and necessary for rhythm-generation in the rodent spinal cord, and that this function cannot be replaced by light activation of inhibitory neurons. Even with the excitatory network still present, activation of inhibitory neurons is unable to access this network to generate locomotor activity. When large areas of the cord are illuminated, the light-induced locomotor-like activity in the Vglut2::Cre; RC-ChR2 mice is robust, with all of the characteristics observed during drug-induced or neural-evoked locomotor-like activity, including the mixed flexor-extensor output in the L4 root (39, 43, 44). Moreover, light-induced locomotor-like activity has a higher burst frequency than seen with other methods (up to 2.7 Hz), is well tuned, and is instantaneously turned on with the appropriate pattern.
Drug-induced locomotor-like activity is readily evoked and can be maintained for hours, permitting long timespan analysis in wild type as well as mutant and lesioned animals (45–50). Although the activity most likely uses the same networks as under in vivo conditions, an obvious drawback is that the drugs act upon every neuron in the spinal cord, not all of which are necessarily components of the locomotor network. Focal optogenetic stimulation of the spinal cord may have a potential to bypass these problems.
A Network with Multiple Rhythmogenic Flexor and Extensor Modules Is the Best Description of the Rodent Hindlimb CPG.
Having established the robustness of optogenetic methods for activation or inactivation of the network, we could directly use it to address the macroscopic architecture of the network. Our study directly refutes several proposed models and favors one in particular.
The classical half-center model posits that the activity in both flexor- and extensor-networks are mutually dependent and both essential for rhythm generation. In this model, the individual half-centers are unable to burst without reciprocal activity. This organization clearly contrasts with our present findings that the flexor and extensor networks on the same side of the cord can be rhythmically active independently. Furthermore, multiple independent flexor and extensor rhythm generators can be activated in isolation. One could envision that the half-center would be structured over the left and right side of the cord instead. Such a structure would be consistent with the drop in locomotion frequency seen when a cord is bisected into two hemicords (38, 39), and with a bilaterally shared network proposed in turtles (51). The frequency change would then depend on a loss of commissural input. A recent study also found that the swimming CPG in the Xenopus tadpole embryo (stage 37/38) relies on bilaterally shared half-center organization for rhythm generation (52). However, our results clearly show that in the rodent CPG, similar to the lamprey (53), locomotor-like bursting can be induced unilaterally without any activity on the contralateral side or after the ventral commissure was cut and, thus, precluding any crossing signals.
The asymmetric flexor-burst model relies on a single rhythmogenic structure that is active during flexor bursts and, at the same time, inhibits tonic activity in extensors. In our results, we find support for the flexor rhythm being both faster and more readily evoked than the extensor rhythm. However, we also find that we can evoke bursting in extensors without any activity in the flexors, concluding that the hierarchical relationship between the two networks is not the characteristic that generates rhythmicity in the extensors but rather allows the endogenous rhythm to proceed in a coordinated fashion.
A modular network with a distributed burst capability is the organization that best accounts for the ability to evoke rhythmic activity in a limited population of the hindlimb motor neuron pools, as observed in the present study. In contrast to modules of synergists or muscles surrounding a single joint, we describe a module as a presynaptic network that appear to be able to drive individual motor neuron pools in isolation. This description is not unlike the unit burst generator model, first hypothesized for the cat locomotor network (18). The present experiments provide a direct demonstration of this model.
The best test for the existence of a modular network is to independently evoke rhythmicity in two closely related modules and observe the locomotor-like activity in the two corresponding motor neurons pools. The tibialis anterior and vastus lateralis motor neuron pools can fire in the flexion phase during normal locomotion and could be successfully separated from the flexor-related bursting in the L2 ventral root. This observation is striking evidence for separate rhythmic modules given that the vastus lateralis motor neuron pool resides in the L3 segment, close to the Iliopsoas motor neuron pool that is recorded from the L2 ventral root.
Our previous experiments have indicated that an inhibitory premotor network organized in a half-center–like fashion can coordinate and produce combined flexor and extensor motor output in response to drugs (50). Moreover, several half-center motifs have been characterized by intracellular recordings during locomotion (54). These elements are probably essential for patterning the rhythmic output and are not disputed by the results in this study. However, this connectivity does not preclude that the rhythmicity itself is locally generated. Intrinsically rhythmic modules may also lead to a more flexible system that allows for different rhythmic patterns of activity, for instance scratching or backward walking (55). We therefore conclude that the most basic units of the locomotor CPG in rodents are independent rhythmogenic modules that are interconnected during locomotion to ensure a coordinated output.
Methods
All experiments were approved by the local ethical committee (Stockholm's Norra Forsöksdjursnämnd) and performed in accordance with European guidelines for the care and use of laboratory animals.
Mice.
The transgenic lines described were as follows: Vglut2::Cre (34), Rosa26-CAG-LSL-eNpHR3.0-EYFP-WPRE, and Rosa26-CAG-LSL-ChR2-EYFP-WPRE (33). The VIAAT::Cre mouse is constructed by using BAC-technology; the production details are published in SI Methods.
In Vitro Experiments.
Mice aged 0–4 d were used in all experiments. The pups were anesthetized in Isoflurane, decapitated, and eviscerated before the spinal cord with or without the brainstem attached was isolated in ice-cold low calcium Ringers solution [oxygenated 95% (vol/vol) O2/5% (vol/vol) CO2] that contained 111 mM NaCl, 3 mM KCl, 11 mM glucose, 25 mM NaHCO3, 3.7 mM MgSO4, 1.1 mM KH2PO4, and 0.25 mM CaCl2 at pH 7.4. In most experiments, the lumbar ventral roots were cut at the exit point from the vertebrate canal. In some experiments, peripheral nerves to the vastus lateralis and tibialis anterior muscles were dissected out before the spinal cord was isolated with the peripheral nerves still attached. The dorsal roots were cut in all cases at the exit point of the vertebrate canal. The isolated spinal cord with nerves attached was transferred to a recording chamber that was continuously perfused with normal Ringers solution and contained 110 mM NaCl, 3 mM KCl, 11 mM glucose, 25 mM NaHCO3, 1.25 mM MgSO4, 1.1 mM KH2PO4, and 2.5 mM CaCl2 oxygenated 95% O2/5% CO2 to obtain a pH of 7.4. All recordings were done at room temperature.
Drugs.
In some experiments, NMDA (7 µM; Sigma) was used in combination with serotonin (5-HT, 8 µM, Sigma) to induce locomotor-like activity in vitro. CNQX (10 µM; Sigma) and AP5 (30 µM; Sigma) were used together to block glutamatergic neurotransmission during ChR2 intracellular recordings.
Neural-Evoked Locomotor Activity.
The protocol for stimulating descending fibers inducing locomotor-like activity was similar to the one used in previous studies (56).
Recording of Locomotor Activity and Analysis.
The locomotor activity was recorded with suction electrodes attached to the L2 and the L5 lumbar roots on either side of the cord or to the cut end of peripheral nerves. The nerve activity was band-passed filtered at 100 Hz to 1 kHz and sampled by using Axoscope 10 (Molecular Devices) at 1 kHz. Time/frequency plots were generated by using the Spinalcore software (57). The software uses morlet wavelet algorithms on rectified data to derive the frequency component of the time series data recorded from each root. The time/frequency data are plotted only when the two signals are significantly coherent. The phase relationship between the signals is then presented as a vector field. The color coding shows the coherence between the two signals.
Intracellular Recordings.
Whole-cell recordings ware performed in acute 300-µm slices. Patch electrodes had resistances of 5–8ΜΩ. Electrodes contained 128 mM K gluconate, 10 mM Hepes, 0.0001 mM CaCl2, 1 mM glucose, 4 mM NaCl, 5 mM ATP, and 0.3 mM GTP at pH 7.4. Whole-cell recordings were performed at room temperature by using a Multiclamp 700B amplifier (Molecular Devices) and acquired by using pClamp software (Clampex v.10, Molecular Devices).
Light Stimulation of Cord.
Light stimulation was executed through a 5×, 10×, or 50× microscope objective through a 450- to 490-nm band-pass filter for ChR2 or a 536- to 556-nm band-pass filter for eNpHR. For more restricted stimulations of ChR2-positive cells, we used a 473-nm laser scanning system (UGA-40; Rapp Optoelectronic) to illuminate spots ranging from 320–1,000 µm2 with an intensity ranging from 0.07–1.5 mW/mm2 as measured with a laser power meter (Coherent).
Supplementary Material
Acknowledgments
We thank Aharon Lev-Tov and Yoav Mor for the use of the Spinal Core software, Ann-Charlotte Westerdahl and Peter Löw for extensive work on the protocols for genotyping, and Natalie Sleiers for animal breeding. This work was supported by the Söderberg Foundation, the Swedish Research Council, Strat-Neuro, and an advanced European Research Council grant (to O.K.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1304365110/-/DCSupplemental.
References
- 1. Forssberg H (1985) Ontogeny of human locomotor control. I. Infant stepping, supported locomotion and transition to independent locomotion. Exp Brain Res 57(3):480–493. [DOI] [PubMed]
- 2.Kudo N, Nishimaru H. Reorganization of locomotor activity during development in the prenatal rat. Ann N Y Acad Sci. 1998;860:306–317. doi: 10.1111/j.1749-6632.1998.tb09058.x. [DOI] [PubMed] [Google Scholar]
- 3.Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG. Descending command systems for the initiation of locomotion in mammals. Brain Res Brain Res Rev. 2008;57(1):183–191. doi: 10.1016/j.brainresrev.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Lev-Tov A, Etlin A, Blivis D. Sensory-induced activation of pattern generators in the absence of supraspinal control. Ann N Y Acad Sci. 2010;1198:54–62. doi: 10.1111/j.1749-6632.2009.05424.x. [DOI] [PubMed] [Google Scholar]
- 5.Kudo N, Yamada T. N-methyl-D,L-aspartate-induced locomotor activity in a spinal cord-hindlimb muscles preparation of the newborn rat studied in vitro. Neurosci Lett. 1987;75(1):43–48. doi: 10.1016/0304-3940(87)90072-3. [DOI] [PubMed] [Google Scholar]
- 6.Smith JC, Feldman JL, Schmidt BJ. Neural mechanisms generating locomotion studied in mammalian brain stem-spinal cord in vitro. FASEB J. 1988;2(7):2283–2288. doi: 10.1096/fasebj.2.7.2450802. [DOI] [PubMed] [Google Scholar]
- 7.Hultborn H, et al. How do we approach the locomotor network in the mammalian spinal cord? Ann N Y Acad Sci. 1998;860:70–82. doi: 10.1111/j.1749-6632.1998.tb09039.x. [DOI] [PubMed] [Google Scholar]
- 8.McCrea DA, Rybak IA. Organization of mammalian locomotor rhythm and pattern generation. Brain Res Brain Res Rev. 2008;57(1):134–146. doi: 10.1016/j.brainresrev.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grillner S, Zangger P. How detailed is the central pattern generation for locomotion? Brain Res. 1975;88(2):367–371. doi: 10.1016/0006-8993(75)90401-1. [DOI] [PubMed] [Google Scholar]
- 10.Orlovsky GN, Deliagina TG, Grillner S. Neuronal Control of Locomotion. Oxford: Oxford Press; 1998. [Google Scholar]
- 11.Kiehn O, et al. Excitatory components of the mammalian locomotor CPG. Brain Res Brain Res Rev. 2008;57(1):56–63. doi: 10.1016/j.brainresrev.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Grillner S, Jessell TM. Measured motion: Searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol. 2009;19(6):572–586. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimaru H, Kakizaki M. The role of inhibitory neurotransmission in locomotor circuits of the developing mammalian spinal cord. Acta Physiol (Oxf) 2009;197(2):83–97. doi: 10.1111/j.1748-1716.2009.02020.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhong G, Shevtsova NA, Rybak IA, Harris-Warrick RM. Neuronal activity in the isolated mouse spinal cord during spontaneous deletions in fictive locomotion: Insights into locomotor central pattern generator organization. J Physiol. 2012;590(Pt 19):4735–4759. doi: 10.1113/jphysiol.2012.240895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stuart DG, Hultborn H. Thomas Graham Brown (1882–1965), Anders Lundberg (1920-), and the neural control of stepping. Brain Res Brain Res Rev. 2008;59(1):74–95. doi: 10.1016/j.brainresrev.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 16. Duysens J, Pearson KG (1976) The role of cutaneous afferents from the distal hindlimb in the regulation of the step cycle of thalamic cats. Exp Brain Res 24:245–255. [DOI] [PubMed]
- 17.Brownstone RM, Wilson JM. Strategies for delineating spinal locomotor rhythm-generating networks and the possible role of Hb9 interneurones in rhythmogenesis. Brain Res Brain Res Rev. 2008;57(1):64–76. doi: 10.1016/j.brainresrev.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grillner S. Control of Locomotion in Bipeds, Tetrapods, and Fish. Handbook of Physiology—The Nervous System II. Bethesda, MD: Am Physiol Soc; 1981. pp. 1179–1236. [Google Scholar]
- 19.Stein PS. Neuronal control of turtle hindlimb motor rhythms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2005;191(3):213–229. doi: 10.1007/s00359-004-0568-6. [DOI] [PubMed] [Google Scholar]
- 20.Grillner S. The motor infrastructure: From ion channels to neuronal networks. Nat Rev Neurosci. 2003;4(7):573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- 21. Grillner S & Zangger P (1979) On the central generation of locomotion in the low spinal cat. Exp Brain Res 34(2):241–261. [DOI] [PubMed]
- 22. Deliagina TG, Orlovsky GN, Pavlova GA (1983) The capacity for generation of rhythmic oscillations is distributed in the lumbosacral spinal cord of the cat. Exp Brain Res 53(1):81–90. [DOI] [PubMed]
- 23. Kjaerulff O, Kiehn O (1996) Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: A lesion study. The J Neurosci 16(18):5777–5794. [DOI] [PMC free article] [PubMed]
- 24.Cowley KC, Schmidt BJ. Regional distribution of the locomotor pattern-generating network in the neonatal rat spinal cord. J Neurophysiol. 1997;77(1):247–259. doi: 10.1152/jn.1997.77.1.247. [DOI] [PubMed] [Google Scholar]
- 25. Cazalets JR, Borde M, Clarac F (1995) Localization and organization of the central pattern generator for hindlimb locomotion in newborn rat. J Neurosci 15(7 Pt 1):4943–4951. [DOI] [PMC free article] [PubMed]
- 26.Delivet-Mongrain H, Leblond H, Rossignol S. Effects of localized intraspinal injections of a noradrenergic blocker on locomotion of high decerebrate cats. J Neurophysiol. 2008;100(2):907–921. doi: 10.1152/jn.90454.2008. [DOI] [PubMed] [Google Scholar]
- 27.Perret C, Cabelguen JM. Main characteristics of the hindlimb locomotor cycle in the decorticate cat with special reference to bifunctional muscles. Brain Res. 1980;187(2):333–352. doi: 10.1016/0006-8993(80)90207-3. [DOI] [PubMed] [Google Scholar]
- 28.Kriellaars DJ, Brownstone RM, Noga BR, Jordan LM. Mechanical entrainment of fictive locomotion in the decerebrate cat. J Neurophysiol. 1994;71(6):2074–2086. doi: 10.1152/jn.1994.71.6.2074. [DOI] [PubMed] [Google Scholar]
- 29.Burke RE, Degtyarenko AM, Simon ES. Patterns of locomotor drive to motoneurons and last-order interneurons: Clues to the structure of the CPG. J Neurophysiol. 2001;86(1):447–462. doi: 10.1152/jn.2001.86.1.447. [DOI] [PubMed] [Google Scholar]
- 30.Lafreniere-Roula M, McCrea DA. Deletions of rhythmic motoneuron activity during fictive locomotion and scratch provide clues to the organization of the mammalian central pattern generator. J Neurophysiol. 2005;94(2):1120–1132. doi: 10.1152/jn.00216.2005. [DOI] [PubMed] [Google Scholar]
- 31.Kiehn O. Development and functional organization of spinal locomotor circuits. Curr Opin Neurobiol. 2011;21(1):100–109. doi: 10.1016/j.conb.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Hägglund M, Borgius L, Dougherty KJ, Kiehn O. Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nat Neurosci. 2010;13(2):246–252. doi: 10.1038/nn.2482. [DOI] [PubMed] [Google Scholar]
- 33.Madisen L, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15(5):793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borgius L, Restrepo CE, Leao RN, Saleh N, Kiehn O. A transgenic mouse line for molecular genetic analysis of excitatory glutamatergic neurons. Mol Cell Neurosci. 2010;45(3):245–257. doi: 10.1016/j.mcn.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 35.Dumoulin A, et al. Presence of the vesicular inhibitory amino acid transporter in GABAergic and glycinergic synaptic terminal boutons. J Cell Sci. 1999;112(Pt 6):811–823. doi: 10.1242/jcs.112.6.811. [DOI] [PubMed] [Google Scholar]
- 36.Kiehn O, Kjaerulff O. Distribution of central pattern generators for rhythmic motor outputs in the spinal cord of limbed vertebrates. Ann N Y Acad Sci. 1998;860:110–129. doi: 10.1111/j.1749-6632.1998.tb09043.x. [DOI] [PubMed] [Google Scholar]
- 37. Stein PS, Victor JC, Field EC, Currie SN (1995) Bilateral control of hindlimb scratching in the spinal turtle: Contralateral spinal circuitry contributes to the normal ipsilateral motor pattern of fictive rostral scratching. J Neurosci 15(6):4343–4355. [DOI] [PMC free article] [PubMed]
- 38. Kjaerulff O Kiehn O (1997) Crossed rhythmic synaptic input to motoneurons during selective activation of the contralateral spinal locomotor network. J Neurosci 17(24):9433–9447. [DOI] [PMC free article] [PubMed]
- 39.Bonnot A, Whelan PJ, Mentis GZ, O’Donovan MJ. Locomotor-like activity generated by the neonatal mouse spinal cord. Brain Res Brain Res Rev. 2002;40(1-3):141–151. doi: 10.1016/s0165-0173(02)00197-2. [DOI] [PubMed] [Google Scholar]
- 40.McHanwell S, Biscoe TJ. The localization of motoneurons supplying the hindlimb muscles of the mouse. Philos Trans R Soc Lond B Biol Sci. 1981;293(1069):477–508. doi: 10.1098/rstb.1981.0082. [DOI] [PubMed] [Google Scholar]
- 41.Hayes HB, Chang YH, Hochman S. An in vitro spinal cord-hindlimb preparation for studying behaviorally relevant rat locomotor function. J Neurophysiol. 2009;101(2):1114–1122. doi: 10.1152/jn.90523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillis GB, Biewener AA. Hindlimb muscle function in relation to speed and gait: in Vivo patterns of strain and activation in a hip and knee extensor of the rat (Rattus norvegicus) J Exp Biol. 2001;204(Pt 15):2717–2731. doi: 10.1242/jeb.204.15.2717. [DOI] [PubMed] [Google Scholar]
- 43.Nishimaru H, Kudo N. Formation of the central pattern generator for locomotion in the rat and mouse. Brain Res Bull. 2000;53(5):661–669. doi: 10.1016/s0361-9230(00)00399-3. [DOI] [PubMed] [Google Scholar]
- 44.Jiang Z, Carlin KP, Brownstone RM. An in vitro functionally mature mouse spinal cord preparation for the study of spinal motor networks. Brain Res. 1999;816(2):493–499. doi: 10.1016/s0006-8993(98)01199-8. [DOI] [PubMed] [Google Scholar]
- 45.Kullander K, et al. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science. 2003;299(5614):1889–1892. doi: 10.1126/science.1079641. [DOI] [PubMed] [Google Scholar]
- 46.Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron. 2004;42(3):375–386. doi: 10.1016/s0896-6273(04)00249-1. [DOI] [PubMed] [Google Scholar]
- 47.Crone SA, et al. Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron. 2008;60(1):70–83. doi: 10.1016/j.neuron.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 48. Crone SA, Zhong G, Harris-Warrick R, Sharma K (2009) In mice lacking V2a interneurons, gait depends on speed of locomotion. J Neurosci 29(21):7098–7109. [DOI] [PMC free article] [PubMed]
- 49.Zhang Y, et al. V3 spinal neurons establish a robust and balanced locomotor rhythm during walking. Neuron. 2008;60(1):84–96. doi: 10.1016/j.neuron.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Talpalar AE, et al. Identification of minimal neuronal networks involved in flexor-extensor alternation in the mammalian spinal cord. Neuron. 2011;71(6):1071–1084. doi: 10.1016/j.neuron.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Stein PS. Alternation of agonists and antagonists during turtle hindlimb motor rhythms. Ann N Y Acad Sci. 2010;1198:105–118. doi: 10.1111/j.1749-6632.2010.05500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moult PR, Cottrell GA, Li WC. Fast silencing reveals a lost role for reciprocal inhibition in locomotion. Neuron. 2013;77(1):129–140. doi: 10.1016/j.neuron.2012.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cangiano L Grillner S (2005) Mechanisms of rhythm generation in a spinal locomotor network deprived of crossed connections: The lamprey hemicord. J Neurosci 25(4):923–935. [DOI] [PMC free article] [PubMed]
- 54.Hultborn H, Illert M, Santini M. Convergence on interneurones mediating the reciprocal Ia inhibition of motoneurones. I. Disynaptic Ia inhibition of Ia inhibitory interneurones. Acta Physiol Scand. 1976;96(2):193–201. doi: 10.1111/j.1748-1716.1976.tb10188.x. [DOI] [PubMed] [Google Scholar]
- 55.Grillner S, Wallen P, Saitoh K, Kozlov A, Robertson B. Neural bases of goal-directed locomotion in vertebrates–an overview. Brain Res Brain Res Rev. 2008;57(1):2–12. doi: 10.1016/j.brainresrev.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 56.Talpalar AE, Kiehn O. Glutamatergic mechanisms for speed control and network operation in the rodent locomotor CpG. Front Neural Circuits. 2010;4(19):1–14. doi: 10.3389/fncir.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mor Y, Lev-Tov A. Analysis of rhythmic patterns produced by spinal neural networks. J Neurophysiol. 2007;98(5):2807–2817. doi: 10.1152/jn.00740.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.