Abstract
From the perspectives of disease transmission and sterility maintenance, the world’s blood supplies are generally safe. However, in multiple clinical settings, red blood cell (RBC) transfusions are associated with adverse cardiovascular events and multiorgan injury. Because ∼85 million units of blood are administered worldwide each year, transfusion-related morbidity and mortality is a major public health concern. Blood undergoes multiple biochemical changes during storage, but the relevance of these changes to unfavorable outcomes is unclear. Banked blood shows reduced levels of S-nitrosohemoglobin (SNO-Hb), which in turn impairs the ability of stored RBCs to effect hypoxic vasodilation. We therefore reasoned that transfusion of SNO-Hb–deficient blood may exacerbate, rather than correct, impairments in tissue oxygenation, and that restoration of SNO-Hb levels would improve transfusion efficacy. Notably in mice, administration of banked RBCs decreased skeletal muscle pO2, but infusion of renitrosylated cells maintained tissue oxygenation. In rats, hemorrhage-induced reductions in muscle pO2 were corrected by SNO-Hb–repleted RBCs, but not by control, stored RBCs. In anemic awake sheep, stored renitrosylated, but not control RBCs, produced sustained improvements in O2 delivery; in anesthetized sheep, decrements in hemodynamic status, renal blood flow, and kidney function incurred following transfusion of banked blood were also prevented by renitrosylation. Collectively, our findings lend support to the idea that transfusions may be causally linked to ischemic events and suggest a simple approach to prevention (i.e., SNO-Hb repletion). If these data are replicated in clinical trials, renitrosylation therapy could have significant therapeutic impact on the care of millions of patients.
Keywords: ethyl nitrite, hemoglobin cysbeta93, nitric oxide, storage lesion
With ∼85 million units of human blood collected each year for therapeutic purposes [World Health Organization Fact Sheet #279 (www.who.int/worldblooddonorday/media/who_blood_safety_factsheet_2011.pdf)], infusion of red blood cells (RBCs) is among the most common procedures in medicine. RBC transfusion is premised on a direct correlation between the O2 carrying capacity of blood (increased by transfusion) and the delivery of O2 to tissues, and thus assumes that transfusion will improve tissue oxygenation. However, it is unclear how often transfusion meets this goal. Although blood transfusion can be life-saving, evidence continues to accumulate that administration of stored RBCs may not always be beneficial and, in some settings, may actually cause harm (1–6), findings of particular concern because even mild anemia is prognostic of adverse outcomes (7, 8).
The range of adverse transfusion sequelae (myocardial infarction, renal injury, multiorgan failure, and death) (9–12), suggest that banked blood may acutely exacerbate rather than correct tissue hypoxia. Although it has long been suggested that storage impairs the ability of RBCs to deliver O2 (13–15), it has not been obvious why this should occur upon infusing a small fraction of overall RBC volume. Reconceptualization of the respiratory cycle as a three-gas system (NO/O2/CO2) (16), which includes a role for bioactive NO derived from RBCs in hypoxia-regulated vasodilation (i.e., O2 delivery) (17), provides a basis for understanding why increasing bulk O2 content alone can fail to improve tissue perfusion (18). In addition to releasing NO bioactivity, RBCs may also stimulate NO production from the endothelium by releasing ATP (19). Endothelial NO is thought to influence settling points in tissues (basal tone), but RBC-derived NO may effect demand-coupled changes in blood flow, which are endothelium independent (20–22). Inasmuch as RBCs tend to traverse the microcirculation “in series,” impaired capillary transit of single cells (as might result from impaired vasodilation) may impede microcirculatory flow at large.
Tissue perfusion is matched with metabolic demand through a physiological response, termed hypoxic vasodilation, in which local blood flow is coupled to desaturation of hemoglobin (Hb) (22–24). Hb within RBCs is thus a principal sensor and transducer of this response (20, 21, 25, 26). Specifically, it is proposed that the conformational changes incurred upon binding and release of O2 from the hemes are intimately linked to binding and release of NO from cysteine residues in Hb, and that the released NO is liberated from RBCs in the form of bioactive S-nitrosothiols (SNOs). There is further appreciation that NO derived from nitrite may participate in RBC vasodilation through its conversion into SNOs (16, 17, 27, 28). [Note, however, that NO itself cannot escape sequestration by excess hemes of Hb (24).] Thus, RBCs may dilate blood vessels through a SNO-based mechanism as blood transits from arteries to veins (20, 29, 30). By extension, blood flow (and O2 delivery) would be negatively impacted by conditions that reduce circulating levels of SNO-Hb.
Hb conformation is regulated not only by O2, but also by CO2 and pH. Notably, banked RBCs are stored in an acidic isotonic solution (∼pH 6.5), which accelerates SNO-Hb decay (31). We and others previously reported that storage of blood leads to marked losses in SNO-Hb within 1 d (32, 33), which are paralleled by losses in the ability of RBCs to effect hypoxic vasodilation (32). Blood is >80% depleted in SNO-Hb by 7 d and remains low thereafter [whereas, nitrite levels do not decline during storage (34)]. We further showed that the defect in RBC-mediated vasodilation could be corrected by selectively repleting SNO-Hb (32, 35), but this has only been demonstrated with very small amounts of blood (1 mL). Restoration of the hypoxic vasodilatory capacity of banked blood raises the possibility that such an intervention might improve tissue oxygenation following transfusion (36). We therefore tested this postulate in four distinct and complementary transfusion paradigms.
Results
Study 1: Top-Up Transfusion in Mice.
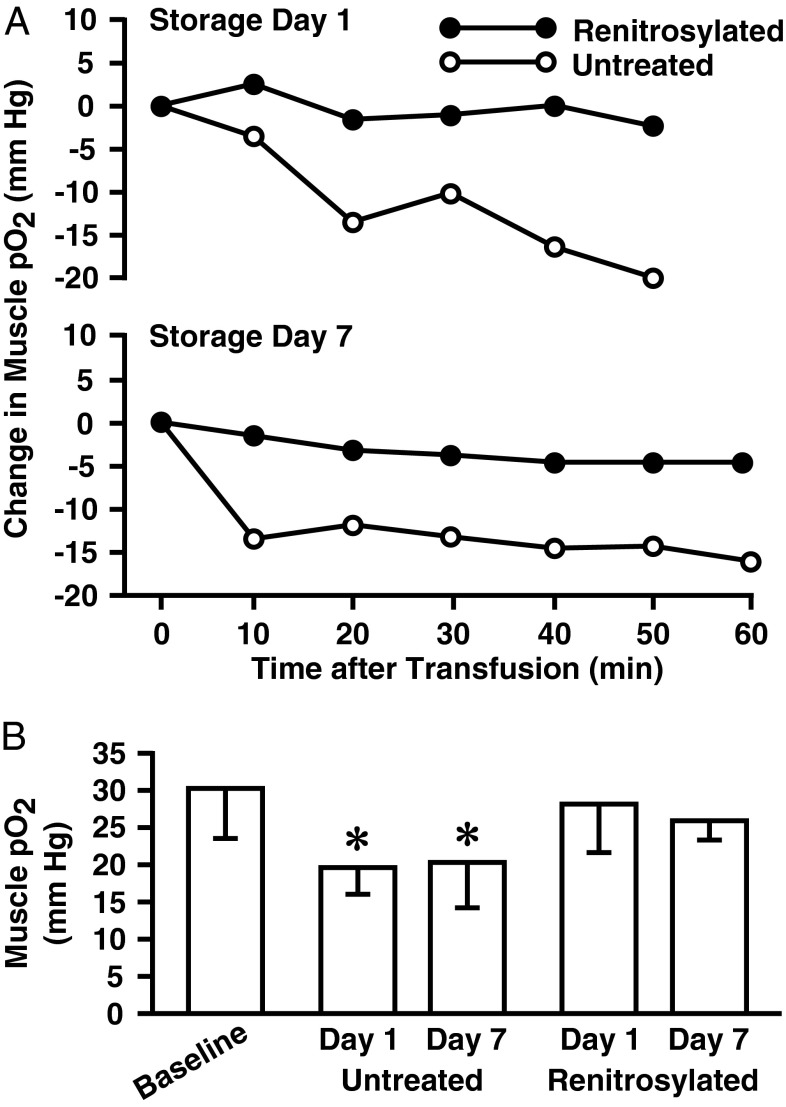
After 1 d of storage, rodent blood is depleted of SNO-Hb by more than 70% (37, 38). As an initial test of the effects of renitrosylation, we measured tissue oxygenation (using pO2 electrodes inserted into skeletal muscle) following transfusion of stored untreated or renitrosylated blood. Renitrosylation of blood in these initial experiments used an established NO methodology (Materials and Methods) that increases SNO-Hb concentrations without increasing nitrite (32, 35, 39). Normovolemic mice received the human equivalent of one unit (200 µL) of RBCs [∼10% of estimated blood volume (40)] that had been stored for 1 or 7 d. Representative and group oxygenation responses of the mouse thigh muscle bed are presented in Fig. 1. Basal muscle pO2 under anesthesia was 32.0 ± 7.3 mm Hg (n = 17). Administration of stored, SNO-depleted RBCs produced progressive declines in muscle pO2. At the end of monitoring, pO2 was 19.9 ± 6.9 mm Hg for 1-d-old blood and 20.2 ± 10.4 for 7-d-old blood (both P < 0.05 vs. baseline). In contrast, RBCs stored for 1 or 7 d, and then renitrosylated immediately before transfusion, produced no significant change in pO2 (28.3 ± 11.3 and 26.1 ± 4.1 mm Hg for RBC days 1 and 7, respectively; P > 0.05). As an additional control, we sought to infuse RBCs procured from “Cysβ93-deficient” mice that should be refractory to SNO-Hb formation (41). However, our on-going characterizations of these strains have revealed that these mice exhibit normal levels of SNO-Hb (Discussion and Fig. S1).
Fig. 1.
Mouse transfusion and muscle pO2. (A) Representative time courses of skeletal muscle pO2 changes following receipt of 200 μL of untreated (○) or renitrosylated (●) allogenic blood stored for 1 or 7 d. (B) Mean pO2 values (±SD) at baseline (n = 17) and 50–60 min after transfusion with 1- or 7-d-old untreated or renitrosylated blood (n = 3 for each condition). An asterisk denotes a significant reduction in pO2 values in the untreated cohorts compared with baseline.
Study 2: Hemorrhage and Transfusion in Rats.
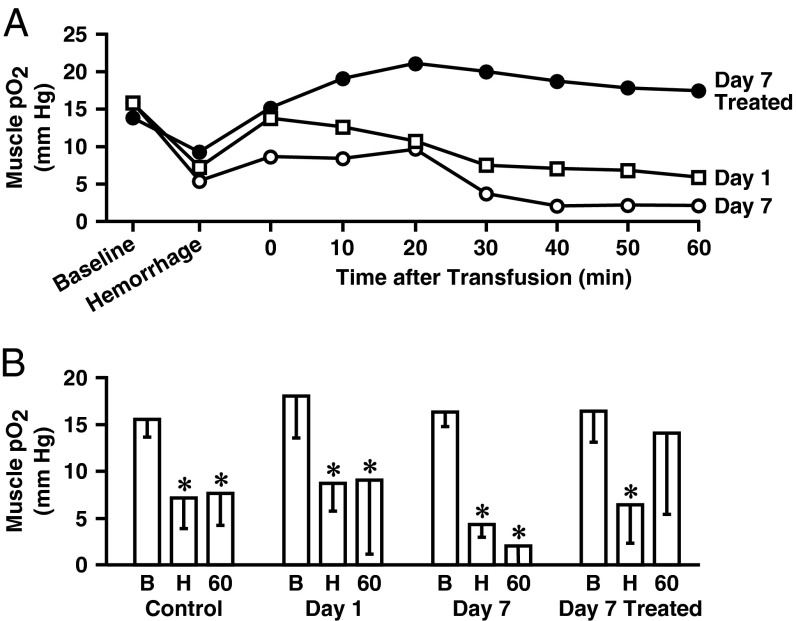
Controlled hemorrhage provides a relevant model of tissue ischemia in which to test the effect of stored blood. In anesthetized rats, 25–30% of the estimated blood volume was removed to reach the target mean arterial pressure (MAP) of 55 mm Hg. Changes in rat skeletal muscle oxygenation in response to blood loss and transfusion are presented in Fig. 2. Hemorrhage produced significant declines in muscle pO2 across all treatment groups (P < 0.05 compared with starting levels). Animals received untreated RBCs stored for 1 or 7 d or renitrosylated RBCs stored for 7 d. Although transfusion of both untreated and renitrosylated RBCs restored MAP, only renitrosylated (SNO-Hb repleted) blood was accompanied by improvements in thigh muscle pO2 (from a nadir of 6.3 ± 4.1 to 13.8 ± 8.5 mm Hg 60 min after transfusion; Fig. 2B). In the groups that received untreated RBCs stored for 1 or 7 d, muscle pO2 remained at or near the hemorrhage-induced lows, and thus were significantly lower than the baseline values (8.9 ± 7.9 mm Hg compared with a starting level of 17.7 ± 4.4 mm Hg for day 1 blood, and 2.0 ± 2.3 mm Hg vs. 16.1 ± 1.5 mm Hg for day 7 blood, both P < 0.05). These differences in tissue oxygenation response were reflected in metabolic parameters measured in snap-frozen hind-limb muscle biopsies procured 60 min after transfusion (Table 1). Notably, muscle lactate and lactate/pyruvate ratio increased after infusion of SNO-depleted RBCs, but not after infusion of SNO-repleted RBCs, and creatine phosphate content was preserved after infusion of SNO-repleted RBCs, but not after infusion of SNO-depleted RBCs.
Fig. 2.
Rat hemorrhage/transfusion and muscle pO2. (A) Representative time courses of skeletal muscle pO2 changes from baseline to hemorrhage and then in response to allogeneic transfusion with untreated RBCs stored for 1 or 7 d (□ and ○, respectively) or renitrosylated RBCs stored for 7 d (treated; ●). Monitoring was conducted for 60 min after transfusion. (B) Mean muscle pO2 values (±SD) at baseline (B), after hemorrhage (H), and 60 min after transfusion with untreated or renitrosylated RBCs (n = 5–7 per group). An asterisk denotes a significant difference in pO2 values compared with baseline within each group.
Table 1.
Rat thigh muscle metabolic parameters
| Control | Hemorrhage | SNO-Hb–depleted | SNO-Hb–repleted | |
| ATP | 5.6 ± 0.7 | 4.8 ± 0.4 | 4.9 ± 0.9 | 4.9 ± 0.7 |
| ADP | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.6 ± 0.2* |
| CrP | 17 ± 3 | 24 ± 6* | 20 ± 7 | 25 ± 2* |
| Cr | 11 ± 3 | 10 ± 3 | 15 ± 3 | 12 ± 2 |
| Pyr | 0.06 ± 0.02 | 0.07 ± 0.02 | 0.05 ± 0.01 | 0.05 ± 0.01 |
| L | 2.5 ± 0.8 | 3.3 ± 1.5 | 4.3 ± 2.6* | 2.6 ± 1.4 |
| L/Pyr | 39 ± 8 | 47 ± 22 | 87 ± 65* | 51 ± 33 |
| CrP+Cr | 30 ± 5 | 35 ± 8 | 37 ± 7* | 38 ± 2* |
Muscle metabolic markers under control (n = 7), hemorrhage (n = 9), and transfusion conditions (n = 10 for both). Values followed by an asterisk (*) indicate a significant difference from control values (P < 0.05). ADP, adenosine diphosphate; ATP, adenosine triphosphate; Cr, creatine; CrP, creatine phosphate; L, lactate; L/Pry, lactate/pyruvate ratio; Pyr, pyruvate.
Study 3: Intraoperative Transfusion of Anemic Sheep.
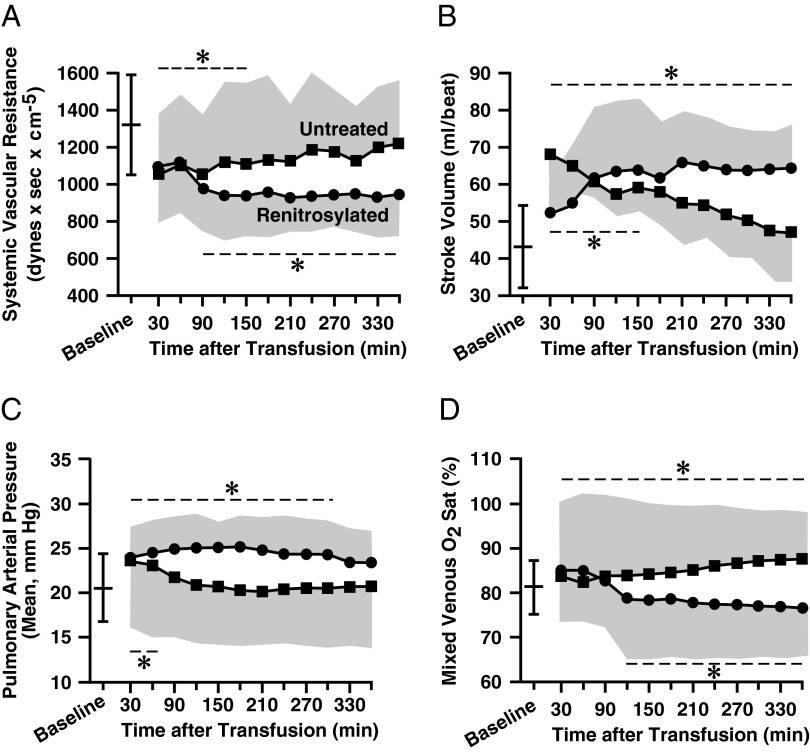
Large animals allow for assessments of hemodynamic responses to transfusion over prolonged periods of time. Two days after bloodletting (target Hb of ∼9 g/dL), adult sheep (n = 7 per group) were anesthetized and instrumented with peripheral and central catheters. Hemodynamic monitoring and organ blood flow determinations were made as each animal received two units of leukocyte-depleted packed ovine RBCs that had been stored for 14 d. After transfusions, there were initial declines in systemic vascular resistance (SVR) in both the control (untreated blood) and treatment (renitrosylated blood) groups (Fig. 3A). However, although vasodilatation persisted in the group that received renitrosylated blood, it reversed in the group that received untreated RBCs. By the end of the monitoring period, SVR had returned to the pretransfusion level in the control group and was significantly higher than in sheep that received SNO-Hb–repleted blood (P < 0.05). These higher SVR values correlated with higher MAP readings in the control animals versus animals receiving renitrosylated blood (Fig. S2). Stroke volume (Fig. 3B) rose in the renitrosylated group, whereas following an initial rise, it returned to baseline in sheep given untreated RBCs. Pulmonary arterial pressure (Fig. 3C) increased above baseline early after transfusion, but by the end of monitoring the within-group (compared with baseline) and between-group values were not significantly different. Similarly, pulmonary vascular resistance did not differ within or between groups (Fig. S2). Arterial pO2 was not different between groups. However, venous O2 saturation (SvO2) declined in the group that received renitrosylated blood (indicating improved O2 extraction by tissues) and increased in the control group (Fig. 3D). This SvO2 response was reflected in group differences in O2 extraction: by the end of the monitoring period the median values (plus first and third quartile deviations) were 15.4% (12.2%, 20.7%) in the treated group but only 7.7% (6.8%, 10.7%) in the control group (P < 0.05).
Fig. 3.
Sheep hemodynamic responses to intraoperative transfusion. Mean time courses (±SDs, shown by the shaded envelopes) for various cardiovascular parameters following receipt of two units of untreated (■) or renitrosylated (●) packed RBCs (n = 5–7). Group mean (±SD) baseline values are demarcated by the free-standing bar in each panel. The dashed lines and asterisk denote within-group datapoints that are significantly different (P < 0.05) from their respective baseline values. (A) SVR initially declined in both groups but only remained reduced in sheep receiving treated RBCs. (B) Stroke volume (SV) in the untreated cohort initially rose then returned to baseline, but it remained elevated in the treated group. (C) Mean pulmonary arterial pressure (mPAP) rose in both groups then returned to baseline. (D) Mixed SvO2 increased in the untreated group and declined in the treated group and these differences persisted throughout the monitoring session.
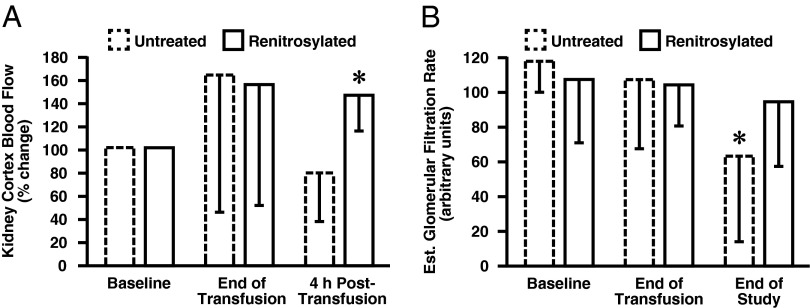
Organ blood flow was assessed using the microsphere technique. Flow to internal organs (liver, adrenals, spleen) (Table S1) had trended upward at the end of transfusion and had returned to baseline by 4 h posttransfusion. The kidney showed the most notable treatment-dependent response. Pretransfusion renal blood flow was 2.9 ± 1.3 and 0.41 ± 0.29 mL⋅min−1⋅g−1 in the cortex and medulla, respectively. In animals that received renitrosylated blood (n = 7), flow to the kidney cortex rose at the end of transfusion (Fig. 4A). At 4 h posttransfusion, cortical flow remained significantly higher than baseline (142 ± 32%); blood flow was also higher than in the untreated control group (n = 6), whose renal blood flow had declined to anemic baseline values (78 ± 42%; P < 0.05). A similar response was seen in the kidney medulla where at 4 h posttransfusion, control blood flow was 122 ± 77% of the pretransfusion level compared with 229 ± 112% for renitrosylated blood (P < 0.05). These differences in blood flow had functional corollaries (Fig. 4B). By the end of the monitoring period, estimated glomerular filtration rate (eGFR) in the controls had declined from 121 ± 43 to 74 ± 45 (P = 0.004; arbitrary units). In contrast eGFR did not change in animals receiving renitrosylated blood (112 ± 29 at the start and 101 ± 37 at the end of monitoring; P = 0.653).
Fig. 4.
Sheep renal responses to intraoperative transfusion. (A) Mean percent change (±SD) from baseline in cortical kidney blood flow (KBF) following receipt of two units of untreated (dashed line, n = 6) or renitrosylated (solid line, n = 7) ovine RBCs that had been stored for 14 d. Blood flow measurements were taken immediately at the end of transfusion and then again 4 h later. KBF in the group that received untreated RBCs was significantly less than flow in the group that received treated cells. (B) Mean (±SD) eGFR at baseline and after transfusion. By study end, eGFR was significantly less than baseline in the group that received untreated RBCs (P = 0.004; arbitrary units). In contrast eGFR did not change in animal receiving renitrosylated blood (P = 0.653).
Study 4: Awake Transfusion of Anemic Sheep.
Awake sheep were transfused with two units of 14-d-old ovine RBCs, 3 d after blood-letting. Transfusion increased mean Hb concentrations to similar levels in control and treated groups: from 9.1 ± 1.7 to 10.4 ± 1.5 g/dL and 9.5 ± 1.0 to 10.3 ± 1.0 g/dL in the untreated and treated groups, respectively. MetHb levels were essentially unchanged by transfusion (e.g., going from 0.70% ± 0.40 to 0.80% ± 0.46 in the treated group). Hemodynamic assessments made over 16 h indicated that all animals were physiologically stable during the posttransfusion monitoring period and clinical chemistries identified no group differences.
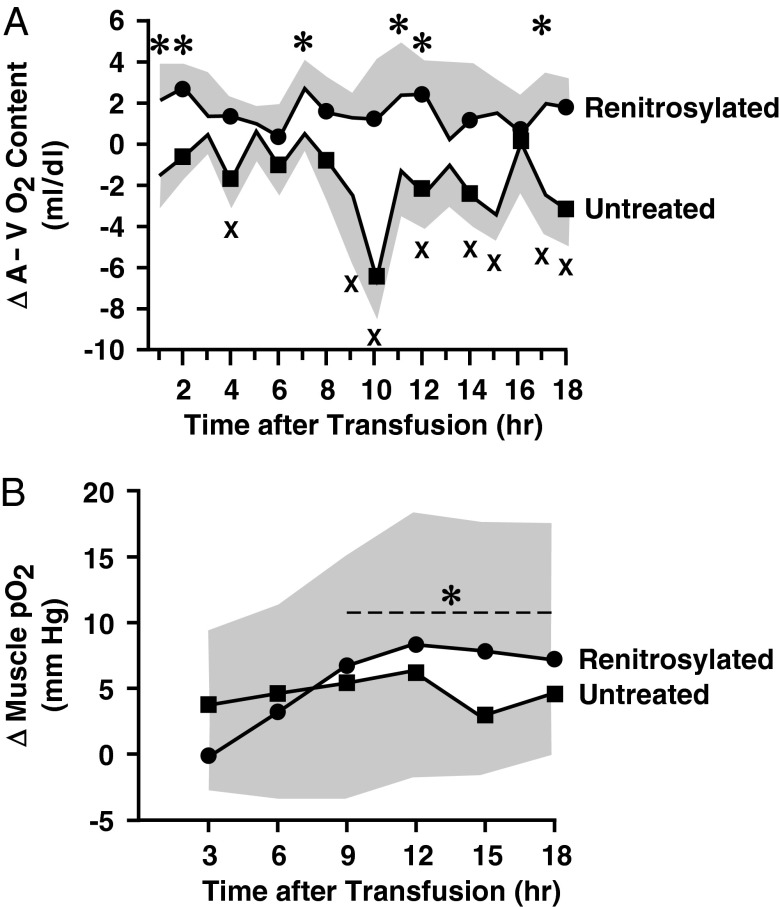
Blood O2 content was used to monitor changes in O2 utilization. Before transfusion, baseline arterial and venous (A-V) blood O2 content (expressed as mL O2/100 mL blood) were similar for the two groups (13.5 ± 1.2 and 9.0 ± 1.8 for the untreated; 13.0 ± 1.5 and 8.9 ± 2.1 for the treated; both n = 5). Transfusion produced the expected rise in arterial blood O2 content in both groups. However, although venous O2 content rose in the untreated group, venous O2 content either stayed constant or declined in the group receiving renitrosylated blood. Thus, A-V O2 differences were significantly lower than baseline (P < 0.05) in sheep receiving control transfusions (Fig. 5A) and significantly higher than baseline following transfusion of renitrosylated blood (P < 0.05).
Fig. 5.
Transfusion-induced changes in sheep oxygenation. (A) Mean time course (±SD, shown by the shaded envelopes) of change in A-V O2 content differences after transfusion of two units of untreated (■, n = 5) or renitrosylated (●, n = 5) ovine RBCs that had been stored for 14 d. An asterisk denotes datapoints that are significantly greater than the baseline value in the renitrosylated group and an X denotes datapoints that are significantly less than baseline in the untreated group (both P < 0.05). (B) Mean changes in skeletal muscle pO2 (±SD) determined in 3-h increments (blocks). The dashed line and asterisk denote data points in the renitrosylated group that are significantly greater than baseline (P < 0.05).
To augment the A-V O2 measurements, we directly recorded tissue pO2 with a needle probe placed in the hock muscle. Pretransfusion muscle pO2 values were similar between the two groups at 22.0 ± 8.1 and 22.5 ± 7.3 mm Hg. A median value was calculated at 3-h intervals to avoid overreliance on single tissue pO2 measurements. Group differences in O2 delivery after transfusion were reflected as change from baseline (Fig. 5B). For the untreated cohort (n = 4), none of the differences in posttransfusion pO2 values were significantly different from baseline. However, in the group receiving renitrosylated-banked blood (n = 5), pO2 values were significantly higher than baseline (four of the six blocks; P < 0.05).
Discussion
The present results build on the earlier discovery (32, 33) that storage diminishes the SNO-Hb–linked vasodilatory activity of blood that subserves hypoxic vasodilation (22). Experiments using four different transfusion paradigms across three different species demonstrated that banked blood deficient in SNO-Hb failed to correct anemia-induced reductions in blood flow and O2 utilization. Furthermore, in some settings, transfusion exacerbated anemia-induced deficits in tissue pO2. Any one paradigm has its limitations, but taken together the data offer compelling support to the clinical evidence that standard transfusion regimens may do little to improve end-organ O2 delivery. In stark contrast, repletion of SNO-Hb at the time of transfusion resulted in sustained improvements in tissue pO2 and related parameters of O2 sufficiency, including blood flow and lactate levels.
Our findings provide evidence that adverse transfusion outcomes may reflect underlying defects in RBC function (e.g., impaired NO-based vasodilation) and are suggestive of a causal relationship between declines in microvascular O2 delivery and impairments in organ function. Renitrosylation could offer a unique approach to improve tissue oxygenation and a compelling strategy to directly address the adverse cardiovascular morbidity associated with transfusion (6). By extension, tissue O2 sufficiency may be a more relevant biomarker for therapeutic testing and posttransfusion outcomes than the current standard of circulating RBC survival time (42). Case in point are the results from anesthetized anemic sheep where transfusion of renitrosylated RBCs, but not untreated blood, increased kidney blood flow and maintained GFR. Intraoperative blood transfusion is a well-recognized risk factor for acute kidney injury (AKI) (43–45), and the incidence of transfusion-associated AKI is amplified by preoperative anemia (44). Thus, occurrence rates of AKI may be a useful measure of the O2 delivery capability of banked blood.
Adverse clinical responses to blood transfusion have been associated with or exacerbated by storage duration. Banked RBCs indeed undergo multiple biochemical changes during storage [loss of molecular modulators of O2 binding, impaired RBC shape/flexibility, increased RBC adhesiveness, and hemolysis (46)], so it is notable that we were able to reverse impairments of oxygenation and organ dysfunction by SNO-Hb repletion, without correcting these other defects. Moreover, at least one clinical study has linked transfusion of 3-d-old RBCs to mortality (2), a time point that is well before most storage-related biochemical changes occur (47), except for declines in SNO-Hb (32, 33). Our present studies and previous findings (47) show that infusion of even 1-d-old blood can decrease tissue oxygenation consistent with this rapid loss in NO bioactivity.
Mechanisms of RBC vasodilation merit comment. We originally described an NO-based mechanism by which RBCs can relax blood vessels under hypoxia (30, 35). In this model, thiols of Hb deploy NO bioactivity: relaxations by human RBCs are (i) inhibited by prior depletion of SNO-Hb, (ii) potentiated by thiols, and (iii) dependent on cGMP (i.e., mediated by SNOs), and yet unaffected by the absence of endothelium or endothelial nitric oxide synthase (37). Furthermore, declines in SNO-Hb in hypoxic RBCs were commensurate with measured release of bioactive SNOs (29). SNO-Hb is thus in equilibrium with the small molecular weight SNOs that mediate vasodilation (16, 17, 20, 30). It was subsequently shown that ATP released from RBCs can also relax blood vessels (48, 49), albeit through an endothelium-dependent mechanism. A role for nitrite in relaxation by RBCs has also gained credence. However, nitrite is a nuance of the SNO-based mechanism (16, 24) [not a rejection of it as originally put forth by Cosby et al. (50)] because nitrite acts as a precursor to SNO-Hb formation (16, 17, 27, 28, 51). Indeed, initial claims that free NO derived from nitrite could escape the hemes in Hb to elicit vasodilation independently of SNOs (50) have not been reproduced (even by the authors themselves) (52–54), and were likely an in vitro artifact of reagents added to the bioassay system (16, 24, 52, 53). These same proponents of a SNO-independent role for nitrite have claimed that hypoxic vasodilation was unaltered in Cysβ93-deficient mice refractory to SNO-Hb formation (41). However, these mice are not true Cys93 knockouts: an extra human Cysγ93 gene was built into the mouse and a mouse fetal Cys93 is also present in the circulating RBCs. Indeed, SNO-Hb levels are in fact unchanged in these mice (strains kindly provided by T. M. Townes, University of Alabama, Birmingham, Birmingham, AL) (Fig. S1). Moreover, hypoxic vasodilation (blood flow and tissue pO2) was not actually tested in these animals, nor did these authors test NO-based relaxations by Cys-mutant RBCs (55). Rather, the authors assayed ATP-mediated (i.e., endothelium dependent) relaxations that have no bearing on SNO-based vasodilation. Most notably, Hb levels were elevated in the Cysβ93 mutant mice, indicative of tissue hypoxia and thus affirming an essential role for Cysβ93. Thus, the most parsimonious explanation for restoration of vessel relaxations by RBCs that are depleted of SNO-Hb [but not nitrite (34)] through storage and then repleted with S-nitrosylating agents, involves a SNO-based mechanism.
The comparative effects of transfusion with SNO-depleted vs. -repleted blood should be interpreted in accordance with the physiology of anemia. In anemic patients, a rise in SvO2 following transfusion is often assumed to reflect improved O2 delivery, but our findings suggest otherwise. In our sheep studies, the A-V O2 content gradient decreased after transfusion of untreated blood (reflecting a rise in SvO2), but increased with administration of renitrosylated RBCs (Fig. 5). Thus, remarkably, renitrosylated RBCs caused the A-V O2 content gradient to increase and SvO2 to decline at constant or increasing cardiac output, which is indicative of an increase in body O2 utilization. The response to renitrosylated blood is thus characteristic of reperfusion (i.e., preservation/restoration of microcirculatory blood flow). The declines in SVR and improvements in kidney blood flow following transfusion of renitrosylated blood provide further support for the idea that peripheral perfusion is being restored.
The responses we documented to administration of untreated stored blood suggest that SNO-Hb–depleted RBCs may cause microcirculatory injury or mitochondrial dysfunction reminiscent of sepsis [high SvO2 (Fig. 3); elevated lactate and lactate/pyruvate ratio (Table 1)]. This interpretation is consistent with clinical studies in which administration of blood to septic patients fails to improve oxygen utilization (56, 57) and may even increase morbidity and mortality (58). Sepsis is characterized in part by increased NO generation and dysregulation of SNO homeostasis (59). On the surface, transfusion of renitrosylated RBCs in this setting could have deleterious hemodynamic effects. However, efforts to block NO production in septic shock have increased mortality (60) and recent evidence suggests that NO may be beneficial in shock-like situations (61). Accordingly, transfusion of renitrosylated RBCs that can subserve vasodilation in a pO2/pCO2-regulated manner, may well improve microcirculatory perfusion in critically ill subjects. Because of the high prevalence of anemia in septic patients (62), this is a concept worth exploring.
In summary, we have used an O2-sensitive allosteric switch in Hb to conditionally deliver NO bioactivity in hypoxic tissues and a first-in-class renitrosylating agent that can harness this allosteric pathway to enhance tissue oxygenation. Our findings support the concept that renitrosylation therapy offers a means to ameliorate a failure of tissue perfusion and oxygenation produced by transfusion of stored RBCs. In this regard, perhaps the most remarkable aspect of our study is the insight that knowledge of the SNO-Hb status of banked blood may be used to judge the efficacy of RBC transfusion. Inasmuch as the world’s supply of banked RBCs is deficient in SNO-Hb, efforts to restore its levels may hold great therapeutic promise.
Materials and Methods
Animals studies were approved by the Duke University and Case Western Reserve University Institutional Animal Care and Use Committees. All procedures complied with The Guide for the Care and Use of Laboratory Animals. Experiments were conducted on young adult male mice (C57/BL6; ∼20 g) and rats (Sprague-Dawley; ∼300 g) obtained from Charles River, and adult mix-breed sheep obtained from local commercial suppliers. All potentially painful procedures were conducted under an appropriate plane of anesthesia. Methods for blood procurement, storage, and renitrosylation, along with details of the animal studies, transfusion protocols, and the data analyses are provided in SI Materials and Methods. RBC renitrosylation occurred within 30 min of transfusion. At the end of the experiments, animals were humanely killed by approved methods listed in the American Veterinary Medical Association Guidelines on Euthanasia.
Supplementary Material
Acknowledgments
This work was sponsored in part by National Institutes of Health Grants R01-HL091876 and R01-HL095463, Defense Advanced Research Projects Agency Contract N66001-10-C-2015, a grant from the Case Western Reserve University/Cleveland Clinic Clinical and Translational Science Award (UL1RR024989), and both the Coulter-Case and the Duke-Coulter Translational Partnerships.
Footnotes
Conflict of interest statement: J.S.S. has a financial interest in N30 Pharma, Adamas Pharma, Life Health, and Vindica Pharm. J.D.R., C.A.P., and J.S.S. hold multiple patents related to renitrosylation of blood.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1306489110/-/DCSupplemental.
References
- 1.Murphy GJ, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation. 2007;116(22):2544–2552. doi: 10.1161/CIRCULATIONAHA.107.698977. [DOI] [PubMed] [Google Scholar]
- 2.Hajjar LA, et al. Transfusion requirements after cardiac surgery: The TRACS randomized controlled trial. JAMA. 2010;304(14):1559–1567. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 3.Kim P, et al. Impact of acute blood loss anemia and red blood cell transfusion on mortality after percutaneous coronary intervention. Clin Cardiol. 2007;30(10) Suppl 2:II35–II43. doi: 10.1002/clc.20231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Netzer G, et al. Association of RBC transfusion with mortality in patients with acute lung injury. Chest. 2007;132(4):1116–1123. doi: 10.1378/chest.07-0145. [DOI] [PubMed] [Google Scholar]
- 5.Kneyber MC, Hersi MI, Twisk JW, Markhorst DG, Plötz FB. Red blood cell transfusion in critically ill children is independently associated with increased mortality. Intensive Care Med. 2007;33(8):1414–1422. doi: 10.1007/s00134-007-0741-9. [DOI] [PubMed] [Google Scholar]
- 6.Carson JL, et al. Clinical Transfusion Medicine Committee of the AABB Red blood cell transfusion: A clinical practice guideline from the AABB. Ann Intern Med. 2012;157(1):49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 7.Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345(17):1230–1236. doi: 10.1056/NEJMoa010615. [DOI] [PubMed] [Google Scholar]
- 8.Wu WC, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007;297(22):2481–2488. doi: 10.1001/jama.297.22.2481. [DOI] [PubMed] [Google Scholar]
- 9.Malone DL, et al. Blood transfusion, independent of shock severity, is associated with worse outcome in trauma. J Trauma. 2003;54(5):898–905. doi: 10.1097/01.TA.0000060261.10597.5C. [DOI] [PubMed] [Google Scholar]
- 10.Rao SV, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292(13):1555–1562. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 11.Vincent JL, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288(12):1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 12.Tinmouth A, Fergusson D, Yee IC, Hébert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46(11):2014–2027. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 13.Valtis DJ, Kennedy AC. Defective gas-transport function of stored red blood-cells. Lancet. 1954;266(6803):119–124. doi: 10.1016/s0140-6736(54)90978-2. [DOI] [PubMed] [Google Scholar]
- 14.Bunn HF, May MH, Kocholaty WF, Shields CE. Hemoglobin function in stored blood. J Clin Invest. 1969;48(2):311–321. doi: 10.1172/JCI105987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugerman HJ, et al. The basis of defective oxygen delivery from stored blood. Surg Gynecol Obstet. 1970;131(4):733–741. [PubMed] [Google Scholar]
- 16.Doctor A, Stamler JS. Nitric oxide transport in blood: A third gas in the respiratory cycle. Comprehensive Physiology. 2011;1(1):541–568. doi: 10.1002/cphy.c090009. [DOI] [PubMed] [Google Scholar]
- 17.McMahon TJ, et al. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8(7):711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 18.Hodges AN, Delaney S, Lecomte JM, Lacroix VJ, Montgomery DL. Effect of hyperbaric oxygen on oxygen uptake and measurements in the blood and tissues in a normobaric environment. Br J Sports Med. 2003;37(6):516–520. doi: 10.1136/bjsm.37.6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellsworth ML. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med Sci Sports Exerc. 2004;36(1):35–41. doi: 10.1249/01.MSS.0000106284.80300.B2. [DOI] [PubMed] [Google Scholar]
- 20.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: The role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 21.Roach RC, Koskolou MD, Calbet JA, Saltin B. Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol. 1999;276(2 Pt 2):H438–H445. doi: 10.1152/ajpheart.1999.276.2.H438. [DOI] [PubMed] [Google Scholar]
- 22.Ross JM, Fairchild HM, Weldy J, Guyton AC. Autoregulation of blood flow by oxygen lack. Am J Physiol. 1962;202:21–24. doi: 10.1152/ajplegacy.1962.202.1.21. [DOI] [PubMed] [Google Scholar]
- 23.Duling BR, Berne RM. Longitudinal gradients in periarteriolar oxygen tension. A possible mechanism for the participation of oxygen in local regulation of blood flow. Circ Res. 1970;27(5):669–678. doi: 10.1161/01.res.27.5.669. [DOI] [PubMed] [Google Scholar]
- 24.Allen BW, Stamler JS, Piantadosi CA. Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation. Trends Mol Med. 2009;15(10):452–460. doi: 10.1016/j.molmed.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: Role of circulating ATP. Circ Res. 2002;91(11):1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- 26.González-Alonso J, Mortensen SP, Dawson EA, Secher NH, Damsgaard R. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: Role of erythrocyte count and oxygenation state of haemoglobin. J Physiol. 2006;572(Pt 1):295–305. doi: 10.1113/jphysiol.2005.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angelo M, Singel DJ, Stamler JS. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc Natl Acad Sci USA. 2006;103(22):8366–8371. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salgado MT, Ramasamy S, Tsuneshige A, Manoharan PT, Rifkind JM. A new paramagnetic intermediate formed during the reaction of nitrite with deoxyhemoglobin. J Am Chem Soc. 2011;133(33):13010–13022. doi: 10.1021/ja1115088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doctor A, et al. Hemoglobin conformation couples erythrocyte S-nitrosothiol content to O2 gradients. Proc Natl Acad Sci USA. 2005;102(16):5709–5714. doi: 10.1073/pnas.0407490102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: A dynamic activity of blood involved in vascular control. Nature. 1996;380(6571):221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 31.Hausladen A, et al. Assessment of nitric oxide signals by triiodide chemiluminescence. Proc Natl Acad Sci USA. 2007;104(7):2157–2162. doi: 10.1073/pnas.0611191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds JD, et al. S-nitrosohemoglobin deficiency: A mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci USA. 2007;104(43):17058–17062. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett-Guerrero E, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci USA. 2007;104(43):17063–17068. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexander JT, et al. Red blood cells stored for increasing periods produce progressive impairments in nitric oxide-mediated vasodilation. Transfusion. 2013 doi: 10.1111/trf.12111. 10.1111/trf.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pawloski JR, Hess DT, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature. 2001;409(6820):622–626. doi: 10.1038/35054560. [DOI] [PubMed] [Google Scholar]
- 36.Reynolds JD, Hess DT, Stamler JS. The transfusion problem: Role of aberrant S-nitrosylation. Transfusion. 2011;51(4):852–858. doi: 10.1111/j.1537-2995.2011.03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diesen DL, Hess DT, Stamler JS. Hypoxic vasodilation by red blood cells: Evidence for an S-nitrosothiol-based signal. Circ Res. 2008;103(5):545–553. doi: 10.1161/CIRCRESAHA.108.176867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMahon TJ, et al. A nitric oxide processing defect of red blood cells created by hypoxia: Deficiency of S-nitrosohemoglobin in pulmonary hypertension. Proc Natl Acad Sci USA. 2005;102(41):14801–14806. doi: 10.1073/pnas.0506957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gow AJ, Stamler JS. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature. 1998;391(6663):169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- 40.Barbee RW, Perry BD, Ré RN, Murgo JP. Microsphere and dilution techniques for the determination of blood flows and volumes in conscious mice. Am J Physiol. 1992;263(3 Pt 2):R728–R733. doi: 10.1152/ajpregu.1992.263.3.R728. [DOI] [PubMed] [Google Scholar]
- 41.Isbell TS, et al. SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation. Nat Med. 2008;14(7):773–777. doi: 10.1038/nm1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48(6):1053–1060. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 43.O'Keeffe SD, et al. Blood transfusion is associated with increased morbidity and mortality after lower extremity revascularization. J Vasc Surg. 2010;51(3):616–621, 621.e1–621.e3. doi: 10.1016/j.jvs.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 44.Karkouti K, et al. Influence of erythrocyte transfusion on the risk of acute kidney injury after cardiac surgery differs in anemic and nonanemic patients. Anesthesiology. 2011;115(3):523–530. doi: 10.1097/ALN.0b013e318229a7e8. [DOI] [PubMed] [Google Scholar]
- 45.Causey MW, Maykel JA, Hatch Q, Miller S, Steele SR. Identifying risk factors for renal failure and myocardial infarction following colorectal surgery. J Surg Res. 2011;170(1):32–37. doi: 10.1016/j.jss.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 46.Glynn SA. The red blood cell storage lesion: A method to the madness. Transfusion. 2010;50(6):1164–1169. doi: 10.1111/j.1537-2995.2010.02674.x. [DOI] [PubMed] [Google Scholar]
- 47.Tsai AG, Cabrales P, Intaglietta M. Microvascular perfusion upon exchange transfusion with stored red blood cells in normovolemic anemic conditions. Transfusion. 2004;44(11):1626–1634. doi: 10.1111/j.0041-1132.2004.04128.x. [DOI] [PubMed] [Google Scholar]
- 48.Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol. 1998;275(5 Pt 2):H1726–H1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Sun CW, Honavar J, Townes T, Patel RP. Role of the b93cys, ATP and adenosine in red cell dependent hypoxic vasorelaxation. Int J Physiol Pathophysiol Pharmacol. 2013;5(1):21–31. [PMC free article] [PubMed] [Google Scholar]
- 50.Cosby K, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9(12):1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 51.Luchsinger BP, et al. Routes to S-nitroso-hemoglobin formation with heme redox and preferential reactivity in the beta subunits. Proc Natl Acad Sci USA. 2003;100(2):461–466. doi: 10.1073/pnas.0233287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dalsgaard T, Simonsen U, Fago A. Nitrite-dependent vasodilation is facilitated by hypoxia and is independent of known NO-generating nitrite reductase activities. Am J Physiol Heart Circ Physiol. 2007;292(6):H3072–H3078. doi: 10.1152/ajpheart.01298.2006. [DOI] [PubMed] [Google Scholar]
- 53.Luchsinger BP, et al. Assessments of the chemistry and vasodilatory activity of nitrite with hemoglobin under physiologically relevant conditions. J Inorg Biochem. 2005;99(4):912–921. doi: 10.1016/j.jinorgbio.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 54.Isbell TS, Gladwin MT, Patel RP. Hemoglobin oxygen fractional saturation regulates nitrite-dependent vasodilation of aortic ring bioassays. Am J Physiol Heart Circ Physiol. 2007;293(4):H2565–H2572. doi: 10.1152/ajpheart.00759.2007. [DOI] [PubMed] [Google Scholar]
- 55.Stamler JS, Singel DJ, Piantadosi CA. SNO-hemoglobin and hypoxic vasodilation. Nat Med. 2008;14(10):1008–1009. doi: 10.1038/nm1008-1008. [DOI] [PubMed] [Google Scholar]
- 56.Fuller BM, et al. Transfusion of packed red blood cells is not associated with improved central venous oxygen saturation or organ function in patients with septic shock. J Emerg Med. 2012;43(4):593–598. doi: 10.1016/j.jemermed.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sadaka F, et al. The effect of red blood cell transfusion on tissue oxygenation and microcirculation in severe septic patients. Ann Intensive Care. 2011;1(1):46. doi: 10.1186/2110-5820-1-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Napolitano LM, et al. Clinical practice guideline: Red blood cell transfusion in adult trauma and critical care. J Trauma. 2009;67(6):1439–1442. doi: 10.1097/TA.0b013e3181ba7074. [DOI] [PubMed] [Google Scholar]
- 59.Trzeciak S, et al. Resuscitating the microcirculation in sepsis: The central role of nitric oxide, emerging concepts for novel therapies, and challenges for clinical trials. Acad Emerg Med. 2008;15(5):399–413. doi: 10.1111/j.1553-2712.2008.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.López A, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32(1):21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 61.Cabrales P, Tsai AG, Intaglietta M. Exogenous nitric oxide induces protection during hemorrhagic shock. Resuscitation. 2009;80(6):707–712. doi: 10.1016/j.resuscitation.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goyette RE, Key NS, Ely EW. Hematologic changes in sepsis and their therapeutic implications. Semin Respir Crit Care Med. 2004;25(6):645–659. doi: 10.1055/s-2004-860979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.