Lang et al. have recently published that innate immune cells, including bone-marrow granulocytes and splenic macrophages, express the plasma-membrane protein Toso as a unique regulator of innate immune responses during bacterial infection and septic shock (1). In contrast, we and others have previously reported that the IgM Fc receptor (FcμR), originally designated as Toso or Fas apoptosis inhibitory molecule 3, is expressed only by B-lineage cells in mice and is involved in IgM homeostasis, B-cell survival, and humoral immune responses (2, 3). Because of these conflicting results, we have extensively reexamined the cellular distribution of Toso/FcμR and wish to provide some new results and comments.
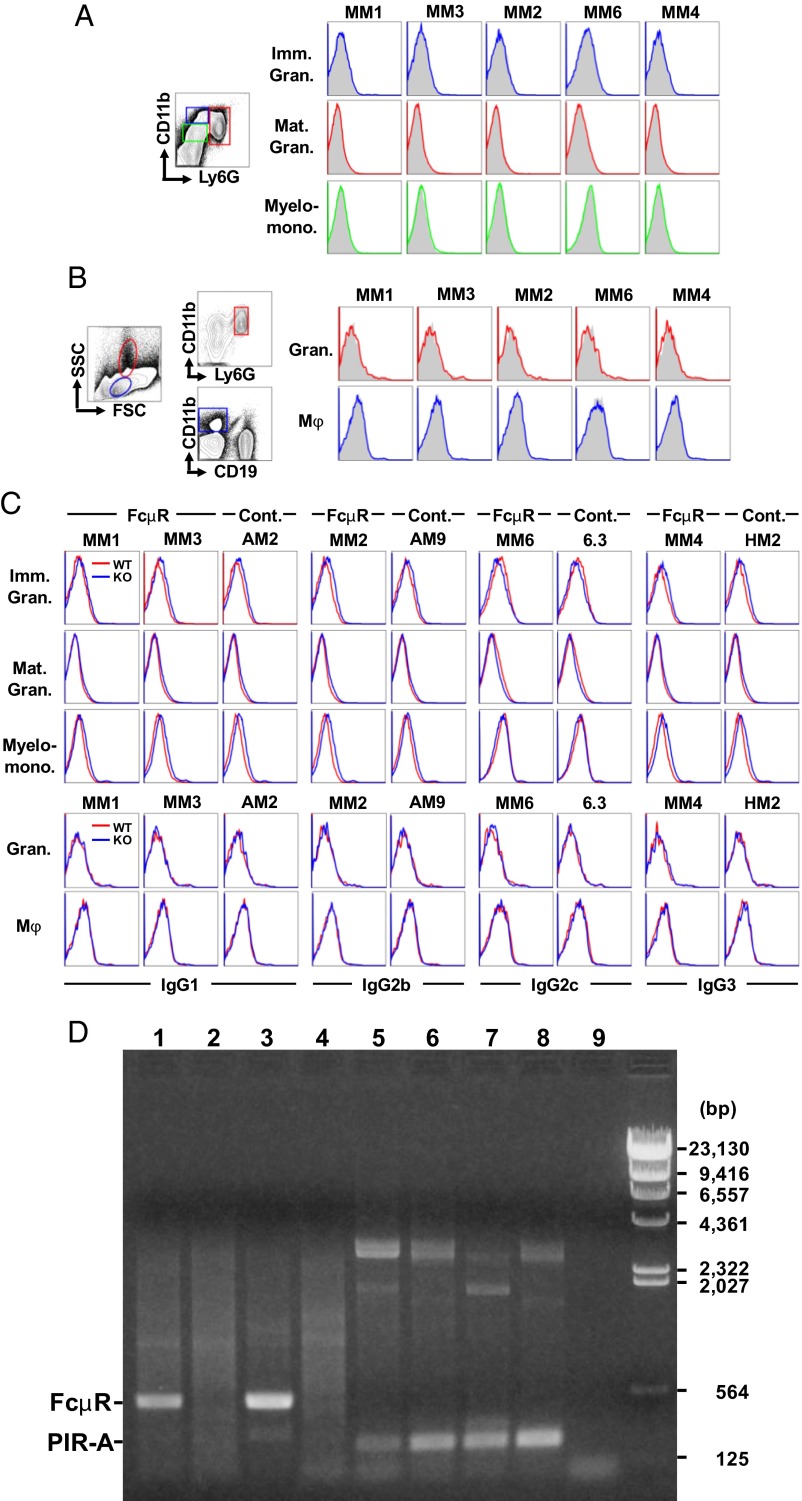
First, we conducted flow cytometric analyses of cell-surface Toso/FcμR. Cells were first incubated with two FcγR-blocking monoclonal antibodies (mAbs), a critical step when staining myeloid cells, and then with a panel of five different murine mAbs with proven specificity for mouse Toso/FcμR (2), followed by fluorochrome-labeled rat anti-mouse κ mAb as the detection reagent. None of the anti-FcμR mAbs demonstrated specific cell-surface staining, compared with their isotype-matched control mAbs, of immature, mature granulocytes or myelomonocytoid cells from wild-type bone-marrow (Fig. 1A). In addition, Toso/FcμR was not expressed on granulocytes or macrophages from wild-type spleen (Fig. 1B). When the cell-surface staining of Toso/FcμR was analyzed using Fcmr-deficient cells as a negative control, as was done by Lang et al. (see figure 1 in ref. 1), there were no significant differences in splenic granulocytes and macrophages, but there were subtle isotype-specific differences observed in bone-marrow myeloid cells (Fig. 1C). Fcmr-deficient marrow myeloid cells had a tendency of less reactivity with mouse γ2cκ, but more reactivity with mouse γ1κ, γ2bκ, and γ3κ isotypes than wild-type myeloid cells. This minimal staining, however, was an artifact because it occurred independently of mAb specificity.
Fig. 1.
Granulocytes and macrophages do not express Toso/FcμR. Flow cytometric analysis of cell surface FcμR (A–C). Single-cell suspensions prepared from C57BL/6, wild-type bone marrow (A) and spleen (B) were first incubated with both rat anti-FcγRII/III (AB93 clone) and hamster anti-FcγRIV (9E9) mAbs at 30 μg/mL to block all FcγRs and then with the indicated mouse anti-mouse FcμR mAbs [clone MM1 and MM3 (both γ1κ isotype), MM2 (γ2bκ), MM6 (γ2cκ), MM4 (γ3κ)] or their isotype-matched control mAbs at 10 μg/mL. The bound mouse mAbs were detected by addition of Alexa 647-labeled rat anti-mouse κ mAb (187.1) and were counterstained with FITC–anti-Ly6G/Gr-1 (1A8), PE/Cy7–anti-CD11b/Mac-1 (M1/70), and PE–anti-CD19 (1D3) mAbs. (The concentrations of anti-FcμR and Alexa 647-anti-mouse κ mAbs were determined based on the staining results with AKR mouse-derived thymoma BW5147 cells stably expressing mouse FcμR on the cell surface.) Stained cells with the light-scatter characteristics of lymphocyte/macrophages or granulocytes were analyzed using an Accuri C6 flow cytometer. Marrow immature (Ly6GLo/CD11bHi/CD19−; blue) and mature (Ly6GHi/CD11b+/CD19−; red) granulocytes and myelomonocytoid (Ly6GLo/CD11bLo/CD19−; green) cells boxed in A and splenic granulocytes (Ly6GHi/CD11b+/CD19−; red) and macrophages (CD11b+/CD19−; blue) gated in B were examined for reactivity of the indicated anti-FcμR (colored lines) and control mAbs (shaded histograms). (C) Cells from wild-type and Fcmr-deficient (KO) bone marrow (Upper three rows) and spleen (Lower two rows) were similarly incubated and the reactivity of anti-FcμR mAbs and the indicated isotype-matched control mAbs with wild-type (red lines) or KO (blue lines) cells was compared in an overlay fashion. Note that FcμR is not expressed on the surface of myeloid cells. RT-PCR analysis of FcμR (D). Total RNA isolated from WT bone marrow CD19+ B-lineage cells (lanes 1 and 5) and Ly6G+ granulocytes (lanes 2 and 6) enriched twice by FACS and from wild-type (lanes 3 and 7) or Rag1-deficient (lanes 4 and 8) splenocytes were converted to first-strand cDNA before PCR amplification using a set of primers: (i) Fcmr exon 2 coding (5′-ccagggaaccatggacttt-3′) and exon 3 noncoding (5′-ctttggctatgactccagaa-3′) (lanes 1–4) and (ii) Pira exon 8 coding (5′-cctgtggagctcacagtctcag-3′) and exon 9 noncoding (5′-cccagagtgtagaacattgaagatg-3′) (lanes 5–8). Lane 9 is a PCR control without a first-strand cDNA template. Each amplification reaction underwent 35 cycles of: denaturation at 94 °C for 30 s, annealing at 56 °C (for FcμR) or at 60 °C (for paired Ig-like receptor-A, PIR-A) for 30 s, and extension at 68 °C for 1 min. The final extension was performed at 68 °C for 10 min. One-tenth of the amplified products was electrophoresed in 0.9% agarose and stained with ethidium bromide. HindIII-digested λ-DNA fragments were used as a size marker (bp). Note that FcμR transcripts of 512 bp are detectable in wild-type bone marrow B-lineage cells and splenocytes (lanes 1 and 3), but not in double-sorted, wild-type bone marrow granulocytes and Rag1-deficient splenocytes (lanes 2 and 4) and that PIR-A transcripts of 253 bp are detected in all RNA samples (lanes 5–8).
Second, we performed RT-PCR assays to detect the expression of the Toso/Fcmr in Ly6G+ granulocyte- and CD19+ B-lineage cell-populations that were enriched from wild-type bone marrow by FACS. Toso/FcμR transcripts were clearly detectable in the B-lineage cells, but not in the double-sorted granulocytes, even after 35 cycles of amplification (Fig. 1D). The lack of Toso/Fcmr expression by phagocytes was also confirmed by RT-PCR analysis of recombination activating gene 1 (Rag1)-deficient splenocytes that are devoid of B and T cells but contain abundant granulocytes and macrophages. As a control, transcripts of paired Ig-like receptors (PIRs), known to be expressed by both B and myeloid cells, were detectable in all RNA samples examined. Collectively, these findings conclusively demonstrate at both protein and RNA levels that Toso/FcμR is not expressed by myeloid cells.
Because IgM is the first Ig isotype to appear during phylogeny, ontogeny, and immune responses, and is the first line of defense against pathogens, we also initially assumed that FcμR might have a broad cellular distribution. Instead, however, the expression of FcμR is actually restricted to adaptive immune cells: B, T and NK cells in humans (4) and B cells in mice (2, 3, 5). This finding suggests a distinct function of FcμR compared with FcRs for switched Ig isotypes and species-related differences.
Acknowledgments
We thank Ms. Enid Keyser for FACS sorting; Drs. John Kearney and Jeffrey Ravetch for FcγR-blocking reagents; Drs. Peter Burrows, John Smith, and Kevin Roth for suggestions and comments; and Ms. Jacquelin Bennett for submitting the letter. This work was supported in part by National Institutes of Health/National Institute of Allergy and Infectious Diseases Grant R21AI094625 (to H.K.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Lang KS, et al. Involvement of Toso in activation of monocytes, macrophages, and granulocytes. Proc Natl Acad Sci USA. 2013;110(7):2593–2598. doi: 10.1073/pnas.1222264110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Honjo K, et al. Altered Ig levels and antibody responses in mice deficient for the Fc receptor for IgM (FcμR) Proc Natl Acad Sci USA. 2012;109(39):15882–15887. doi: 10.1073/pnas.1206567109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouchida R, et al. Critical role of the IgM Fc receptor in IgM homeostasis, B-cell survival, and humoral immune responses. Proc Natl Acad Sci USA. 2012;109(40):E2699–E2706. doi: 10.1073/pnas.1210706109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubagawa H, et al. Identity of the elusive IgM Fc receptor (FcμR) in humans. J Exp Med. 2009;206(12):2779–2793. doi: 10.1084/jem.20091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shima H, et al. Identification of TOSO/FAIM3 as an Fc receptor for IgM. Int Immunol. 2010;22(3):149–156. doi: 10.1093/intimm/dxp121. [DOI] [PubMed] [Google Scholar]