Abstract
Preeclampsia (PE) is a pregnancy-specific disorder characterized by sudden onset of hypertension and proteinuria in the second half of pregnancy (>20 wk). PE is strongly associated with abnormal placentation and an excessive maternal inflammatory response. Galectin-1 (Gal-1), a member of a family of carbohydrate-binding proteins, has been shown to modulate several processes associated with placentation and to promote maternal tolerance toward fetal antigens. Here, we show that Gal-1 exhibits proangiogenic functions during early stages of pregnancy, promoting decidual vascular expansion through VEGF receptor 2 signaling. Blocking Gal-1–mediated angiogenesis or lectin, galactoside-binding, soluble, 1 deficiency results in a spontaneous PE-like syndrome in mice, mainly by deregulating processes associated with good placentation and maternal spiral artery remodeling. Consistent with these findings, we observed a down-regulation of Gal-1 in patients suffering from early onset PE. Collectively, these results strengthen the notion that Gal-1 is required for healthy gestation and highlight Gal-1 as a valuable biomarker for early PE diagnosis.
Pregnancy constitutes a major challenge for the mother’s immune and cardiovascular systems. Indeed, it is highly dependent on the proper temporal coordination of several vascular processes including angiogenesis at the fetal–maternal interface and the remodeling of the spiral arteries in the decidua. Disturbances in these processes lead to adverse pregnancy outcomes and pregnancy-related disorders like preeclampsia (PE) (1). As a syndrome, the clinical manifestations of PE comprise hypertension, proteinuria, systemic inflammation, endothelial dysfunction, and intrauterine growth restriction (IUGR), present in different grades of severity. Even though the maternal symptoms resolve after delivery, mothers and children who have undergone a PE pregnancy suffer from an increased long-term cardiovascular risk (2). The placenta plays a crucial role in the pathogenesis of this condition as elevated circulating levels of antiangiogenic factors soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng) released from the placenta have been reported (3, 4). PE is also characterized by a shallow trophoblast invasion with less favorable remodeling of narrow spiral arteries. This leads to disrupted uteroplacental flow and placental injury with releasing of molecules (such as sFlt-1 and sEng) that mediate the endothelial dysfunction and maternal signs, especially in early onset PE (<34 wk) (5). In addition, dysregulation of the renin–angiotensin system (RAS) together with increased autoantibodies against the angiotensin II receptor type 1 (AT1AA) contribute to the development of PE (6, 7). Despite being a leading cause of maternal death and a major contributor to maternal and perinatal morbidity (2), as well as a threat for future cardiovascular disease, the mechanisms responsible for the pathogenesis of PE are poorly understood.
Galectin-1 (Gal-1), an evolutionarily conserved glycan-binding protein, is abundant in the female reproductive tract, where it displays an increased expression during pregnancy in mammals (8, 9). Circulating Gal-1 levels increase during the first trimester compared with nonpregnant women and reach a maximum in the second trimester, which is maintained until term (10). Gal-1 exerts important functions on the maternal immune tolerance toward the fetus by promoting tolerogenic dendritic cells (DC), IL-10+ regulatory T (Treg) cells (11), and the apoptosis of alloreactive T cells (12), as well as by regulating the expression of human leukocyte antigen-G (HLA-G) on trophoblasts during the early stages of human pregnancy (10). Of note, the invasion process that characterizes human placentation seems to be fine tuned by Gal-1 (13, 14). Besides its role in immune modulation, Gal-1 regulates angiogenesis processes by directly binding to neuropilin-1 (NRP-1) on endothelial cells and promoting activation of the vascular endothelial growth factor receptor 2 [VEGFR2, also known as Fetal Liver Kinase 1 (Flk-1)] signaling pathway (15). Although the role played by Gal-1 in tumor angiogenesis and the possibility to apply Gal-1 inhibitors as angiostatic therapy has been extensively studied (16, 17), little information is available on its angiogenic function during physiological events like pregnancy.
Here, we show that Gal-1 is able to rescue pregnancy favoring the angiogenesis process via VEGFR2 signaling. Inhibition of Gal-1–mediated angiogenesis leads to the development of PE-like symptoms including hypertension, proteinuria, increased levels of AT1AA, elevated sEng in the circulation, and IUGR. In addition, in vitro studies showed that invasive and adhesive capacities of human extravillous trophoblast (EVT), properties needed for proper spiral artery modification, are dysregulated upon Gal-1 inhibition. Finally, we defined that lectin, galactoside-binding, soluble, 1 (Lgals1) is the only galectin member to be down-regulated in patients who developed the PE syndrome. Differences were observed in placental and circulating Gal-1 levels between early and late onset PE and we identify Gal-1 as a valuable biomarker that could anticipate the development of PE. Thus, our work identifies Gal-1 as a key regulator of the angiogenesis process during early gestation in mice and provides evidence that dysregulation of Gal-1 could contribute to the PE development.
Results
Gal-1 Exhibits Proangiogenic Properties During Early Gestation.
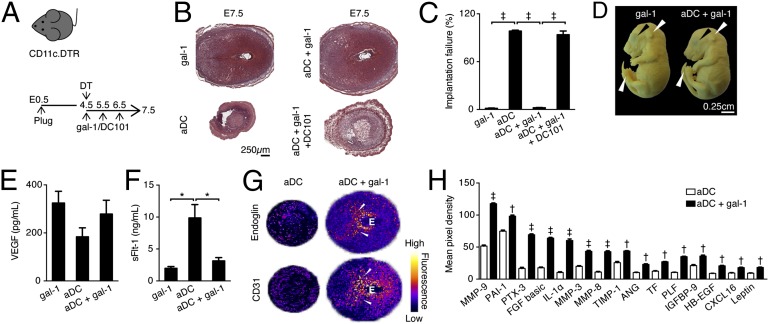
To investigate the contribution of Gal-1 during the angiogenesis process associated with gestation, we used a well-characterized model of impaired vascular expansion (18). In this model, a transient ablation of dendritic cells (aDC) on embryonic day 4.5 (E4.5) leads to complete embryo resorption on E7.5 (Fig. 1 A and B). Administration of recombinant Gal-1 from E4.5–6.5 into aDC pregnant female mice promoted embryo survival (Fig. 1 B and C). Fetuses carried by aDC+Gal-1–treated females developed normally past E16.5, as evidenced by the pinna covering completely the external auditory meatus, fused eyelids, and disappeared umbilical hernia typically seen at Theiler stage (TS) 25 (Fig. 1D).
Fig. 1.
Galectin-1 exhibits proangiogenic functions during early gestation. (A) Schematic diagram for the experimental design. Pregnant CD11c.DTR (diphtheria toxin receptor) female mice were treated with DT on E4.5 and hrGal-1 (10 µg) were administered i.p. from E4.5 to E6.5. Mice were killed on E7.5. (B) Representative H&E stained whole implantation sites from Gal-1–treated control, ablated DC (aDC), aDC+Gal-1 and aDC+Gal-1+neutralizing VEGFR2 antibody (DC101). (C) Implantation failure (IF) was calculated on E7.5 as follows: IF = V × 100/T, where V = nonviable embryos and T = total embryos. (D) Images from fetuses carried by control and aDC+Gal-1 dams on E16.5. Upper white arrows show pinna covering completely the external auditory meatus, Upper black arrows show eyelids closed and Lower white arrows absent umbilical hernia. (E) Systemic VEGF levels measured by ELISA on E7.5. (F) sFlt-1 serum levels analyzed by ELISA on E7.5. (G) Representative 3D surface plot from endoglin and CD31 stainings on E7.5 analyzed by ImageJ. Fluorescence scale is shown to illustrate intensities. White arrows point to the decidual vascular zone. E = embryo. (H) Relative changes in angiogenesis related proteins between aDC and aDC+Gal-1. Pixel densities were analyzed by ImageJ on developed X-ray films. †P < 0.01 and ‡P < 0.001 using two-tailed t test. In the rest of the figures, data are plotted as mean ± SEM *P < 0.05 and ‡P < 0.001 (one-way ANOVA, Tukey´s test). Data shown are mean values ± SEM derived from six to eight mice per group, each analyzed in duplicate.
Gal-1 binds to NRP-1, which has been shown to enhance activation and signaling of VEGFR2. Thus, we next analyzed if Flk-1 phosphorylation (p-Flk-1) changes upon Gal-1 supplementation. As depicted in Fig. S1, we found a higher number of p-Flk-1+ cells in the mesometrial decidua (MD) in the aDC+Gal-1–treated animals compared with control mice, indicating that Gal-1 promotes Flk-1 signaling. This was confirmed by treating aDC–Gal-1 female mice with a well-characterized Flk-1 neutralizing antibody (19), which led to complete embryo resorption (Fig. 1 B and C).
To provide insights into the contribution of Gal-1 to vascular expansion, we evaluated the circulating levels of VEGF and sFlt-1 on E7.5 by ELISA. Although no significant changes were found in VEGF circulating levels between aDC and aDC+Gal-1 mice (Fig. 1E), Gal-1 supplementation reduced sFlt-1, thereby enhancing VEGF bioavailability (Fig. 1F). Furthermore, the expression of endoglin and CD31 was reduced in the decidua derived from aDC implantations and restored by Gal-1 supplementation as shown by surface plots of immunofluorescence staining (Fig. 1G). To directly define the angiogenic status in aDC+Gal-1 mice, we simultaneously assessed the relative levels of 53 angiogenesis-related proteins on E7.5. As shown in Fig. 1H and Fig. S1, proangiogenic proteins such as angiogenin (ANG), tissue factor (TF), heparin-binding epidermal growth factor (HB-EGF), plasminogen activator inhibitor-1 (PAI-1), leptin, proliferin (PLF), fibroblast growth factor-basic (FGF), pentraxin-3 (PTX-3), and chemokine (C-X-C motif) ligand 16 (CXCL16) were substantially increased upon Gal-1 administration. In addition, proteins involved in matrix remodeling such as matrix metallopeptidase 9 (MMP-9), MMP-3, MMP-8, tissue inhibitor of metalloproteinase 1 (TIMP-1), IGF binding protein-9 (IGFBP-9), and cytokines inducing MMP expression (e.g., IL-1α) were also up-regulated by Gal-1 in aDC mice.
Neutralizing Gal-1–Mediated Angiogenesis Provokes a PE-Like Syndrome.
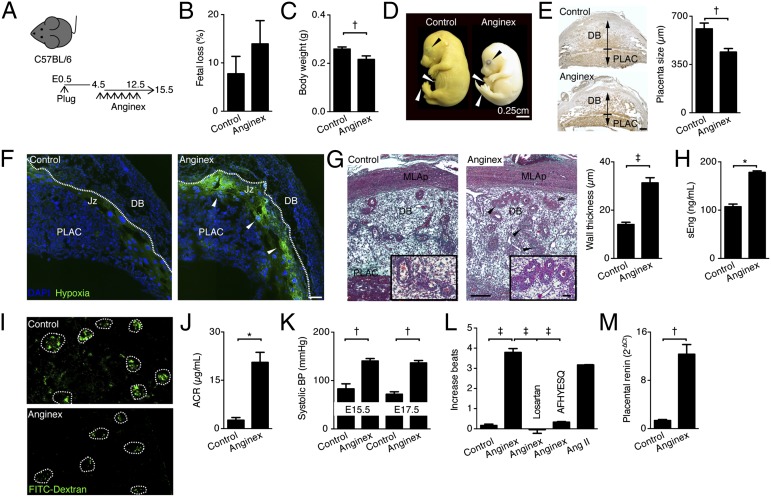
To examine the effect of neutralizing Gal-1–mediated angiogenesis during early gestation, we used anginex, a 33-mer cytokine-like artificial β-peptide that inhibits endothelial cell growth by specifically blocking the proangiogenic functions of Gal-1 (17) (Fig. 2A). Although fetal loss rates were not modified compared with control mice (Fig. 2B), inhibiting Gal-1 with anginex from E4.5 to E12.5 resulted in IUGR. In particular, the body weight (BW) of fetuses carried by treated dams was significantly reduced and an immature phenotype corresponding to TS23 characterized by eyelids open, umbilical hernia, and no-parallel fingers and toes (Fig. 2 C and D) was evident. As shown in Fig. 2E, Gal-1 inhibition also reduced the placenta size as analyzed on isolectin-B4 (IB4) staining. Moreover, the treated placentas displayed an elevated hypoxic status in the junction zone (Fig. 2F) and a reduction in placental lactogen I (PL-I) expression, a placental hormone important for the supply of energy to the developing fetus (Fig. S1). In concordance with that, the wall thickness of spiral arteries within the decidua basalis of treated mice was significantly enlarged (Fig. 2G). This was accompanied by elevated circulating levels of sEng (Fig. 2H), whereas no differences were found for sFlt-1 (Fig. S1). These findings suggest that inhibiting Gal-1–mediated angiogenesis leads to a failure in placentation and fetal growth, features typically observed in the human PE syndrome. Thus, we next evaluated whether Gal-1 inhibition in mice resulted in other PE-like symptoms in late pregnancy. As depicted in Fig. 2 I and J, anginex treatment during pregnancy caused kidney dysfunction and proteinuria as seen by a reduction in FITC-labeled dextran filtration in vivo and an increased albumin-to-creatinine ratio (ACR). Furthermore, treated dams displayed a significantly higher blood pressure (BP) compared with controls (Fig. 2K). In addition, we observed an increased placental renin expression and autoantibodies against the AT1AA in treated dams (Fig. 2 L and M). Taken together, these results indicate that inhibition of Gal-1–mediated angiogenic pathways during the vascular and placental development period provokes PE-like symptoms.
Fig. 2.
Inhibition of Gal-1–mediated angionenesis provokes PE-like symptoms in mice. (A) Experimental design: C57BL/6 pregnant female mice were treated with anginex (0.5 mg/kg) from E4.5 to E12.5. Mice were killed on E15.5. (B) Fetal loss rate was registered on E13.5. (C) Fetal body weight (in grams) was registered on E15.5. (D) Images from fetuses carried by control and anginex-treated females on E16.5. Top black arrow shows eyelids; Middle white arrow denotes umbilical hernia; Bottom white arrow points out fingers and toes. (E) Placental size (in micrometers) was calculated on E13.5 using AxioX software on isolectin-B4 (IB4)-stained whole implantation site sections. (Scale bar, 250 µm.) (F) Placental hypoxia was evaluated on E10.5 using the HypoxiaProbeTM. (Scale bar, 500 µm.) (G) Wall thickness (in micrometers) of the maternal spiral arteries as evaluated by Masson-trichrome staining on E13.5. (H) sEng serum levels analyzed by ELISA on E15.5. (I) Representative images of maternal kidney perfusion as analyzed with FITC-dextran (green) on E15.5; white encircled glomeruli are shown. (J) Ratio of albumin to creatinine in urine collected on E15.5. (K) Systolic blood pressure (BP) as measured on E15.5 and E17.5. (L) Autoantibodies against angiotensin II receptor type 1 (AT1-AA) were determined in maternal sera on E15.5. (M) Quantitative real-time PCR analysis of placental expression of renin in control and anginex-treated dams at E17.5. In all figures, data are expressed as mean ± SEM. *P < 0.05, †P < 0.01, and ‡P < 0.001 using two-tailed t test. Data shown are mean values ± SEM derived from six to eight mice per group, each analyzed in duplicate. MLAp, mesometrial lymphoid aggregate of pregnancy; DB, decidua basalis; PLAC, placenta.
Gal-1 Inhibition Deregulates Vital Human Trophoblast Functions.
To provide further insights into the mechanism by which Gal-1 inhibition provokes PE-like symptoms, we treated the EVT-derived cell line (Saint George's Hospital placental cell line-4; SGHPL-4) with anginex. As shown in Fig. S2, the number of networks and total length of capillaries were significantly reduced in SGHPL-4 cells treated with anginex (10 μM) or (20 μM) compared with control. Next, we examined the adhesion ability of SGHPL-4 to an endothelial (Saint George's Hospital endothelial cell line-7; SGHEC-7) monolayer. Anginex significantly reduced the adhesion of SGHPL-4 cells to the SGHEC-7 monolayer (Fig. S2). To investigate whether the observed decrease in adhesion was directly due to induction of apoptosis of SGHPL-4 cells, caspase-3 was quantified. As depicted in Fig. S2, treatment did not reduce tube formation or adhesion by increasing rates of apoptosis in SGHPL-4 cells. Finally, to identify additional signals potentially involved in placentation failure triggered by Gal-1 inhibition, a microarray analysis was performed on SGHPL-4 cells collected upon 5-h or 24-h treatment. Statistical analysis at a false discovery rate (FDR) of 5% revealed 315 genes with higher mRNA levels and 138 genes with decreased expression in treated SGHPL-4 (24 h) samples (Table S1). Most of the up-regulated genes encode proteins well known to be induced under hypoxia or oxidative stress conditions, by inflammatory cytokines, and upon immune activation. Down-regulated genes encode proteins specific for trophoblast functions such as pregnancy-specific glycoproteins. In addition, 38 genes were transiently up-regulated upon Gal-1 inhibition after 5-h treatment. Finally, 107 genes were up-regulated and 67 genes down-regulated following Gal-1 inhibition (Fig. S2 and Table S1).
Lgals1-Deficient Dams Develop a Spontaneous PE-Like Syndrome.
To further evaluate the contribution of Gal-1 to the pathogenesis of the PE syndrome, we compared the reproductive fitness of Lgals1 wild-type (WT) and deficient (KO) dams. As shown in Fig. S3, similar fetal loss rates and litter sizes were observed between Lgals1 WT and KO dams. However, fetuses carried by Lgals1 KO dams depicted a small body weight compared with Lgals1 WT fetuses (Fig. S3). In addition, we confirmed that Gal-1 is a receptor for the angiogenesis inhibitor anginex, because anginex treatment in Lgals1 KO mice did not lead to any weight differences with respect to nontreated dams (Fig. S3). Evaluation on E16.5 demonstrated that fetuses carried by Lgals1 WT dams depicted a typical phenotype TS25, whereas fetuses carried by Lgals1 KO mice showed a TS23, evidenced by eyelids open, umbilical hernia, and no-parallel fingers and toes, which was not further modified upon anginex treatment (Fig. S3). However, once pups were born, the observed differences in fetal growth were no longer seen after 2 wk postnatal (Fig. S4). The diminished fetal body weights were accompanied by 30%–40% reductions in the placental masses of Lgals1 KO dams at midgestation (E13.5) (Fig. S3). Further analysis revealed an overexpression of hypoxia-inducible factor-2α (HIF-2α) mRNA, which responds to hypoxia, inflammation, and oxidative stress in Lgals1-deficient placentas (Fig. S3). In contrast, placental growth factor (PGF) and VEGF expression on placental tissues derived from Lgals1-deficient dams were down-regulated compared with Lgals1 WT mice (Fig. S3). Moreover, increased placental mRNA expression of angiotensin II receptor type 1 (AT1) was observed in Lgals1 KO mice (Fig. S3). Given the vascular abnormalities seen in Lgals1 KO placentas, we sought to evaluate whether this translated into PE-like symptoms in vivo. As shown in Fig. S3, a diminished FITC-labeled dextran renal filtration and an increased ACR characterized Lgals1-deficient mice later on in gestation (E17.5). Furthermore, increased mean arterial blood pressure was observed in Lgals1 KO mice compared with Lgals1 WT females (Fig. S3). In contrast, no differences were found when serum levels of sFlt-1 and sEng on E15.5 were compared between Lgals1 WT and KO mice (Fig. S4).
Early Onset PE Syndrome Is Characterized by Dysregulation of Placental Gal-1 Expression.
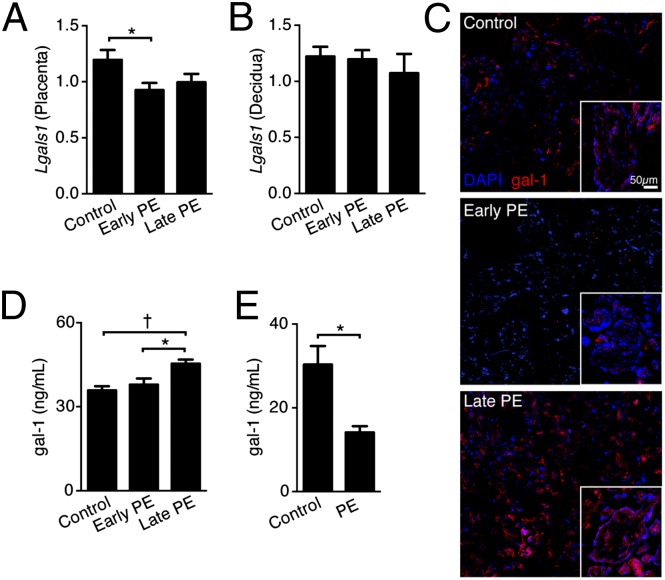
To investigate the possible link between deregulation of Gal-1 expression and the naturally occurring PE syndrome in humans, we examined the galectin profile in uteroplacental samples of 25 preeclamptic women and 23 women with uneventful pregnancies using a microarray approach. We analyzed a unique patient cohort, where only nonlaboring patients without membrane breakage and no uterine contractions were included at elective cesarean sections (c-sections). Table S2 shows that from the 14 galectins analyzed, only Lgals1 was significantly down-regulated (1.3-fold) in the preeclamptic group compared with the control group. Next, we confirmed Lgals1 expression in decidua and placenta samples by quantitative PCR (qPCR). Here, we discriminated between early (<34 wk, severe PE) and late onset PE (>34 wk), because they have been proposed to result from different disease mechanisms (20). As depicted in Fig. 3A, we observed a down-regulation of Lgals1 expression in placenta samples from early onset PE patients compared with the control group. No differences in Lgals1 expression between early, late onset PE, and control groups were found when decidua samples were analyzed (Fig. 3B). Next, we confirmed Gal-1 protein expression in placental villous tissue. Fig. 3C shows that Gal-1 was down-regulated in early onset PE, but up-regulated in late onset PE placental villi compared with control patients (Fig. 3C and Fig. S4). In addition, circulating Gal-1 levels were significantly up-regulated in late onset PE compared with control and early onset PE patients (Fig. 3D). Finally, we analyzed the circulating levels of Gal-1 in a prospective study of healthy pregnant women. Of note, patients included in this study were recruited at 22 wk of gestation, where no clinical signs of PE (such as hypertension or proteinuria) were found. As shown in Fig. 3E, serum Gal-1 levels were significantly reduced in the second trimester in pregnant women who subsequently developed the PE syndrome compared with women that had an uneventful pregnancy. This result suggests that screening of systemic Gal-1 levels during the second trimester could represent a valuable tool to anticipate PE symptoms.
Fig. 3.
Gal-1 dysregulation characterizes early onset PE. (A and B) RT-PCR analysis of placental (A) and decidual (B) expression of Gal-1 in control (n = 36), early (n = 18), and late (n = 17) onset patients with PE. (C) Representative frozen sections were prepared from control, early, and late onset patients with PE (n = 5) and stained for Gal-1 by immunofluorescence (red, Gal-1; blue, DAPI nuclear staining). Pictures were imaged at 20× magnification on a confocal fluorescence microscope. (D) Circulating Gal-1 levels as analyzed by ELISA in a cohort of 15 patients per group, early (<34 wk), late onset PE (>34 wk), and control (32–37 wk) were included in this analysis. (E) Peripheral Gal-1 levels evaluated in a cohort of patients during the first trimester (n = 8 per group), where some women subsequently developed PE during the second trimester. *P < 0.05 using two-tailed t test. In A and D, data are expressed as mean ± SEM. *P < 0.05 and †P < 0.01 using one-way ANOVA followed by Bonferroni´s test.
Discussion
Preeclampsia is a major contributor to perinatal morbidity and mortality worldwide and a leading cause of maternal death. Despite the considerable research efforts to understand the primary events that precede clinical symptoms of the disease, few significant advances have been made in the last decades. Clinically diagnosed most often in the late second or third trimester, the only current available treatment for PE is placental delivery by labor induction or c-section. Therefore, the identification of biomarkers in early stages of the PE syndrome could help to target women at elevated preeclampsia risk for closer follow-up, optimizing delivery timing, and avoiding unnecessary premature deliveries. In this study, we provide unique insights into the role of Gal-1 during the angiogenesis process associated with pregnancy and show that deregulation of this lectin antedates the clinical symptoms of PE in a pilot study.
We have previously shown that Gal-1 exerts potent proangiogenic properties (17, 21). Gal-1 binds to NRP-1, activates VEGFR2 signaling and modulates the migration of vascular endothelial cells, promoting angiogenesis (15). Here, we demonstrated that Gal-1 is able to rescue gestation in a model of reduced vascular expansion (18) by regulating angiogenesis and blood vessel function as evidenced by the acquisition of classical endothelial cell markers (such as CD31 and endoglin) on the decidual vascular zone. Whereas Gal-1 can directly stimulate endothelial cells (15, 21), it is important to note we observed that it can also enhance VEGF bioavailability in vivo by reducing sFlt-1, which could indirectly promote angiogenesis. Furthermore, we show that the protective effect of Gal-1 is abrogated by blocking VEGFR2 signaling, suggesting that this pathway is important for Gal-1–mediated angiogenesis during early gestation. This is further supported by the fact that higher frequencies of p-Flk-1+ decidual cells were found upon Gal-1 supplementation. Interestingly, VEGFR2 and NRP-1 expression is abundant between E4.5 and E6.5 at the mesometrial pole, which is the primary site of decidual angiogenesis (22) and administration of a single dose of anti-VEGFR2 antibody during the peri-implantation period caused embryo resorption (23). In addition, VEGFR2 is the major transducer of VEGF signals in endothelial cells (24) and therefore we propose that the abortogenic effect observed in aDC+Gal-1+DC101 mice could be a consequence of a dual inhibition of Gal-1 and VEGF signaling through VEGFR2. By restoring decidual vascular density during the early postimplantation period, Gal-1 promotes embryonic and subsequent fetal development. Indeed, Gal-1 is able to restore vascular permeability and angiogenesis, which is important for the establishment of a first exchange apparatus until the placenta becomes functionally competent (25). In addition, our results from protein array analysis strengthened the relevance of Gal-1 during angiogenesis associated with gestation. Thus, Gal-1 directly enhanced the expression of classical proangiogenic factors (e.g., ANG, CXCL16, HB-EGF) and proteins involved in matrix remodeling (e.g., MMP-9, TIMP-1) and therefore indirectly regulated angiogenesis (26).
We previously identified Gal-1 as the prime receptor of the angiostatic peptide anginex (17) and here we extend this observation by demonstrating that Lgals1-deficient dams do not show any additional anomalies in pregnancy progression following anginex treatment. Our study further revealed that upon in vivo interference with the angiogenic properties of Gal-1, progression of pregnancy is not precluded. This is in agreement with our previous finding showing that Lgals1-deficient mice had litter sizes similar to their WT counterparts (11). However, Gal-1 deficiency can pose a threat to pregnancy because Lgals1 KO mice or dams in which Gal-1 is blocked develop PE symptoms. In addition, fetuses carried by anginex-treated or Lgals1 KO dams were characterized by reduced body weights and a delayed Theiler stage of development, features resembling the IUGR commonly associated with PE syndrome. Clinically, IUGR is defined as a failure to reach the anticipated birth weight, and restricted growth can be of fetal, maternal, and placental origins (27). However, the majority of IUGR cases are associated with placental pathology, including abnormalities of the villous parenchyma, the umbilical cord, and PE-related changes, which reduces nutritional supply from the uteroplacental circulation to the fetus. As a proxy for IUGR, small for gestational age (SGA) (usually defined as below 10% of the sex-specific gestational age weight percentiles) is often used, as it represents a simpler clinical entity. The lower pup weight in our study (SGA) is indicative of IUGR, and we showed that the smaller pup weight was associated with a decrease in placental mass, which is consistent with the description of smaller placentas in patients with severe PE and IUGR (28). Inadequate angiogenic factor expression in Gal-1 blocked or Lgals1 KO placentas is correlated with thickening of the arterial walls in the decidua. It has been strongly suggested that defects in placenta differentiation that lead to uterine invasion result in a rudimentary arterial remodeling causing hypoperfusion of the placenta in severe cases of PE (1). This is also consistent with our in vivo findings showing an increased wall thickness in maternal spiral arteries and in vitro results demonstrating that Gal-1 inhibition affects vascular-like properties of human EVT by decreasing tube formation and more importantly, adhesion to endothelial cell layers. This suggests that disruption of Gal-1 signaling renders EVT no longer able to migrate, resulting in a shallow interstitial invasion and poor remodelling of maternal uteroplacental arteries. Because NRP-1 is expressed in villi and invading EVT (29) and particularly important for cell motility and chemotactic migration of endothelial cells (30), we speculate that the vascular-like properties of human trophoblast are dependent on Gal-1/NRP-1 signaling pathways. Furthermore, recent studies have demonstrated that Gal-1 regulates trophoblast invasion, differentiation, and immunomodulatory functions (such as HLA-G expression) in vitro (10, 13, 14). Therefore, it is attractive to speculate that defective Gal-1 expression contributes to the impaired placentation and vascular abnormalities observed in anginex-treated or Lgals1 KO placentas. For instance, our results and previous studies indicate that interfering with Gal-1 function could (i) influence maternal vascular adaptation associated with early phases of gestation; (ii) prevent a normal placentation (10, 13, 14), (iii) abrogate maternal immune tolerance toward fetal antigens (11, 12), and (iv) threaten gestation by favoring the development of PE, implying that Gal-1 function is essential for a healthy gestation.
Anomalies in Gal-1 circulating levels or alteration of Gal-1 expression in the placental villi have been associated with the physiopathology of early pregnancy loss (10, 31). In addition, we have recently identified a reduced frequency of Gal-1 expressing T and natural killer (NK) cells in the peripheral blood of women with PE (32), which might contribute to the excessive inflammatory responses during the course of PE syndrome (33). In the present study, we showed that Gal-1 is the only member of the galectin family whose expression is dysregulated during PE as indicated by our microarray analysis, which designates Gal-1 as a unique lectin involved on the pathogenesis of PE. Interestingly, a recent study has shown that plasma VEGFR2 concentration is reduced in PE patients compared with levels in normal pregnancies (34), which is thought to result from decreased ligand-induced shedding due to endothelial dysfunction. Because the Gal-1/NRP-1 interaction enhances VEGFR2 activation and signaling, we hypothesize that the persistently lower plasma concentrations of VEGFR2 in PE women might result from impaired receptor trafficking at the endothelial cell surfaces due both to the reduced VEGF bioavailability and a decrease in the availability of Gal-1 to bind NRP-1. This hypothesis is further supported by the fact that circulating Gal-1 levels during the second trimester were reduced in the patients who subsequently develop the PE syndrome. Thus, Gal-1 emerges as a valuable biomarker to predict PE syndrome, giving possibilities to clinicians to intensify the surveillance for optimizing delivery timing for improved maternal and offspring health.
As a syndrome, PE manifests as several clinically recognizable features [e.g., hypertension, proteinuria, excessive edema, IUGR, HELLP syndrome (Hemolysis, Elevated Liver enzymes, Low Platelet count), eclampsia, pulmonary edema, visual disturbance, and blindness] and more importantly, in different grades of severity (35). The distinction between mild (late onset, >34 wk) and severe (early onset, <34 wk) PE is important because the clinical management strategies are very different, especially because fetal lung maturation is insufficient for an optimal outcome of a baby born before week 34. It has been speculated that mild PE is the consequence of inadequate maternal adaptation to gestation, whereas severe PE is due to a placenta dysfunction. Here, we showed a differential Gal-1 expression in early versus late onset PE syndrome. This is in agreement with the study from Than and coworkers reporting increased Gal-1 expression in late onset PE (36). The fact that placental Gal-1 expression is different in early versus late onset PE supports the hypothesis that they have a different etiology. Thus, the finding that placental Gal-1 expression is increased in late onset PE could be the consequence of inadequate maternal adaptation to gestation. Under these circumstances, the overexpression of Gal-1 is linked to the placental hypoperfusion or dysfunctional perfusion as a compensatory mechanism. In addition, this increased Gal-1 expression may symbolize a further placental mechanism to control the exacerbated maternal immune response that normally accompanies the PE (33). In the case of early onset PE, we speculate that the defective placental Gal-1 expression negatively influences angiogenic factor production, migration, and invasion of EVT, resulting later in the rudimentary remodeling of maternal spiral arteries and abnormal uteroplacental artery blood flow typically associated with IUGR and PE (37). Indeed, a study in mice has shown that severe abnormalities in placentation precede the development of PE (38). Our study is clinically relevant as it provides important insights into the role of Gal-1 in gestation, reinforcing the concept of its unique properties in supporting pregnancy. Taken together, we conclude that Gal-1 fine tunes different essential processes during early (angiogenesis) or mid (placentation) gestation and emerges as an important lectin required for healthy pregnancy.
Materials and Methods
Mice and Tissue Collection.
All mice strains in the experimentation were acquired and maintained and timed pregnancies were established as described (11, 39). Care of mice was taken following institutional guidelines under a protocol approved by the state authority for Animal Use in Research and Education, Berlin (Landesamtes für Gesundheit und Soziales Berlin, LaGeSo). After cohabitation, the presence of a vaginal plug was denoted as embryonic day 0.5 (E0.5). i.p. injections of diphtheria toxin (DT) (2 ng/g; Sigma-Aldrich) was used on E4.5 to deplete DC, human recombinant Gal-1 (hrGal-1) (10 µg; PetroTech) and neutralizing Flk-1 antibody (clone DC101; 1.32 mg per mouse per d) were administrated i.p. from E4.5 to E6.5, whereas anginex (0.5 mg/kg BW) was injected from E4.5–E12.5. Pregnant female mice were killed on E7.5, E10.5, E13.5, E15.5, or E17.5 (n = 6–8 per d per group) depending on the experiment. Whole implantations were frozen for cryostat sections or formalin fixed for paraffin sections on E7.5, E10.5, and E13.5. Placental and decidual tissues were separated on E15.5 and frozen for isolation of total protein according to standard procedures. Placental weight was recorded in late gestation (from E15.5 to E17.5). Fetal development was analyzed by Theiler stage and body weight as described previously (39). Details are available in SI Materials and Methods.
Human Samples.
For analyses of Gal-1 levels, patient samples from the research bio-bank collection at Oslo University Hospital, Oslo, approved by the Regional Committee of Medical Research Ethics in Eastern Norway, were used as described (40). The clinical characteristics of the human cohort are summarized in SI Materials and Methods and Table S3. For measurement of circulating Gal-1 levels during the second trimester, samples from a prospective cohort study conducted by the Department of Pediatrics, Women and Infants Hospital, Providence, were used. Written informed consent was obtained from all of the women, and the study was approved by the institutional review boards of Women and Infants Hospital. The characteristics of recruited participants are summarized in Table S4.
Morphological Analyses.
Serial cryostat or paraffin sections of whole implantations at E7.5, E10.5, and E13.5 to evaluate tissue morphology by H&E, Masson-Goldner’s trichrome, vascular development by CD31, endoglin and placental development by PL-I were carried out as described (11, 39). Oxygen tensions at placentation sites were evaluated with Hypoxyprobe-1 Plus Kit as described (41). Gal-1 indirect immunofluorescence analysis in human placenta tissue with a Gal-1–specific antibody (Santa Cruz Biotechnology; sc-28248) was carried out as described previously (10). Details are available in SI Materials and Methods.
ELISAs.
Quantification of VEGF, sFlt-1, and sEng serum levels were performed using the mouse VEGF DuoSet ELISA (R&D Systems; DY493), mouse soluble Flt-1 Quantikine Immunoassay (R&D Systems; MVR100), and mouse Endoglin DuoSet ELISA (R&D Systems; DY3120), respectively, following the manufacturer’s recommendations. Circulating Gal-1 levels in human serum were measured with a noncommercial ELISA (12). Details are available in SI Materials and Methods.
Angiogenesis Array.
We assessed the levels of angiogenesis-related proteins using whole implantations on E7.5 with a Mouse Angiogenesis Array kit (R&D Systems; ARY015) following the manufacturer’s recommendations. Details are available in SI Materials and Methods.
Kidney Function and Blood Pressure Evaluation.
FITC-labeled dextran (Sigma-Aldrich; FD-2000S) and the ACR were used to determine renal function on E15.5 or E17.5 as described (41). Blood pressure was measured in the tail artery in pregnant females from E10.5 to E17.5. Systolic and diastolic pressure measurements were performed with a computerized, noninvasive tail-cuff acquisition system (CODA System; Kent Scientific) as described (41). Details are available in SI Materials and Methods.
qRT-PCR.
Total RNA isolation, subsequent cDNA synthesis, and quantitative real-time PCR were performed using our standard protocol (11) described in SI Materials and Methods. Mouse primer sequences are given in Table S5. Details for the qRT-PCR of human specimens are available in SI Materials and Methods.
AT1AA.
We measured AT1AA levels in mouse serum samples as described previously (42). Details are available in SI Materials and Methods.
Statistical Analysis.
Data are expressed as mean ± SEM. We analyzed mouse and human data by nonparametric Mann–Whitney u test and ANOVA. Differences among groups were evaluated using the Kruskal–Wallis, Tukey’s, or Bonferroni test. The in vitro data were analyzed by t test. We considered a P value less than 0.05 as statistically significant as analyzed by GraphPad Prism 5.0.
Supplementary Material
Acknowledgments
We thank P. Moschansky (S.M.B.’s laboratory), I. Kamer, J. Anders (R.D.’s laboratory), and Dr. S. Kalkunte (S.S.’s laboratory) for their excellent technical assistance in generating this work; Dr. K. Mayo for providing anginex; L. Øhra Levy and W. Norris for their assistance with the biobanks at Oslo University Hospital and Women and Infants Hospital, respectively. This work was mainly supported by research grants from the Fritz Thyssen Stiftung (Az. 10.10.2.125) and Bundesministerium für Bildung und Forschung (BMBF)–Deutschen Zentrum für Luft–und Raumfahrt (DLR) (01DM1208) (to S.M.B.). N.F. received a doctoral fellowship and I.T-G. a Habilitation scholarship from the Charité, Universitätsmedizin Berlin. G.B. received a postdoctorate fellowship from the Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina. F.H. is supported by the DFG (HE6249/1-1) and V.L.J.L.T. by grants from the Dutch Cancer Research Society (UM-2008-4101 and VU2009-4358). A.C.S. and S.M.W.-F. received support from the University of Oslo and Oslo University Hospital grants.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. F.W.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303707110/-/DCSupplemental.
References
- 1.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JM, Lain KY. Recent insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23(5):359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 3.Maynard SE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venkatesha S, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 5.Naicker T, Khedun SM, Moodley J, Pijnenborg R. Quantitative analysis of trophoblast invasion in preeclampsia. Acta Obstet Gynecol Scand. 2003;82(8):722–729. doi: 10.1034/j.1600-0412.2003.00220.x. [DOI] [PubMed] [Google Scholar]
- 6.Herse F, et al. Dysregulation of the circulating and tissue-based renin-angiotensin system in preeclampsia. Hypertension. 2007;49(3):604–611. doi: 10.1161/01.HYP.0000257797.49289.71. [DOI] [PubMed] [Google Scholar]
- 7.Zhou CC, et al. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14(8):855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips B, et al. Differential expression of two beta-galactoside-binding lectins in the reproductive tracts of pregnant mice. Biol Reprod. 1996;55(3):548–558. doi: 10.1095/biolreprod55.3.548. [DOI] [PubMed] [Google Scholar]
- 9.von Wolff M, Wang X, Gabius HJ, Strowitzki T. Galectin fingerprinting in human endometrium and decidua during the menstrual cycle and in early gestation. Mol Hum Reprod. 2005;11(3):189–194. doi: 10.1093/molehr/gah144. [DOI] [PubMed] [Google Scholar]
- 10.Tirado-González I, et al. Galectin-1 influences trophoblast immune evasion and emerges as a predictive factor for the outcome of pregnancy. Mol Hum Reprod. 2013;19(1):43–53. doi: 10.1093/molehr/gas043. [DOI] [PubMed] [Google Scholar]
- 11.Blois SM, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13(12):1450–1457. doi: 10.1038/nm1680. [DOI] [PubMed] [Google Scholar]
- 12.Kopcow HD, et al. T cell apoptosis at the maternal-fetal interface in early human pregnancy, involvement of galectin-1. Proc Natl Acad Sci USA. 2008;105(47):18472–18477. doi: 10.1073/pnas.0809233105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer I, et al. Stimulation of syncytium formation in vitro in human trophoblast cells by galectin-1. Placenta. 2010;31(9):825–832. doi: 10.1016/j.placenta.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 14.Kolundžić N, et al. Galectin-1 is part of human trophoblast invasion machinery—a functional study in vitro. PLoS ONE. 2011;6(12):e28514. doi: 10.1371/journal.pone.0028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh SH, et al. Galectin-1, a novel ligand of neuropilin-1, activates VEGFR-2 signaling and modulates the migration of vascular endothelial cells. Oncogene. 2008;27(26):3746–3753. doi: 10.1038/sj.onc.1211029. [DOI] [PubMed] [Google Scholar]
- 16.Griffioen AW, et al. Anginex, a designed peptide that inhibits angiogenesis. Biochem J. 2001;354(Pt 2):233–242. doi: 10.1042/0264-6021:3540233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thijssen VL, et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc Natl Acad Sci USA. 2006;103(43):15975–15980. doi: 10.1073/pnas.0603883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plaks V, et al. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest. 2008;118(12):3954–3965. doi: 10.1172/JCI36682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller DW, et al. Rapid vessel regression, protease inhibition, and stromal normalization upon short-term vascular endothelial growth factor receptor 2 inhibition in skin carcinoma heterotransplants. Am J Pathol. 2005;167(5):1389–1403. doi: 10.1016/S0002-9440(10)61226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huppertz B. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension. 2008;51(4):970–975. doi: 10.1161/HYPERTENSIONAHA.107.107607. [DOI] [PubMed] [Google Scholar]
- 21.Thijssen VL, et al. Tumor cells secrete galectin-1 to enhance endothelial cell activity. Cancer Res. 2010;70(15):6216–6224. doi: 10.1158/0008-5472.CAN-09-4150. [DOI] [PubMed] [Google Scholar]
- 22.Halder JB, et al. Differential expression of VEGF isoforms and VEGF(164)-specific receptor neuropilin-1 in the mouse uterus suggests a role for VEGF(164) in vascular permeability and angiogenesis during implantation. Genesis. 2000;26(3):213–224. [PubMed] [Google Scholar]
- 23.Douglas NC, et al. Vascular endothelial growth factor receptor 2 (VEGFR-2) functions to promote uterine decidual angiogenesis during early pregnancy in the mouse. Endocrinology. 2009;150(8):3845–3854. doi: 10.1210/en.2008-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 25. Matsumoto H, et al. (2002) Cyclooxygenase-2 differentially directs uterine angiogenesis during implantation in mice. J Biol Chem 277(32):29260–29267. [DOI] [PubMed]
- 26.Torry DS, et al. Angiogenesis in implantation. J Assist Reprod Genet. 2007;24(7):303–315. doi: 10.1007/s10815-007-9152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox P, Marton T. Pathological assessment of intrauterine growth restriction. Best Pract Res Clin Obstet Gynaecol. 2009;23(6):751–764. doi: 10.1016/j.bpobgyn.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 28.McMaster MT, Zhou Y, Fisher SJ. Abnormal placentation and the syndrome of preeclampsia. Semin Nephrol. 2004;24(6):540–547. doi: 10.1016/s0270-9295(04)00124-x. [DOI] [PubMed] [Google Scholar]
- 29.Baston-Buest DM, et al. Expression of the vascular endothelial growth factor receptor neuropilin-1 at the human embryo-maternal interface. Eur J Obstet Gynecol Reprod Biol. 2011;154(2):151–156. doi: 10.1016/j.ejogrb.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 30.Pan Q, et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11(1):53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Liu AX, et al. Proteomic analysis on the alteration of protein expression in the placental villous tissue of early pregnancy loss. Biol Reprod. 2006;75(3):414–420. doi: 10.1095/biolreprod.105.049379. [DOI] [PubMed] [Google Scholar]
- 32.Molvarec A, et al. Peripheral blood galectin-1-expressing T and natural killer cells in normal pregnancy and preeclampsia. Clin Immunol. 2011;139(1):48–56. doi: 10.1016/j.clim.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 33.Redman CW, Sacks GP, Sargent IL. Preeclampsia: An excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180(2 Pt 1):499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 34.Munaut C, et al. Differential expression of Vegfr-2 and its soluble form in preeclampsia. PLoS ONE. 2012;7(3):e33475. doi: 10.1371/journal.pone.0033475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Redman CW, Sargent IL (2009) Placental stress and pre-eclampsia: A revised view. Placenta 30 Suppl A:S38–42. [DOI] [PubMed]
- 36.Than NG, et al. Severe preeclampsia is characterized by increased placental expression of galectin-1. J Matern Fetal Neonatal Med. 2008;21(7):429–442. doi: 10.1080/14767050802041961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwata M, et al. Prenatal detection of ischemic changes in the placenta of the growth-retarded fetus by Doppler flow velocimetry of the maternal uterine artery. Obstet Gynecol. 1993;82(4 Pt 1):494–499. [PubMed] [Google Scholar]
- 38.Dokras A, et al. Severe feto-placental abnormalities precede the onset of hypertension and proteinuria in a mouse model of preeclampsia. Biol Reprod. 2006;75(6):899–907. doi: 10.1095/biolreprod.106.053603. [DOI] [PubMed] [Google Scholar]
- 39. Barrientos G, et al. (2012) CXCR4(+) dendritic cells promote angiogenesis during embryo implantation in mice. Angiogenesis 16(2):417–427. [DOI] [PubMed]
- 40.Herse F, et al. Cytochrome P450 subfamily 2J polypeptide 2 expression and circulating epoxyeicosatrienoic metabolites in preeclampsia. Circulation. 2012;126(25):2990–2999. doi: 10.1161/CIRCULATIONAHA.112.127340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed A, Singh J, Khan Y, Seshan SV, Girardi G. A new mouse model to explore therapies for preeclampsia. PLoS ONE. 2010;5(10):e13663. doi: 10.1371/journal.pone.0013663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallukat G, et al. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103(7):945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.