Abstract
Caseinolytic proteases (ClpPs) are large oligomeric protein complexes that contribute to cell homeostasis as well as virulence regulation in bacteria. Although most organisms possess a single ClpP protein, some organisms encode two or more ClpP isoforms. Here, we elucidated the crystal structures of ClpP1 and ClpP2 from pathogenic Listeria monocytogenes and observe an unprecedented regulation principle by the catalytic triad. Whereas L. monocytogenes (Lm)ClpP2 is both structurally and functionally similar to previously studied tetradecameric ClpP proteins from Escherichia coli and Staphylococcus aureus, heptameric LmClpP1 features an asparagine in its catalytic triad. Mutation of this asparagine to aspartate increased the reactivity of the active site and led to the assembly of a tetradecameric complex. We analyzed the heterooligomeric complex of LmClpP1 and LmClpP2 via coexpression and subsequent labeling studies with natural product-derived probes. Notably, the LmClpP1 peptidase activity is stimulated 75-fold in the complex providing insights into heterooligomerization as a regulatory mechanism. Collectively, our data point toward different preferences for substrates and inhibitors of the two ClpP enzymes and highlight their structural and functional characteristics.
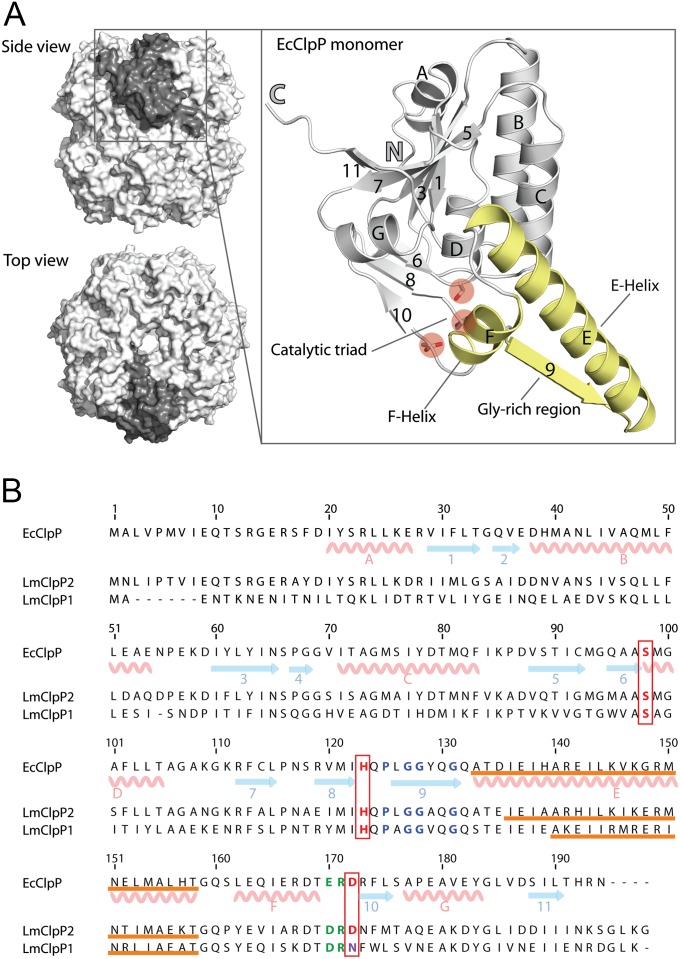
The caseinolytic protease P (ClpP) is a highly conserved enzyme present in bacteria and higher organisms (1–3). ClpP is responsible for cell homeostasis and among other duties for the regulation of bacterial virulence in several pathogens including Staphylococcus aureus and Listeria monocytogenes (4, 5). Early structural studies revealed the topology of the Escherichia coli ClpP complex that consists of two heptameric rings building up a 300 kDa cylinder (Fig. 1A) (6). The interior of this proteolytic machinery exhibits 14 active sites flanked by axial pores that allow protein substrates to enter the hydrolytic chamber. ClpP gains its catalytic activity in complex with AAA+-chaperones (such as ClpC, ClpE, and ClpX in the case of L. monocytogenes). These ATP-dependent enzymes bind to the axial pores of ClpP, unfold the protein prone to degradation, and direct it into the proteolytic chamber (7–9).
Fig. 1.
Main structural elements of ClpP. (A) Top and side view of the tetradecameric ClpP complex from E. coli (10) (EcClpP, PDB ID code 1TYF, surface representation) with one subunit highlighted in dark gray. Each subunit (close-up, ribbon diagram) is made up of seven α-helices (denoted with letters) and 11 β-strands (denoted with numbers) and contains a catalytic triad (highlighted with red circles). Relevant secondary structures (α-helices E and F, β-strand 9) are highlighted in gold. (B) Sequence alignment of EcClpP with LmClpP1 and LmClpP2. The secondary structure elements are depicted for EcClpP. The catalytic triad is framed in red, the residues forming the E-helix are underlined in orange, the conserved proline and the glycins in the Gly-rich loop are colored blue, and the Asp/Arg sensor is shown in green.
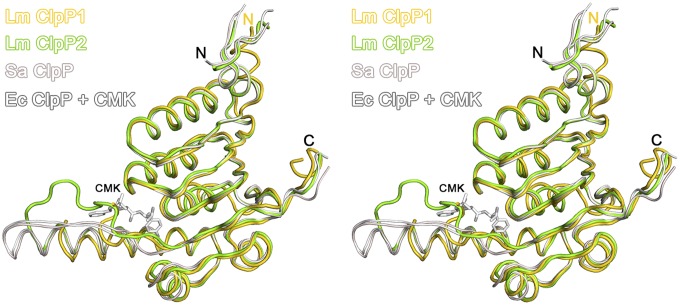
A close-up view of a single ClpP monomer reveals several characteristic structural features that are conserved among this class of proteases. To harmonize the ClpP nomenclature for all subsequent discussions, we use a general sequence numbering based on the first determined crystal structure of ClpP from E. coli [EcClpP, Protein Data Bank (PDB) ID code 1TYF] (10) (Fig. 1B). According to this nomenclature, a catalytic triad (Ser98, His123, Asp172) essential for proteolysis, a central E-helix with a Gly-rich loop region essential for interring contacts between the two heptamers, and a N-terminal region essential for interaction with a AAA+-chaperone can be observed in all published X-ray structures to date (Fig. 1A, Fig. S1B) (10–18). Cocrystallization of E. coli ClpP with an irreversible dipeptide chloromethylketone inhibitor confirmed the reactivity of the catalytic triad residues Ser98 and His123 and illustrate a binding site for the dipeptide within the Gly-rich loop region that adopts an antiparallel beta-strand (19) (Fig. 2). Recently, two conformations of ClpP from S. aureus have been reported that are thought to represent physiologically important states with an active and an inactive catalytic triad corresponding to an extended and a bent E-helix, respectively (Fig. S2) (11, 12). In addition, a highly conserved aspartate/arginine sensor (Asp170/Arg171) links oligomerization to the catalytic activity and exhibits characteristic conformations in both states (Fig. S2) (12). In agreement with this model, ClpP heptamers lack the interaction of the sensor residues with their counterparts on the adjacent ring and thus have an inactive triad. In the tetradacameric state, the senor feedbacks the correct assembly to the active sites, thereby ensuring controlled proteolysis.
Fig. 2.
Stereo-representation of ClpP monomers. Structural superposition of LmClpP1 (gold), LmClpP2 (green), SaClpP (PDB ID code 3V5E, pale red), and EcClpP (PDB ID code 2FZS, gray) with covalently bound CMK inhibitor.
Although most organisms possess a single ClpP protein with a conserved fold (6, 11, 13–16, 18, 20), the genomes of some organisms encode two or more ClpP isoforms (21–24). For a cyanobacterial system, heptameric rings of mixed composition have been reported that interact with different chaperones (22). In contrast, ClpP proteins from L. monocytogenes (LmClpP1 and LmClpP2) as well as from Mycobacterium tuberculosis have been found to assemble into heterooligomeric complexes composed of two homoheptamers (25, 26). Inhibition of LmClpP2 with lactone-based inhibitors led to down-regulation of virulence without affecting viability (27). In contrast, both mycobacterial ClpP subunits are essential for bacterial survival, emphasizing defined functional roles of ClpP proteins among species (26, 28).
Interestingly, LmClpP2 shares a high-sequence homology with ClpP enzymes of various organisms that feature one ClpP (Fig. S1 A and C). LmClpP1 exhibits only 41% sequence identity with LmClpP2, raising the question of how these two distinct isoforms interact and how they differ functionally. Furthermore, there is a distinct difference between the two ClpP homologs in the composition of their catalytic triad: Asp172 of LmClpP2 is replaced by an asparagine in LmClpP1, an unusual observation within serine proteases that is, however, conserved in several uncharacterized homologs (Fig. S1 A and C). Although the replacement of an aspartate with an asparagine represents only a moderate structural alteration, it significantly influences the strength of the catalytic triad charge-relay system. The nucleophilicity of the active site Ser98 in LmClpP1 and LmClpP2 was previously monitored and compared by β-lactone activity-based probes (25, 29). Although all monocyclic β-lactones selectively labeled LmClpP2 either as a homooligomer or as part of the heterooligomeric complex, a probe derived from the bicyclic natural product vibralactone (VLP) was able to interact with both LmClpP1 and LmClpP2 catalytic sites. Importantly, binding of the ligand to LmClpP1 was only observed in the presence of LmClpP2 (25).
Results
In our studies we performed a two-tiered strategy that first evaluates the chemical reactivity of the enzyme active site followed by the investigation of the corresponding molecular arrangement at the atomic level.
Redesign of the Catalytic Triad in LmClpP1 by a N172D Mutation Leads to Increased Reactivity and Tetradecameric Oligomerization.
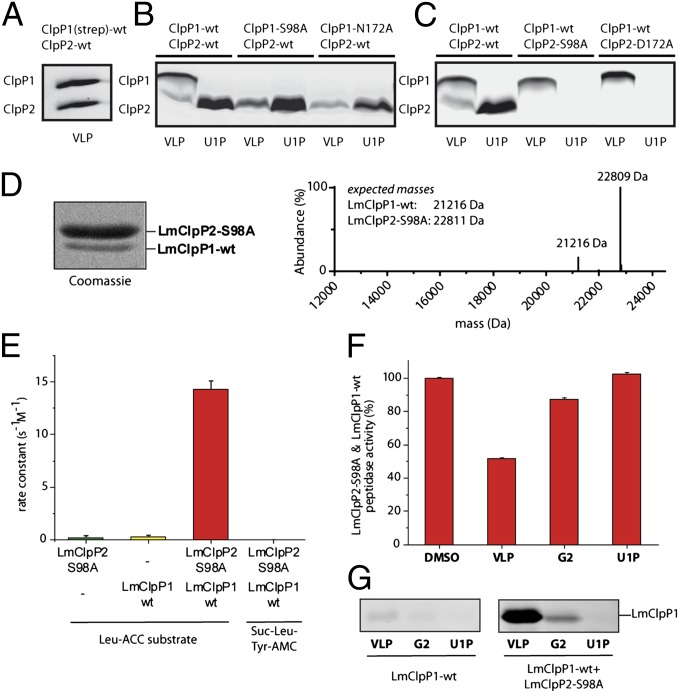
To investigate the reactivities of the two different triads, we performed several in-depth chemical and biochemical assays with LmClpP1 and LmClpP2. Considering the electronic layout of a charge-relay system, it is expected that the natural Asp172Asn exchange in LmClpP1 reduces the strength of hydrogen bonds within the active site, thus lowering the nucleophilicity of Ser98. This is in accordance with previous findings that this mutation impairs LmClpP1 to open monocyclic β-lactones but still allows binding to bicyclic VLP (25). To test if the LmClpP1 triad can be reactivated, we mutated Asn172 to Asp and subsequently monitored the active site acylation by the following procedure (Fig. 3 A and B): (i) Living E. coli cells recombinantly expressing the mutant LmClpP1 construct were incubated with various lactone probes in situ (Fig. S3A). (ii) Following lysis, Huisgen–Sharpless–Meldal click-chemistry was used to attach a fluorescent rhodamine-azide to the alkyne handle (Fig. 3A) (30–32). (iii) The proteome was separated by SDS/PAGE and lactone modified ClpPs were visualized via fluorescent scanning. Whereas VLP only showed moderate labeling of wild-type LmClpP1, mutation of Asn172 to alanine prevented binding to VLP completely (Fig. 3B). This strongly indicates that Asn172 is an essential member of the catalytic triad and that it is required for proper acylation reactivity during peptide bond hydrolysis. Interestingly, the N172D mutant enzyme reacted not only with VLP but also with monocyclic probes G2 and E2 that did not bind to wild-type LmClpP1 (Fig. 3B). These findings emphasize that the N172D redesign of the triad led to an increased reactivity of LmClpP1. We thus performed assays with fluorogenic substrates to quantitatively compare the catalytic activities of the LmClpP1 wild-type and mutant enzymes. The frequently used substrate Suc–Leu–Tyr–7-amino-4-methylcoumarin is only hydrolyzed by LmClpP2 but not by LmClpP1 (Fig. 3C). We therefore used a tripeptide substrate with leucine at the P1 site (Leu–7-amino-4-carbamoylmethylcoumarin; SI Methods), which is cleaved by both LmClpP enzymes (for structural details on the fluorogenic substrate Leu–ACC, see Fig. S5B). Remarkably, using this substrate, LmClpP1–N172D showed a ∼20-fold increase in peptidase activity compared with wild-type LmClpP1 (Fig. 3D).
Fig. 3.
Activity based protein profiling experiments with β-lactone probes and peptidase activity of different LmClpP constructs. (A) Schematic illustration of a labeling experiment with a ClpP subunit containing the Ser–His–Asp catalytic triad. For R1 and R2, please refer to Fig. S3A. (B) Fluorescent SDS/PAGE analysis of LmClpP1 separately as wild-type and with mutated N172 residue, labeled with VLP and the monocyclic β-lactones (VLP, G2, E2, U1P) in situ. (C) Peptidase activity of LmClpP proteins (3 µM) measured with 200 µM Suc–Leu–Tyr–AMC at 32 °C. (D) Peptidase activity of LmClpP proteins (3 µM) recorded with 100 µM of a custom-synthesized, fluorogenic Leu–ACC (for structural details on the fluorogenic substrate Leu–ACC, see Fig. S5A) substrate at 32 °C. Note, LmClpP1–N172D is 20-fold more active than wild-type LmClpP1. (E) Fluorescent SDS/PAGE analysis of ClpP1 and ClpP1–N172D in comparison with ClpP2, labeled in situ with VLP and monocyclic β-lactones (VLP, U1P, E2, G2, D3, 120P, A1, M1, N1, P1, Q1).
It was previously suggested that the activity of ClpP is coupled to the oligomeric state with heptamers as inactive and tetradecamers as active species (12, 25). Recombinantly purified wild-type LmClpP1 exists predominantly as a heptamer. In contrast, the LmClpP1–N172D mutant formed a tetradecamer as shown by size exclusion chromatography (SEC) (Fig. S3B). These findings indicate that the active site mutation affects both the reactivity as well as the oligomerization of the protease.
Next, we elucidated the β-lactone binding preferences of LmClpP1, LmClpP2, and LmClpP1–N172D with a collection of probes via in situ labeling experiments. Although wild-type LmClpP2 accommodates aromatic rings as well as medium to long aliphatic chains (e.g., E2, G2, U1P, 120P, and D3, respectively), LmClpP1–N172D favors small P1 sites as present in G2 and E2 (Fig. 3E), indicating significant differences in the substrate binding channel. To quantitatively assess the amount of lactone binding, we applied intact protein mass spectrometry (MS) and determined the influence of all lactones on peptidase activity (Fig. S4 A–C). For LmClpP1–N172D, solely VLP and, to a minor extent, G2 were able to reduce peptidase activity by 50% and 12%, respectively. Correspondingly, VLP was identified by MS to react covalently with LmClpP1–N172D. In case of LmClpP2, lactone-induced inhibition of peptidase activity corresponded well with the proportion of covalently modified active sites as well as with results of gel labeling (Fig. 3E, Fig. S4B). Additionally, we examined the kinetics for the reaction of LmClpP2 with the representative lactones G2 and U1P according to our previously established protocol (33) (Fig. S5). The results revealed a one-step reaction for G2 and a reversible precomplex of enzyme and U1P before irreversible covalent attachment.
Heterooligomeric Complex Formation Stimulates Hydrolytic Activity of LmClpP1.
Labeling of coexpressed and copurified LmClpP1 and LmClpP2 (in which only one of the two proteins contains a Strep-tag) with VLP yielded bands for both isoforms suggesting the presence of a heterooligomeric complex (Fig. 4A) (25). However, this complex represents only a minor species in vitro as shown by analytical SEC experiments and native PAGE (Fig. S6). We therefore investigated the activity of several LmClpP1 and LmClpP2 active site mutants by in situ proteome labeling. As expected, the LmClpP1–S98A and N172A mutants were not modified by VLP due to disruption of the catalytic triad (Fig. 4B). The corresponding mutations in LmClpP2 (S98A and D172A) resulted in inactive enzymes as well (Fig. 4C). Interestingly, wild-type LmClpP1 retained acylation activity in complexes with both LmClpP2 mutants, demonstrating that LmClpP2 serves as an effector for LmClpP1. These observations are in agreement with recent findings on MtClpP2 (26) and emphasize the mutually stimulating influence of both LmClpP isoforms.
Fig. 4.
ClpP1P2 heteroconstructs illustrating distinct substrate specificities. (A) Labeling profile of the isolated tetradecameric complex of LmClpP1(strep)/LmClpP2 with VLP. (B and C) Fluorescent SDS/PAGE analysis of recombinantely coexpressed ClpP1P2 either with ClpP2 wild-type (wt) and a ClpP1 mutant (B) or ClpP2 wild-type and a mutated ClpP1 (C). The labeling was performed with VLP and the monocyclic β-lactone U1P. (D) Copurified Strep-tagged LmClpP2–S98A and tag-free LmClpP1 (ratio 5:1) identified by intact protein MS. (E) Heterooligomerization of LmClpP1 stimulates its peptidolytic activity 75-fold. Rate constants of the heterooligomeric complex were calculated with respect to the concentration of the LmClpP1 subunits. (F) Peptidase activities of the heterooligomeric complex after incubation with 50 µM of inhibitor at room temperature. (G) Binding of isolated LmClpP1 and in the presence of LmClpP2–S98A with β-lactones (VLP, G2, U1P).
To elucidate this aspect in more detail we analyzed a putative activation of LmClpP1 in the presence of LmClpP2 by peptidolytic assays. To ensure that the activity exclusively originates from LmClpP1, we used a copurified mixture of inactive LmClpP2–S98A and LmClpP1. Quantification through MS and coomassie gel yielded a ratio of 3:1–9:1 of LmClpP2–S98A to LmClpP1 varying with different preparations (Fig. 4D). Interestingly, the complex of LmClpP2–S98A and LmClpP1 showed a 75-fold increase in peptidase activity compared with pure LmClpP1 (Fig. 4E). Notably, LmClpP1 was even ninefold more active than the LmClpP1–N172D mutant in the heterooligomeric complex (Fig. 3D). Because Suc–Leu–Tyr–AMC is not cleaved in the assay, side activity by E. coli ClpP can be ruled out (Fig. 4E). As expected for LmClpP1-mediated activity, only VLP but not U1P reduced the peptidase turnover by 50% (Fig. 4F). In agreement with β-lactone labeling (Fig. 4G), these results point toward ClpP1 regulation dependent on the heterooligomer formation and, furthermore, display substrate preferences between LmClpP1 and LmClpP2.
Crystal Structures of LmClpP1 and LmClpP2.
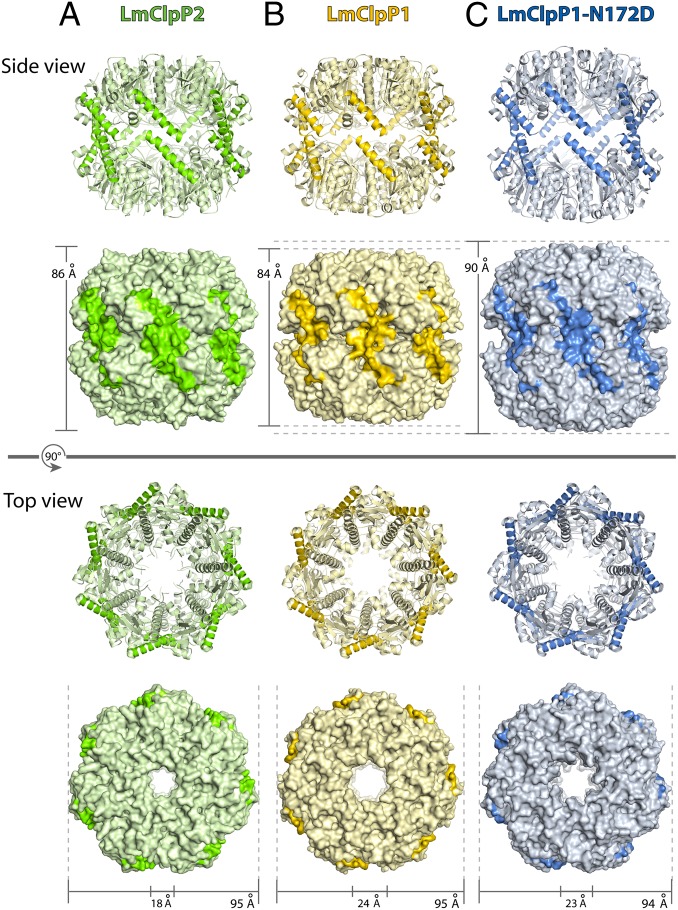
To gain insight into the molecular interactions that account for the observed differences in chemical reactivity, we determined the crystal structures of LmClpP1 and LmClpP2. Both enzymes were purified, crystallized, and the structures were determined by molecular replacement (Table S1). LmClpP2 was elucidated at 2.6 Å resolution and consists of two heptameric rings that form a tetradecameric barrel (Fig. 5A). These two rings are interconnected by E helices, resulting in a cylindrical shape with a height of 86 Å. The helix is similar to previously reported structures in the active and extended form (10, 12, 17, 18); the complex height, however, resembles the inactive and bent form (11, 13, 15, 34). The residues forming the catalytic triad (Ser98, His123, and Asp172) of LmClpP2 are misaligned in all 14 subunits (Fig. S7A). This is further supported by the tilted position of the arginine sensor (Arg171) that engages in an intramolecular contact with Asp168 (Fig. S7B). In analogy to inactive ClpP from S. aureus (SaClpP; Fig. S2), Arg171 hence lacks binding to Asp170 across the heptamer interface, which likely induces the misalignment of the proximal catalytic triad (Fig. S7) (12).
Fig. 5.
Structure of LmClpP tetradecamers. Top and side view of the tetradecameric complex LmClpP2 (A, green), LmClpP1 (B, gold), and LmClpP1–N172D (C, blue). The E-helix is highlighted with a dark color.
The observation of an extended E-helix accompanied by an inactive triad is not present in many structures. A structural overlay between single subunits of extended SaClpP (active state), chloromethylketone-bound E. coli ClpP (CMK–EcClpP), and LmClpP2 reveals significant differences in the orientation and length of the Gly-rich region that precedes the central E-helix (Fig. 2). In LmClpP2 the Gly-rich segment forms a long, unstructured loop accompanied by a partially unfolded and thus significantly shorter E-helix. In SaClpP and CMK–EcClpP, however, the Gly-rich region adopts beta strand conformation leading to a well-defined substrate binding channel (Fig. 2) (10, 19). Of note, the beta strand mediates ring–ring contacts in the active structure of SaClpP and thus stabilizes tetradecamer formation (Fig. 2, Fig. S7).
The structure of LmClpP1 was elucidated at 2.0 Å resolution (Fig. 5B). Surprisingly, the overall structural fold of LmClpP1 matches by 88% and a root-mean-square deviation of Cα-atoms of 0.9 Å despite the low sequence homology with LmClpP2 (Fig. S7B). This finding suggests that both enzymes evolved under phylogenetic pressure to conserve their overall architecture that is likely a prerequisite for recognition and heterooligomer formation. Again, the Arg171 sensor motif is tilted and the catalytic triad inactive (Fig. S7B). Interestingly, structural alignments show an even shorter E-helix of LmClpP1 compared with LmClpP2 as well as an extended loop that is similar to the one observed for LmClpP2 (Fig. 2, Fig. S7A). Within this loop alternative conformations are visible in the electron density maps, indicating structural flexibility in this region. Furthermore, the sequence comparison between LmClpP1 and LmClpP2 shows no identity in its primary structure and length within the N-terminal amino acids (Fig. 1B). LmClpP1 thus exhibits a larger axial pore compared with LmClpP2 and other bacterial ClpPs (Fig. 5), which is in line with results of previous electron microscopy studies (25).
Crystal Structure of the LmClpP1–N172D Mutant.
To understand the mechanistic basis for the reactivation of LmClpP1, we crystallized the LmClpP1–N172D mutant and determined its crystal structure to 2.0 Å resolution (Table S1). The enzyme assembles into a tetradecameric complex (Fig. 5C), however with several striking differences compared with the wild-type (wt) LmClpP1 structure, (i) the catalytic triad is aligned as in the active SaClpP or EcClpP structures (Fig. S7A), (ii) the E-helix of LmClpP1–N172D is extended by four amino acids, and (iii) the loop region is four amino acids shorter as in the wild type, which is due to a transition of loop residues I136–A140 into the corresponding E-helix. As in the wild-type structure, no electron density was obtained for the Gly-rich loop.
The conformation of the catalytic triad—that is, the position of Asn/Asp172—is directly linked to an Asp–Arg sensor as previously described for SaClpP (12). The interactions of the sensor residues Asp170 and Arg171 with their counterparts on the opposite ring (Fig. 6 A and B) as illustrated in LmClpP1–N172D enable the interaction of the catalytic residues His123 and Asp172 in contrast to LmClpP1 wild type (Fig. 6B). Hereby, several hydrogen bonds within the equatorial plane between the two heptameric rings are formed, stabilizing the tetradecameric complex. Interestingly, a conserved Pro125 undergoes major structural rearrangements that correspond to an aligned and a misaligned triad. The distinct states of Pro125 keep its trans configuration. This tilted position is indicative for an active triad, while rotation of the proline by 58°(backbone rearrangement C-α, 1.3 Å; N, 1.9 Å) as observed in wild-type LmClpP1 depicts an inactive state. Furthermore, this proline is at a central position that links the alignment of the active site via the flexible glycine-rich loop with the E-helix length.
Fig. 6.
Mechanism that links activity to oligomerization. (A) Tetradecameric complex of LmClpP1–N172D (blue) structurally superimposed with wt (gold, only two opposite monomers are shown). The monomers superposition of ClpP1 wt (gold) and ClpP1–N172D (blue) show the key elements involved in the catalytic triad. When the active site is aligned in the case of LmClpP1–N172D, proline 125 induces a conformational switch toward the E-helix (A140–T158), which results in the extension of the E-helix (I136–T158) and thus in an extended complex. Furthermore, the alignment of the triad induces the down position of the Asp/Arg (D170/R171) sensor. (B) The close-up displays the Asp/Arg sensor (D170/R171) and the interaction to the sensor of the adjacent monomer of the opposite ring (D′170/R′171), which is absent in wt ClpP1.
Structural Expansion of Wild-Type and Mutant LmClpP1.
Another striking difference between wild-type and mutant LmClpP1 is the lateral dimension of both complexes with 7% (6 Å) expansion of the mutant (Fig. S8). The basis for the increased length is the prolonged E-helix in the mutant enzyme, which displaces both heptamers apart from each other, preventing a clash with helix F (residues Tyr162–Thr169; Fig. S8). Interestingly, the lateral dimension of LmClpP2 is just in between these two states, contributing to the view that ClpP proteases are able to sample many different conformations (35).
Discussion
Whereas ClpPs from bacteria that exhibit only one isoform have been studied in great detail, the function and regulation of systems with more than one ClpP isoform are still poorly understood. Pioneering work in mycobacteria revealed a heterooligomeric assembly of ClpP1 and ClpP2 in which the interaction of both heptameric rings stimulated activity and exhibited specialized substrate preferences (26). Here we elucidate the high-resolution X-ray structures of LmClpP1, LmClpP1–N172D, as well as LmClpP2 and evaluate their activity by customized probes as well as substrate turnover assays. These chemical and structural experiments provide unprecedented insight into the regulation principle of the catalytic triad, which influences proteolytic activity, oligomerization, helix length, and complex size via several coordinated conformational changes.
Isolated LmClpP1 is almost inactive in peptidolytic and acylation assays. However, an active site redesign by mutagenesis induced both oligomerization and significant activation in peptide hydrolysis as well as lactone binding. In addition, LmClpP1 is significantly stimulated in a heterooligomeric complex with LmClpP2. These two observations of our biochemical studies identify an important regulatory role of the catalytic triad. We thus determined the key players of a complex structural network by comparing the wild-type and mutant LmClpP1 structures that revealed pronounced differences in the arrangement of the catalytic triad residue Asn172. The orientation of this amino acid influences two adjacent structural core features, the Asp170/Arg171 sensor, important for oligomerization, and the Pro125 switch. This switch links the N-terminal end of the E-helix with His123 of the catalytic triad and thus helix length with enzyme activity (Fig. 7). In the N172D mutant, this region is in a relaxed mode with the central Pro125 residue in a tilted position (58°) (Fig. 6A). This structural rearrangement provides the helix more flexibility to expand. If this Pro125 rotates back, as observed in the wt structure, the loop residues pull at the helix end, leading to its partial unfolding. In turn, the position of the proline is directly coupled to the orientation of the catalytic triad His123 that adopts in two conformational states as well as reflecting the activity status of the enzyme. Therefore, the mutant structure corresponds to an active ClpP species with an aligned catalytic triad. By contrast, the LmClpP1 wild-type enzyme, which contains an Asn172, exhibits a weaker H-bond network with His123, likely causing a conformational change toward an unfolded E-helix as well as an inactive triad (Fig. 7).
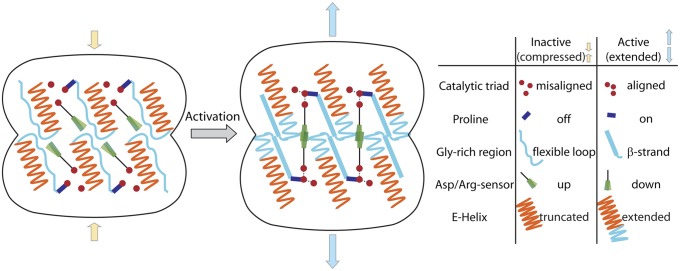
Fig. 7.
Extension of both heptameric rings in the ClpP tetradecamer. Depicted is a model displaying all key residues within the inactive (compressed) and active (extended) tetradecamer. Activation proceeds through an extension of the E-helix, proline rearrangement, active site alignment, and interaction of the Asp/Arg residues across the heptamer interface.
Further properties of LmClpP1 are the lack of all conserved residues that are important for the interaction with ATP-dependent chaperones such as ClpX as well as an enlarged entry channel that might allow chaperone-independent accessibility of protein substrates (36). This has been observed with N-terminal deletion mutants and with acyldepsipeptide-bound ClpPs that exhibit an enlarged entry pore and therefore unregulated proteolytic activity (18, 36–38). However, a ClpX-independent proteolytic machinery would require tight regulation in vivo. Thus, the naturally occurring Asp to Asn substitution accounting for the reduced activity of LmClpP1 in combination with the restricted ligand specificity, as shown by our acylation and peptidolytic assays, seems to be an evolutionary invention ensuring controlled degradation processes.
Additionally, the proteolytic activity of LmClpP1 is governed by the need for heterooligomerization with LmClpP2. According to our data, we propose that activation of LmClpP1 is achieved by the interaction of its relaxed E-helix with the matching E-helix of the adjacent LmClpP2 heptameric ring across the tetradecamer interface. This interaction extends both helices, pushes the Pro125 switch, aligns the Arg/Asp network, and activates the LmClpP1 triad for substrate processing.
One prerequisite for LmClpP1/LmClpP2 heterooligomerization is dissociation of LmClpP2 into heptamers and an appropriate recognition by LmClpP1. Although LmClpP1 and LmClpP2 share low sequence identity, converged evolution of the two enzymes is a likely explanation that ensures a conserved overall structural fold and facilitates recognition of different heptamers for coordinated complex formation. We modeled a heterooligomer structure (Fig. S7C), confirming that the interaction between the two different rings provides sufficient contacts and stability, which is in line with our biochemical as well as EM studies (25).
Taken together, we elucidate striking features of the two ClpP isoforms of L. monocytogenes, revealing an unprecedented regulation principle of LmClpP1 activity. Despite low sequence homology, LmClpP1 and LmClpP2 have evolved a similar overall structural fold. However, a major difference is the nature of LmClpP1’s unusual Asn–His–Ser catalytic triad (39). Our mutational and structural experiments emphasize that this triad represents a key regulatory element that keeps LmClpP1 heptameric and inactive in absence of LmClpP2. In contrast, LmClpP2 is an active tetradecamer that is able to operate independently of LmClpP1. Moreover, our biochemical data show that both isoforms exhibit preferences for specific ligands and substrates in vitro.
Methods
Diffraction datasets were collected using synchrotron radiation of λ = 1.0 Å at the beamline X06SA, Swiss Light Source (SLS). Datasets were processed using the program package XDS (40). Table S1 summarizes data collection and refinement statistics. Crystal structure analysis was performed by molecular replacement using the program PHASER (41). For further crystallographic details, synthesis, cloning, mutagenesis, protein expression, purification and biochemical assays, see SI Methods.
Supplementary Material
Acknowledgments
E.Z. was supported by the Sonderforschungsbereich (SFB)749 and by the Technische Universität München Graduate School (TUM-GS). M. Gersch is a member of the TUM-GS and was supported by the Fonds der chemischen Industrie. S.A.S. was supported by the Deutsche Forschungsgemeinschaft [SFB749, SFB1035, and Forschergruppe (FOR)1406], a European Research Council starting grant, and the Center for Integrated Protein Science. M.D. was supported by the Foundation for Polish Science.
Footnotes
Conflict of interest statement: M. Gersch, M.P., M.D., and S.A.S. are named inventors on a patent application describing fluorogenic substrates suitable for ClpP activity measurements.
This article is a PNAS Direct Submission. C.S.C. is a guest editor invited by the Editorial Board.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 4JCQ, 4JCR, and 4JCT).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219125110/-/DCSupplemental.
References
- 1.Gottesman S, Roche E, Zhou Y, Sauer RT. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 1998;12(9):1338–1347. doi: 10.1101/gad.12.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottesman S, Maurizi MR, Wickner S. Regulatory subunits of energy-dependent proteases. Cell. 1997;91(4):435–438. doi: 10.1016/s0092-8674(00)80428-6. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman S, Maurizi MR. Regulation by proteolysis: Energy-dependent proteases and their targets. Microbiol Rev. 1992;56(4):592–621. doi: 10.1128/mr.56.4.592-621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frees D, Qazi SN, Hill PJ, Ingmer H. Alternative roles of ClpX and ClpP in Staphylococcus aureus stress tolerance and virulence. Mol Microbiol. 2003;48(6):1565–1578. doi: 10.1046/j.1365-2958.2003.03524.x. [DOI] [PubMed] [Google Scholar]
- 5.Frees D, Sørensen K, Ingmer H. Global virulence regulation in Staphylococcus aureus: Pinpointing the roles of ClpP and ClpX in the sar/agr regulatory network. Infect Immun. 2005;73(12):8100–8108. doi: 10.1128/IAI.73.12.8100-8108.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito S, et al. Combination of borane-dimethyl sulfide complex with catalytic sodium tetrahydroborate as a selective reducing agent for α-hydroxy esters. Chem Lett. 1984;13(8):1389–1392. [Google Scholar]
- 7.Saito S, Bunya N, Inabe M, Moriwake T, Torii S. A facile cleavage of oxirane with hydrazoic acid in DMF: A new route to chiral β-hydroxyl-α-amino acids. Tetrahedron Lett. 1985;26(43):5309–5312. [Google Scholar]
- 8.Maglica Z, Kolygo K, Weber-Ban E. Optimal efficiency of ClpAP and ClpXP chaperone-proteases is achieved by architectural symmetry. Structure. 2009;17(4):508–516. doi: 10.1016/j.str.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Reid BG, Fenton WA, Horwich AL, Weber-Ban EU. ClpA mediates directional translocation of substrate proteins into the ClpP protease. Proc Natl Acad Sci USA. 2001;98(7):3768–3772. doi: 10.1073/pnas.071043698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Hartling JA, Flanagan JM. The structure of ClpP at 2.3 A resolution suggests a model for ATP-dependent proteolysis. Cell. 1997;91(4):447–456. doi: 10.1016/s0092-8674(00)80431-6. [DOI] [PubMed] [Google Scholar]
- 11.Geiger SR, Böttcher T, Sieber SA, Cramer P. A conformational switch underlies ClpP protease function. Angew Chem Int Ed Engl. 2011;50(25):5749–5752. doi: 10.1002/anie.201100666. [DOI] [PubMed] [Google Scholar]
- 12.Gersch M, List A, Groll M, Sieber SA. Insights into structural network responsible for oligomerization and activity of bacterial virulence regulator caseinolytic protease P (ClpP) protein. J Biol Chem. 2012;287(12):9484–9494. doi: 10.1074/jbc.M111.336222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Bakkouri M, et al. The Clp chaperones and proteases of the human malaria parasite Plasmodium falciparum. J Mol Biol. 2010;404(3):456–477. doi: 10.1016/j.jmb.2010.09.051. [DOI] [PubMed] [Google Scholar]
- 14.Gribun A, et al. The ClpP double ring tetradecameric protease exhibits plastic ring-ring interactions, and the N termini of its subunits form flexible loops that are essential for ClpXP and ClpAP complex formation. J Biol Chem. 2005;280(16):16185–16196. doi: 10.1074/jbc.M414124200. [DOI] [PubMed] [Google Scholar]
- 15.Ingvarsson H, et al. Insights into the inter-ring plasticity of caseinolytic proteases from the X-ray structure of Mycobacterium tuberculosis ClpP1. Acta Crystallogr D Biol Crystallogr. 2007;63(Pt 2):249–259. doi: 10.1107/S0907444906050530. [DOI] [PubMed] [Google Scholar]
- 16.Kang SG, Maurizi MR, Thompson M, Mueser T, Ahvazi B. Crystallography and mutagenesis point to an essential role for the N-terminus of human mitochondrial ClpP. J Struct Biol. 2004;148(3):338–352. doi: 10.1016/j.jsb.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Kim DY, Kim KK. The structural basis for the activation and peptide recognition of bacterial ClpP. J Mol Biol. 2008;379(4):760–771. doi: 10.1016/j.jmb.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 18.Lee BG, et al. Structures of ClpP in complex with acyldepsipeptide antibiotics reveal its activation mechanism. Nat Struct Mol Biol. 2010;17(4):471–478. doi: 10.1038/nsmb.1787. [DOI] [PubMed] [Google Scholar]
- 19.Szyk A, Maurizi MR. Crystal structure at 1.9A of E. coli ClpP with a peptide covalently bound at the active site. J Struct Biol. 2006;156(1):165–174. doi: 10.1016/j.jsb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Kim SJ, Matsuoka S, Patti GJ, Schaefer J. Vancomycin derivative with damaged D-Ala-D-Ala binding cleft binds to cross-linked peptidoglycan in the cell wall of Staphylococcus aureus. Biochemistry. 2008;47(12):3822–3831. doi: 10.1021/bi702232a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schelin J, Lindmark F, Clarke AK. The clpP multigene family for the ATP-dependent Clp protease in the cyanobacterium Synechococcus. Microbiology. 2002;148(Pt 7):2255–2265. doi: 10.1099/00221287-148-7-2255. [DOI] [PubMed] [Google Scholar]
- 22.Stanne TM, Pojidaeva E, Andersson FI, Clarke AK. Distinctive types of ATP-dependent Clp proteases in cyanobacteria. J Biol Chem. 2007;282(19):14394–14402. doi: 10.1074/jbc.M700275200. [DOI] [PubMed] [Google Scholar]
- 23.Viala J, Mazodier P. ClpP-dependent degradation of PopR allows tightly regulated expression of the clpP3 clpP4 operon in Streptomyces lividans. Mol Microbiol. 2002;44(3):633–643. doi: 10.1046/j.1365-2958.2002.02907.x. [DOI] [PubMed] [Google Scholar]
- 24.Viala J, Rapoport G, Mazodier P. The clpP multigenic family in Streptomyces lividans: Conditional expression of the clpP3 clpP4 operon is controlled by PopR, a novel transcriptional activator. Mol Microbiol. 2000;38(3):602–612. doi: 10.1046/j.1365-2958.2000.02155.x. [DOI] [PubMed] [Google Scholar]
- 25.Zeiler E, et al. Vibralactone as a tool to study the activity and structure of the ClpP1P2 complex from Listeria monocytogenes. Angew Chem Int Ed Engl. 2011;50(46):11001–11004. doi: 10.1002/anie.201104391. [DOI] [PubMed] [Google Scholar]
- 26.Akopian T, et al. The active ClpP protease from M. tuberculosis is a complex composed of a heptameric ClpP1 and a ClpP2 ring. EMBO J. 2012;31(6):1529–1541. doi: 10.1038/emboj.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Böttcher T, Sieber SA. Beta-lactones decrease the intracellular virulence of Listeria monocytogenes in macrophages. ChemMedChem. 2009;4(8):1260–1263. doi: 10.1002/cmdc.200900157. [DOI] [PubMed] [Google Scholar]
- 28.Raju RM, et al. Mycobacterium tuberculosis ClpP1 and ClpP2 function together in protein degradation and are required for viability in vitro and during infection. PLoS Pathog. 2012;8(2):e1002511. doi: 10.1371/journal.ppat.1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Böttcher T, Sieber SA. β-lactones as specific inhibitors of ClpP attenuate the production of extracellular virulence factors of Staphylococcus aureus. J Am Chem Soc. 2008;130(44):14400–14401. doi: 10.1021/ja8051365. [DOI] [PubMed] [Google Scholar]
- 30.Huisgen R. 1,3 Dipolar Cylcoaddition Chemistry. New York: Wiley; 1984. pp. 1–176. [Google Scholar]
- 31.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed Engl. 2002;41(14):2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 32.Tornøe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67(9):3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 33.Gersch M, et al. The mechanism of caseinolytic protease (ClpP) inhibition. Angew Chem Int Ed Engl. 2013;52(10):3009–3014. doi: 10.1002/anie.201204690. [DOI] [PubMed] [Google Scholar]
- 34.Lee BG, Kim MK, Song HK. Structural insights into the conformational diversity of ClpP from Bacillus subtilis. Mol Cells. 2011;32(6):589–595. doi: 10.1007/s10059-011-0197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimber MS, et al. Structural and theoretical studies indicate that the cylindrical protease ClpP samples extended and compact conformations. Structure. 2010;18(7):798–808. doi: 10.1016/j.str.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Bewley MC, Graziano V, Griffin K, Flanagan JM. Turned on for degradation: ATPase-independent degradation by ClpP. J Struct Biol. 2009;165(2):118–125. doi: 10.1016/j.jsb.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brötz-Oesterhelt H, et al. Dysregulation of bacterial proteolytic machinery by a new class of antibiotics. Nat Med. 2005;11(10):1082–1087. doi: 10.1038/nm1306. [DOI] [PubMed] [Google Scholar]
- 38.Li DH, et al. Acyldepsipeptide antibiotics induce the formation of a structured axial channel in ClpP: A model for the ClpX/ClpA-bound state of ClpP. Chem Biol. 2010;17(9):959–969. doi: 10.1016/j.chembiol.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snijder HJ, et al. Structural investigations of the active-site mutant Asn156Ala of outer membrane phospholipase A: Function of the Asn-His interaction in the catalytic triad. Protein Sci. 2001;10(10):1962–1969. doi: 10.1110/ps.17701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kabsch W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J Appl Cryst. 1993;26(6):795–800. [Google Scholar]
- 41.McCoy AJ, et al. Phaser crystallographic software. J Appl Cryst. 2007;40(Pt 4):658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.