Abstract
Epstein-Barr virus (EBV) is a human oncogenic herpesvirus associated with epithelial cell and B-cell malignancies. EBV infection of B lymphocytes is essential for acute and persistent EBV infection in humans; however, the role of epithelial cell infection in the normal EBV life cycle remains controversial. The rhesus lymphocryptovirus (LCV) is an EBV-related herpesvirus that naturally infects rhesus macaques and can be used experimentally to model persistent B-cell infection and B-cell lymphomagenesis. We now show that the rhesus LCV can infect epithelial cells in immunosuppressed rhesus macaques and can induce epithelial cell lesions resembling oral hairy leukoplakia in AIDS patients. Electron microscopy, immunohistochemistry, and DNA-RNA in situ hybridization were used to identify the presence of a lytic rhesus LCV infection in these proliferative, hyperkeratotic, or parakeratotic epithelial cell lesions. These studies demonstrate that the rhesus LCV has tropism for epithelial cells, in addition to B cells, and is a relevant animal model system for studying the role of epithelial cell infection in EBV pathogenesis.
Epstein-Barr virus (EBV) can infect both lymphocytes and epithelial cells. EBV infection of B lymphocytes is a key step in the pathogenesis of acute EBV infection, persistent EBV infection, and EBV-associated B-cell malignancies (4, 7). The role of epithelial cell infection in EBV biology is more controversial. The association of EBV infection with nasopharyngeal and gastric carcinomas clearly demonstrates that EBV can infect epithelial cells, and latent EBV infection is believed to contribute to the malignant transformation of epithelial cells in these instances (14, 21, 23, 25). EBV infection and lytic replication in lingual epithelial cells can also cause oral hairy leukoplakia in immunosuppressed AIDS patients (8). However, it is less certain whether EBV infection of epithelial cells is a routine, or required, component of the virus life cycle in all individuals or whether EBV infection of epithelial cells is an atypical event associated with immunosuppression or an increased risk of oral malignancies.
The traditional paradigm for primary EBV infection places virus infection and replication in oral epithelial cells as a critical step between oral inoculation of virus and invasion of the peripheral blood B-cell compartment (22). However, this model has been challenged by the inability to detect EBV infection in the tonsillar epithelium of infectious mononucleosis patients (10, 13). This has led some to speculate that EBV infection of epithelial cells is not an important step for primary EBV infection and that orally inoculated virus may bypass epithelial cells and directly infect B cells. The apparent lack of persistent EBV infection and virus in oral secretions of patients with X-linked agammaglobulinemia who lack B cells is also consistent with the possibility that epithelial cell infection is not important and that the principal source of infectious EBV in oral secretions is from EBV-infected B cells in tonsillar tissue rather than infected epithelial cells (4).
Other explanations may contribute to the apparent inability to detect EBV infection in epithelial cells of immunocompetent hosts. The tonsil may not be the optimal site for EBV infection of oral epithelium, and patients presenting with clinically advanced infectious mononucleosis requiring tonsillectomy may be beyond the optimal time points for peak epithelial cell infection. Persistent EBV infection in epithelial cells may be cryptic in immunocompetent hosts, with only occasional latently infected cells dispersed in various anatomical locations. Patterns of viral gene expression may be different in epithelial cells than in B cells. For example, in situ hybridization (ISH) for the abundant small EBV-encoded RNAs (EBERs) is frequently used as a sensitive assay for EBV infection in B cells, but EBERs are not commonly expressed in the EBV-infected epithelial cells of oral hairy leukoplakia (6). Reactivation of lytic EBV infection in epithelial cells should result in easily detectable viral gene expression, but reactivation may be occurring in sites that are difficult to access and biopsy in otherwise healthy individuals. Thus, it remains uncertain whether epithelial cell infection with EBV is a normal component of the acute and persistent EBV life cycle.
Studies of nonhuman primate herpesviruses closely related to and in the same lymphocryptovirus (LCV) genus as EBV can provide an animal model system to study EBV pathogenesis (12). The rhesus LCV genome has been completely sequenced, and the viral gene repertoire is identical to that of EBV (18). Like EBV, the rhesus LCV efficiently immortalizes B cells in tissue culture (15). As is the case for humans, nearly all rhesus monkeys raised in conventional domestic colonies have a persistent, asymptomatic rhesus LCV infection that can be detected in the peripheral blood lymphocytes of healthy, seropositive animals (16). Rhesus LCV is also associated with the development of B-cell lymphomas in immunosuppressed monkeys (9, 15). Thus, there is considerable genetic and biologic evidence that rhesus LCV infection of B cells is very similar to EBV infection. Viral DNA can be detected in the oral secretions of persistently infected monkeys, and naive rhesus macaques can be successfully infected by experimental oral virus inoculation, suggesting that the mode of transmission is also similar to that of EBV infection in humans (12). However, to date there has been no definitive evidence that the rhesus LCV can infect epithelial cells. In the present study, we describe naturally occurring rhesus LCV-infected epithelial cell lesions in immunosuppressed macaques that resemble oral hairy leukoplakia. These studies demonstrate that like EBV, rhesus LCV has tropism for both B cells and epithelial cells in vivo and that this animal model system should be useful for exploring the role of epithelial cells in EBV infection.
MATERIALS AND METHODS
Animals.
Histopathologic reports for 585 simian immunodeficiency virus (SIV)-infected rhesus macaques necropsied between 1997 and 2002 at the New England Primate Research Center were reviewed, and eight cases with ballooning degeneration of mucosal or epidermal epithelial cells containing intranuclear inclusion bodies suggestive of herpesvirus infection were identified. All macaques were assigned to studies funded by the National Institutes of Health that were conducted in accordance with federal and institutional guidelines for animal care. The monkeys in this report were experimentally inoculated with SIVmac251, SIVmac239, SIVM5, or SIVmac239/YE, which have been previously described (3, 11, 17). Briefly, SIVmac251 is an uncloned pathogenic virus. SIVmac239 is a molecularly cloned pathogenic virus, SIVM5 lacks five N-glycan attachment sites from SIVmac239 Env, and SIVmac239/YE has two amino acid changes in the Nef region of SIVmac239. All animals in this study acquired LCV infection naturally. The macaques were euthanized either at the study endpoint or on the basis of established criteria for SIV-infected macaques with end-stage AIDS. Thorough necropsies were performed, and tissue specimens of all organs were collected for fixation in 10% buffered formalin.
Electron microscopy.
A 5.0-μm section of formalin-fixed esophagus (A02-303) immunostained with anti-BZLF1 was processed for transmission electron microscopy as previously described (2). In brief, toluene was used to remove the coverslip and in the preparation of the fixative and resin. The fixed tissue was postfixed in 1% osmium tetroxide (Stevens Metallurgical Corp., New York, N.Y.), dehydrated, and embedded in eponate 12 resin (Ted Pella, Redding, Calif.). Ultrathin sections were cut on a Leica Ultracut R ultramicrotome and stained with uranyl acetate and Sato's lead stain (19). The sections were examined on a JEOL 1010 transmission electron microscope.
Rhesus LCV serology.
Serum antibodies to the rhesus LCV small viral capsid antigen (sVCA) were detected by using an sVCA peptide enzyme immunoassay as previously described (16).
EBER ISH.
RNA ISH for EBER expression was performed as previously described (20).
CISH.
Chromogenic ISH (CISH) for the presence of LCV DNA was performed on formalin-fixed, paraffin-embedded tissue after pretreatment with methanol-0.5% peroxide for 20 min followed by proteinase K (1:5 dilution; Dako, Carpinteria, Calif.) at room temperature for 10 min. Rhesus LCV cosmids CC1, QA15, and LV28 (18) were mixed in equal portions, and the rhesus LCV DNA was labeled with digoxigenin (Roche). Hybridization with the digoxigenin-labeled rhesus LCV DNA probe was carried out for 10 min at 75°C followed by 4 h at 37°C. Slides were washed three times and incubated with 10% goat serum in 50 mM Tris-Cl (pH 7.4) for 20 min to block nonspecific binding sites. Next, rabbit antidigoxigenin (1:150 dilution; Dako) was applied in 50 mM Tris-HCl (pH 7.4) with 3% goat serum at room temperature for 1 h. Slides were washed in 50 mM Tris-HCl (pH 7.4), and goat anti-rabbit horseradish peroxidase-conjugated antibody (Envision+ detection kit; Dako) was applied for 30 min. After further washing, immunoperoxidase staining was developed by using a diaminobenzidine (DAB) chromogen kit (Dako) according to the instructions of the manufacturer and counterstained with methyl green counterstain.
Anti-BZLF1 and anti-EBNA-2 immunohistochemistry.
Immunohistochemistry for LCV immediate-early viral lytic protein (BZLF1, clone BZ.1; Dako) and EBV-encoded nuclear antigen 2 (EBNA-2, clone PE2; Dako) was performed on 5.0-μm sections from formalin-fixed, paraffin-embedded tissues. Both antibodies are known to cross-react with the respective rhesus LCV homologues based on the detection of an appropriately sized protein by Western blot analysis of cells transfected with recombinant expression plasmids and expression of the respective recombinant rhesus LCV genes. The sections were deparaffinized and rehydrated. Endogenous peroxidase activity was quenched with a hydrogen peroxide block for 5 min. Antigen retrieval was achieved by microwaving for 20 min. The sections were treated with Dako Protein Block for 10 min and incubated at 4°C overnight with the primary antibody (BZLF1 diluted 1:80 or EBNA-2 diluted 1:6400). The secondary antibody, biotinylated horse anti-mouse immunoglobulin G (Vector Laboratories, Burlingame, Calif.) diluted 1:200, was applied for 30 min, followed by a 30-min incubation with Vectastain ABC Elite. The reaction was visualized by using DAB chromogen (Dako), and the slides were counterstained with Mayer's hematoxylin.
Anti-sVCA immunohistochemistry.
Immunohistochemistry for LCV sVCA (anti-sVCA-p18; BFRF3) was performed with 5.0-μm-thick formalin-fixed, paraffin-embedded tissue sections. Briefly, slides were deparaffinized and pretreated with 10 mM sodium citrate buffer (pH 6.0) (Zymed, South San Francisco, Calif.) in a steam pressure cooker (Decloaking Chamber; BioCare Medical, Walnut Creek, Calif.) according to the manufacturer's instructions, followed by washing in distilled water. All further steps were performed at room temperature in a hydrated chamber. Slides were pretreated with Peroxidase Block (Dako) for 5 min to quench endogenous peroxidase activity followed by a 1:5 dilution of goat serum in 50 mM Tris-HCl (pH 7.4) for 20 min to block nonspecific binding sites. Primary rat anti-sVCA antibody (OT15E, anti-sVCA-p18) (BFRF3; Cyto-Barr BV, Bergen, The Netherlands) was applied at a 1:250 dilution in 50-mM Tris-HCl (pH 7.4) with 3% goat serum for 1 h. After washing in 50 mM Tris-HCl (pH 7.4), secondary rabbit anti-rat antibody (Dako) was applied at a 1:7,500 dilution in 50 mM Tris-HCl (pH 7.4) with 3% goat serum for 30 min. Slides were washed again in 50 mM Tris-HCl (pH 7.4), and goat anti-rabbit horseradish peroxidase-conjugated antibody (Envision+ detection kit; DAKO) was applied for 30 min. After further washing, immunoperoxidase staining was developed by using a DAB chromogen kit (Dako) according to the instructions of the manufacturer and counterstained with Harris hematoxylin.
RESULTS
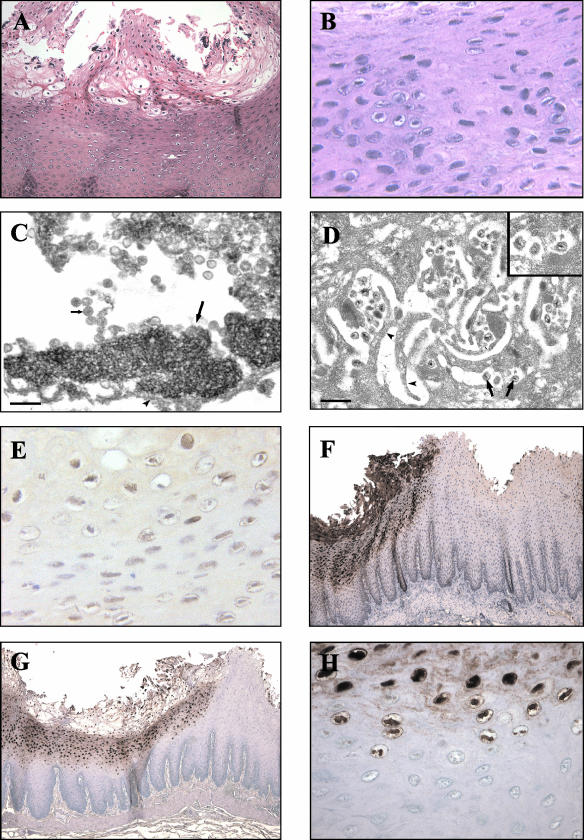
Epithelial abnormalities suggestive of viral infection were noted by light microscopic examination of hematoxylin- and eosin-stained sections from routine necropsies of SIV-infected rhesus macaques at the New England Primate Research Center from 1997 to 2002. These lesions were found most frequently within the mucosal tissues of the upper digestive tract (tongue in four cases and esophagus in five cases); however, in one case (A98-563), a hyperkeratotic lesion of the skin of the penis was grossly apparent (Table 1). Similar lesions have not been observed in routine necropsies of immunocompetent animals. The histologic findings were similar to those seen in oral hairy leukoplakia, a proliferative epithelial cell lesion most frequently seen on the tongue and associated with EBV infection in AIDS patients (8). The macaque epithelial cell lesions were characterized by irregularities or corrugations in a hyperplastic epithelium with mounds of parakeratotic cells at the tips of prominent papillae (Fig. 1A). Within the prickle and functional cell layers of the esophagus or stratum spinosum of the epidermis, a patchy band of lightly stained “balloon cells” or koilocytes was present (Fig. 1A and B). Nuclei within these koilocytic cells demonstrated markedly condensed chromatin with vacuolization of the surrounding nucleoplasm. No obvious abnormality in the basal epithelial cell layer was identified, and accompanying inflammation was not present.
TABLE 1.
Clinical data and viral phenotypic findings for LCV-associated epithelial lesions in SIV-infected macaques
| Monkey | Age | Virus | Mo post- infection | LCV serology | CD4+ cell count | Cause of death | Site of lesion | DNA ISH | BZLF1 | EBNA-2 | sVCA | EBERs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A97-123 | 11 yr, 4 mo | SIVmac239YE | 30 | + | NDa | Disseminated lymphoma | Esophagus | + | + | ND | ND | + |
| A02-303 | 5 yr, 6 mo | SIV M5 mutant | 28 | + | 165 | Lymphoproliferative disease, EPECb colitis | Tongue, esophagus | + | + | + | + | − |
| A02-563 | 3 yr, 4 mo | SIVmac239 | 22 | + | 378 | Mural thrombus right ventricle, SIV pulmonary arteriopathy, lymphoproliferative disease | Tongue, esophagus | + | + | + | + | − |
| A98-563 | 3 yr, 3 mo | SIVmac239 | 22 | + | 72 | Disseminated Mycobacterium avium | Penis | + | + | − | + | − |
| A01-643 | 4 yr, 5 mo | SIVmac239 | 20 | ND | ND | Lymphoproliferative disease, cholangiohepatitis | Esophagus | + | + | + | + | − |
| A99-75 | 1 yr, 10 mo | SIVmac239 | 8 | + | 1,045 | Disseminated cryptosporidiosis, cytomegalovirus meningitis and neuritis, simlan virus 40 nephritis | Tongue | + | + | + | + | − |
| A00-467 | 1 yr, 3 mo | SIVmac239 | 7 | + | ND | Disseminated lymphoma | Tongue | ND | + | + | ND | ND |
| A01-66 | 4 yr, 1 mo | SIVmac251 | 13 | + | 33 | Lymphoma, transitional cell carcinoma | Esophagus | ND | + | − | ND | ND |
ND, not done.
EPEC, enteropathogenic Escherichia coli.
FIG. 1.
Morphological, electron microscopic, and viral phenotypic findings for a representative mucosal lesion in an SIV-infected rhesus macaque (A02-303). (A and B) Hematoxylin- and eosin-stained section of a mucosal lesion with hyperparakeratosis and prominent balloon cells within the upper epithelial cell layers. Magnifications, ×100 (A) and ×400 (B). (C) Transmission electron micrograph showing the nucleus of a degenerative mucosal epithelial cell undergoing karyolysis with marginated chromatin (long arrow) and numerous intranuclear herpesvirus nucleocapsids (short arrow). The arrowhead indicates the nuclear membrane. Bar, 200 nm. (D) Transmission electron micrograph showing an area of mucosa revealing herpesvirus virions (arrows) within the intercellular spaces between interdigitating cytoplasmic projections (arrowheads) of adjacent epithelial cells. Bar, 500 nm. The inset shows a higher magnification of virions which measure 160 nm in diameter. (E and F) Immunohistochemical staining showing expression of BZLF1 (magnification, ×400) (E) and sVCA (magnification, ×50) (F). (G and H) CISH demonstrating strong nuclear staining with a probe specific for LCV DNA. Magnifications, ×50 (G) and ×400 (H).
Sections from A02-303 were examined by electron microscopy to identify potential virus particles. High magnification of a cell nucleus from the prickle cell layer (Fig. 1C) shows marginated chromatin in a nucleus filled with nucleocapsids compatible in size (approximately 100 nm) with a herpesvirus. Structures resembling enveloped virions with a size of approximately 160 nm were visible in regions consistent with the interdigitating cytoplasmic processes of adjacent epithelial cells (Fig. 1D). Thus, electron microscopy confirmed the presence of marginated chromatin, nucleocapsids, and enveloped viruses consistent with an active herpesvirus infection.
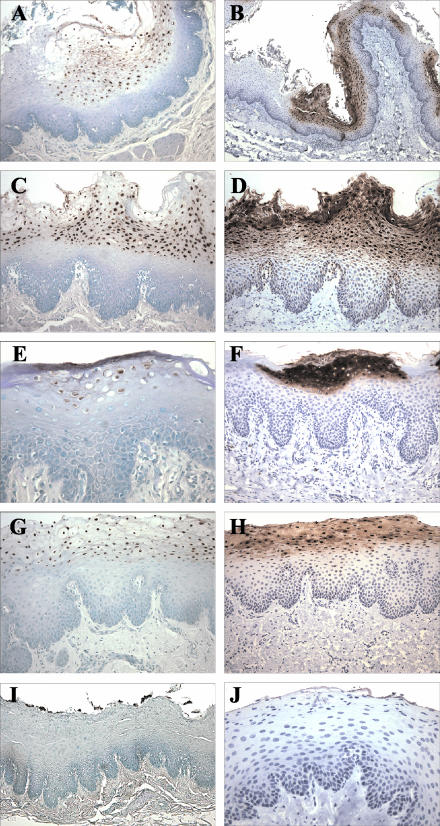
Since the hyperplastic mucosal epithelium and evidence for active herpesvirus infection were reminiscent of oral hairy leukoplakia, additional studies were focused on definitively identifying whether infection with an EBV-related herpesvirus was associated with the macaque epithelial lesions. Testing of available serum samples showed that all animals had serologic evidence of infection with the EBV-related LCV that naturally infects rhesus macaques (Table 1). Histologic sections were stained with an EBV BZLF1 monoclonal antibody that was known to cross-react with the rhesus LCV BZLF1 homologue. All lesions were positive for the immediate-early viral lytic protein, BZLF1 (Fig. 1E and Table 1). To provide confirmation and further characterization of lytic infection in these lesions, a second monoclonal antibody, OT15E, which is specific for the EBV sVCA, was identified based on its ability to cross-react with an 18-kDa protein in rhesus LCV-infected cells induced for lytic viral replication (data not shown). Immunohistochemistry with the OT15E antibody showed strong expression of sVCA in all lesions examined, demonstrating active lytic infection throughout the histologically abnormal areas of all lesions (Fig. 1F and 2B, D, F, and H; Table 1). Both BZLF1 and sVCA antibodies detected viral gene expression in virtually all cells with intranuclear inclusions and were restricted to cells in the upper stratified epithelium. There was a sharp demarcation with no staining of adjacent normal epithelium, and control sections from animals without epithelial cell lesions did not stain with the BZLF1 and sVCA antibodies, demonstrating the specificity of the immunohistochemical staining (Fig. 2J).
FIG. 2.
CISH for LCV DNA (A, C, E, G, and I) and immunohistochemical staining for sVCA (anti-sVCA-p18; BFRF3) (B, D, F, H, and J) in epithelial lesions from SIV-infected rhesus macaques A01-643 (A and B), A02-563 (C and D), A98-563 (E and F), and A99-75 (G and H) and in control nonlesional tissue from macaque A98-118 (I and J). Magnifications, ×100 (A, C, D, F, G, and H), ×50 (B and I), and ×200 (E and J).
Infection with rhesus LCV was also confirmed by DNA ISH with cloned rhesus LCV DNA probes. Cells with intranuclear inclusions were strongly positive by DNA ISH, consistent with viral DNA replication (Fig. 1G and H and 2A, C, E, and G). Histologically normal epithelium was negative (Fig. 2I). DNA ISH with the rhesus LCV DNA probes is specific for rhesus LCV infection, cross-reacting only weakly with EBV-infected cells and not at all with cells infected with the closely related gamma-2 herpesvirus rhesus rhadinovirus (data not shown). DNA CISH and immunohistochemistry (BZLF1 and sVCA) generally stained identical areas of abnormal epithelial cell histology; i.e., most cells were positive for LCV DNA, BZLF1, and sVCA expression (Fig. 2). DNA CISH and sVCA immunohistochemistry showed a gradient of increasing intensity in the upper levels of the stratified epithelium, consistent with progressive lytic infection (Fig. 1F and G and 2), and BZLF1 expression was generally more uniform throughout the epithelial cell layers (Fig. 1E and data not shown). Thus, in five cases, there is strong expression of an immediate-early lytic viral protein, a structural viral capsid protein, and abundant viral DNA, which definitively identify an active rhesus LCV infection in these epithelial cell lesions. There was no convincing evidence for latent LCV infection in the more undifferentiated cells of the basal layer, i.e., cells that were positive for an episomal pattern of viral DNA and negative for BZLF1 expression; however, at this time, technical issues limit the ability to perform DNA CISH and BZLF1 immunohistochemistry on the same tissue sections, and rare cells of this type may be missed by studies of serial tissue sections.
Five of seven lesions were also positive for EBNA-2 expression by using a monoclonal antibody that is known to detect a conserved epitope in the carboxy-terminal acidic transactivating domain of the EBV, baboon, and rhesus LCV EBNA-2 homologues (Table 1). EBNA-2 is an EBV latent infection protein and is not typically associated with lytic replication. However, aberrant EBNA-2 expression has also been described in association with active viral replication in oral hairy leukoplakia (24). ISH for the rhesus LCV EBER homologues was negative in five of six cases, and in one case epithelial cells were positive for EBER expression (Table 1). These small, nonpolyadenylated RNAs are typically expressed at very high copy numbers in latently infected cells, and EBER ISH is commonly used to detect EBV infection in tissue sections. However, the frequent lack of EBER expression in the rhesus epithelial cell lesions is similar to the lack of EBER expression reported in EBV-associated oral hairy leukoplakia from AIDS patients (6). In addition, EBER ISH, EBNA-2 immunohistochemistry, and DNA CISH failed to detect LCV-positive lymphocytes infiltrating the subepithelial cell layers associated with these lesions.
DISCUSSION
These are the first studies to unequivocally identify that rhesus LCV can infect and is associated with disease in epithelial cells. These studies show that the rhesus LCV is similar to EBV, with cell tropism and a disease spectrum that includes epithelial cells, in addition to B lymphocytes. The role of epithelial cell infection in the normal life cycle of EBV infection is controversial. EBV infection can clearly spread to and cause disease in epithelial cells, but whether EBV routinely infects and persists in epithelial cells in healthy, immunocompetent hosts is unclear. The presence of rhesus LCV infection in epithelial cells demonstrates that the epithelial cell tropism is not peculiar to EBV infection of humans but is likely shared by other EBV-related herpesviruses in the LCV genus.
The histologic appearance of these proliferative, hyperkeratotic, and parakeratotic epithelial cell lesions is very similar to that of oral hairy leukoplakia in AIDS patients. The electron microscopic appearance, immunohistochemical staining for an immediate-early protein and a viral capsid protein, and strong DNA ISH clearly demonstrate the association of these epithelial cell lesions with rhesus LCV infection in multiple cases. Furthermore, they characterize the rhesus LCV infection as an active lytic process in the lesions, similar to that of EBV infection in oral hairy leukoplakia. Baskin et al. had previously observed squamous epithelial lesions with intranuclear inclusions in eight SIV-infected macaques (1). Hyperkeratosis and acanthosis were present in lesions on external epithelial cell surfaces (e.g., penis, chest skin, and hand), and lesions on internal epithelial surfaces (e.g., tongue and esophagus) were characterized by intranuclear inclusions and ballooning degeneration but without hyperkeratosis. Bacterial and fungal infections with inflammation were also frequently found associated with these lesions. The data implicating infection with an EBV-related herpesvirus were limited to one of four tissue samples that stained with a monoclonal antibody against the EBV LMP1 protein and positive DNA ISH with a 3.1-kb EBV DNA probe in one of three cases. In our hands, the EBV LMP1 monoclonal antibodies do not cross-react with the rhesus LCV LMP1 homologue. In addition, the rhesus LCV genome sequence reveals that the LMP1 gene is one of the least well-conserved genes between EBV and rhesus LCV, with no significant amino acid homology in the carboxy terminus targeted by various EBV LMP1 monoclonal antibodies (5). Overall, the virologic data linking an EBV-related herpesvirus to these lesions were limited. We did not find any characteristic epithelial cell lesions that failed to test positive for rhesus LCV DNA, but we cannot rule out the possibility that other viruses or pathogens may be associated with similar lesions in immunosuppressed hosts.
The presence of lesions on external epithelial cell surfaces suggests that infection may have occurred as a result of biting and secondary infection from oral secretions containing virus. In six of seven of our cases, the rhesus LCV-positive lesions occurred on internal sites, i.e., in the esophagus and/or tongue, eliminating self-induced trauma as a potential portal of infection. The frequent involvement of the esophagus in SIV-infected macaques raises the question of whether this might also be a common site for EBV-induced epithelial cell abnormalities in AIDS patients. At these internal sites, epithelial cell infection most likely originated from viremia and infection through the basal epithelial cell surfaces, but one cannot rule out the possibility of infection from virus in oral secretions bathing and infecting the apical epithelial cell surface. The DNA ISH technique used here has the sensitivity to detect single-copy episomes in paraffin-embedded tissue (J. L. Kutok et al., unpublished data). Thus, it is interesting that no low-copy viral infection was noted in the basal epithelial cell layers, suggesting that these lesions are not associated with persistent, latent rhesus LCV infection in the basal epithelium.
Multiple questions regarding EBV infection of epithelial cells in immunocompetent, healthy hosts remain unresolved. Are oral epithelial cells the first cells infected with incoming virus in primary EBV infection? Is viral replication in oral epithelial cells required for entry into the peripheral blood compartment during primary EBV infection? Does EBV persist and latently infect oral epithelial cells in convalescent, seropositive hosts? Is viral replication in oral epithelial cells the source of virus shed in the oral secretions of persistently infected hosts? The rhesus LCV model can be helpful for investigating these questions by allowing for more thorough dissection of the oral and upper digestive tracts of naturally and experimentally infected animals to search for reservoirs of virus infection. In addition, the experimental rhesus LCV model is conducive for kinetic studies immediately after viral infection, whereas studies with humans are limited to clinical presentation with infectious mononucleosis as the earliest time point for potential study. The development of genetic systems for rhesus LCV will provide an experimental system for studying alterations in viral genes that may affect epithelial cell infection or for using markers to more easily identify isolated foci of viral infection. The development of reagents and probes to detect rhesus LCV infection will be particularly important for detecting reservoirs of LCV infection in the oral cavity, as these sites are likely to be subtle in immunocompetent hosts. The complete rhesus LCV genome sequence is useful for identifying well-conserved targets where existing EBV-specific monoclonal antibodies, e.g., the EBV BZLF1 and sVCA monoclonal antibodies used in the present studies, might cross-react. In addition, the development of a sensitive rhesus LCV DNA ISH assay for paraffin-embedded tissue provides an important assay for detection of low-copy rhesus LCV infection independent of viral gene expression. These are important new tools for demonstrating epithelial cell tropism in rhesus LCV infection and will aid in the use of this model system to investigate the role of epithelial cell infection in EBV pathogenesis.
Acknowledgments
This work was supported by Public Health Service grants DE14388 and CA68051. Services from the New England Primate Research Center were supported by a base grant to the institution (Public Health Service grant P51RR00168).
We thank Kristen Toohey for photographic assistance and Janice Williams for histologic work.
REFERENCES
- 1.Baskin, G. B., E. D. Roberts, D. Kuebler, L. N. Martin, B. Blauw, J. Heeney, and C. Zurcher. 1995. Squamous epithelial proliferative lesions associated with rhesus Epstein-Barr virus in simian immunodeficiency virus-infected rhesus monkeys. J. Infect. Dis. 172:535-539. [DOI] [PubMed] [Google Scholar]
- 2.Chien, K., R. Van de Velde, and R. Heusser. 1982. A one-step method for re-embedding paraffin embedded specimens for electron microscopy, p. 356-357. In G. W. Bailey (ed.), Fortieth Annual Proceedings of the EMSA Meeting. Claitor’s Publishing Co., Baton Rouge, La.
- 3.Du, Z., S. M. Lang, V. G. Sasseville, A. A. Lackner, P. O. Ilyinskii, M. D. Daniel, J. U. Jung, and R. C. Desrosiers. 1995. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell 82:665-674. [DOI] [PubMed] [Google Scholar]
- 4.Faulkner, G. C., S. R. Burrows, R. Khanna, D. J. Moss, A. G. Bird, D. H. Crawford, J. W. Gratama, M. A. Oosterveer, F. E. Zwaan, J. Lepoutre, G. Klein, and I. Ernberg. 1999. X-linked agammaglobulinemia patients are not infected with Epstein-Barr virus: implications for the biology of the virus. J. Virol. 73:1555-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franken, M., O. Devergne, M. Rosenzweig, B. Annis, E. Kieff, and F. Wang. 1996. Comparative analysis identifies conserved tumor necrosis factor receptor-associated factor 3 binding sites in the human and simian Epstein-Barr virus oncogene LMP1. J. Virol. 70:7819-7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilligan, K., P. Rajadurai, L. Resnick, and N. Raab-Traub. 1990. Epstein-Barr virus small nuclear RNAs are not expressed in permissively infected cells in AIDS-associated leukoplakia. Proc. Natl. Acad. Sci. USA 87:8790-8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gratama, J. W., M. A. Oosterveer, F. E. Zwaan, J. Lepoutre, G. Klein, and I. Ernberg. 1988. Eradication of Epstein-Barr virus by allogeneic bone marrow transplantation: implications for sites of viral latency. Proc. Natl. Acad. Sci. USA 85:8693-8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenspan, J. S., D. Greenspan, E. T. Lennette, D. I. Abrams, M. A. Conant, V. Petersen, and U. K. Freese. 1985. Replication of Epstein-Barr virus within the epithelial cells of oral “hairy” leukoplakia, an AIDS-associated lesion. N. Engl. J. Med. 313:1564-1571. [DOI] [PubMed] [Google Scholar]
- 9.Habis, A., G. B. Baskin, M. Murphey-Corb, and L. S. Levy. 1999. Simian AIDS-associated lymphoma in rhesus and cynomolgus monkeys recapitulates the primary pathobiological features of AIDS-associated non-Hodgkin's lymphoma. AIDS Res. Hum. Retrovir. 15:1389-1398. [DOI] [PubMed] [Google Scholar]
- 10.Karajannis, M. A., M. Hummel, I. Anagnostopoulos, and H. Stein. 1997. Strict lymphotropism of Epstein-Barr virus during acute infectious mononucleosis in nonimmunocompromised individuals. Blood 89:2856-2862. [PubMed] [Google Scholar]
- 11.Kestler, H., T. Kodama, D. Ringler, M. Marthas, N. Pedersen, A. Lackner, D. Regier, P. Sehgal, M. Daniel, N. King, et al. 1990. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science 248:1109-1112. [DOI] [PubMed] [Google Scholar]
- 12.Moghaddam, A., M. Rosenzweig, D. Lee-Parritz, B. Annis, R. P. Johnson, and F. Wang. 1997. An animal model for acute and persistent Epstein-Barr virus infection. Science 276:2030-2033. [DOI] [PubMed] [Google Scholar]
- 13.Niedobitek, G., A. Agathanggelou, H. Herbst, L. Whitehead, D. H. Wright, and L. S. Young. 1997. Epstein-Barr virus (EBV) infection in infectious mononucleosis: virus latency, replication and phenotype of EBV-infected cells. J. Pathol. 182:151-159. [DOI] [PubMed] [Google Scholar]
- 14.Pathmanathan, R., U. Prasad, R. Sadler, K. Flynn, and N. Raab-Traub. 1995. Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N. Engl. J. Med. 333:693-698. [DOI] [PubMed] [Google Scholar]
- 15.Rangan, S. R., L. N. Martin, B. E. Bozelka, N. Wang, and B. J. Gormus. 1986. Epstein-Barr virus-related herpesvirus from a rhesus monkey (Macaca mulatta) with malignant lymphoma. Int. J. Cancer 38:425-432. [DOI] [PubMed] [Google Scholar]
- 16.Rao, P., H. Jiang, and F. Wang. 2000. Cloning of the rhesus lymphocryptovirus viral capsid antigen and Epstein-Barr virus-encoded small RNA homologues and use in diagnosis of acute and persistent infections. J. Clin. Microbiol. 38:3219-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reitter, J. N., and R. C. Desrosiers. 1998. Identification of replication-competent strains of simian immunodeficiency virus lacking multiple attachment sites for N-linked carbohydrates in variable regions 1 and 2 of the surface envelope protein. J. Virol. 72:5399-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivailler, P., H. Jiang, Y. G. Cho, C. Quink, and F. Wang. 2002. Complete nucleotide sequence of the rhesus lymphocryptovirus: genetic validation for an Epstein-Barr virus animal model. J. Virol. 76:421-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato, T. 1968. A modified method for lead staining of thin sections. J. Electron Microsc. (Tokyo) 17:158-159. [PubMed] [Google Scholar]
- 20.Schmidtko, J., R. Wang, C. L. Wu, S. Mauiyyedi, N. L. Harris, P. Della Pelle, N. Brousaides, L. Zagachin, J. A. Ferry, F. Wang, T. Kawai, D. H. Sachs, B. A. Cosimi, and R. B. Colvin. 2002. Posttransplant lymphoproliferative disorder associated with an Epstein-Barr-related virus in cynomolgus monkeys. Transplantation 73:1431-1439. [DOI] [PubMed] [Google Scholar]
- 21.Shibata, D., L. M. Weiss, K. Takada, H. zur Hausen, H. Schulte-Holthausen, G. Klein, W. Henle, G. Henle, P. Clifford, and L. Santesson. 1992. Epstein-Barr virus-associated gastric adenocarcinoma. Am. J. Pathol. 140:769-774. [PMC free article] [PubMed] [Google Scholar]
- 22.Sixbey, J. W., J. G. Nedrud, N. Raab-Traub, R. A. Hanes, and J. S. Pagano. 1984. Epstein-Barr virus replication in oropharyngeal epithelial cells. N. Engl. J. Med. 310:1225-1230. [DOI] [PubMed] [Google Scholar]
- 23.Takada, K. 2000. Epstein-Barr virus and gastric carcinoma. Mol. Pathol. 53:255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walling, D. M., A. G. Perkins, J. Webster-Cyriaque, L. Resnick, and N. Raab-Traub. 1994. The Epstein-Barr virus EBNA-2 gene in oral hairy leukoplakia: strain variation, genetic recombination, and transcriptional expression. J. Virol. 68:7918-7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.zur Hausen, H., H. Schulte-Holthausen, G. Klein, W. Henle, G. Henle, P. Clifford, and L. Santesson. 1970. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature 228:1056-1058. [DOI] [PubMed] [Google Scholar]