Significance
Porin channels in the outer membrane of Gram-negative bacteria were originally thought to be nonspecific channels that discriminate among solutes only by their gross physicochemical properties, such as size, hydrophobicity, and charge. Since the discovery of the ampicillin-binding site inside one porin channel, however, such “specific” interactions are hypothesized to play significant roles especially for the permeation of drugs. We show, by the careful examination of influx and efflux of ampicillin and benzylpenicillin, that the original nonspecific model is still valid and that it is misleading to overemphasize the role of specific binding sites.
Keywords: AcrB, OmpC
Abstract
Small, hydrophilic compounds such as β-lactams diffuse across the outer membrane of Gram-negative bacteria through porin channels, which were originally thought to be nonspecific channels devoid of any specificity. However, since the discovery of an ampicillin-binding site within the OmpF channel in 2002, much attention has been focused on the potential specificity of the channel, where the binding site was assumed either to facilitate or to retard the penetration of β-lactams. Since the earlier studies on porin permeability were done without the knowledge of the contribution of multidrug efflux pumps in the overall flux process across the cell envelope, in this study we have carefully studied both the porin permeability and active efflux of ampicillin and benzylpenicillin. We found that the influx occurs apparently by a spontaneous passive diffusion without any indication of specific binding within the concentration range relevant to the antibiotic action of these drugs, and that the higher permeability for ampicillin is totally as expected from the gross property of this drug as a zwitterionic compound. The active efflux by AcrAB was more effective for benzylpenicillin due to the stronger affinity and high degree of positive cooperativity. Our data now give a complete quantitative picture of the influx, efflux, and periplasmic degradation (catalyzed by AmpC β-lactamase) of these two compounds, and correlate closely with the susceptibility of Escherichia coli strains used here, thus validating not only our model but also the parameters obtained in this study.
The permeability of Escherichia coli porins OmpF and OmpC was studied soon after the discovery of their pore-forming functions, by reconstitution of purified proteins and by using β-lactams as probes in intact cells (1–3). The results suggested that the porin channels lack specificity, and that the permeation rates of solutes were determined by their gross physicochemical properties such as size, hydrophobicity, and charges. This conclusion was important at that time because there were indeed claims that porin channels had some specificity (4).
These 30-y-old conclusions, however, currently need reexamination because of two reasons. The first is the discovery of constitutively expressed multidrug efflux pumps that function synergistically with the barrier function of the outer membrane (5). The second was the discovery in 2002 that ampicillin (AMP) at high concentrations can produce transient blockage of OmpF channel in planar bilayer assays, a result suggesting the presence of a binding site for AMP within the channel (6). This result, reported in an article entitled “Designed to penetrate: Time-resolved interaction of single antibiotic molecules with bacterial pores” (6), led many scientists to hypothesize that the porin channels are not entirely nonspecific, and that the binding to this site accelerated the permeation of certain β-lactams (7, 8) or are even necessary for their permeation (8, 9). For example, ref. 8 states, “It has become clear that the transport of β-lactams or fluoroquinolones through OmpF-type porins is not by passive diffusion through an inert tube, but involves specific interactions with porin channels.” On the other hand, some scientists argued that this binding must cause significant retardation in AMP influx, and reported that benzylpenicillin (PEN), which cannot bind to this site, diffuses faster than AMP through OmpF (10). Unfortunately, the permeability of AMP and PEN was estimated in the era when active efflux of drugs was not known (11). Although permeability determination was possible with AMP using porin-containing liposomes (12), this system could not be used with PEN because it permeates across the lipid bilayers rapidly (13). Our previous efflux study of various acid-stable penicillins, including AMP, determined both the permeability and efflux kinetics; however, PEN could not be included because of its instability in the acidic conditions used for the termination of the reaction (14). Furthermore, an unnatural E. coli construct with a much wider mutant porin channel had to be used to facilitate the uptake of some penicillins (14).
In view of this situation, a quantitative study with high precision appeared necessary to clarify the porin-mediated influx of both AMP and PEN in E. coli. In this study, we first determined, using intact cells of efflux-deficient mutants, the influx process across the outer membrane of these drugs by coupling it with their subsequent hydrolysis by a β-lactamase in the periplasm (15), by stopping the β-lactamase reaction with a brief heating in a boiling water bath. Our results showed that the very low-affinity binding site within the porin channel does not visibly affect the permeation of AMP, and that porin channels should be treated as nonspecific channels as proposed in 1983, at least for the first approximation. We then used cells expressing the major efflux pump AcrAB, which is mostly responsible for active efflux of β-lactams (16, 17), to determine the efflux kinetics as was previously done for cephalosporins (18) and acid-stable penicillins (14), and evaluated the movement of these drugs across the cell envelope.
Results
Setting Up the Assay Conditions.
Previous studies for β-lactam influx and efflux were conducted with strains that were modified extensively to optimize their behavior during the assay, such as the overexpression of plasmid-coded β-lactamase or the mutational alteration of channel size of porin (3, 14, 15, 18). The former may lead to the leakage of significant amounts of the overproduced β-lactamase into the medium, which may affect the accuracy of the assay. The latter directly affects the permeability. It was thus desirable to use a strain with minimal modifications. In this study, we chose E. coli LA51 (19), a K-12 derivative with wild-type porins but possessing an “up-mutation” in the promoter region of chromosomal ampC β-lactamase gene, producing a 10-fold increase in AmpC activity (11). The increased hydrolysis rates in this strain were sufficient for the assay of permeability and efflux for both AMP and PEN (see below).
We constructed LA51A, the acrAB deletion mutant of LA51 by transducing ΔacrAB::kan marker from AG100A (17), and used this strain for the influx assay, and used LA51 for the efflux assay caused by AcrAB. As is well known, AcrAB is responsible for the major part of efflux of β-lactams in E. coli (17, 20). Although AcrD can make a significant contribution to β-lactam efflux when overexpressed, it is expressed at a very low level in uninduced cells (21).
Our assays always involved the determination of the velocity of hydrolysis (Vh) of penicillins by the periplasmic AmpC in intact cells. The prerequisite conditions for the assay were (i) no leakage of AmpC enzyme, (ii) the movement of the substrates is in a steady state, so that we can consider that Vh = Vin in LA51A, and Vh = Vin – Ve in LA51, where Vin and Ve denote the rate of influx and efflux, respectively. If the assay condition satisfies both, Vin or Ve can be determined from the value of Vh.
To meet condition (i), 5 mM MgCl2 was added to growth media and assay mixture, to increase the outer membrane stability and to prevent the undesirable leakage of AmpC. We have examined the possible leakage as well as the time course of hydrolysis (SI Text and Fig. S1), and the results clearly indicated that there was no significant leakage of the enzyme and that we are justified in assuming a steady-state condition during the assay.
Quantitative Analysis of Influx.
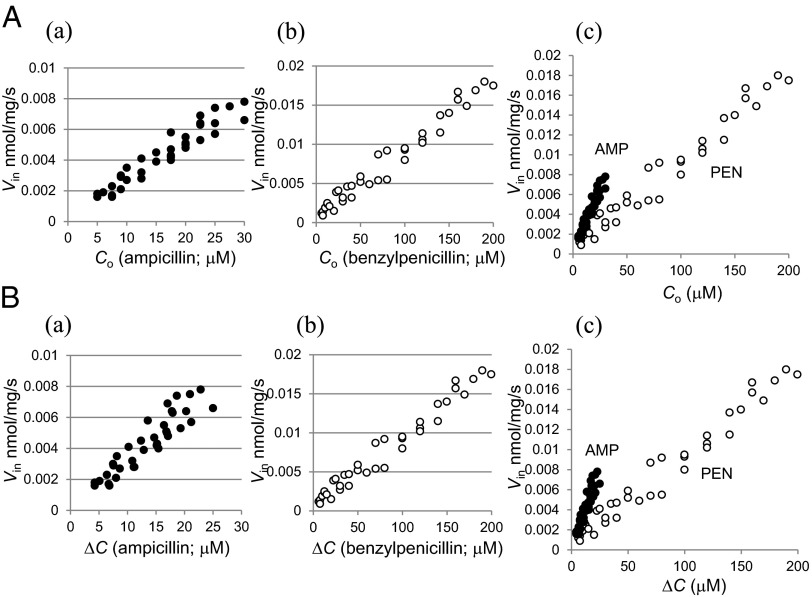
Fig. 1A shows the relationship of Vin and extracellular substrate concentration (Co) of AMP and PEN in LA51A, in which the active efflux was abolished. For both of them, Vin showed linear increase proportional to Co, displaying the characteristic of passive diffusion process. This was refined further by analyzing the correlation between Vin and the concentration gradient across the outer membrane (ΔC) at each point of Co (Fig. 1B). The periplasmic substrate concentration (Cp) was calculated by Michaelis–Menten equation of Cp = Vh × Km/(Vmax – Vh), using Vmax (0.014 and 0.255 nmol⋅mg−1⋅s−1 for AMP and PEN, respectively) and Km (4.93 and 6.21 µM for AMP and PEN) of AmpC determined with sonicated cell extract (Table 1). The results clearly showed a linear correlation between Vin and ΔC, with no sign of saturation that might be expected from the specific drug-binding site within the channel. Fick’s first law of diffusion, Vin = P × A × ΔC, where P is permeability coefficient of the outer membrane, and A is the surface area of the cell [132 cm2/mg [cell dry weight] (22)], is thus obviously applicable to describe the influx process of AMP and PEN. P values for AMP and PEN were 0.28 × 10−5 cm/s and 0.07 × 10−5 cm/s, respectively. The permeability of AMP is therefore shown to be four times higher than PEN.
Fig. 1.
Influx kinetics of AMP and PEN in LA51A. (A) Relationship between Vin and Co of AMP (a) and PEN (b). These were combined in graph (c). (B) Relationship between Vin and ΔC of AMP (a) and PEN (b). These were combined in graph (c). Abbreviations: ΔC, concentration gradient across the outer membrane (=Co – Cp); Vin, rate of influx.
Table 1.
Kinetic constants of AmpC β-lactamase
| AMP |
PEN |
|||
| Strain | Km, µM | Vmax, nmol⋅mg−1⋅s−1 | Km, µM | Vmax, nmol⋅mg−1⋅s−1 |
| LA51 | 4.48 | 0.019 | 5.28 | 0.330 |
| LA51A | 4.93 | 0.014 | 6.21 | 0.255 |
Evaluation of the Influence of Diffusion Across the Cytoplasmic Membrane.
The monoanionic, hydrophobic β-lactams were shown to diffuse across the lipid bilayer and the cytoplasmic membrane rather rapidly (13). It was thus necessary to evaluate the potential influence that this may have on the result of influx assay of PEN. When the diffusion rate across the cytoplasmic membrane, Vout, is taken into consideration of the influx assay, Vh should be described as Vh = Vin – Vout. In this equation, Vin can be derived from POM × AOM × (Co – Cp), and Vout from PCM × ACM × (Cp – Cc), where OM and CM stand for outer membrane and cytoplasmic membrane, respectively, and Cc denotes the substrate concentration in cytoplasm. Substitution and rearrangement give the following:
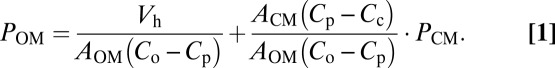 |
The degree of influence by diffusion across the cytoplasmic membrane is thus expressed in the second term of right side of Eq. 1. Within this term, AOM/ACM should be close to 1 because there should not be any significant difference between AOM and ACM, and in our influx assay of PEN, the ratio of Cp/Co was always less than 1/200. PCM can be estimated from the half-equilibration time of PEN across the cytoplasmic membrane, which was previously determined to be around 3 min (13). In E. coli cell, the half-equilibration time of 3 min corresponds to ∼1 × 10−7 cm/s of permeability coefficient (23). Hence, this term is estimated to be as follows:
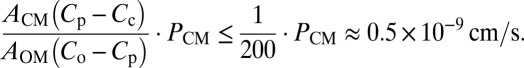 |
This corresponds to less than 0.1% of first term in the right side of Eq. 1. Thus, the influence of the diffusion across the cytoplasmic membrane seems insignificant and will not be considered in our study.
Determination of Influx Route.
For the understanding of the influx process, determining the influx route is essential. The β-lactams have been shown to diffuse mainly through the OmpF channel (2, 12, 24). We confirmed this by analyzing Vin in the LA51A with ompF or ompC deletion (Fig. 2). Deletion of ompF gene caused drastic decrease in Vin for both of AMP and PEN. Deletion of ompC, however, caused only slight decreases of Vin. The majority of AMP and PEN were thus shown to diffuse through OmpF channel, consistent with previous research.
Fig. 2.
Influx route of AMP and PEN. Vin of AMP (A) and PEN (B) was measured in LA51A, LA51A ΔompF, and LA51A ΔompC. Abbreviations: Co, extracellular substrate concentration; Vin, rate of influx.
Quantitative Analysis of Efflux.
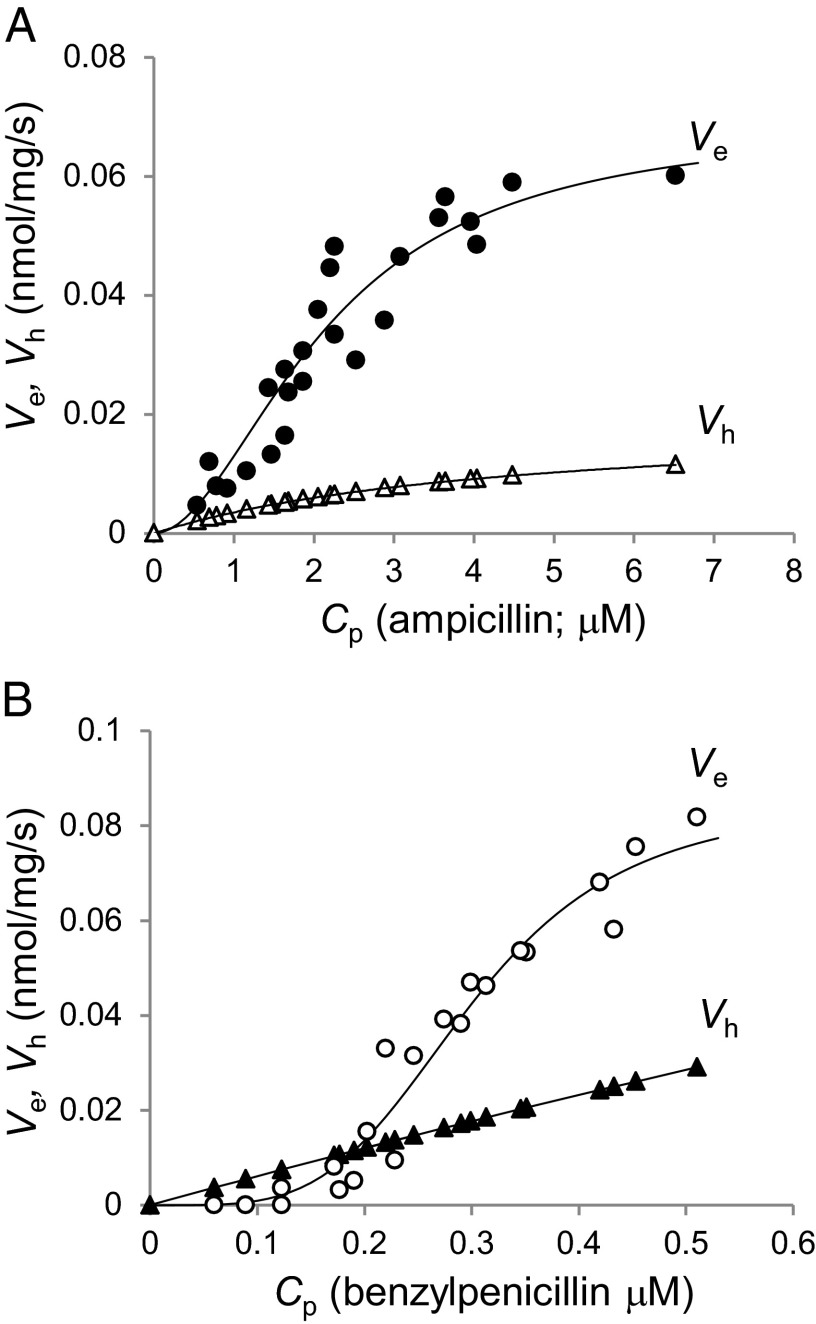
With P values obtained as above, we were now able to analyze the efflux activity using LA51, which expresses acrAB at its wild-type, constitutive level, and the equation of Ve = Vin – Vh, where Vin can be calculated from Fick’s first law of diffusion, Vin = P × A × ΔC, using the value of P obtained in the assay with LA51A (14, 18). Fig. 3 shows the efflux kinetics of AcrAB for AMP and PEN. There was significant cooperativity, and the best fit was obtained by assuming a Hill coefficient of 1.9 and 4.0 for AMP and PEN, respectively. The K0.5 value, where the efflux attains a half-maximal velocity, was 2.16 µM for AMP but was exceptionally low for PEN, 0.30 µM. Vmax was 0.069 and 0.085 nmol⋅mg cells−1⋅s−1 for AMP and PEN. The efflux kinetics for AMP showed good agreement with the previous result (14), except Vmax, which was about sixfold lower. This difference, however, seems reasonable because the previous study was conducted in a strain with deletion of acrR and the cells from stationary phase were used, conditions that enhance the expression of acrAB by about sixfold (25).
Fig. 3.
Efflux kinetics of AcrB for AMP (A) and PEN (B). Abbreviations: Cp, periplasmic substrate concentration; Ve, rate of efflux; Vh, rate of hydrolysis.
Periplasmic Drug Concentration Required for Growth Inhibition.
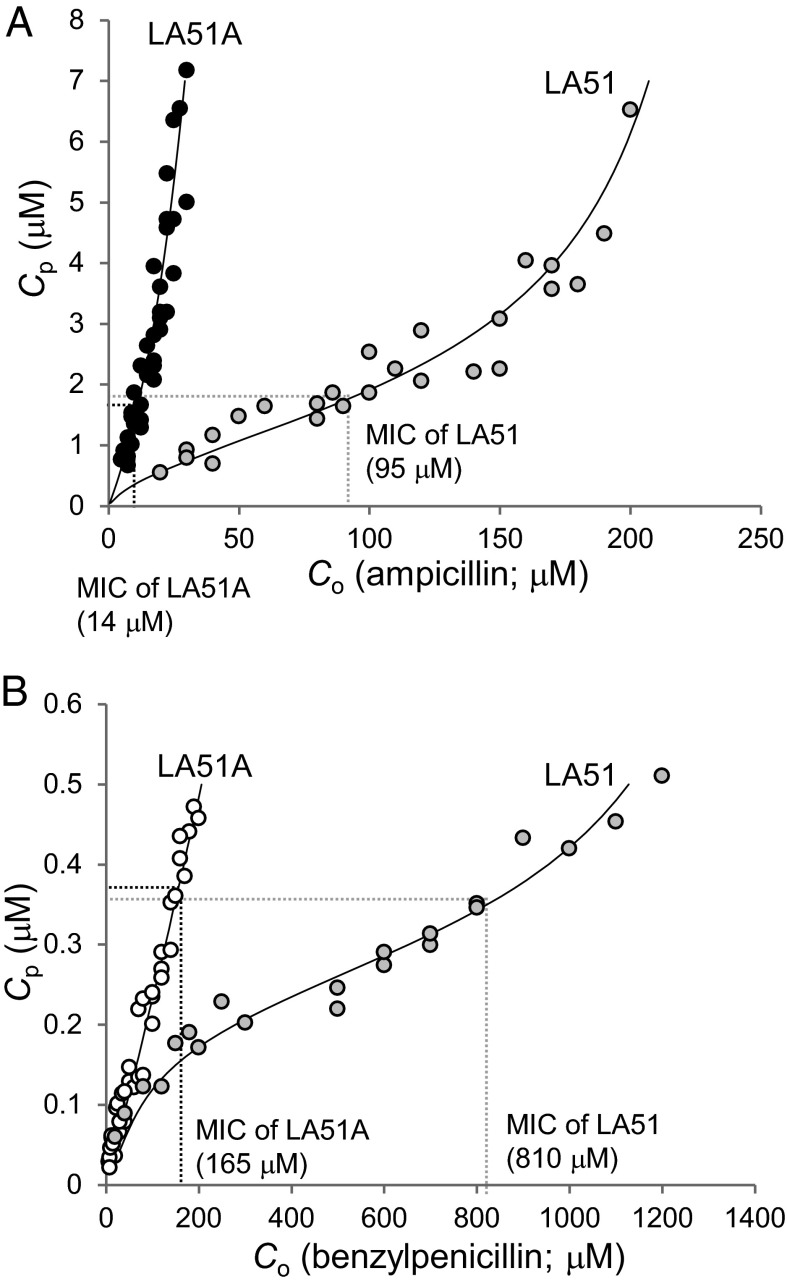
The data obtained in the experiments of Figs. 1 and 3 were replotted to show Cp values at various values of Co (Fig. 4). In this manner, we can assess the periplasmic drug concentration needed for growth inhibition. When the external concentration equals minimum inhibitory concentration (MIC), we assume that the drug reaches a critical concentration (Cinh) needed to inhibit growth (11). Determination of MIC of AMP and PEN was done first by the microdilution method, and produced values of 32 and 4 µg/mL AMP and 512 and 64 µg/mL PEN for LA51 and LA51A, respectively. The gradient plate method also produced similar values of MIC, i.e., 35 and 5 µg/mL AMP and 300 and 60 µg/mL PEN (Fig. S2). Because the twofold microdilution method cannot give precise values, we assumed that the MICs determined by gradient plate method represent true MICs. The values of Cinh can be estimated from Fig. 4. It is important that two strains, LA51 and LA51A, produced similar values of Cinh, serving as a further confirmation of both our approach and our data. The estimated values of Cinh were 1.7 μM for AMP and 0.37 μM for PEN. The Cinh values determined here are similar to those estimated on the basis of affinities for penicillin-binding proteins (11) in the case of AMP, but much lower than that for PEN.
Fig. 4.
Quantitative monitoring of periplasmic concentration (Cp) of AMP (A) and PEN (B), in LA51 and LA51A. Combined with MIC values, which are shown in graphs, Cp that is lethal for the cells can be obtained. Theoretical relationship between Co and Cp, which satisfies the equation of Vin = Vh – Ve, is shown as curves in each graph. Abbreviation: Co, extracellular substrate concentration.
The Parameters Obtained Are Consistent with the MIC Values.
One possible way to assess the validity of parameters obtained in this study is to calculate the movement of AMP and PEN when Co = MIC. Here, Cp reaches Cinh, and Ve and Vh in LA51 can be derived using the parameters obtained earlier (Tables 1 and 2). Calculated values are shown in Table 3. The influx of the drug at this condition, however, should provide the sufficient rate to overcome the sum of the activities of efflux and hydrolysis. Vin at Co = MIC is described as Vin = P × A × (Co – Cinh). The calculated Vin of AMP and PEN in LA51 are 0.034 and 0.075 nmol⋅mg−1⋅s−1, respectively. For both of AMP and PEN, Vin is close to the sum of Ve and Vh, confirming the validity of our approach as well as the parameters obtained.
Table 2.
Parameters for the drug flux in the E. coli cell envelope
| Influx | Efflux |
|||
| Drug | P, cm/s | Vmax, nmol⋅mg−1⋅s−1 | K0.5, μM | h |
| AMP | 0.28 × 10−5 | 0.069 | 2.16 | 1.9 |
| PEN | 0.07 × 10−5 | 0.085 | 0.30 | 4.0 |
Table 3.
Calculated values (in nmol⋅mg−1⋅s−1) of Vin, Ve, and Vh in LA51 at Co = MIC
| Rate | AMP | PEN |
| Vin | 0.034 | 0.075 |
| Ve | 0.028 | 0.057 |
| Vh | 0.005 | 0.022 |
Discussion
Porin Channels Are Essentially Nonspecific.
A major purpose of this study was to examine the outer membrane permeation of two penicillins, AMP and PEN, in view of the presence of an AMP-binding site within the OmpF channel. We performed the study with an acrAB deletion mutant so that the results would not be affected by the active efflux process.
We found that both AMP and PEN diffused across the outer membrane apparently by a spontaneous passive diffusion process, without any hint of saturation kinetics (Fig. 1). This is perhaps not surprising, because the affinity of AMP to the binding site is extremely weak, with the dissociation constant reported as 1.9 M (by planar bilayer assay) (6) and 1 M (by molecular dynamics simulation) (10). Thus, in the range of concentrations we used (less than 30 µM for AMP), the drug was at least four orders of magnitude below its KD.
We can examine first if our permeation data fit with the model of the porin channels as nonspecific channels. We found that AMP permeated about 2.2 times faster than PEN through the OmpF channel (Fig. 2). This is precisely what is expected from the effect the gross physicochemical properties of the solute exert on its penetration rate through this nonspecific channel: monoanionic compounds diffuse at one-half of the rates of uncharged analogs (for example compare lactobionic acid with lactose in ref. 2), and zwitterionic compounds appeared to diffuse somewhat faster than uncharged compounds of a similar molecular weight [for example, l-Ser-l-Ala, Mr of 176, diffused 50% faster than d-glucose, Mr of 180 (2)]. [It is theoretically possible that the interior-negative Donnan potential could retard the permeation of anionic PEN. However, under our assay conditions (50 mM KPi buffer, 5 mM MgSO4), the Donnan potential would be quenched to about 10 mV (26) and is unlikely to affect the permeation rates strongly.] These results thus fit the notion that diffusion through porins may be treated, at the level of first approximation, as a process through a large, open channel, which is affected by the gross physicochemical properties of the solute, i.e., size, charge, and lipophilicity (2, 3).
More recently, however, models have been published that assumes a major role for the “specific” binding of AMP and other drugs within the porin for drug penetration, and we can examine whether our result fits with such models. There are two classes for these models. Some workers assume that permeation of a drug (such as AMP) is retarded by the specific binding (10). Such a model predicts that drugs that do not bind to the binding site (such as PEN) will permeate more rapidly than those that bind (such as AMP). Our data (Figs. 1 and 2) show that PEN permeates more slowly than AMP and clearly contradict the model. Although PEN has been claimed to penetrate faster than AMP (10) in liposome-swelling assays, this was apparently caused by the improper application of the technique, without the knowledge that PEN diffuses through the phospholipid bilayers quite rapidly (2, 13). Another class of the specific binding models assumes that the binding site facilitates drug diffusion (7, 8). In extreme cases, it has been claimed that drug permeability can be calculated simply from its on-constant to the binding site (8). Our results clearly do not fit with such a model, because PEN, which has never been shown to bind to the AMP-binding site, nevertheless permeates at a significant rate that corresponds to 25–45% of the diffusion rate of AMP (Figs. 1 and 2). This model also does not fit with the observation that many dozens of solutes, such as simple sugars, that are most unlikely to bind to the AMP-binding site, nevertheless diffuse through the porin channels quite rapidly (1). The proponents of this model made a mistake when they applied the model for LamB (27), the phage lambda receptor, which has a narrow channel and requires the tight binding of ligands to slide them through it, to general-purpose, wide-open porins that allow a rapid, nonspecific diffusion of any small solutes. In conclusion, the specific binding site may produce subtle influence on the diffusion rates of drugs, but this effect is minor, and the rates are predominantly determined by the gross physicochemical properties of the drug. Overemphasizing the role of the specific binding site [for example, in an article entitled “Toward screening for antibiotics with enhanced permeation properties through bacterial porins” (10)] is misleading and does not contribute to the progress in the field.
The binding of drugs within the channel, however, can produce subtle effects on the permeation rates. A recent study by Ziervogel and Roux (28) is instructive. They found by cocrystallization that AMP binds to the OmpF channel at a site just proximal to its narrowest point.
Site-directed mutagenesis of several residues inside OmpF channel was shown to produce alterations of MIC of various antibiotics (29–31). These data, although interesting, cannot be interpreted immediately as the consequence of the changes in the binding process, because no structural data are available for the interaction of the drugs with the mutant channel. Additionally, Ziervogel and Roux (28) also showed that conversion of several crucial residues (D121, S125, R167, R168) to others decreased AMP MIC from 2 to 1 µg/mL, presumably by a slight increase in its permeation rate. However, they pointed out that some of the mutations could cause significant changes in the size of the channel. Indeed, mutations of often targeted residues, such as D113 or R132, were reported to produce increased permeability for sugars, which are not known to bind to the AMP-binding site (32, 33).
Active Efflux of AMP and PEN by AcrAB-TolC System.
In the cells with functional AcrAB pump, the drug molecules that penetrated into the periplasm are either pumped out by the AcrB pump or hydrolyzed by the periplasmic AmpC β-lactamase. Determination of efflux kinetics for AMP and PEN showed that efflux plays a predominant role, in comparison with hydrolysis, for the removal of drugs from the periplasm (Fig. 3). The efflux of PEN was especially effective due to the low K0.5 (0.3 µM) and a high degree of cooperativity (Hill coefficient of 4.0). This value of K0.5 is exceptionally low among β-lactams that have so far been studied. Thus, the K0.5 value ranged from about 5 (nitrocefin) to 288 µM (cephaloridine) among cephalosporins (18), and averaged at 0.98 µM among acid-stable penicillins (14). The Hill coefficient of 4.0 is similar to the average value, 3.8, for the acid-stable penicillins previously studied (except AMP) (14), and are higher than those found for cephalosporins, which ranged from 1.0 to 3.2 (18).
In Fig. 3, we can see quantitatively the relative importance of efflux over enzymatic hydrolysis, when the drugs are present at MIC concentrations (producing Cp of 1.7 and 0.36 µM for AMP and PEN, respectively, from Fig. 4). We note that this situation is found even in LA51, where the level of AmpC β-lactamase has been increased about 10-fold over the wild-type level. Thus, we can safely assume that the poorly expressed wild-type levels of AmpC contribute very little to the AMP and PEN susceptibility in the strains with the wild-type level expression of AcrAB.
Finally, we can compare the present results with the analysis, performed in 1987, without the knowledge of active efflux (11). First, in the earlier study, the kinetic parameters of AmpC β-lactamase were not correct. The Km values obtained in this study (4.9 and 4.5 µM for AMP and PEN, respectively) are close to those reported by Galleni and Frère (3.5 and 4.4 µM) (34) and appear to be more reliable than those used in 1987, 0.9 and 1.9 µM. Presumably because efflux was not considered, the permeability coefficients published in 1987 (9.8 × 10−5 and 0.2 × 10−5 cm/s for AMP and PEN) are very far from our values of 0.28 × 10−5 and 0.07 × 10−5 cm/s. The values of Cinh, which in this study were estimated from the influx and efflux parameters, and validated by the concurrence of values from two strains, were 1.7 and 0.36 µM for AMP and PEN, whereas in the 1987 study they had to be estimated from the penicillin-binding protein binding data and were assumed to be 1.9 and 2.2 µM, and the large difference between these two compounds was not detected. With all these problems, it is not surprising that, for AMP and PEN, the MIC values predicted from the influx rate and the hydrolysis rate did not fit with the observed values in our 1987 study. However, the fit was somewhat better for cephalosporins, where efflux may not play such a major role especially in concentrations at or below MIC values (18).
Materials and Methods
Bacterial Strains.
LA51 is an E. coli K-12 derivative, in which the expression level of chromosomal AmpC is substantially increased due to the mutated promoter of ampC (19). LA51A, a ΔacrAB derivative of LA51, was constructed by transducing the acrAB::Kmr gene from strain AG100A (17), selecting transductants with 35 μg/mL kanamycin. LA51A ΔompC was constructed by introducing the ompC null genotype by cotransduction with zei-298::Tn10 that is located nearby, from strain CS1253 (35). Tn10 was then removed as described by Bochner et al. (36). To construct LA51A ΔompF, we first transduced ΔacrAB::Kmr gene from RAM1337 (37), and kanamycin resistance gene was removed by using FLP recombinase from pCP20 (38). Then the ompF gene was disrupted by transducing ompF::Tn5 from strain MH450 (39). Transduction was done using P1cml,clr100 phage according to the standard protocols (40).
Influx Assay.
Cells were precultured overnight in LB broth containing 5 mM MgCl2 at 37 °C with shaking. This culture was diluted 50-fold in a fresh medium, and cultivated further at 37 °C with shaking until it reached an optical density at 600 nm (OD600) of 1.0∼1.2. Cells were harvested by centrifugation at 3,000 × g for 10 min at room temperature (RT) and washed once with KP buffer [50 mM potassium phosphate buffer (pH 7.0) containing 5 mM MgCl2]. Cells were resuspended in KP buffer, and OD600 of the cell suspension was adjusted to 4.0 (corresponding to 1.5 mg [dry weight]/mL). For the assay, 0.4 mL of the cell suspension was mixed with 0.6 mL of KP buffer containing desired concentration of AMP or PEN, and incubated at RT. The reaction was done in a glass test tube (18 mm × 150 mm). After the incubation period of 0, 5, 10, and 15 min, the reaction was stopped by heating the test tube in a boiling water bath for 50 s with constant shaking. The test tube was then cooled by plunging it into a water bath at RT.
The hydrolysis product was quantified by microiodometry (41) as follows. After the reaction as above, 1 mL of KP buffer and 1 mL of starch–iodine solution were added to the reaction mixture, and OD592 was determined after 30 min at RT. Complete hydrolysis of 1 μM substrate decreases OD592 by 0.128 in a cuvette with a 10-mm light path (41). Using this value, the amount of hydrolysis product ([P]) was calculated. The rate of hydrolysis was obtained by linear fitting of [P] vs. reaction period (s) data.
To obtain the kinetic parameter of the AmpC β-lactamase, the assay was done using sonicated cell extracts (30-s rest and 30-s pulse, eight times). The activity was measured as described above, but with the following modifications: (i) reaction was done in 2 mL of total volume, (ii) the volume of cell extract used in the assay was adjusted according to the level of hydrolytic activity that differs significantly depending on the substrates, and (iii) incubation period was fixed to 5 min. Vmax and Km values were derived by curve fitting by CurveExpert (version 1.4), using Michaelis–Menten equation.
Efflux Assay.
The hydrolysis rates by the intact cell of LA51, which expresses acrAB, were determined under various extracellular substrate concentrations, Co, according to the same protocol for influx assay as described above. The rate of efflux, Ve, was obtained using the equation of Ve = Vin − Vh, where Vin denotes the rate of influx. Vin was calculated by Fick’s first law of diffusion, Vin = P × A × (Co – Cp), where P, A, and Cp denote permeability coefficient, surface area of the cell [=132 cm2/mg [dry cell weight] (22)], and periplasmic substrate concentration. Cp was derived from Michaelis–Menten equation using the observed value of Vh and the kinetic parameters of AmpC, and P was determined in the influx assay using LA51A. Because the efflux activity showed sigmoidal curves when plotted against Cp, we used the Michaelis–Menten equation modified for cooperativity, Ve = Vmax·(Cp)h/[(Cp)h + (K0.5)h], where h is the Hill coefficient, and K0.5 is the substrate concentration that gives the half-maximum velocity. These constants were derived by curve fitting using CurveExpert (version 1.4).
MIC Determination.
Linear gradient plates containing AMP or PEN in the bottom layer were made in square petri dishes, using LB agar supplemented with 5 mM MgCl2 (42). Cells were cultured in LB broth containing 5 mM MgCl2 at 37 °C with shaking, and at a late exponential phase (OD600 = 1.0) the culture was diluted to OD600 of 0.1 and used as inoculum. One microliter of inoculum was streaked as a line across the plate, in parallel to the gradient. The plates were incubated for 18 h at 37 °C.
Microdilution method was done using a series of 200 μL of LB broth supplemented with 5 mM MgCl2 and containing twofold serial deletion of AMP or PEN, which were prepared in the 96-well microtiter plates. Approximately 104 cells from the inoculum as described above were inoculated. The plates were incubated for 18 h for 37 °C.
Supplementary Material
Acknowledgments
This study was supported in part by Public Health Service Grant R01 AI-009644 (to H.N.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1310333110/-/DCSupplemental.
References
- 1.Nikaido H, Rosenberg EY. Effect of solute size of diffusion rates through the transmembrane pores of the outer membrane of Escherichia coli. J Gen Physiol. 1981;77(2):121–135. doi: 10.1085/jgp.77.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nikaido H, Rosenberg EY. Porin channels in Escherichia coli: Studies with liposomes reconstituted from purified proteins. J Bacteriol. 1983;153(1):241–252. doi: 10.1128/jb.153.1.241-252.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikaido H, Rosenberg EY, Foulds J. Porin channels in Escherichia coli: Studies with beta-lactams in intact cells. J Bacteriol. 1983;153(1):232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikaido H, Luckey M, Rosenberg EY. Nonspecific and specific diffusion channels in the outer membrane of Escherichia coli. J Supramol Struct. 1980;13(3):305–313. doi: 10.1002/jss.400130304. [DOI] [PubMed] [Google Scholar]
- 5.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178(20):5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nestorovich EM, Danelon C, Winterhalter M, Bezrukov SM. Designed to penetrate: Time-resolved interaction of single antibiotic molecules with bacterial pores. Proc Natl Acad Sci USA. 2002;99(15):9789–9794. doi: 10.1073/pnas.152206799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danelon C, Nestorovich EM, Winterhalter M, Ceccarelli M, Bezrukov SM. Interaction of zwitterionic penicillins with the OmpF channel facilitates their translocation. Biophys J. 2006;90(5):1617–1627. doi: 10.1529/biophysj.105.075192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James CE, et al. How beta-lactam antibiotics enter bacteria: A dialogue with the porins. PLoS One. 2009;4(5):e5453. doi: 10.1371/journal.pone.0005453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pagès JM, James CE, Winterhalter M. The porin and the permeating antibiotic: A selective diffusion barrier in Gram-negative bacteria. Nat Rev Microbiol. 2008;6(12):893–903. doi: 10.1038/nrmicro1994. [DOI] [PubMed] [Google Scholar]
- 10.Hajjar E, et al. Toward screening for antibiotics with enhanced permeation properties through bacterial porins. Biochemistry. 2010;49(32):6928–6935. doi: 10.1021/bi100845x. [DOI] [PubMed] [Google Scholar]
- 11.Nikaido H, Normark S. Sensitivity of Escherichia coli to various beta-lactams is determined by the interplay of outer membrane permeability and degradation by periplasmic beta-lactamases: A quantitative predictive treatment. Mol Microbiol. 1987;1(1):29–36. doi: 10.1111/j.1365-2958.1987.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimura F, Nikaido H. Diffusion of β-lactam antibiotics through the porin channels of Escherichia coli K-12. Antimicrob Agents Chemother. 1985;27(1):84–92. doi: 10.1128/aac.27.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li XZ, Ma D, Livermore DM, Nikaido H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: Active efflux as a contributing factor to β-lactam resistance. Antimicrob Agents Chemother. 1994;38(8):1742–1752. doi: 10.1128/aac.38.8.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim SP, Nikaido H. Kinetic parameters of efflux of penicillins by the multidrug efflux transporter AcrAB-TolC of Escherichia coli. Antimicrob Agents Chemother. 2010;54(5):1800–1806. doi: 10.1128/AAC.01714-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmermann W, Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma D, et al. Molecular cloning and characterization of acrA and acrE genes of Escherichia coli. J Bacteriol. 1993;175(19):6299–6313. doi: 10.1128/jb.175.19.6299-6313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okusu H, Ma D, Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J Bacteriol. 1996;178(1):306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagano K, Nikaido H. Kinetic behavior of the major multidrug efflux pump AcrB of Escherichia coli. Proc Natl Acad Sci USA. 2009;106(14):5854–5858. doi: 10.1073/pnas.0901695106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaurin B, Grundström T, Edlund T, Normark S. The E. coli β-lactamase attenuator mediates growth rate-dependent regulation. Nature. 1981;290(5803):221–225. doi: 10.1038/290221a0. [DOI] [PubMed] [Google Scholar]
- 20.Sulavik MC, et al. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob Agents Chemother. 2001;45(4):1126–1136. doi: 10.1128/AAC.45.4.1126-1136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirakawa H, Nishino K, Yamada J, Hirata T, Yamaguchi A. Beta-lactam resistance modulated by the overexpression of response regulators of two-component signal transduction systems in Escherichia coli. J Antimicrob Chemother. 2003;52(4):576–582. doi: 10.1093/jac/dkg406. [DOI] [PubMed] [Google Scholar]
- 22.Smit J, Kamio Y, Nikaido H. Outer membrane of Salmonella typhimurium: Chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob Agents Chemother. 1989;33(11):1831–1836. doi: 10.1128/aac.33.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harder KJ, Nikaido H, Matsuhashi M. Mutants of Escherichia coli that are resistant to certain β-lactam compounds lack the ompF porin. Antimicrob Agents Chemother. 1981;20(4):549–552. doi: 10.1128/aac.20.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma D, Alberti M, Lynch C, Nikaido H, Hearst JE. The local repressor AcrR plays a modulating role in the regulation of acrAB genes of Escherichia coli by global stress signals. Mol Microbiol. 1996;19(1):101–112. doi: 10.1046/j.1365-2958.1996.357881.x. [DOI] [PubMed] [Google Scholar]
- 26.Sen K, Hellman J, Nikaido H. Porin channels in intact cells of Escherichia coli are not affected by Donnan potentials across the outer membrane. J Biol Chem. 1988;263(3):1182–1187. [PubMed] [Google Scholar]
- 27.Benz R, Schmid A, Vos-Scheperkeuter GH. Mechanism of sugar transport through the sugar-specific LamB channel of Escherichia coli outer membrane. J Membr Biol. 1987;100(1):21–29. doi: 10.1007/BF02209137. [DOI] [PubMed] [Google Scholar]
- 28.Ziervogel BK, Roux B. The binding of antibiotics in OmpF porin. Structure. 2013;21(1):76–87. doi: 10.1016/j.str.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simonet V, Malléa M, Pagès JM. Substitutions in the eyelet region disrupt cefepime diffusion through the Escherichia coli OmpF channel. Antimicrob Agents Chemother. 2000;44(2):311–315. doi: 10.1128/aac.44.2.311-315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bredin J, et al. Alteration of pore properties of Escherichia coli OmpF induced by mutation of key residues in anti-loop 3 region. Biochem J. 2002;363(Pt 3):521–528. doi: 10.1042/0264-6021:3630521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vidal S, Bredin J, Pagès JM, Barbe J. Beta-lactam screening by specific residues of the OmpF eyelet. J Med Chem. 2005;48(5):1395–1400. doi: 10.1021/jm049652e. [DOI] [PubMed] [Google Scholar]
- 32.Saint N, et al. Structural and functional characterization of OmpF porin mutants selected for larger pore size. II. Functional characterization. J Biol Chem. 1996;271(34):20676–20680. [PubMed] [Google Scholar]
- 33.Phale PS, et al. Role of charged residues at the OmpF porin channel constriction probed by mutagenesis and simulation. Biochemistry. 2001;40(21):6319–6325. doi: 10.1021/bi010046k. [DOI] [PubMed] [Google Scholar]
- 34.Galleni M, Frère JM. A survey of the kinetic parameters of class C beta-lactamases. Penicillins. Biochem J. 1988;255(1):119–122. doi: 10.1042/bj2550119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnaitman CA, McDonald GA. Regulation of outer membrane protein synthesis in Escherichia coli K-12: Deletion of ompC affects expression of the OmpF protein. J Bacteriol. 1984;159(2):555–563. doi: 10.1128/jb.159.2.555-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bochner BR, Huang HC, Schieven GL, Ames BN. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HS, Nagore D, Nikaido H. Multidrug efflux pump MdtBC of Escherichia coli is active only as a B2C heterotrimer. J Bacteriol. 2010;192(5):1377–1386. doi: 10.1128/JB.01448-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97(12):6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall MN, Silhavy TJ. The ompB locus and the regulation of the major outer membrane porin proteins of Escherichia coli K12. J Mol Biol. 1981;146(1):23–43. doi: 10.1016/0022-2836(81)90364-8. [DOI] [PubMed] [Google Scholar]
- 40.Millar JH. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1972. pp. 201–205. [Google Scholar]
- 41.Novick RP. Micro-iodometric assay for penicillinase. Biochem J. 1962;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takatsuka Y, Nikaido H. Threonine-978 in the transmembrane segment of the multidrug efflux pump AcrB of Escherichia coli is crucial for drug transport as a probable component of the proton relay network. J Bacteriol. 2006;188(20):7284–7289. doi: 10.1128/JB.00683-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.