Abstract
The low effectiveness of morphine and related mu opioid analgesics for the treatment of chronic inflammatory pain is a result of opioid-induced release of proinflammatory cytokines and glutamate that lower the pain threshold. In this regard, the use of opioids with metabotropic glutamate-5 receptor (mGluR5) antagonist has been reported to increase the efficacy of morphine and prevent the establishment of adverse effects during chronic use. Given the presence of opioid receptors (MORs) and mGluR5 in glia and neurons, together with reports that suggest coexpressed MOR/mGluR5 receptors in cultured cells associate as a heteromer, the possibility that such a heteromer could be a target in vivo was addressed by the design and synthesis of a series of bivalent ligands that contain mu opioid agonist and mGluR5 antagonist pharmacophores linked through spacers of varying length (10–24 atoms). The series was evaluated for antinociception using the tail-flick and von Frey assays in mice pretreated with lipopolysaccharide (LPS) or in mice with bone cancer. In LPS-pretreated mice, MMG22 (4c, 22-atom spacer) was the most potent member of the series (intrathecal ED50 ∼9 fmol per mouse), whereas in untreated mice its ED50 was more than three orders of magnitude higher. As members of the series with shorter or longer spacers have ≥500-fold higher ED50s in LPS-treated mice, the exceptional potency of MMG22 may be a result of the optimal bridging of protomers in a putative MOR-mGluR5 heteromer. The finding that MMG22 possesses a >106 therapeutic ratio suggests that it may be an excellent candidate for treatment of chronic, intractable pain via spinal administration.
Keywords: MPEP, tolerance, respiratory depression
The excitatory neurotransmitter, glutamate, in the central nervous system (CNS) is an important mediator of opioid nociception, dependence, and withdrawal (1). Glutamate exerts its effect via two different classes of glutamate receptors: ionotropic and metabotropic. Among the metabotropic receptors (mGluRs), the receptor-5 subtype (mGluR5) is widely distributed in the CNS (2), where it modulates synaptic transmission, neuronal excitability, and plasticity. The mGluR5 is a class C G protein-coupled receptor (GPCR), the activation of which is mediated by binding of glutamate to its extracellular Venus flytrap domain. It is noteworthy that the selective mGluR5 antagonist, 2-methyl-6-(phenylethynyl) pyridine (MPEP), acts allosterically by binding to the seven transmembrane (7TM) domain of the receptor (3).
Reports of presence of the mu opioid receptor (MOR) and mGluR5 in the spinal cord and the ability of coadministered MPEP and morphine to enhance morphine antinociception and suppress morphine-induced tolerance and dependence suggest a design strategy for the development of potent analgesics based on the targeting of both MOR and mGluR5 (4–6). Given evidence (7) that supports the physical association of coexpressed MOR and mGluR5 as heteromer (MOR-mGluR5) in cultured cells, and the presence of MOR and mGluR5 in the dorsal horn and glia of the spinal cord, our design approach involved the synthesis of bivalent ligands that contain mu opioid agonist and mGluR5 antagonist pharmacophores in a single molecule (bivalent ligand). An analogous approach has been used previously to target mu-delta (8), mu-kappa (9), and delta-kappa (10) opioid heteromers.
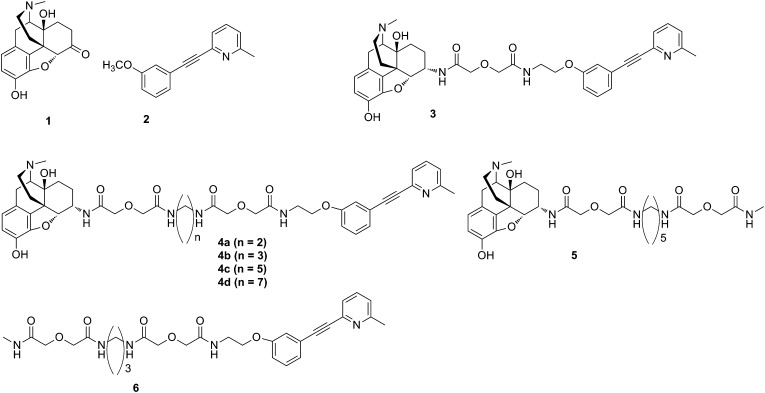
The bivalent ligands (3, 4a–4d) in the present study contain pharmacophores derived from the mu opioid agonist, oxymorphone (11) (Fig. 1), and the mGluR5 antagonist, m-methoxy-MPEP (M-MPEP) (3). Here we show that in lipopolysaccharide (LPS)-pretreated mice and in C3H mice, the antinociceptive potency of intrathecal (i.t.)-administered members of the series is highly dependent on spacer length, with MMG22 (4c, 22 atoms) having unprecedented potency without tolerance (Fig. 2).
Fig. 1.
Mu agonist, oxymorphone 1, and mGluR5 antagonist, M-MPEP2, as models for the pharmacophores in compounds 3, 4a–4d, 5, and 6.
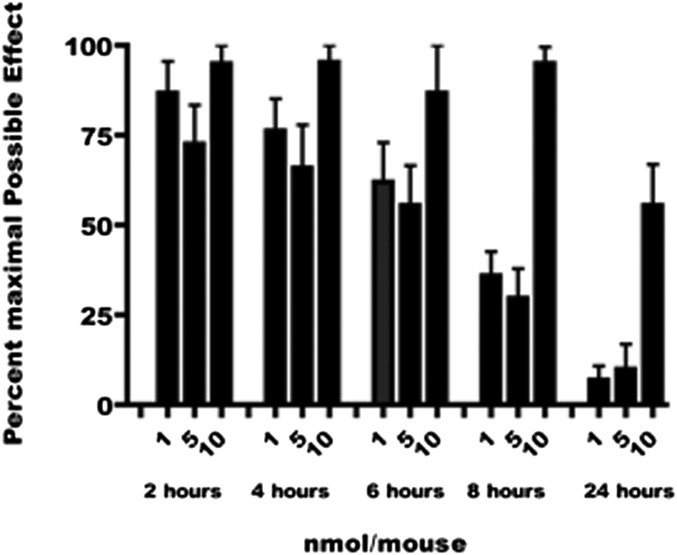
Fig. 2.
Time-course of antinociception for MMG22 in mice pretreated with LPS.
Results
Ligand Design and Synthesis.
The pharmacophores for targeting MOR-mGluR5 heteromer were derived from the mu agonist, oxymorphone 1 (11), and the mGluR5 antagonist, m-methoxy-MPEP 2 (M-MPEP) (3). The use of 2 both as a radioligand and as a selective pharmacologic antagonist for the mGluR5 suggests that the phenoxy oxygen can serve as the point of attachment for a spacer that links the mGluR5 antagonist pharmacophore (12) to the mu agonist pharmacophore. Thus, replacement of the m-methoxy group of 2 with an m-ethoxyethylamine substituent permitted facile attachment of the spacer through the amino group to permit synthesis of bivalent ligand 3 and series 4. In addition, we prepared the monovalent ligands 5 and 6 as controls.
Antinociception Studies Using Normal and LPS-Treated Mice.
The target compounds (3, 4a–4d, 5, 6) were tested on untreated (control) mice and LPS-pretreated mice, using the tail-flick method; the results are presented in Table 1. In control mice, i.t. administration of 4a–4c was approximately threefold more potent than administration of 4d or 5. Metabotropic antagonist 6 was ∼70-fold less potent than 4a–4c. In contrast, on i.c.v. administration, 4a, 4b, and 5 had comparable antinociceptive potencies, which were greater than the other ligands. In this mode of administration, 4c was a partial agonist and 4d was 10-fold less potent. Profound differences were observed when mice were pretreated with LPS. The bivalent ligands 4b and 4d also were devoid of tolerance formation, with ED50 values of 10.08 and 0.25 pmol per mouse, respectively, whereas 4a produced tolerance in this study. Tolerance was also observed on i.t. administration of bivalent 3, with monovalent agonist 5, and with a mixture of 5 and antagonist monovalent 6. The bivalent 4c (MMG22) was exceptionally potent (ED50 = 9 fmol per mouse) and without tolerance 24 h after i.t. administration. The 24-h tolerance test was carried out using 25 pmol per mouse, which is 4 × ED50. This test gave the following result: controls = 94 ± 4.4, and with the same dose, 24 h tolerance, resulting in 82.2 ± 11.9% maximal possible effect (MPE), respectively. The potencies of the MMG series relative to a mixture of monovalent ligands are listed in Table 2. It is noteworthy that the most potent member of the series is nearly 48,000× more potent than the mixture of monovalents 5 and 6.
Table 1.
Comparison of the antinociceptive activity (pmol per mouse) of bivalent ligands and corresponding monovalent ligands
| Compound | Control | LPS pretreated (1 mg/kg 24 h i.p.) | ||||
| ED50 (confidence interval) | ED50 (confidence interval) | ED50 (confidence interval) | ED50 (confidence interval) | |||
| i.t. | i.c.v. | i.c.v./i.t. ratio | i.t. | i.c.v. | i.c.v./i.t. ratio | |
| Morphine | 27 (21.21–35.56) | 301 (224–406) | 11 | 35 (24.27–50.40) | 301 (224–406) | 8.6 |
| 2 | 250; 13.27 ± 3.39%* | 250; 14.59 ± 4.63%* | 1 | 250; 21.96 ± 10.50%* | — | — |
| 3 (MMG10) | 114.7 (66.3–198.3)† | 529.6 (397.9–704.9)‡ | 4.62 | 8.17 (5.90–11.34)‡ | 500; 56.86 ± 8.95%* | — |
| 4a (MMG19) | 34.31 (23.47–50.14)‡ | 119.50 (76.97–185.4)‡ | 3.48 | 20.57 (17.66–23.97)‡ | 80.85 (59.08–110.6)‡ | 3.93 |
| 4b (MMG20) | 36.08 (25.86–50.33)† | 113.3 (74.23–173.0)† | 3.14 | 10.08 (4.95–20.52)† | 64.36 (42.09–98.42)† | 6.38 |
| 4c (MMG22) | 38.70 (25.48–58.77)† | 250, 71 ± 10%*,† | — | 0.00883 (0.003–0.026)† | 374.9 (215.6–652.0)† | 42,457 |
| 4d (MMG24) | 132.7 (75.77–232.4)† | 1,000; 53.76 ± 16.36%* | — | 0.25 (0.13–0.49)† | 411.9 (274.2–618.6) | 1,648 |
| 5 | 110.06 (81.44–153.16)‡ | 89.81 (61.20–126.26)‡ | 0.82 | 21.29 (13.55–33.75)‡ | 168.9 (124.0–230.2)‡ | 7.93 |
| 6 | 2,500; 26.13 ± 7.27%* | 250; 24.87 ± 11.77%* | — | 548.7 (216.1–1,394)† | 2,500; 38.83 ± 11.59%* | — |
The highest dose measured for antinociception and the corresponding percentage maximal possible effect for that dose.
No tolerance observed 24 h after the initial dose–response curve.
Tolerance observed 24 h after the initial dose–response curve.
Table 2.
Relative potencies of bivalent (3, 4a–4d) and monovalent (5 + 6) ligands in LPS- pretreated mice
The potencies are relative to 5 + 6.
The synergy of the two monovalents (5 + 6) was evaluated; as 5 was 25× more potent than 6, the mixture was one part 5 to 25 parts 6. The theoretical ED50 was calculated to be 285 pmol per mouse, and the observed ED50 was 332.9 pmol per mouse (281–396). There is therefore no significant synergy between the two compounds (13).
Table 3.
Respiratory depression* of intrathecal morphine and MMG22 (4c) in LPS-pretreated mice
| Compound | Dose, nmol per mouse | Mice with O2 saturation less than 90%, % | Therapeutic ratio‡ |
| Morphine | 5 | 50† | 182 |
| 4c | 5 | 0 | 566,251 |
| 4c | 10 | 100† | 1,132,503 |
Respiratory depression was considered positive if the O2 saturation decreased from the control level (96-99%) to less than 90%.
The average O2 saturation levels in mice that fell below the 90% level were 85%.
The dose administered divided by the ED50 for antinociception.
The most potent compound, MMG22, was evaluated further for its effectiveness in a time-course study using the doses 1, 5, and 10 nmol (i.t.) in LPS-pretreated mice during a period of 24 h. These studies revealed no adverse effect at those doses and duration of action greater than 50% MPE 24 h after the highest dose. Control mice treated with 5 nmol MMG22 exhibited a time-course profile indistinguishable from the LPS-pretreated mice, but displayed tonic seizures. Given the >4,000-fold greater i.t. ED50 for control mice vs. LPS-pretreated mice, it appears that inflammation greatly enhances the potency of MMG22 with a concomitant decrease in toxicity.
Complete Freund’s Adjuvant Administration and Antinociception in the Behavioral Studies in Mice.
Recently, Liu et al. (14) used complete Freund’s adjuvant (CFA) to evaluate mGluR5 receptor contribution to inflammatory tongue pain, which suggests mGluR5 is involved in signaling in the development of mechanical and heat hypersensitivity. This study revealed that phosphorylated extracellular signal-regulated kinase (pERK)-immunoreactive cells are increased in upper cervical spinal cord (C1–C2), indicating up-regulation of mGluR5 (14). In our studies, the left hind paws of mice were injected (intraplantar) with a 50% solution of CFA (10 µg, Sigma-Aldrich) in PBS while the mice were under isoflurane anesthesia. After i.t. administration of MMG22, an ED50 of 7.66 fmol per mouse was observed, showing effectiveness comparable with that obtained in LPS-pretreated mice. Similar potency without tolerance was also observed in the bone cancer mouse model, in that MMG22 had an ED50 of 3.81 fmol per mouse on day 3 after inoculation.
Discussion
Reports on the presence of MOR and mGluR5 in the spinal cord, and the existence of MOR-mGluR5 heteromer in HEK293 cells coexpressed with MOR and mGluR5, has raised the likelihood that a putative heteromer may be relevant to the pharmacology of morphine, particularly in the presence of inflammation (7). Thus, targeting such a heteromer with bivalent ligands that activate MOR with concomitant antagonism of mGluR5, could represent a promising strategy to develop analgesics with enhanced potency and reduced adverse effects on spinal administration. Such ligands should be particularly effective in the treatment of inflammatory pain arising from spinal cord injury (15), transient nerve root compression (16), and other conditions in which glutamate is up-regulated in neurons and glia. In this regard, as intrathecal administration of mGluR5 antagonist is known to inhibit activation of spinal astrocytes in a mouse model and in vitro, targeting a MOR-mGluR5 heteromer may be a useful strategy in the pharmacotherapy of inflammatory pain (17).
The ligands synthesized for this study contain pharmacophores related to the mu agonist, oxymorphone 1, and mGluR5 antagonist, M-MPEP 2 (Fig. 1). This involved connecting derivatives of 1 and 2 through different length spacers (10–24 atoms) to afford the target bivalent ligands in the MMG series (3, 4a–4d). The selection of spacer lengths was based on prior studies that revealed a range of 18–22 atoms for effective bridging of G protein-coupled receptor protomers (8–10, 18–20).
Members of the MMG series were administered by i.t. or i.c.v. routes to both normal and LPS-pretreated mice. LPS was used because this inflammation model is well established (21). The MMG ligands 3, 4a, 4b, and 4d produced antinociception in the pmol range when administered i.t. to LPS-pretreated mice, whereas MMG22 (4c, 22-atom spacer) had greater than 1,000-fold antinociceptive potency (ED50 8.8 fmol per mouse) than its lower homolog MMG20 (4b) (Table 1). Apparently, the 22-atom spacer length is well suited to optimally bridge MOR and mGluR5 protomers in the putative heteromer, as suggested by the 25-fold lower potency of its higher homolog, MMG24, with a 24-atom spacer. The observation that MMG22 is >42,000× more potent by the i.t. vs. the i.c.v. route of administration in LPS-pretreated mice suggests that its putative target, MOR-mGluR5, is localized in the spinal cord. Significantly, MMG22 has ∼37,000× greater potency than a mixture of monovalent ligands 5 and 6 in LPS-pretreated mice (Table 2), further supporting the importance of a 22-atom spacer to promote optimal bridging to a putative MOPR-mGluR5 heteromer.
In contrast to the exceptional i.t. potency of MMG22 in LPS-pretreated mice, untreated control exhibited an i.t. ED50 that was ∼4,000× greater, illustrating the dramatic effect of inflammation in enhancing antinociception of MMG22 (Table 1). The magnitude of this effect is associated with MMG22 only, as relatively small changes in potency as a function of spacer length occur in control mice. This suggests that the spinal level of putative MOR-mGluR5 heteromer in normal mice is substantially lower than that in LPS-pretreated mice. Thus, up-regulation of MOR (22–26), mGluR5 (14), and glutamate (21, 27) resulting from inflammation suggest a possible explanation for the extraordinary increase in antinociception produced by MMG22. In LPS-pretreated mice, greatly increased spinal expression of cell-surface neuronal or glial MOR and mGluR5 could lead to elevated levels of MOR-mGluR5 heteromer, which would in turn lead to enhanced potency of MMG22.
The most potent member of the series, MGG22, was evaluated further for the inhibition of nociception, using the von Frey assay in C3H/He mice treated with CFA. After i.t. administration of MMG22, its potency (ED50 ∼8 fmol per mouse) was comparable to that obtained with LPS-treated mice, using the tail-flick assay. The von Frey procedure subsequently was used in testing MMG22, using C3H/He mice with bone cancer, and was found to have comparable potency (∼4 fmol per mouse) on day 3. These data support the validity of the tail-flick LPS-pretreatment assay as a model of inflammatory pain and suggest that spinally administered MMG22 may be of use in the pharmacotherapy of a variety of conditions in which conventional analgesics have reduced efficacy. Finally, the finding that MMG22 produced no respiratory depression at a dose that is 1/2 million-fold greater than its ED50, suggests this side effect would not be relevant in the therapeutic range (Table 3).
Conclusions
In light of reports on the up-regulation of spinal MOR and mGluR5 in inflammation, and evidence for the existence of MOR-mGluR5 heteromer in cultured cells, the structure–activity relationship of the MMG series suggests that MMG22 (4c) may be effective for the treatment of inflammatory pain. The extraordinary i.t. antinociception produced by MMG22 in LPS-pretreated, but not in control, mice is consistent with the optimal targeting of a putative MOR-mGluR5 heteromer, particularly as its lower and higher homologs exhibit substantially lower potency. The results suggest that the opioid and MPEP pharmacophores of MMG22 optimally bridge their respective protomers of such a heteromer. Given the unprecedented i.t. potency of MMG22 and the absence of tolerance in the tail-flick and von Frey assays under inflammatory conditions, this ligand has potential as a spinally administered analgesic for intractable pain that is refractory to morphine or other analgesics. This could include chronic pain associated with bone cancer and other conditions involving severe inflammatory pain. It is noteworthy that because MMG22 possesses >106 therapeutic ratio (Table 3), it may be an excellent candidate for treatment of chronic, intractable pain via spinal administration. In addition, MMG22 may be useful as a pharmacologic tool to investigate MOR-mGluR5 in vivo and in vitro. Finally, the likelihood of MOR-mGluR5 as a target suggests that high-throughput screening may lead to additional agents for treatment of inflammatory pain.
Materials and Methods
Animals.
Male ICR-CD1 mice (17–25g; Harlan), or male C3H/He mice (15–20g, National Cancer Institute) were housed in groups of four in a temperature- and humidity-controlled environment with unlimited access to food and water. They were maintained on a 12-h light/dark cycle. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Minnesota in Minneapolis.
Drug Administration.
Compounds 2–6 were dissolved in 10% (wt/vol) DMSO and then diluted to less than 1% DMSO in the test solutions. DMSO when given i.c.v. or i.t. in a 1% or less concentrated solution did not show any antinociception. Compounds were administered in a 5-μL volume in conscious mice, either i.c.v. (Haley and McCormick, ref. 28) or i.t. (Hylden and Wilcox, ref. 29). Peak times were determined by comparing the percentage MPE (%MPE) at 5, 10, 20, and 60 min after injection.
Pain Models.
Mice were pretreated with LPS (1 mg/kg, i.p., from Escherichia coli 0111:B4; Sigma-Aldrich) for 24 h before testing for antinociception of compounds 2–6 (30). This group was compared with a group without LPS pretreatment.
The most potent ligand, 4c, was evaluated using CFA as a second pain model to measure antinociception. The left hindpaws were injected (intraplantar) with a 50% solution of CFA (10 µg, Sigma-Aldrich) in PBS while the mice were under isoflurane anesthesia. Forty-eight hours after the paws were injected, antinociception was measured after administration of 4c (31).
The mouse hind paw model of bone cancer was also used to measure the antinociception of 4c. The cancer cells and implantation of the cells have been described in detail elsewhere (32, 33). Briefly, the mouse is anesthetized using 3% isoflurane in 3 L/min oxygen. Tumor cells (National Collection of Type Cultures clone 2472 fibrosarcoma cells) were manually injected by boring into the calcaneus bone, using a 291/2-gauge needle connected to a sterile 0.3-mL insulin syringe. After the injection, mice were allowed to recover in cages on a heating pad.
Assays.
Radiant tail flick.
The tail-flick assay was used first to test for antinociception, as described by D’Amour and Smith (34) and modified by Dewey et al. (35). For the measurement of tail-flick latency, mice are wrapped in a light cloth and held gently in one hand with the tail positioned in the apparatus (Tail Flick Analgesia Meter, Columbus Instruments) for radiant heat stimulus. The tail-flick response is elicited by applying radiant heat to the dorsal side of the tail. The intensity of the heat is set so that the mouse flicks its tail within 2–3 s. The test latency is measured before drug treatment (control) and again after the drug treatment (test) at the peak time of the compound; a 10-s maximum cutoff time is used to prevent damage to the tail. Antinociception is quantified, according to the method of Harris and Pierson (36), as the %MPE, which is calculated as %MPE = (Test − Control/10 – Control) × 100.
Mechanical hypersensitivity.
C3H mice were placed under a clean glass cup on a wire mesh grid and allowed to acclimate for 30 min. Mechanical hypersensitivity was tested using a von Frey filament 3.61 (which produces a force of 0.7 g) to the plantar surface of both the left and right hind paw with enough force to cause it to bow slightly. Starting with the right hind paw, the number of positive responses out of a total of 10 applications was recorded, followed by the left hind paw. Positive response was indicated by a sharp withdrawal or flinching behavior of the paw. Baseline von Frey measurements were obtained before treatment with CFA, tumor, and saline controls. Control animals did not differ from untreated mice. Subsequent measurements were taken at various points after i.t. injection of test compounds on days 3, 7, 10, 14, 17, and 21. The %MPE was calculated [(Time-point value – Day# baseline)/(Day 0 baseline – Day# baseline)] × 100 (32).
Acute tolerance.
Acute tolerance, using the radiant tail-flick assay, was measured by comparing the ED80–90 dose on day 1 with the same dose measured 24 h later on the same mouse. For the LPS-pretreated mice, no additional LPS was given to the mice for the acute tolerance test. To confirm that the LPS was still fully effective after 48 h, a compound (4c) that is known not to produce tolerance was tested 48 h after the initial LPS injection and was found not to differ from the 24 h value.
Respiratory depression.
Respiratory depression was measured in mice using the Mouse Ox (small animal oximeter from STARR Life Sciences Corp). A CollarClip (unanesthetized neck sensor) for mice was used as the site for measuring the data. Day 1 of each experiment involved training the animals, using a disposable CollarClip. The collar was left on for approximately 1 h. Respiratory depression was considered positive if the O2 saturation decreased from 96–99% to less than 90%. Mice were monitored for a 60-min period, with measurements at 10, 20, 30, and 60 min.
Statistics.
At least three groups of four to 10 mice were used for each dose–response curve. ED50 values with 95% confidence intervals are computed with GraphPad Prism 4 by using nonlinear regression methods. Ratios were considered significant if the confidence interval did not overlap.
Synthesis and characterization.
Preparation and characterization data for all of the compounds are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank Prof. Wicox for use of the oximeter. This work was supported by National Institutes of Health Grant R01DA030316 from the National Institute on Drug Abuse.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.W.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305461110/-/DCSupplemental.
References
- 1.Guo Y, Wang HL, Xiang XH, Zhao Y. The role of glutamate and its receptors in mesocorticolimbic dopaminergic regions in opioid addiction. Neurosci Biobehav Rev. 2009;33(6):864–873. doi: 10.1016/j.neubiorev.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Patel S, et al. Species differences in mGluR5 binding sites in mammalian central nervous system determined using in vitro binding with [18F]F-PEB. Nucl Med Biol. 2007;34(8):1009–1017. doi: 10.1016/j.nucmedbio.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Gasparini F, et al. [(3)H]-M-MPEP, a potent, subtype-selective radioligand for the metabotropic glutamate receptor subtype 5. Bioorg Med Chem Lett. 2002;12(3):407–409. doi: 10.1016/s0960-894x(01)00767-3. [DOI] [PubMed] [Google Scholar]
- 4.Lee JS, Ro JY. Peripheral metabotropic glutamate receptor 5 mediates mechanical hypersensitivity in craniofacial muscle via protein kinase C dependent mechanisms. Neuroscience. 2007;146(1):375–383. doi: 10.1016/j.neuroscience.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Aoki T, Narita M, Shibasaki M, Suzuki T. Metabotropic glutamate receptor 5 localized in the limbic forebrain is critical for the development of morphine-induced rewarding effect in mice. Eur J Neurosci. 2004;20(6):1633–1638. doi: 10.1111/j.1460-9568.2004.03609.x. [DOI] [PubMed] [Google Scholar]
- 6.Gabra BH, Smith FL, Navarro HA, Carroll FI, Dewey WL. mGluR5 antagonists that block calcium mobilization in vitro also reverse (S)-3,5-DHPG-induced hyperalgesia and morphine antinociceptive tolerance in vivo. Brain Res. 2008;1187:58–66. doi: 10.1016/j.brainres.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schröder H, et al. Allosteric modulation of metabotropic glutamate receptor 5 affects phosphorylation, internalization, and desensitization of the micro-opioid receptor. Neuropharmacology. 2009;56(4):768–778. doi: 10.1016/j.neuropharm.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Daniels DJ, et al. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci USA. 2005;102(52):19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S, Yekkirala A, Tang Y, Portoghese PS. A bivalent ligand (KMN-21) antagonist for mu/kappa heterodimeric opioid receptors. Bioorg Med Chem Lett. 2009;19(24):6978–6980. doi: 10.1016/j.bmcl.2009.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniels DJ, Kulkarni A, Xie Z, Bhushan RG, Portoghese PS. A bivalent ligand (KDAN-18) containing delta-antagonist and kappa-agonist pharmacophores bridges delta2 and kappa1 opioid receptor phenotypes. J Med Chem. 2005;48(6):1713–1716. doi: 10.1021/jm034234f. [DOI] [PubMed] [Google Scholar]
- 11.Lewenstein MJ, Weiss U. 1957. US Patent US2806033.
- 12.Alagille D, et al. Functionalization at position 3 of the phenyl ring of the potent mGluR5 noncompetitive antagonists MPEP. Bioorg Med Chem Lett. 2005;15(4):945–949. doi: 10.1016/j.bmcl.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 13.Liu MG, et al. Metabotropic glutamate receptor 5 contributes to inflammatory tongue pain via extracellular signal-regulated kinase signaling in the trigeminal spinal subnucleus caudalis and upper cervical spinal cord. J Neuroinflammation. 2012;9:258. doi: 10.1186/1742-2094-9-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gwak YS, Hulsebosch CE. Upregulation of Group I metabotropic glutamate receptors in neurons and astrocytes in the dorsal horn following spinal cord injury. Exp Neurol. 2005;195(1):236–243. doi: 10.1016/j.expneurol.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson KJ, Guarino BB, Winkelstein BA. Transient nerve root compression load and duration differentially mediate behavioral sensitivity and associated spinal astrocyte activation and mGLuR5 expression. Neuroscience. 2012;209:187–195. doi: 10.1016/j.neuroscience.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 16.Ren BX, et al. Intrathecal injection of metabotropic glutamate receptor subtype 3 and 5 agonist/antagonist attenuates bone cancer pain by inhibition of spinal astrocyte activation in a mouse model. Anesthesiology. 2012;116(1):122–132. doi: 10.1097/ALN.0b013e31823de68d. [DOI] [PubMed] [Google Scholar]
- 17.Aceto MD, et al. MDAN-21: A bivalent opioid ligand containing mu-agonist and delta-antagonist pharmacophores and its effects in rhesus monkeys. Int J Med Chem. 2012 doi: 10.1155/2012/327257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Z, Bhushan RG, Daniels DJ, Portoghese PS. Interaction of bivalent ligand KDN21 with heterodimeric delta-kappa opioid receptors in human embryonic kidney 293 cells. Mol Pharmacol. 2005;68(4):1079–1086. doi: 10.1124/mol.105.012070. [DOI] [PubMed] [Google Scholar]
- 19.Bhushan RG, Sharma SK, Xie Z, Daniels DJ, Portoghese PS. A bivalent ligand (KDN-21) reveals spinal delta and kappa opioid receptors are organized as heterodimers that give rise to delta(1) and kappa(2) phenotypes. Selective targeting of delta-kappa heterodimers. J Med Chem. 2004;47(12):2969–2972. doi: 10.1021/jm0342358. [DOI] [PubMed] [Google Scholar]
- 20.Takaki J, et al. L-glutamate released from activated microglia downregulates astrocytic L-glutamate transporter expression in neuroinflammation: The ‘collusion’ hypothesis for increased extracellular L-glutamate concentration in neuroinflammation. J Neuroinflammation. 2012;9:275. doi: 10.1186/1742-2094-9-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mousa SA, Machelska H, Schäfer M, Stein C. Immunohistochemical localization of endomorphin-1 and endomorphin-2 in immune cells and spinal cord in a model of inflammatory pain. J Neuroimmunol. 2002;126(1-2):5–15. doi: 10.1016/s0165-5728(02)00049-8. [DOI] [PubMed] [Google Scholar]
- 22.Morinville A, Cahill CM, Kieffer B, Collier B, Beaudet A. Mu-opioid receptor knockout prevents changes in delta-opioid receptor trafficking induced by chronic inflammatory pain. Pain. 2004;109(3):266–273. doi: 10.1016/j.pain.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Ballet S, et al. Expression and G-protein coupling of mu-opioid receptors in the spinal cord and dorsal root ganglia of polyarthritic rats. Neuropeptides. 2003;37(4):211–219. doi: 10.1016/s0143-4179(03)00045-3. [DOI] [PubMed] [Google Scholar]
- 24.Zaringhalam J, Manaheji H, Mghsoodi N, Farokhi B, Mirzaiee V. Spinal μ-opioid receptor expression and hyperalgesia with dexamethasone in chronic adjuvant-induced arthritis in rats. Clin Exp Pharmacol Physiol. 2008;35(11):1309–1315. doi: 10.1111/j.1440-1681.2008.05009.x. [DOI] [PubMed] [Google Scholar]
- 25.Puehler W, et al. Rapid upregulation of μ opioid receptor mRNA in dorsal root ganglia in response to peripheral inflammation depends on neuronal conduction. Neuroscience. 2004;129(2):473–479. doi: 10.1016/j.neuroscience.2004.06.086. [DOI] [PubMed] [Google Scholar]
- 26.Shaqura MA, Zöllner C, Mousa SA, Stein C, Schäfer M. Characterization of μ opioid receptor binding and G protein coupling in rat hypothalamus, spinal cord, and primary afferent neurons during inflammatory pain. J Pharmacol Exp Ther. 2004;308(2):712–718. doi: 10.1124/jpet.103.057257. [DOI] [PubMed] [Google Scholar]
- 27.Inquimbert P, et al. Peripheral nerve injury produces a sustained shift in the balance between glutamate release and uptake in the dorsal horn of the spinal cord. Pain. 2012;153(12):2422–2431. doi: 10.1016/j.pain.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haley TJ, McCormick WG. Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Br Pharmacol Chemother. 1957;12(1):12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hylden JL, Wilcox GL. Intrathecal morphine in mice: A new technique. Eur J Pharmacol. 1980;67(2-3):313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 30.Seo YJ, et al. The differential effects of acetaminophen on lipopolysaccharide induced hyperalgesia in various mouse pain models. Pharmacol Biochem Behav. 2008;91(1):121–127. doi: 10.1016/j.pbb.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 31.Sorge RE, et al. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci. 2011;31(43):15450–15454. doi: 10.1523/JNEUROSCI.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smeester BA, Al-Gizawiy M, Beitz AJ. Effects of different electroacupuncture scheduling regimens on murine bone tumor-induced hyperalgesia: Sex differences and role of inflammation. Evidence-Based Complementary and Alternative Medicine. 2012 doi: 10.1155/2012/671386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wacnik PW, et al. Functional interactions between tumor and peripheral nerve: Morphology, algogen identification, and behavioral characterization of a new murine model of cancer pain. J Neurosci. 2001;21(23):9355–9366. doi: 10.1523/JNEUROSCI.21-23-09355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D'Amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72(1):74–79. [Google Scholar]
- 35.Dewey WL, Harris LS, Howes JF, Nuite JA. The effect of various neurohumoral modulators on the activity of morphine and the narcotic antagonists in the tail-flick and phenylquinone tests. J Pharmacol Exp Ther. 1970;175(2):435–442. [PubMed] [Google Scholar]
- 36.Harris LS, Pierson AK. Narcotic antagonists in the benzomorphan series. J Pharmacol Exp Ther. 1964;143(2):141–148. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.