Abstract
Understanding disease transmission dynamics in multihost parasite systems is a research priority for control and potential elimination of many infectious diseases. In China, despite decades of multifaceted control efforts against schistosomiasis, the indirectly transmitted helminth Schistosoma japonicum remains endemic, partly because of the presence of zoonotic reservoirs. We used mathematical modeling and conceptual frameworks of multihost transmission ecology to assess the relative importance of various definitive host species for S. japonicum transmission in contrasting hilly and marshland areas of China. We examine whether directing control interventions against zoonotic reservoirs could further reduce incidence of infection in humans or even eliminate transmission. Results suggest that, under current control programs, infections in humans result from spillover of transmission among zoonotic reservoirs. Estimates of the basic reproduction number within each species suggest that bovines (water buffalo and cattle) maintained transmission in the marshland area and that the recent removal of bovines from this area could achieve local elimination of transmission. However, the sole use of antifecundity S. japonicum vaccines for bovines, at least at current efficacies, may not achieve elimination in areas of comparable endemicity where removal of bovines is not a feasible option. The results also suggest that rodents drive transmission in the hilly area. Therefore, although targeting bovines could further reduce and potentially interrupt transmission in marshland regions of China, elimination of S. japonicum could prove more challenging in areas where rodents might maintain transmission. In conclusion, we show how mathematical modeling can give important insights into multihost transmission of indirectly transmitted pathogens.
Most human pathogens are zoonoses (i.e., diseases that can be transmitted, directly or indirectly, from animals to humans) and often involve a multiplicity of reservoirs and vectors (1). However, most epidemiological theory of parasitic infections has been developed for the case of single host–parasite and host–vector–parasite systems. Meanwhile, mathematical models of zoonoses have tended to consider just one phase of the zoonotic process, focusing on either dynamics in the reservoirs or outbreaks in the human population (2). Such studies have also been dominated by viral diseases, whereas indirectly transmitted zoonotic pathogens in general and helminth parasites in particular have been neglected (2). In addition, although some conceptual frameworks have been proposed for multihost parasite systems to help identify key zoonotic reservoirs (3) and classify zoonotic disease threats (4), these frameworks have been rarely applied to empirical data and mathematical modeling studies (5).
One important multihost parasite is Schistosoma japonicum, the helminth responsible for intestinal schistosomiasis in parts of East and Southeast Asia. The parasite is indirectly transmitted by snail intermediate hosts between definitive hosts, which include not only humans but also many other mammals that may act as zoonotic reservoirs. Given the fillip that large-scale control programs against human helminthiases have received (6), studies that aim to elucidate the transmission ecology of zoonotic parasites among multiple host species are especially timely and have been identified as a priority by the World Health Organization Disease Reference Group for Helminth Infections (5). In China, after five decades of concerted and multifaceted interventions (including chemotherapy, mollusciciding, health education, and sanitation and environmental improvement), levels of S. japonicum infection among humans are now often low in areas where schistosomiasis remains endemic (7). However, progress to further control and elimination seems to be slowing down (8). Transmission has even reemerged in some areas where schistosomiasis was thought to have been eliminated (9).
In this paper, we combine mathematical modeling with a conceptual approach for the study of S. japonicum transmission in two ecologically distinct (marshland and hilly) areas of China, where we conducted parasitological surveys and population genetics studies (10, 11) that inform model design. We apply a Bayesian approach for the estimation of infection prevalence in each host species and estimate the intrinsic potential for transmission (the basic reproduction number or R0) for each species in each environment, taking into account uncertainty in model parameters. Applying conceptual frameworks proposed for multihost systems (3, 4), we elucidate the most important host species (through identifying maintenance and essential hosts for transmission, which are defined in Materials and Methods) in each region and show that, as a zoonosis, S. japonicum could be classified as a spillover pathogen, an apparent multihost pathogen, or a true multihost pathogen depending on the location and scale at which the multihost parasite community is observed. We also explore the potential impact of interventions targeting different zoonotic reservoirs for control and elimination of S. japonicum in each region.
Therefore, our work is not only relevant for the control of schistosomiasis japonica in China but also shows how mathematical models and conceptual frameworks can be applied to empirical data to characterize multihost parasite systems and the uncertainties therein.
Results
Estimates of 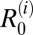 and Overall R0.
and Overall R0.
The mathematical model (Fig. S1) tracks the prevalence of infection in each definitive host species, i, and snail intermediate hosts. Based on the findings from our previous field and molecular epidemiological studies (10–12), the definitive host species incorporated into the transmission model were humans (H), cattle (C), water buffaloes (W), goats (G), dogs (D), cats (F), and rodents (R); thus, i
 [H, C, W, G, D, F, R]. The basic reproduction number, R0, is defined here as the average number of definitive hosts that would become infected (by snail intermediate hosts) from a typical primary case in a completely susceptible population. If R0 < 1, the infection cannot persist within the population, whereas if R0 > 1, the infection will be able to spread within the population and may reach a stable endemic equilibrium (13). For each study village and region, based on the assumptions of the model, we calculated the overall R0 across all host species and the component
[H, C, W, G, D, F, R]. The basic reproduction number, R0, is defined here as the average number of definitive hosts that would become infected (by snail intermediate hosts) from a typical primary case in a completely susceptible population. If R0 < 1, the infection cannot persist within the population, whereas if R0 > 1, the infection will be able to spread within the population and may reach a stable endemic equilibrium (13). For each study village and region, based on the assumptions of the model, we calculated the overall R0 across all host species and the component 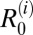 for each definitive host species i (Table 1). We assessed which definitive host species are maintenance hosts in each area based on whether
for each definitive host species i (Table 1). We assessed which definitive host species are maintenance hosts in each area based on whether 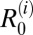 > 1. In addition, we estimated overall R0 values after removal of each host species from the model, with values below one suggesting that the removed host species is an essential host for transmission in a given area (Table 2). Because most parameters were assigned a plausible distribution of values rather than a point estimate, we present here the distributions of model outcomes in terms of their medians and interpercentile ranges [henceforth referred to as 95% confidence intervals (95% CIs)].
> 1. In addition, we estimated overall R0 values after removal of each host species from the model, with values below one suggesting that the removed host species is an essential host for transmission in a given area (Table 2). Because most parameters were assigned a plausible distribution of values rather than a point estimate, we present here the distributions of model outcomes in terms of their medians and interpercentile ranges [henceforth referred to as 95% confidence intervals (95% CIs)].
Table 1.
Estimates of the basic reproduction number for S. japonicum transmission within each definitive host species [R0(i)] and overall (R0) for each village and region in Anhui, China
| Marshland region (Tongling) |
Hilly region (Shitai) |
|||||||
| GH | HP | XZ | Overall | LQ | LS | YT | Overall | |
| Humans | 0.0 (0.0, 0.1) | 0.0 (0.0, 0.3) | 0.0 (0.0, 0.1) | 0.0 (0.0, 0.1) | 0.0 (0.0, 0.2) | 0.3 (0.0, 0.9) | 0.2 (0.0, 0.8) | 0.1 (0.0, 0.7) |
| Cattle | 1.8 (1.2, 2.8) | 1.1 (0.3, 1.7) | 2.9 (1.5, 5.8) | 2.1 (1.3, 3.3) | 0.0 (0.0, 0.2) | 0.1 (0.0, 0.7) | — | 0.0 (0.0, 0.4) |
| Water buffalo | 0.1 (0.0, 1.0) | 0.3 (0.1, 1.0) | 0.1 (0.0, 0.9) | 0.1 (0.0, 0.9) | — | — | — | — |
| Bovines* | 1.9 (1.5, 2.8) | 1.2 (0.6, 1.7) | 2.9 (1.6, 5.6) | 2.1 (1.6, 3.3) | 0.0 (0.0, 0.2) | 0.1 (0.0, 0.7) | — | 0.0 (0.0, 0.4) |
| Goats | — | — | 0.1 (0.0, 1.0) | 0.0 (0.0, 0.2) | — | — | — | — |
| Dogs | 0.0 (0.0, 0.3) | 0.0 (0.0, 0.2) | 0.0 (0.0, 0.1) | 0.0 (0.0, 0.1) | 0.2 (0.0, 1.6) | 0.0 (0.0, 0.3) | 0.1 (0.0, 1.3) | 0.1 (0.0, 0.8) |
| Cats | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.3) | 0.0 (0.0, 0.1) | 0.0 (0.0, 0.1) | 0.0 (0.0, 00) |
| Rodents | 0.0 (0.0, 0.4) | 0.2 (0.0, 1.3) | 0.0 (0.0, 0.3) | 0.0 (0.0, 0.4) | 1.8 (1.1, 6.2) | 1.3 (0.6, 2.9) | 1.5 (0.9, 4.8) | 1.5 (0.9, 2.9) |
| Overall† | 1.9 (1.5, 2.8) | 1.3 (1.2, 1.8) | 2.9 (1.9, 5.7) | 2.1 (1.6, 3.3) | 2.0 (1.3, 6.3) | 1.4 (1.1, 2.9) | 1.6 (1.1, 4.8) | 1.6 (1.3, 2.9) |
Values represent medians (and interpercentile ranges) from a multivariate uncertainty analysis. R0(i) values above one (bold) indicate maintenance host species. GH, Guanghui; HP, Heping; LQ, Longquan; LS, Longshang; XZ, Xingzhuang; YT, Yuantou.
Values for bovines represent those values for cattle and water buffalo combined (calculated from the largest eigenvalue of a next generation matrix of transmission among cattle and water buffalo only).
Overall R0 values calculated from the largest eigenvalue of the next generation matrix for transmission among all host species.
Table 2.
Estimates of the overall basic reproduction number (R0) for S. japonicum transmission within each village after removal of transmission from each definitive host species
| Removed host species | Marshland region (Tongling) |
Hilly region (Shitai) |
||||||
| GH | HP | XZ | Overall | LQ | LS | YT | Overall | |
| Humans | 1.9 (1.5, 2.8) | 1.3 (1.1, 1.8) | 2.9 (1.8, 5.7) | 2.1 (1.6, 3.3) | 2.0 (1.3, 6.3) | 1.4 (0.7, 2.9) | 1.5 (0.9, 4.8) | 1.5 (1.1, 2.9) |
| Cattle | 0.2 (0.0, 1.1) | 0.6 (0.1, 1.4) | 0.3 (0.1, 1.3) | 0.2 (0.0, 1.1) | 2.0 (1.3, 6.3) | 1.4 (0.9, 2.9) | — | 1.6 (1.2, 2.9) |
| Water buffalo | 1.9 (1.3, 2.8) | 1.2 (0.4, 1.8) | 2.9 (1.6, 5.6) | 2.1 (1.4, 3.3) | — | — | — | — |
| Bovines* | 0.0 (0.0, 0.4) | 0.3 (0.0, 1.3) | 0.1 (0.0, 1.1) | 0.1 (0.0, 0.5) | 2.0 (1.3, 6.3) | 1.4 (0.9, 2.9) | — | 1.6 (1.2, 2.9) |
| Goats | — | — | 2.9 (1.7, 5.6) | 2.1 (1.6, 3.3) | — | — | — | — |
| Dogs | 1.9 (1.5, 2.8) | 1.3 (1.1, 1.8) | 2.9 (1.8, 5.6) | 2.1 (1.6, 3.3) | 1.9 (1.1, 6.3) | 1.4 (1.0, 2.9) | 1.5 (0.9, 4.8) | 1.5 (1.1, 2.9) |
| Cats | 1.9 (1.5, 2.8) | 1.3 (1.1, 1.8) | 2.9 (1.8, 5.7) | 2.1 (1.6, 3.3) | 1.9 (1.3, 6.3) | 1.4 (1.1, 2.9) | 1.6 (1.1, 4.8) | 1.6 (1.3, 2.9) |
| Rodents | 1.9 (1.5, 2.8) | 1.2 (0.7, 1.7) | 2.9 (1.8, 5.6) | 2.1 (1.6, 3.3) | 0.2 (0.0, 1.6) | 0.4 (0.1, 1.0) | 0.3 (0.0, 1.3) | 0.3 (0.0, 0.9) |
Values represent medians (and interpercentile ranges) from a multivariate uncertainty analysis. R0 values less than one (bold) indicate that the removed host species is essential for transmission. GH, Guanghui; HP, Heping; LQ, Longquan; LS, Longshang; XZ, Xingzhuang; YT, Yuantou.
Values for bovines represent overall R0 values when transmission from both cattle and water buffaloes is removed.
Overall R0 values were fairly comparable across villages and regions, with medians ranging from 1.3 to 2.9 (Table 1). Estimates for 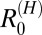 were very low, with 95% CIs below one across all villages. This finding suggests that there is little human contribution to overall transmission and that, at least under current control programs, transmission could not be maintained by humans in the absence of nonhuman mammalian reservoirs. Furthermore, removal of human transmission in the model had little impact on the overall R0 (Table 2), suggesting that humans are nonessential hosts. In the marshland villages, estimates of
were very low, with 95% CIs below one across all villages. This finding suggests that there is little human contribution to overall transmission and that, at least under current control programs, transmission could not be maintained by humans in the absence of nonhuman mammalian reservoirs. Furthermore, removal of human transmission in the model had little impact on the overall R0 (Table 2), suggesting that humans are nonessential hosts. In the marshland villages, estimates of 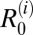 were by far highest for cattle, with medians for
were by far highest for cattle, with medians for 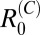 ranging from 1.2 to 2.9 and 95% CIs that were above one in two of three marshland study villages. Estimates for
ranging from 1.2 to 2.9 and 95% CIs that were above one in two of three marshland study villages. Estimates for 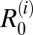 were generally below one for all other species in the marshland region, although the upper 95% CIs were above one for
were generally below one for all other species in the marshland region, although the upper 95% CIs were above one for 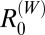 in two villages and
in two villages and 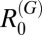 and
and 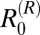 in one village each. Thus, the possibility that water buffalo, goats, and rodents are also maintenance hosts in some villages, in addition to cattle, cannot be ruled out. However, when the model was fitted to pooled data at the regional level,
in one village each. Thus, the possibility that water buffalo, goats, and rodents are also maintenance hosts in some villages, in addition to cattle, cannot be ruled out. However, when the model was fitted to pooled data at the regional level, 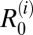 in the marshland region was only significantly above one for cattle, whereas all other
in the marshland region was only significantly above one for cattle, whereas all other 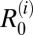 distributions were significantly below one (Table 1).
distributions were significantly below one (Table 1).
In the hilly villages, 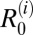 was highest for rodents, suggesting that rodents are driving transmission and likely to be maintenance hosts in these villages (although this finding was not quite robust to parameter uncertainty in two villages, where the lower bound of the 95% CI for
was highest for rodents, suggesting that rodents are driving transmission and likely to be maintenance hosts in these villages (although this finding was not quite robust to parameter uncertainty in two villages, where the lower bound of the 95% CI for 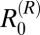 was less than one) (Table 1). If transmission from rodents is removed, the overall R0 in the hilly villages is predicted to fall below one, suggesting that rodents are also essential hosts in this region (Table 2). Estimates for
was less than one) (Table 1). If transmission from rodents is removed, the overall R0 in the hilly villages is predicted to fall below one, suggesting that rodents are also essential hosts in this region (Table 2). Estimates for 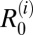 were generally below one for all other species in the hilly villages, although values were particularly uncertain for dogs, with the upper 95% CI for
were generally below one for all other species in the hilly villages, although values were particularly uncertain for dogs, with the upper 95% CI for 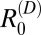 above one in two villages (Table 1).
above one in two villages (Table 1).
Impact of Reducing Bovine Transmission in the Marshland Region.
Transmission-blocking vaccines that reduce the fecundity of adult schistosomes in bovines may soon be deployed (14, 15). In some regions, bovines are even being removed, driven by the “machines replacing cattle” program (8, 10), in the hope of eliminating transmission. Fig. 1 shows the predicted impact of reducing the rate of transmission from bovines (both cattle and water buffalo) to snails on the overall R0 and the incidence in humans in the marshland region. The percentage reduction could correspond, for example, to a proportion of bovines being removed from the region or the efficacy of an antifecundity vaccine (with 100% coverage) at reducing the rate of S. japonicum egg excretion by infected bovines. The results show that reducing bovine transmission could considerably reduce R0 and further reduce the (already low) incidence among humans. However, there was uncertainty around the percentage reduction required to bring R0 below one, with a median estimate of 55% (95% CI = 43–72%). Thus, it is uncertain whether (but perhaps unlikely that) a vaccine that reduces the rate of egg excretion by infected bovines by 50% (the approximate efficacy of current antifecundity vaccines) (14) would eliminate transmission. The model predicts that complete elimination of bovines from the region, which has recently taken place, will reduce R0 to below one (R0 after elimination of bovines = 0.04, 95% CI = 0.01–0.72). Furthermore, the predicted percentage reduction in R0 after elimination of bovines suggests that these animals (cattle and water buffalo together) were responsible for 97% (95% CI = 71–99%) of transmission in the region.
Fig. 1.
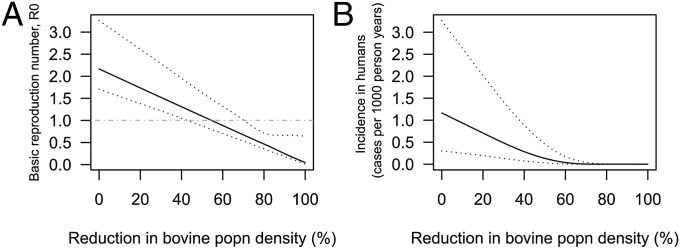
The predicted impact of reducing the population density of bovines in the marshland region of Anhui, China, on (A) the overall R0 for S. japonicum transmission and (B) the steady state incidence of infection in humans. Solid lines represent medians, and dashed lines represent 95% CIs estimated from an uncertainty analysis.
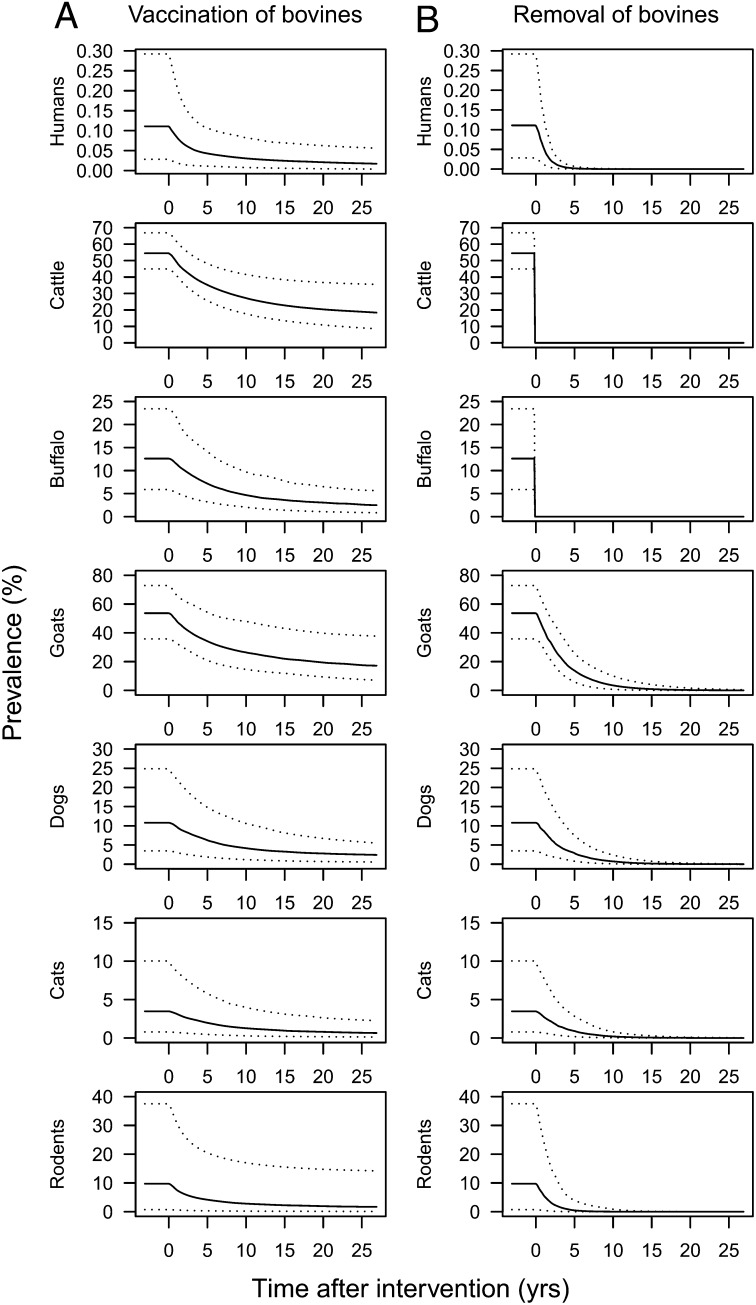
Fig. 2 shows the predicted impact of vaccination (Fig. 2A) and removal of bovines from the marshland region (Fig. 2B) on infection prevalence in each definitive host species over time. (This scenario was not investigated for the hilly region, because there were only eight bovines present across all three study villages in this region.) The vaccination scenario assumes that a vaccine that halves the rate of egg excretion among bovines is introduced at 95% coverage and that this coverage is maintained throughout the simulation. The model predicts that, although this intervention could considerably reduce the prevalence of infection in all host species, particularly within the first 5 y after its introduction, the prevalence is expected to remain fairly high (at around 20%) in cattle and goats, even after 20 y. After complete removal of bovines from the region, prevalence among all other definitive host species is predicted to fall below 1%, the operational threshold of transmission control (16), within around 10–20 y, ultimately leading to elimination of transmission.
Fig. 2.
The predicted impact of (A) vaccinating and (B) eliminating bovines in the marshland region of Anhui, China, on the prevalence of S. japonicum infection in each definitive host species over time. Vaccination assumes 95% coverage with a 50% efficacious antifecundity vaccine.
Impact of Reducing Rodent Transmission in the Hilly Region.
The potential impact of reducing the rodent population density in the hilly region was also investigated (Fig. 3) because of the high contribution of these animals to transmission indicated by the 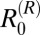 estimates in this region. There was a large degree of uncertainty surrounding the reduction needed in rodent population density that would be sufficient to eliminate transmission from the hilly region, with the 95% CI of estimates ranging from 23% to 85% reduction (Fig. 3B). This wide range is largely because of uncertainty surrounding the baseline rodent population density and rate of egg excretion by infected rodents. Nevertheless, a 20% reduction in rodent density is predicted to reduce the incidence in humans by around 40–50% (Fig. 3B). Given that incidence in humans is already fairly low, this amount essentially translates into less than 10 human cases per 1,000 person-y being prevented. The reduction in R0 predicted by complete elimination of rodent transmission suggests that rodents are responsible for around 83% of transmission in the hilly region (95% CI = 35–98%) and likely to be essential hosts for transmission in this region (Table 2).
estimates in this region. There was a large degree of uncertainty surrounding the reduction needed in rodent population density that would be sufficient to eliminate transmission from the hilly region, with the 95% CI of estimates ranging from 23% to 85% reduction (Fig. 3B). This wide range is largely because of uncertainty surrounding the baseline rodent population density and rate of egg excretion by infected rodents. Nevertheless, a 20% reduction in rodent density is predicted to reduce the incidence in humans by around 40–50% (Fig. 3B). Given that incidence in humans is already fairly low, this amount essentially translates into less than 10 human cases per 1,000 person-y being prevented. The reduction in R0 predicted by complete elimination of rodent transmission suggests that rodents are responsible for around 83% of transmission in the hilly region (95% CI = 35–98%) and likely to be essential hosts for transmission in this region (Table 2).
Fig. 3.
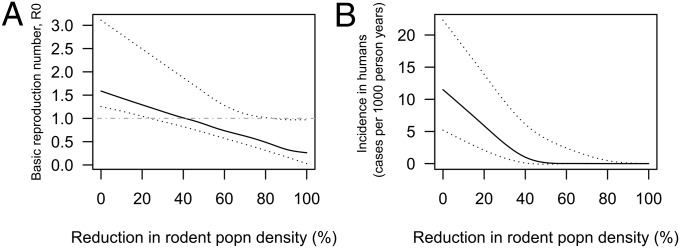
The predicted impact of reducing the rodent population density in the hilly region of Anhui, China, on (A) the overall R0 for S. japonicum transmission and (B) the steady state incidence of infection in humans. Solid lines represent medians, and dashed lines represent 95% CIs estimated from an uncertainty analysis.
Discussion
With the prevalence of S. japonicum among humans now at low levels in many endemic areas of China, the goals of control programs are increasingly shifting from control of schistosomiasis to elimination of transmission. Goals to eliminate schistosomiasis japonica have also been recently articulated by the World Health Organization in its roadmap for accelerating work to overcome the global impact of neglected tropical diseases (NTDs) (17) and the London Declaration of the NTD coalition, pledging to contribute to the elimination or control of 10 NTDs by the end of the decade. For the feasibility of elimination to be assessed and optimum control strategies to be devised, an increased understanding of the transmission dynamics among potential reservoir hosts is paramount.
The very low values for 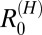 estimated in this study suggest that, under the current multipronged control measures, humans are not maintenance hosts for transmission in the study villages (i.e., they cannot sustain transmission of S. japonicum in the absence of zoonotic reservoirs). Furthermore, the results provide quantitative evidence that humans are not essential hosts for transmission of S. japonicum in China, because even by eliminating transmission from humans, transmission can persist within other definitive host species. This finding highlights that transmission cannot be eliminated from these areas by focusing control efforts on humans alone (for example, by chemotherapy and health education). Snail control, the other major intervention currently in place in many endemic regions of China, is costly, potentially environmentally damaging, and often only partially effective because of the resilience and widely dispersed distribution of snail populations (18). Directing interventions to transmission among definitive host reservoirs could, therefore, be crucial to consolidate and build on current achievements of S. japonicum control programs.
estimated in this study suggest that, under the current multipronged control measures, humans are not maintenance hosts for transmission in the study villages (i.e., they cannot sustain transmission of S. japonicum in the absence of zoonotic reservoirs). Furthermore, the results provide quantitative evidence that humans are not essential hosts for transmission of S. japonicum in China, because even by eliminating transmission from humans, transmission can persist within other definitive host species. This finding highlights that transmission cannot be eliminated from these areas by focusing control efforts on humans alone (for example, by chemotherapy and health education). Snail control, the other major intervention currently in place in many endemic regions of China, is costly, potentially environmentally damaging, and often only partially effective because of the resilience and widely dispersed distribution of snail populations (18). Directing interventions to transmission among definitive host reservoirs could, therefore, be crucial to consolidate and build on current achievements of S. japonicum control programs.
Importantly, our results suggest that bovines, specifically cattle, were likely to be the only species able to maintain transmission alone in our marshland study area and also likely to be essential for transmission to be maintained (Table 2). This result is consistent with a previous modeling study (19), and moreover, it suggests that it is largely robust to parameter uncertainty, the contribution of other definitive host species (such as dogs and rodents), and heterogeneous mixing between species. However, the possibility that goats were also maintenance hosts in one of our villages cannot be ruled out (upper bound for R0(G) was above one). Thus, elimination of bovines from lake and marshland areas where goats are also present may not suffice to eliminate transmission.
Because removal of bovines will not be a feasible solution in many other regions, the potential impact of an antifecundity vaccine against S. japonicum in bovines was also investigated. Studies have shown such vaccines to be ∼50% efficacious at reducing the rate of egg excretion from infected water buffaloes (14, 15). Assuming that these vaccines would have a similar efficacy in cattle, our model predicts that, as a sole intervention, vaccines might be unlikely to eliminate transmission in our marshland study area (assuming that bovines had not since been removed), even if applied at a high coverage. However, such vaccines could prove valuable for improving morbidity control in lake and marshland areas, where human incidence is still relatively high, and may be successful at achieving elimination if coupled with a reduction (rather than complete elimination) of bovine populations and/or intensification of other measures as part of integrated control.
In contrast to the marshland/lake regions, the potential importance of zoonotic reservoirs in hilly regions of China (where density of bovine populations tends to be lower) has previously received relatively little attention. Our results suggest that rodents are probably maintenance host species, which may at least partly explain the reemergence of human and snail infections in some hilly/mountainous regions of China where S. japonicum was thought to have been eliminated, including our study region (9, 20). In theory, reducing the rodent population density could eliminate transmission in such areas, although the feasibility and desirability of rodent control are questionable because of the high reproductive rate of rodents, the notorious difficulties of trapping and poisoning rodents, and the potentially damaging impact on ecosystems. Elimination of S. japonicum in areas where rodents are maintaining transmission could, therefore, be very challenging, and it may be necessary for control programs in such areas to focus on sustaining measures, such as health education and mollusciciding, to minimize or prevent infections in humans.
Because of considerable uncertainty surrounding model parameters for rodents (particularly relating to their population density), the hypothesis that rodents are maintenance hosts in the hilly region was not quite robust to the uncertainty analysis. However, there was generally no evidence that any other host species could maintain transmission in this region. Thus, if rodent densities are in reality at the lower end of the range allowed in the uncertainty analysis, it is possible that a species complex (e.g., rodents, dogs, and perhaps, humans) rather than any single host species is maintaining transmission in this region. The generalist nature of S. japonicum would be, evolutionarily, a greatly advantageous and even essential trait for the survival of this parasite in such situations.
The model results suggest that dogs and cats are unlikely to be maintenance or essential hosts for transmission in either region. Nevertheless, we should not rule out an important role of dogs in the transmission of S. japonicum in China. Our previous molecular analyses suggest that dogs as well as rodents may contribute substantially to snail infections in some hilly areas (12). Furthermore, given their free-roaming nature, dogs could potentially facilitate the spread of infection between otherwise largely isolated rodent habitats within an endemic hilly area or perhaps act as a connecting host between rodent transmission foci and human exposure sites.
S. japonicum is often considered to be a true multihost pathogen because of the wide range of mammalian host species in which natural infections have been found (21). However, by applying a framework proposed for classifying disease threats (4), which is summarized in SI Text, to our model results, this term is perhaps only partly accurate for S. japonicum. Certainly, in the absence of current control efforts, humans probably could sustain transmission alone, in which case S. japonicum would meet the criteria of a true multihost pathogen. Furthermore, both bovines and rodents are likely to be maintenance hosts (albeit in different regions), which again, makes it intuitive to classify S. japonicum as a true multihost pathogen. However, when looking at each habitat type separately, it seems that only one species in each region could currently be considered a maintenance host, whereas all other definitive host species are likely to be nonmaintenance hosts, despite infections seeming endemic in the latter because of substantial transmission from the maintenance host. Thus, within each of our two study regions, S. japonicum would actually be classified as an apparent multihost pathogen according to the proposed framework (4). Furthermore, because infected humans (the target hosts) were not detected in some villages during our parasitological surveys (10), infections may only be transient in human populations in some villages, in which case S. japonicum could even be classified as a spillover pathogen in some areas. Thus, the present study provides an empirical example of how the classification of a specific zoonotic disease threat can vary depending on local epidemiological conditions and the scale at which the multihost–pathogen community is considered.
The study results are subject to limitations relating to the simplifying assumptions of the model, which are discussed in Materials and Methods. For example, we assumed that the probability of a single parasite egg excreted by a definitive host causing a snail infection (defined by parameter-η) is constant across all definitive host species. In reality, this assumption is unlikely because of the among-species variability in factors, such as hatching rates of S. japonicum eggs and spatial interaction of hosts with snail habitats. However, it seems likely that, in the marshland region, η would be highest for bovines, because these animals are reared on snail-inhabited marshy areas of the land, whereas in the hilly areas, rodents were observed to inhabit irrigation ditches where snail populations are concentrated. Furthermore, η would likely be relatively low for humans, given that, at least in our study villages, human stool is often deposited in toilets especially constructed for biogas production (10). Thus, allowing η to be higher for bovines and rodents and lower for humans would only strengthen the findings pertaining to the relative importance of different host species.
In conclusion, taking S. japonicum as a model multihost multiparasite system, this study shows how mathematical modeling and conceptual frameworks can be applied to gain important insights into the transmission ecology of multihost parasite systems. In particular, our results elucidate the relative contribution of different host species to S. japonicum transmission in different habitat types in China and the potential for transmission maintenance in each species in the absence of other definitive host populations. The results suggest that, under current control programs, human incidence is largely caused by spillover from transmission among reservoir hosts and that the main species of the latter varies between different habitat types (i.e., rodents in hilly areas in contrast to bovines in lake/marshland regions) in Anhui, China. Directing interventions to the definitive host reservoirs that are most influential in transmission could be crucial for additional progress to elimination of S. japonicum.
Materials and Methods
Field Data Collection and Analysis.
Details on the local field data used in this study are given in ref. 10. Briefly, three study villages (Guanghui, Heping, and Xingzhuang) were selected from Tongling County, a marshland area within Anhui Province, China. Another three villages (Longquan, Longshang, and Yuantou) were selected from Shitai County, located in a hilly region of the same province. Systematic snail surveys were carried out in each village in 2006 according to standard protocols to estimate the density and prevalence of infection among Oncomelania hupensis snail intermediate hosts (10, 22). Human surveys were carried out in the same year embedded within the ongoing schistosomiasis control program. The indirect hemagglutination assay blood test for antibodies to S. japonicum was performed on all consenting individuals between ages 3 and 65 y in each study village. Stool samples obtained from seropositive humans were tested for S. japonicum infection using the miracidia hatching method and Kato–Katz smears (23). Fecal samples were also collected from cattle, water buffalo, goats, pigs, dogs, cats, and rodents and tested for miracidia hatching as described in ref. 10. The results of the definitive and snail host surveys are given in Tables S1 and S2, respectively. A Bayesian model was applied to estimate posterior densities for the true prevalence of infection in host species in each village accounting for uncertain and imperfect sensitivity and specificity of the diagnostic tests using the methods in previous studies (24–26). SI Text, section 1 and Tables S3, S4, and S5 have full details on this analysis.
Transmission Model.
A schematic representation of the multihost transmission model and a list parameter definitions and values are given in Fig. S1 and Table S6, respectively. The model is based on the prevalence framework of schistosomiasis transmission by Barbour (27), which has been adapted to the transmission dynamics of S. japonicum in both China (19, 28) and the Philippines (29, 30). Additional justification for the use of this framework is given in SI Text, section 2. Here, we extend the model by Barbour (27) by allowing for heterogeneous mixing between definitive host species. This model extension is motivated by our previous molecular analyses that, although generally indicating high levels of parasite gene flow across host species within villages, also suggested at least some degree of substructuring of S. japonicum populations according to host species (11). To incorporate such heterogeneity, the model tracks not only infection prevalence in each definitive host species but also the prevalence among snails according to which definitive host species caused the infection (Fig. S1). Thus, with seven classes of definitive host species, the prevalence among snails was partitioned into seven groups, with yi corresponding to the proportion of the total snail population infected by definitive host species i. Therefore, the rate of change in yi is defined as
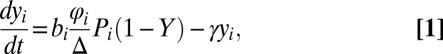 |
where bi is the rate at which an infected definitive host of species i causes snail infections, 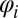 is the population density of definitive host i, Pi is the prevalence of infection in definitive host i, Δ is the snail density, γ is the mortality rate of infected snails, and Y is the overall prevalence of infection among snails (i.e.,
is the population density of definitive host i, Pi is the prevalence of infection in definitive host i, Δ is the snail density, γ is the mortality rate of infected snails, and Y is the overall prevalence of infection among snails (i.e., 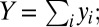 therefore, 1 − Y is the proportion of snails that are susceptible to infection). As in the original model by Barbour (27), snail density Δ is assumed to be constant (and is subsumed within transmission rate bi; hence, the appearance of 1/Δ in Eq. 1).
therefore, 1 − Y is the proportion of snails that are susceptible to infection). As in the original model by Barbour (27), snail density Δ is assumed to be constant (and is subsumed within transmission rate bi; hence, the appearance of 1/Δ in Eq. 1).
By partitioning the prevalence among snails in Eq. 1, the rate of infection from an infected snail to a definitive host of species i can vary depending on the definitive host species, j, that infected the snail. This heterogeneity was implemented by introducing a mixing matrix, Ω. Each element, ωij, of matrix Ω scales the incidence in definitive host species i from a snail infected by a different host species j (i.e., when j
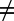 i) relative to the incidence from a snail infected by the same host species (i.e., when j = i). Thus, the rate of change in prevalence, Pi, of definitive host species i is defined as
i) relative to the incidence from a snail infected by the same host species (i.e., when j = i). Thus, the rate of change in prevalence, Pi, of definitive host species i is defined as
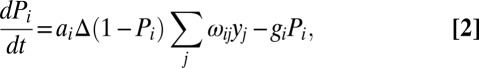 |
where ai is the incidence for a definitive host of species i per unit density of snails that were infected by the same species (when i = j), ωij is the relative incidence in host species i per unit density of snails that were infected by host species j (with ωij for i = j set to one), and gi is the reciprocal of the mean duration of infection in the definitive host.
Thus, all interhost species (off-diagonal) elements of matrix Ω (i.e., all ωij for j
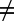 i) are relative to the intrahost species (diagonal) elements, which were set to one. If all interhost species values of ωij are also set to one, the model is equivalent to the model by Barbour (27), with no structuring of transmission according to host species. If these values are <1, there is heterogeneous mixing between definitive host species (such that, if all other things were equal, transmission within species would be greater than between host species). If all interhost species values for ωij are zero, there is no spatial overlap between definitive host species and their respective snail-inhabited contact sites, and therefore, there is no transmission between host species. Because it was not possible to infer the interhost species values for ωij from any data, these parameters were allowed to vary independently between a wide range of 0.01 and 1 (SI Text, section 5).
i) are relative to the intrahost species (diagonal) elements, which were set to one. If all interhost species values of ωij are also set to one, the model is equivalent to the model by Barbour (27), with no structuring of transmission according to host species. If these values are <1, there is heterogeneous mixing between definitive host species (such that, if all other things were equal, transmission within species would be greater than between host species). If all interhost species values for ωij are zero, there is no spatial overlap between definitive host species and their respective snail-inhabited contact sites, and therefore, there is no transmission between host species. Because it was not possible to infer the interhost species values for ωij from any data, these parameters were allowed to vary independently between a wide range of 0.01 and 1 (SI Text, section 5).
It should be noted that time-variable environmental factors (31) and connectivity between villages (32) are also potentially important in determining the transmission dynamics of S. japonicum. However, such complexities were not included in our model because of lack of sufficient data for parameterizing their effects on transmission, and investigation of such factors is beyond the scope of the present study, which focuses on the relative importance of different host species.
Estimation of Transmission Rates and the Basic Reproduction Number.
Transmission rates in the model were estimated from the field data, assuming that the dynamic processes involving loss and gain of infection are at a steady state (i.e., that the observed measures of prevalence of infection among snails and definitive hosts represent equilibrium values in each village) (SI Text, section 3, has full details). Despite the presence of control interventions, such as chemical mollusciciding and chemotherapy of humans and (to some extent) bovines in our study villages, this assumption was deemed reasonable given that these control measures have been occurring for many years. Moreover, longitudinal national surveillance data from sentinel villages within Anhui show comparable snail densities and prevalence of infection among snails and humans across at least 3 y before our field surveys in 2006, suggesting that, under the current, long-standing control measures, the system may have reached an approximately quasisteady state. [Modeling studies have shown that, after repeated treatment and conditioned to nonelimination, the prevalence distribution in those villages where infection persists at a very low level reaches a quasistationary distribution (33, 34).] The basic reproduction number within each species, 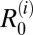 , and the overall R0 for each multihost community were then estimated using a next generation matrix (35) constructed from the system described by Eqs. 1 and 2 (SI Text, section 4, has full details). It should be stressed that R0 values derived from this analysis are estimates of the basic reproduction number for transmission in the presence of current interventions and under the assumption of quasistationary distribution of prevalence, which is particularly relevant for humans, who have been regularly treated.
, and the overall R0 for each multihost community were then estimated using a next generation matrix (35) constructed from the system described by Eqs. 1 and 2 (SI Text, section 4, has full details). It should be stressed that R0 values derived from this analysis are estimates of the basic reproduction number for transmission in the presence of current interventions and under the assumption of quasistationary distribution of prevalence, which is particularly relevant for humans, who have been regularly treated.
Parameter Values and Uncertainty Analysis.
The majority of model parameters was assigned a plausible distribution of values rather than single-point estimates. Values and probability distributions of parameters were chosen based on local parasitological survey data (including the posterior distributions of prevalence data estimated from the Bayesian analysis) (Table S5) and a review of the relevant literature. This approach acknowledges that considerable uncertainty exists surrounding parameter values, because of not only limited knowledge of schistosome and intermediate host biology but also sampling and measurement error (for example, in our field survey data used to estimate R0). Parameter value distributions and their sources are shown in Table S6, and additional details on their selection are given in SI Text, section 5. To efficiently sample the parameter space, the Latin Hypercube Sampling method was used. Longitudinal simulations of the model were run to investigate the impact of various control scenarios on the prevalence of infection across all definitive host species, incidence in humans, and R0 values. Scenarios included removal and vaccination of bovines and reduction in rodent population densities, assuming that all current interventions (e.g., annual treatment of humans and mollusciciding) remain in place throughout the duration of the simulation.
Applying Conceptual Frameworks of Multihost Transmission Ecology.
To complement the modeling analysis, we applied a conceptual framework proposed for identifying the key reservoir hosts in multihost systems (3). Humans can be considered the target population in this study, because this species is of most concern from a public health perspective. All other domesticated and wild animals in which S. japonicum naturally occurs are the nontarget population, and those species constitute potential reservoir hosts, where a reservoir is defined as “one or more epidemiologically connected populations or environments in which the pathogen can be permanently maintained and from which infection is transmitted to the defined target population” (3). Both target and nontarget host species can be classified as either maintenance or nonmaintenance species depending on whether the parasite can persist in that species in the absence of transmission from other host species (assuming, in this case, that sufficient densities of snail intermediate hosts are present). In our study, this classification was determined according to whether estimates of 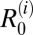 were ≥1 (maintenance host) or <1 (nonmaintenance host). Each host species can also be classified as either essential or nonessential to transmission according to whether transmission can be sustained in the absence of transmission from that species. This classification was determined from estimates of overall R0 after removal of transmission from each species in the model; if it fell below one, then the removed species was classified as an essential host.
were ≥1 (maintenance host) or <1 (nonmaintenance host). Each host species can also be classified as either essential or nonessential to transmission according to whether transmission can be sustained in the absence of transmission from that species. This classification was determined from estimates of overall R0 after removal of transmission from each species in the model; if it fell below one, then the removed species was classified as an essential host.
In addition, we applied a framework proposed by Fenton and Pederson (4) for describing the configurations of multihost–pathogen communities. Based on a pathogen’s within- and between-species transmission rates, this framework allows disease threats to be classified into one of four categories (with respect to the target host population)—specifically (i) spillover pathogen, (ii) apparent multihost pathogen, (iii) true multihost pathogen, and (iv) potentially emerging infectious disease. Definitions of each of these categories are summarized in SI Text, section 6.
Supplementary Material
Acknowledgments
We thank Valerie Isham and the Helminth Research Group at Imperial College London for useful discussions on the model and the Anhui Institute of Parasitic Diseases and the Schistosomiasis Control Stations in Shitai and Tongling for fieldwork support. This study was funded by the Medical Research Council of the United Kingdom through a doctoral studentship (J.W.R.), The Royal Society (J.P.W.), a Kwok studentship (D.-B.L.), and the Wellcome Trust (M.-G.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221509110/-/DCSupplemental.
References
- 1.Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356(1411):983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Smith JO, et al. Epidemic dynamics at the human-animal interface. Science. 2009;326(5958):1362–1367. doi: 10.1126/science.1177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haydon DT, Cleaveland S, Taylor LH, Laurenson MK. Identifying reservoirs of infection: A conceptual and practical challenge. Emerg Infect Dis. 2002;8(12):1468–1473. doi: 10.3201/eid0812.010317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenton A, Pedersen AB. Community epidemiology framework for classifying disease threats. Emerg Infect Dis. 2005;11(12):1815–1821. doi: 10.3201/eid1112.050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basáñez MG, et al. A research agenda for helminth diseases of humans: Modelling for control and elimination. PLoS Negl Trop Dis. 2012;6(4):e1548. doi: 10.1371/journal.pntd.0001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boatin BA, et al. A research agenda for helminth diseases of humans: Towards control and elimination. PLoS Negl Trop Dis. 2012;6(4):e1547. doi: 10.1371/journal.pntd.0001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou XN, et al. Epidemiology of schistosomiasis in the People’s Republic of China, 2004. Emerg Infect Dis. 2007;13(10):1470–1476. doi: 10.3201/eid1310.061423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang LD, et al. A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med. 2009;360(2):121–128. doi: 10.1056/NEJMoa0800135. [DOI] [PubMed] [Google Scholar]
- 9.Liang S, Yang C, Zhong B, Qiu D. Re-emerging schistosomiasis in hilly and mountainous areas of Sichuan, China. Bull World Health Organ. 2006;84(2):139–144. doi: 10.2471/blt.05.025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu DB, et al. Contrasting reservoirs for Schistosoma japonicum between marshland and hilly regions in Anhui, China—a two-year longitudinal parasitological survey. Parasitology. 2010;137(1):99–110. doi: 10.1017/S003118200999103X. [DOI] [PubMed] [Google Scholar]
- 11.Rudge JW, et al. Parasite genetic differentiation by habitat type and host species: Molecular epidemiology of Schistosoma japonicum in hilly and marshland areas of Anhui Province, China. Mol Ecol. 2009;18(10):2134–2147. doi: 10.1111/j.1365-294X.2009.04181.x. [DOI] [PubMed] [Google Scholar]
- 12.Lu DB, et al. Transmission of Schistosoma japonicum in marshland and hilly regions of China: Parasite population genetic and sibship structure. PLoS Negl Trop Dis. 2010;4(8):e781. doi: 10.1371/journal.pntd.0000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson RM, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford: Oxford Univ Press; 1991. [Google Scholar]
- 14.Da’dara AA, et al. DNA-based vaccines protect against zoonotic schistosomiasis in water buffalo. Vaccine. 2008;26(29–30):3617–3625. doi: 10.1016/j.vaccine.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McManus DP, Loukas A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev. 2008;21(1):225–242. doi: 10.1128/CMR.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spear RC, et al. The challenge of effective surveillance in moving from low transmission to elimination of schistosomiasis in China. Int J Parasitol. 2011;41(12):1243–1247. doi: 10.1016/j.ijpara.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization . Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases: A Roadmap for Implementation. Geneva: World Health Organization; 2012. [Google Scholar]
- 18.Yuan Y, Xu X-J, Dong H-F, Jiang M-S, Zhu H-G. Transmission control of schistosomiasis japonica: Implementation and evaluation of different snail control interventions. Acta Trop. 2005;96(2–3):191–197. doi: 10.1016/j.actatropica.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Gray DJ, Williams GM, Li Y, McManus DP. Transmission dynamics of Schistosoma japonicum in the lakes and marshlands of China. PLoS One. 2008;3(12):e4058. doi: 10.1371/journal.pone.0004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang TP, et al. [Outbreaks of acute schistosomiasis in Anhui province in 2003] Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25(7):568–71. [PubMed] [Google Scholar]
- 21.He YX, Salafsky B, Ramaswamy K. Host—parasite relationships of Schistosoma japonicum in mammalian hosts. Trends Parasitol. 2001;17(7):320–324. doi: 10.1016/s1471-4922(01)01904-3. [DOI] [PubMed] [Google Scholar]
- 22.Frandsen F, Christensen NO. An introductory guide to the identification of cercariae from African freshwater snails with special reference to cercariae of trematode species of medical and veterinary importance. Acta Trop. 1984;41(2):181–202. [PubMed] [Google Scholar]
- 23.Yu JM, de Vlas SJ, Jiang QW, Gryseels B. Comparison of the Kato-Katz technique, hatching test and indirect hemagglutination assay (IHA) for the diagnosis of Schistosoma japonicum infection in China. Parasitol Int. 2007;56(1):45–49. doi: 10.1016/j.parint.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Joseph L, Gyorkos TW, Coupal L. Bayesian estimation of disease prevalence and the parameters of diagnostic tests in the absence of a gold standard. Am J Epidemiol. 1995;141(3):263–272. doi: 10.1093/oxfordjournals.aje.a117428. [DOI] [PubMed] [Google Scholar]
- 25.Wang XH, Wu XH, Zhou XN. Bayesian estimation of community prevalences of Schistosoma japonicum infection in China. Int J Parasitol. 2006;36(8):895–902. doi: 10.1016/j.ijpara.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Carabin H, et al. Estimating sensitivity and specificity of a faecal examination method for Schistosoma japonicum infection in cats, dogs, water buffaloes, pigs, and rats in Western Samar and Sorsogon Provinces, The Philippines. Int J Parasitol. 2005;35(14):1517–1524. doi: 10.1016/j.ijpara.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Barbour AD. Modeling the transmission of schistosomiasis: An introductory view. Am J Trop Med Hyg. 1996;55(5 Suppl):135–143. doi: 10.4269/ajtmh.1996.55.135. [DOI] [PubMed] [Google Scholar]
- 28.Williams GM, et al. Mathematical modelling of schistosomiasis japonica: Comparison of control strategies in the People’s Republic of China. Acta Trop. 2002;82(2):253–262. doi: 10.1016/s0001-706x(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 29.Ishikawa H, Ohmae H, Pangilinan R, Redulla A, Matsuda H. Modeling the dynamics and control of Schistosoma japonicum transmission on Bohol island, the Philippines. Parasitol Int. 2006;55(1):23–29. doi: 10.1016/j.parint.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Riley S, et al. Multi-host transmission dynamics of Schistosoma japonicum in Samar province, the Philippines. PLoS Med. 2008;5(1):e18. doi: 10.1371/journal.pmed.0050018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang S, et al. Environmental effects on parasitic disease transmission exemplified by schistosomiasis in western China. Proc Natl Acad Sci USA. 2007;104(17):7110–7115. doi: 10.1073/pnas.0701878104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurarie D, Seto EY. Connectivity sustains disease transmission in environments with low potential for endemicity: Modelling schistosomiasis with hydrologic and social connectivities. J R Soc Interface. 2009;6(35):495–508. doi: 10.1098/rsif.2008.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nåsell I. On the quasi-stationary distribution of the stochastic logistic epidemic. Math Biosci. 1999;156(1–2):21–40. doi: 10.1016/s0025-5564(98)10059-7. [DOI] [PubMed] [Google Scholar]
- 34.Ray KJ, et al. A rationale for continuing mass antibiotic distributions for trachoma. BMC Infect Dis. 2007;7(2007):91. doi: 10.1186/1471-2334-7-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts MG, Heesterbeek JA. A new method for estimating the effort required to control an infectious disease. Proc Biol Sci. 2003;270(1522):1359–1364. doi: 10.1098/rspb.2003.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.