Abstract
Vaccinia virus wild-type strains such as Ankara and WR synthesize proteins capable of inhibiting the activation of host NF-κB, a family of transcription factors that regulate the expression of inflammatory genes. In contrast, an infection by the attenuated MVA strain, whose genome lacks many immunoregulatory genes present in the DNA of its Ankara parent, induces NF-κB activation. Insertion of NF-κB inhibitory genes into the MVA DNA, then, would alter the MVA phenotype. By this method, a 5.2-kb region of Ankara DNA containing the K1L gene and two other genes that are absent in the MVA genome that was identified as NF-κB was inhibited in cells infected with the MVA/5.2kb virus. To determine if K1L was responsible, the relevant biological properties of both a recombinant MVA containing a copy of the WR strain's K1L (MVA/K1L) and a WR deletion mutant lacking the K1L gene (ΔK1L) were examined. Indeed, unlike its progenitor, the altered MVA halted degradation of the host regulatory protein IκBα—a key event in the pathway of transcriptional activation by NF-κB factors. Moreover, MVA/K1L gained the ability to repress artificially contrived and natural NF-κB-regulated expression of a transfected luciferase and the cellular tumor necrosis factor gene, respectively. In contrast, although these functions could also be performed by WR, the ΔK1L virus lost these abilities. Thus, one apparent molecular function of K1L is to prevent IκBα degradation. This impediment to NF-κB-induced host proinflammatory gene expression, in turn, might enhance virus survival.
The NF-κB/Rel transcription factor family regulates the expression of genes involved in immune responses, inflammation, apoptosis, and proliferation (for a review, see references 18 and 24). As the inflammatory responses resulting from NF-κB activation can be detrimental to healthy cells, its activation is tightly controlled by a family of proteins (IκBs) that interact with NF-κB. One such NF-κB inhibitory protein is IκBα, which binds via ankyrin repeats to the p65 subunit of NF-κB dimers (composed of p65 and p50 proteins) located in the cytoplasm. Originally, these interactions were thought to mask the nuclear localization signal of the p65 NF-κB subunit and, thus, to inhibit NF-κB translocation to the nucleus and prevent transcription stimulation (19). Recent studies, however, have shown that IκBα and NF-κB shuttle between the nucleus and cytoplasm, indicating that a dynamic nucleocytoplasmic complex exists (3, 9).
NF-κB activation is initiated through many signals provided by proinflammatory cytokines, pathogen-associated molecular patterns, UV light exposure, and reactive oxygen intermediates (19). When any of these signals is present, the IκB kinase (IKK) is phosphorylated by active cellular protein kinase(s) and subsequently phosphorylates IκBα at serine residues 32 and 36 (6, 8, 14, 15, 25, 42). The phosphorylated IκBα is then polyubiquitinated at lysines 21 and 22 and is targeted for degradation by the 26S proteosome, thereby releasing NF-κB to stimulate transcription of its target genes in collaboration with other factors (2, 13, 14, 37). While other control mechanisms do exist, the key step in the classical NF-κB activation pathway is the phosphorylation of IκBα by IKK (18, 24).
Many viruses purposefully activate NF-κB for use as a transcriptional factor to express viral genes (reviewed in references 23 and 36). However, NF-κB is potentially dangerous to virus-infected cells. For example, NF-κB induces the expression of multiple host proteins such as antiviral cytokines, immune receptor molecules that present viral antigen to cytotoxic T lymphocytes, and inflammatory chemokines that attract immune cells to an area of virus infection, thereby hindering virus survival (19). Thus, viruses must carefully control the timing and duration of NF-κB activation to ensure its usefulness as a transcription factor while restricting its antiviral effects (16, 23, 36). Among the viruses capable of inhibiting NF-κB is vaccinia virus, the prototypic poxvirus, which expresses a large number of proteins that repress innate immune responses (28, 29, 30). In contrast, an attenuated strain of vaccinia virus (MVA), which has lost approximately 15% of its genome compared to that of its Ankara parent, lacks this nullifying ability (1).
Since NF-κB regulates the expression of antiapoptosis genes (27), initial observations in our laboratory led to the hypothesis that resistance to cell death during poxvirus infection was due to NF-κB activation. However, this hypothesis was subsequently disproved when poxviral genes encoding NF-κB inhibitory proteins were discovered. In the present study, it was determined that insertion of a 5.2-kb region of Ankara DNA restored the ability of MVA to deter NF-κB activation. Further dissection of this region found that the K1L gene product was responsible for the observed inhibition; MVA-induced NF-κB activation was halted in cells infected with a recombinant MVA virus containing the vaccinia virus WR K1L gene. Further analysis using a WR-based K1L deletion mutant showed that K1L was necessary for inhibiting NF-κB activity in cultured RK13 cells. These findings are the first report of a molecular function for K1L. Thus, in addition to being necessary for virus reproduction, K1L may also aid in subverting antiviral immune responses.
MATERIALS AND METHODS
Cells and viruses.
Human (293T and Jurkat) and rabbit kidney (RK13) cell lines were obtained from the American Type Culture Collection. Vaccinia virus wild-type (WR and Ankara) and attenuated (MVA) strains were obtained from Bernard Moss (Laboratory of Viral Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health). The MVA/5.2kb virus is an MVA virus whose genome contains the 5.2-kb EcoRI fragment of the parental Ankara DNA (26). This fragment includes the full-length K1L transcriptional unit, along with six other genes (26). The recombinant viruses MVA/44.1, MVA/47.1, MVA/51.1, and MVA/44.1+47.1 contain Ankara left-end genomic segments generated from a cosmid library (44). The MVA/K1L virus, a gift from Gerd Sutter (Institute for Molecular Virology, Munich, Germany), contains the entire K1L gene and its promoter that were amplified from the WR genome by using PCR (41). The amplicon was inserted into the delIII region of MVA, an area commonly used for placement of genetic material into the MVA genome (41). Thus, the MVA/K1L virus contains a leftover fragmented MVA K1L sequence and the entire wild-type WR K1L gene. The ΔK1L virus (a gift from Bernard Moss) is the WR strain of vaccinia virus that has had its K1L gene replaced with the Escherichia coli gpt gene by homologous recombination.
Detection of cell death by using TUNEL.
Cytolysis of poxvirus-infected cells was detected as described previously (38). Briefly, 2 × 106 Jurkat cells were infected with 20 PFU of either WR, MVA, or recombinant MVA viruses (MVA/44.1, MVA/47.1, MVA/51.1, MVA/44.1+47.1, and MVA/5.2kb) per cell. A higher multiplicity of infection (MOI) was used for this cell line because a suspension of cells was infected. At 6 h postinfection, the medium was replaced with one either lacking or containing 50 μM etoposide (Sigma), an exogenous apoptosis inducer. Twelve hours later, cells were fixed, permeabilized, and incubated with a fluorescein-based in situ cell death detection kit (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling [TUNEL] assay; Roche) to detect individual dead cells and then incubated with a mouse monoclonal antibody recognizing the early E3L protein of vaccinia virus, followed by incubation with a Cy5-conjugated anti-mouse antibody (Jackson Immunoresearch). Using a FACScalibur apparatus (Becton Dickinson), 10,000 individual cells positive for E3L staining were analyzed for cell death.
Detection of IκBα levels by using immunoblotting.
Cytoplasmic lysates were made according to a modified version of methods described previously (30). Jurkat cells were infected at an MOI of 20 with MVA, WR, Ankara, or recombinant MVA viruses. Eighteen hours after infection, cells were lysed in cytoplasmic extraction (CE) buffer. RK13 and 293T cell monolayers were infected at an MOI of 10 with WR, MVA, MVA/5.2kb, or MVA/K1L and then lysed in CE buffer 8 h after infection. The protein concentration of each sample was determined using the bicinchoninic acid assay (Pierce) (40). An equal amount of protein (30 μg) for each sample was loaded onto separate lanes of a 12% polyacrylamide gel. After electrophoresis, the proteins were transferred onto a polyvinyl difluoride membrane (Millipore). To detect IκBα, blots were probed with rabbit anti-IκBα antibody (Santa Cruz), a pan-specific polyclonal antibody, followed by goat anti-rabbit immunoglobulin conjugated to horseradish peroxidase (Fisher Scientific). Immunoblots were developed by using chemilluminescence (Pierce). Similar blots were probed with antiactin antibody (Santa Cruz) or antibody to SPI-1 rabbitpox virus protein (a gift from Richard Moyer, Department of Molecular Genetics, University of Florida) (7) to verify equal protein loading and successful virus infection, respectively.
Measurement of NF-κB activation by using the firefly luciferase reporter assay.
Subconfluent monolayers of RK13 cells in a 12-well plate were transfected with 100 ng of pRL-TK (Promega) and 900 ng of pNF-κBluc (Stratagene) using the Lipofectamine 2000 transfection reagent (Life Technologies). Likewise, 50 ng of pRL-null and 450 ng of pNF-κBluc were transfected into 293T cells by using FuGene6 (Roche). The pRL-TK plasmid contains the Renilla reniformis (sea pansy) luciferase gene under the transcriptional control of the herpesvirus thymidine kinase promoter and constitutively expresses low levels of sea pansy luciferase. The pRL-null plasmid is identical to pRL-TK except that it lacks the herpesvirus thymidine kinase promoter. The pRL-null plasmid was substituted for pRL-TK in assays with 293T cells to reduce the luminescent backgrounds. The pNF-κBluc plasmid contains the firefly luciferase gene under the transcriptional control of a synthetic promoter containing five direct repeats of the NF-κB binding element. As a result, firefly luciferase expression is induced by activated NF-κB. At 18 to 24 h posttransfection, cells were mock infected or infected with viruses (MOI of 10). At 8 h postinfection, cell monolayers were lysed in 100 to 250 μl of passive lysis buffer (Promega), followed by cell lysate analysis for both luciferase activities by using the Dual Luciferase Reporter assay (Promega).
Luciferase activity was measured as relative light units (RLUs) by using the Luminoskan microplate luminometer (Labsystems). For all assays, experiments were performed in triplicate. For each experimental point, the average of the firefly luciferase activity was divided by the average of the sea pansy luciferase activity to correct for differences in transfection efficiencies. The resultant ratios were used to compare the expression of the firefly luciferase gene in virus-infected cells to that present in uninfected cells.
Detection of NF-κB-regulated host TNF gene transcription by using reverse transcription-PCR (RT-PCR).
RK13 and 293T cells were infected with WR, MVA, MVA/K1L, or ΔK1L at an MOI of 10. At 6 h postinfection, cells were collected, total RNA was extracted from infected cells using the Qiagen RNeasy kit (Qiagen), and 3 μg in a 50-μl reaction mixture was reverse transcribed into single-stranded cDNA with Superscript II reverse transcriptase (Invitrogen) and oligo(dT) primers. For all PCRs, 1 μl of template cDNA was used. Amplifications of tumor necrosis factor (TNF) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA were performed in parallel by using PCR with primers specific for rabbit (RK13) or human (293T) sequences. Primers for rabbit TNF were 5′-CAAGCCTCTAGCCCACGTA-3′ and antisense oligonucleotide 5′-GGCAATGATCCCAAAGTAG-3′, yielding a 438-bp product (5). The PCR conditions were 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s. Primers for rabbit GAPDH were 5′-GCGCCTGGTCACCAGGGCTGCTT-3′ and antisense oligonucleotide 5′-TGCCGAAGTGGTCGTGGATGACCT-3′, yielding a 465-bp product (5). The PCR was run at 95°C for 30 s, 62°C for 1 min, and 72°C for 30 s. Primers for human TNF were 5′-GAGTGACAAGCCTGTAGCCCATGTTGTAGCA-3′ and antisense oligonucleotide 5′-GGCAATGATGATCCCAAAGTAGACCTGCCCAGACT-3′ and yielded a 480-bp product (22). PCR conditions were 95°C for 45 s, 60°C for 45 s, and 72°C for 2 min. Primers for human GAPDH were 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ and antisense oligonucleotide 5′-CATGTGGGCCATGAGGTCCACCAC-3′ and yielded a 921-bp product (22). PCR conditions were 95°C for 30 s, 61°C for 1 min, and 72°C for 1 min. TNF and GAPDH cDNAs were amplified for either 35 cycles (rabbit) or 25 cycles (human). A portion of each PCR was analyzed by agarose gel electrophoresis, and amplicons were visualized by ethidium bromide staining.
RESULTS
Insertion of a 5.2-kb region of Ankara DNA into the MVA genome eliminates the ability of MVA to induce IκBα degradation.
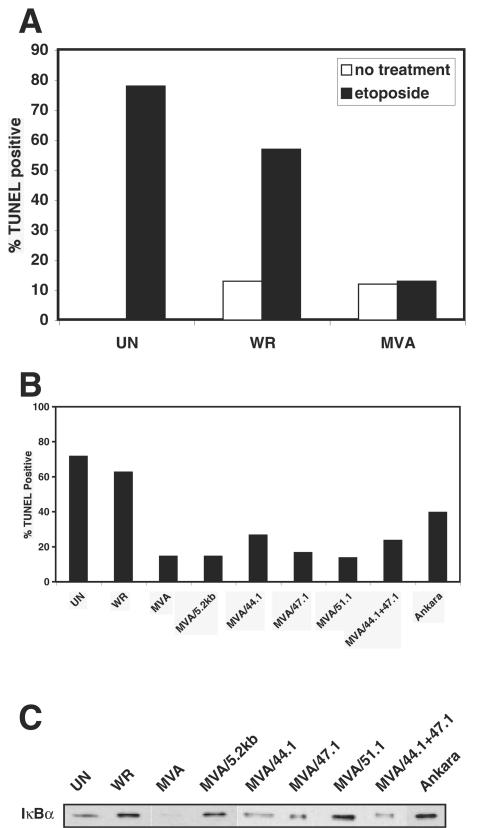
This study was originally designed to discover poxvirus proteins that were novel inhibitors of caspase-9 (mitochondrion)-mediated cell death. For this purpose, the vaccinia virus attenuated MVA strain was examined because it has lost many immunoevasion genes and possibly could be lacking genes encoding antiapoptosis products as well. Surprisingly, when a TUNEL assay was used to detect etoposide-induced cell death, the percentage of MVA-infected cells undergoing this event was significantly lower than that of WR- or Ankara-infected cells (Fig. 1A and B). Despite this difference, both MVA and WR infection equally elicited death in a small population of cells that were not incubated with etoposide. As expected, etoposide treatment induced caspase-9-mediated cytolysis in a majority of mock-infected cells (17).
FIG. 1.
Resistance to etoposide-induced cytolysis does not correlate with the ability of MVA/5.2kb virus to inhibit IκBα degradation. Jurkat cells were mock infected (UN) or infected at an MOI of 20 with the indicated viruses. (A and B) At 6 h postinfection, cells were incubated with medium alone or medium containing 50 μM etoposide. At 12 h after treatment, cells were fixed and cytolysis was detected by using a fluorescence-based TUNEL assay. Only results with etoposide treatment are shown in panel B, as cytolysis only occurred in a minority of untreated cells. (C) Eighteen hours postinfection, Jurkat cells were collected by centrifugation and lysed in CE buffer. Equal amounts of protein from each lysate were subjected to sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis and subsequent Western immunoblotting with anti-IκBα antibody.
In contrast to the WR strain, MVA has been shown to activate host NF-κB (30). Since this factor up-regulates transcription of antiapoptosis genes (27), we hypothesized that NF-κB activation arising from MVA infection would indirectly enhance resistance to etoposide-induced death. Conversely, the inhibition of NF-κB activation by WR proteins would render host cells susceptible to etoposide toxicity. Further, we posited that we could discover a viral gene(s) responsible for NF-κB inhibition by identifying Ankara DNA that, when reinserted into the MVA genome, protected against cytolysis during virus infection. To test this idea, Jurkat cells were infected with recombinant MVA viruses containing segments of the left end of the Ankara genome, and infected cells were assayed for resistance to etoposide-induced cell death and NF-κB activation states (26, 44). For this purpose, death was detected in etoposide-treated, infected cells by using the TUNEL assay. To indirectly detect NF-κB activation, IκBα was detected and compared among virus-infected cell lysates. An equal amount of protein from each virus-infected cytoplasmic extract was analyzed by immunoblotting to ensure that the absence of IκBα in a sample was not due to uneven protein loading. While this method does not precisely determine the levels of NF-κB activation in cells, it is a useful method to determine whether NF-κB is activated. By this manner, cells separately infected with the MVA/5.2kb, MVA/44.1, MVA/47.1, MVA/51.1, or MVA/44.1+47.1 recombinant MVA viruses were found to be more resistant to etoposide-induced toxicity than mock-, WR-, or Ankara-infected cells (Fig. 1B). Moreover, as reported above, MVA-infected cells did not undergo detectable levels of etoposide-induced cytolysis (Fig. 1B), whereas infection in the absence of etoposide triggered death in a minority of cells (data not shown). Since IκBα was detected in uninfected as well as WR- and Ankara-infected cells (Fig. 1C), NF-κB did not appear to be active under these conditions. In contrast, the inability to measure IκBα in MVA-infected lysates signified that NF-κB was probably activated as a result of this loss of IκBα. These data are comparable to those previously reported for infected 293 cells (30). The presence of IκBα in cytoplasmic extracts prepared from cells infected with recombinant MVA/5.2kb and MVA/51.1, and also from MVA/44.1-, MVA/47.1-, and MVA/44.1+47.1-infected cells, albeit at lower levels, indicated that proteins expressed by the inserted Ankara DNA blocked host NF-κB. Since MVA/5.2kb-, MVA/51.1-, and MVA/47.1-infected cells were resistant to etoposide-induced death despite the apparent inhibition of host NF-κB activity, vaccinia virus-induced etoposide resistance is not dependent on activation of host NF-κB.
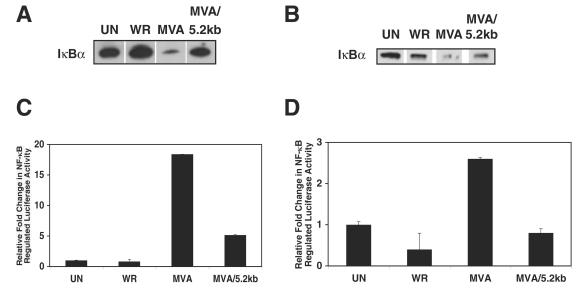
Among the recombinant MVA viruses capable of inhibiting host NF-κB activity, MVA/5.2kb has the least number of Ankara virus genes (26). Therefore, further analyses focused on this virus. Since up to this point only Jurkat cells had been used, the ability of this virus to impede NF-κB activation in other cell lines was examined. In both 293T and RK13 cells, the amount of IκBα was reduced in MVA-infected cells compared to that in WR-infected and uninfected cells (Fig. 2A and B). In agreement, a similar degradation of IκBα has been observed in the human cell line 293, the parent of 293T cells, after infection with MVA (30). Again, inclusion of the 5.2-kb Ankara DNA fragment into the MVA genome reversed this trend, as evidenced by the increased quantity of IκBα in MVA/5.2kb-infected cells. Apparently, virus replication is not required for modulation of IκBα activity, since MVA does not produce progeny in either cell line and only RK13 cells are permissive for MVA/5.2kb (11).
FIG. 2.
The MVA/5.2kb virus inhibits IκBα degradation and NF-κB activation in 293T and RK13 cells. (A and B) 293T cells (A) and RK13 cells (B) were either mock infected (UN) or infected at an MOI of 10 with either the WR, MVA, or MVA/5.2kb virus. At 8 h postinfection, cells were collected and lysed in CE buffer. Equal amounts of cytoplasmic extracts were analyzed on sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis and then by Western immunoblotting with anti-IκBα antisera. (C and D) 293T cells were transfected with pRL-null and pNF-κBluc (C) and RK13 cells were transfected with pRL-TK and pNF-κBluc (D). For both cell types, at 24 h posttransfection cells were either mock infected (UN) or infected at an MOI of 10. Eight hours postinfection, cells were collected and lysed, and firefly and sea pansy luciferase activities were measured. The luciferase assays were performed in triplicate, and each bar represents the averaged data from one representative experiment. The relative fold changes were determined by normalizing the ratios of firefly luciferase activity in virus-infected cells to sea pansy luciferase activity in each virus-infected cell group to the value obtained for uninfected cells (see Table 1 for raw data).
The above experiment detected IκBα at 8 h postinfection. A time course experiment revealed that IκBα was completely degraded in MVA-infected RK13 cells by 4 h postinfection (data not shown). As poxvirus infection inhibits host cell protein synthesis, IκBα levels would not be expected to return late in MVA infection. Indeed, IκBα was absent in MVA-infected 293T and RK13 cells 8 h postinfection (Fig. 2 and 3) and in MVA-infected Jurkat cells 18 h postinfection (Fig. 1C).
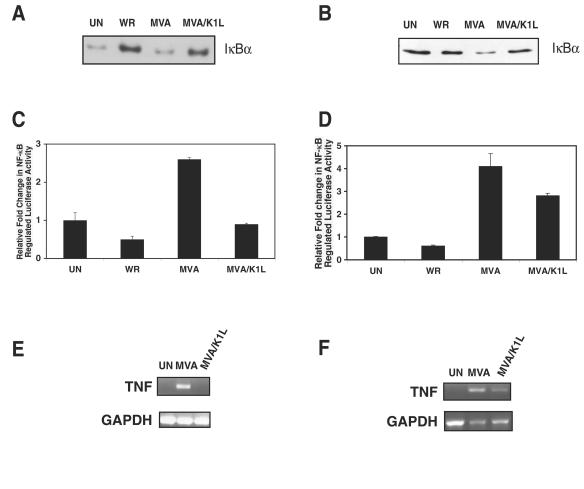
FIG. 3.
K1L inhibits IκBα degradation and NF-κB activation of reporter and cellular genes when expressed during MVA infection. (A and B) RK13 cells (A) or 293T cells (B) were infected at an MOI of 10 with either the WR, MVA, or MVA/K1L virus. At 8 h postinfection, cells were collected and lysed in CE buffer. Cytoplasmic extracts were analyzed on sodium dodecyl sulfate-12% polyacrylamide gels and then probed for IκBα levels by Western immunoblotting. (C and D) RK13 cells were transfected with pRL-TK and pNF-κBluc (C), and 293T cells were transfected with pRL-null and pNF-κBluc (D). For both cell types, at 24 h posttransfection cells were infected at an MOI of 10. At 8 h postinfection, cells were lysed. The ratios of firefly to sea pansy luciferase activities were determined and normalized to that of uninfected cells. Results are expressed as an average of three experiments. (E and F) RK13 cells (E) and 293T cells (F) were infected with MVA or MVA/K1L at an MOI of 10. At 6 h postinfection, total RNA was isolated and reverse transcribed into cDNA. TNF and GAPDH cDNAs were amplified by using PCR, and the resultant DNA products were separated by electrophoresis on a 2% agarose gel and visualized with ethidium bromide. In all cases, mock-infected (UN) cells served as a control.
A 5.2-kb region of Ankara DNA is associated with repression of transcription of a transfected gene regulated by a promoter containing NF-κB binding sites.
It has been shown that the African swine fever virus A238L product inhibits host NF-κB activation independent of IκBα degradation (32, 34). Thus, we considered the inability to detect IκBα in the preceding immunoblotting might not accurately reflect the degree of NF-κB activation. To verify the immunoblotting results, the expression of an NF-κB-transcriptionally regulated firefly luciferase gene in virus-infected and uninfected cells (Fig. 2C and D; Table 1) was measured in a manner similar to that previously used to successfully ascertain NF-κB activation in poxvirus-infected cells (30). As expected, WR infection did not modulate firefly luciferase activity in 293T cells (Fig. 2C). The overall increase in luciferase activity compared to that in uninfected 293T cells may be due to global up-regulation of gene expression during WR infection (10, 35, 39, 43). MVA-infected cells, in contrast, exhibited approximately 18-fold-more firefly luciferase activity than did the mock-infected cells. In carefully examining the luciferase activities in MVA-infected cells, it was noticed that the sea pansy RLUs were lower than for other infected cells, raising the concern that MVA-induced NF-κB activation as measured by a reporter assay is misleading. However, supporting immunoblotting and RT-PCR data (see below) eliminated this possibility. MVA/5.2kb lysates, while retaining higher luciferase activity than mock- or WR-infected cells, still had a threefold-decreased amount of luciferase compared to MVA-infected cells, indicating that a gene product from the inserted Ankara DNA prevents NF-κB activation.
TABLE 1.
Sea pansy and firefly luciferase activites in virus-infected and uninfected 293T and RK13 cells
| Virus | RLUa
|
Firefly/sea pansy ratio | Normalized value | |
|---|---|---|---|---|
| Sea pansy | Firefly | |||
| 293T | ||||
| Uninfected | 133 ± 18 | 854 ± 44 | 6 | 1.0 |
| WR | 296 ± 16 | 1,572 ± 52 | 5 | 0.81 |
| MVA | 86 ± 20 | 10,305 ± 205 | 118 | 19.7 |
| MVA/5.2 kb | 124 ± 20 | 4,095 ± 536 | 33 | 5.5 |
| RK13 | ||||
| Uninfected | 16 ± 6 | 568 ± 132 | 36 | 1.0 |
| WR | 46 ± 4 | 736 ± 109 | 16 | 0.4 |
| MVA | 5 ± 0.2 | 469 ± 104 | 94 | 2.6 |
| MVA/5.2 kb | 39 ± 9 | 1,103 ± 291 | 28 | 0.8 |
Results are the average of three measurements with standard deviations shown.
Examination of RK13 cells yielded similar results (Table 1 and Fig. 2D), although the enhancement of expression of the NF-κB-regulated luciferase gene by MVA was much lower than in infected 293T cells. This anomaly could be due to the greater ratio of firefly to sea pansy luciferase activity in uninfected RK13 compared to 293T cells, resulting in lower normalized values for all infected RK13 cell samples. Importantly, luciferase activity in MVA/5.2kb-infected cells was approximately threefold less than in MVA-infected cells (the same ratio as in 293T cells), demonstrating that indeed MVA/5.2kb infection inhibited NF-κB activation. Moreover, while MVA-infected lysates expressed 2.6-fold-more luciferase activity than uninfected cells, WR infection resulted in a 2-fold decrease.
Insertion of the WR K1L gene into the MVA genome is sufficient for inhibiting degradation of host IκBα and expression of a transfected NF-κB-transcriptionally regulated gene.
The Ankara EcoRI 5.2-kb DNA fragment contains the following genes: N2L, M1L, M2L, K1L, K2L, K3L, and K4L (26). Since intact K1L, M1L, and M2L genes are present in the Ankara 5.2-kb region but fragmented or absent in the MVA genome (26), one or more of these gene products might inhibit MVA-induced NF-κB activation. Of the three, K1L, a host-range gene necessary for vaccinia virus replication in RK13 cells (31, 33), was further examined because it was also present in the genome of two other recombinants (MVA/51.1 and MVA/44.1+47.1) (44) that acquired the ability to inhibit host IκBα degradation (Fig. 1C). Further, the K1L product contains six ankyrin repeats, a motif present in IκBα. Given the role of ankyrin repeats for IκBα in NF-κB binding and inhibition, it was attractive to hypothesize that the vaccinia virus K1L product functioned as an IκBα homolog.
To test the function of K1L, the biological activity of a recombinant MVA containing a copy of the WR K1L gene (MVA/K1L) was examined (41). When cytoplasmic extracts of RK13 cells individually infected with WR, MVA, or MVA/K1L were probed for IκBα by immunoblotting, IκBα was readily detected in WR- and MVA/K1L-infected cells. In contrast, relatively lower amounts were present in MVA-infected and uninfected cells (Fig. 3A). Based on the increase of IκBα in MVA/K1L-infected cells compared to MVA-infected cells, the introduction of K1L was sufficient for ensuring NF-κB inhibition. In this particular experiment, the quantity of IκBα in uninfected compared to WR-infected cells was probably a reflection of the observed increase in IκBα levels early during WR infection (Fig. 4A). Likewise, MVA-induced IκBα degradation also was inhibited by the K1L product in 293T cells (Fig. 3B); i.e., IκBα was degraded in MVA-infected cell lysates but intact in MVA/K1L- or WR-infected cell lysates.
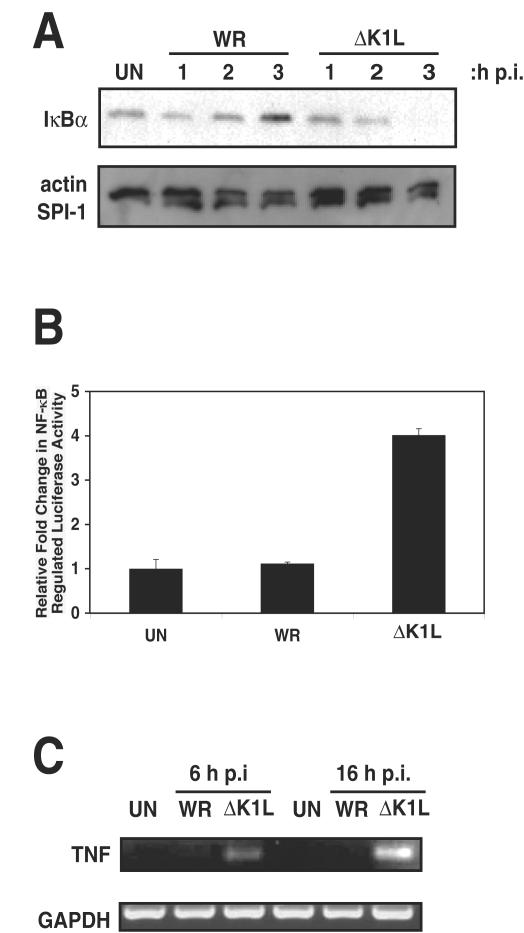
FIG. 4.
K1L is necessary for inhibition of NF-κB activation in RK13 cells. (A) RK13 cells were infected with WR or ΔK1L at an MOI of 10. At the times indicated postinfection, cells were collected and lysed in CE buffer. Equal amounts of cytoplasmic extracts were analyzed by using sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis and subsequent Western immunoblotting with an IκBα antibody. In comparable blots, lysates were analyzed for actin levels to ensure equal protein loading and SPI-1 levels to ensure active virus infection. (B) RK13 cells were transfected with pRL-TK and pNF-κBluc, and 24 h posttransfection cells were infected (MOI = 10) with WR or ΔK1L. At 8 h postinfection, cells were lysed. The ratios of firefly to sea pansy activities were determined and normalized to that of uninfected cells. (C) RK13 cells were infected with WR or ΔK1L (MOI = 10). At 6 or 16 h postinfection, total RNA was isolated and mRNA was reverse transcribed into cDNA. TNF and GAPDH cDNAs were amplified by using PCR, and the resultant DNA products were separated by electrophoresis on a 2% agarose gel and visualized with ethidium bromide.
MVA/K1L virus also repressed expression of the NF-κB-controlled firefly luciferase gene in both RK13 and 293T cells (Fig. 3C and D, respectively). Luciferase activity was 0.5-fold lower in WR-infected cells compared to uninfected RK13 cells. In contrast, NF-κB activation was 2.5-fold higher in MVA-infected cells compared to mock-infected cells. MVA/K1L-infected RK13 cell lysates contained less than half of the luciferase activity compared to extracts from MVA-infected cells. For 293T cells, the RLUs from WR-infected cells were similar to those from uninfected cells. Conversely, this activity was fourfold higher in MVA-infected cells and only threefold higher in MVA/K1L-infected cells.
Recombinant MVA virus containing the WR K1L gene represses transcription of the naturally NF-κB-regulated cellular TNF gene.
In the host cell, the NF-κB transcription factor family regulates expression of the TNF gene as well as that of other genes whose products stimulate inflammatory and immune responses (19). To detect whether the K1L product could inhibit transcription of the endogenous NF-κB-regulated TNF gene during infection, the presence of TNF mRNA was assayed by RT-PCR. Following RT of total RNA from mock-, MVA-, or MVA/K1L-infected RK13 cells, the resultant TNF cDNA, if present, was amplified by using PCR with primers specific for the rabbit TNF gene (Fig. 3E). Since TNF RNA was detected in uninfected or MVA/K1L-infected cells, NF-κB was activated under these conditions. In contrast, successful amplification of TNF RNA from MVA-infected cells demonstrated that a nonproductive MVA infection could still induce NF-κB transcriptional activation. Similar results were obtained when the host cells were replaced by 293T cells (Fig. 3F). However, in this situation, expression of the TNF gene was detected during MVA/K1L infection, albeit at a reduced amount in comparison to that occurring in the MVA-infected cells. Thus, the K1L gene product acted to retard the transcriptional regulatory ability of NF-κB. It should be noted that the inability to amplify TNF cDNA from uninfected cells of either origin was not due to inadequate technique, since the control GAPDH RNA was readily detected in all samples tested.
K1L is necessary for inhibiting NF-κB activation in RK13 cells.
The above studies demonstrated that insertion of the K1L gene into the MVA genome was sufficient to alter the biological effects of MVA on NF-κB activation. To confirm the role of K1L in inhibiting NF-κB activation, a deletion mutant of the WR strain lacking the K1L gene (ΔK1L) was assayed for biological function in RK13 cells. As before, immunoblotting was performed to detect IκBα in the cytoplasmic extracts of RK13 cells infected with WR or ΔK1L (Fig. 4A). In WR-infected cell lysates, IκBα was present in cell extracts harvested 1, 2, and 3 h postinfection, with IκBα levels increasing slightly as the infection progressed. IκBα was found in ΔK1L-infected cell extracts 1 h postinfection but had disappeared after an additional 2 h. This disappearance was not due to global protein degradation, since actin levels remained unaltered during the 3-h postinfection period. Likewise, the amount of SPI-1 (an early viral protein) was unchanged.
To further characterize the NF-κB inhibitory function of K1L, ΔK1L-infected RK13 cells were analyzed for expression of the NF-κB-controlled transfected luciferase gene. Although infection with the parental WR virus did not affect transcription of the firefly gene, elimination of the K1l gene in the ΔK1L virus was associated with a fourfold enhancement of expression (Fig. 4B). The transcriptional activation state of the host cell TNF gene also was examined in ΔK1L-infected cells by using RT-PCR, and the results complemented the above luciferase data (Fig. 4C). In this case, a positive result was only obtained when using RNA from ΔK1L-infected cells at 6 or 16 h postinfection. As before, the inability to amplify TNF RNAs from uninfected or WR-infected cells was not due to degraded RNA, since GAPDH amplicons were readily obtained.
DISCUSSION
Initially, a 5.2-kb EcoRI region of the Ankara genome was shown to restore the ability of the attenuated MVA virus to inhibit NF-κB activation. Of the genes present in this fragment, K1L was considered to be the modulator, based on its presence in other recombinant MVA viruses that also prevented IκBα degradation. To substantiate this inhibitory function of the K1L product, the biological activity of a recombinant MVA (MVA/K1L) containing a copy of the WR strain K1L gene was studied. As predicted, IκBα degradation was inhibited in cells infected with this virus. Further, compared to MVA, MVA/K1L gained the ability to inhibit transcription of both a luciferase and TNF gene, whose expressions are regulated by an artificial and natural promoter, respectively, containing NF-κB binding sites. Thus, a new role can be defined for K1L, namely that of inhibiting NF-κB activation.
Interestingly, MVA/K1L infection did not reduce NF-κB-regulated firefly luciferase production to the extent observed in WR-infected cells (Fig. 3). Likewise, TNF gene expression was not completely diminished in MVA/K1L-infected 293T cells, as was the case for WR-infected cells. These data suggest that other poxvirus proteins may work independently or cooperatively with K1L to block NF-κB activation. Two obvious candidates that might appear to function with K1L to inhibit MVA-induced NF-κB activity are the homologs of the vaccinia virus strain WR A52R and A46R proteins, which prevent interleukin-1 and toll-like receptor-induced NF-κB activation (4). However, these two virus products probably do not inhibit MVA-induced NF-κB activation, because open reading frames with 100% identity to the A52R gene and 50% homology to the A46R gene are present in the MVA genome and presumably are transcriptionally active.
K1L is defined as a host range factor because it is necessary for a productive WR infection in RK13 cells. Infection of RK13 cells with a K1L deletion mutant results in an abrupt halt of host and early viral protein synthesis; however, the molecular mechanism K1L provides to permit virus replication has not yet been identified (31, 33). On the surface, inhibition of host NF-κB activation does not appear to be related to the host range function, as the MVA/K1L virus inhibited NF-κB in both nonpermissive 293T cells and permissive RK13 cells.
The signal transduction pathway that induces NF-κB activation has been well characterized (18, 24). Activated IKK phosphorylation of IκBα targets it for ubiquitination and degradation, thereby releasing NF-κB for subsequent interaction with cellular DNA containing its binding sites. Given that K1L contains ankyrin repeats present in IκB family members, an initial prediction would be that K1L displaces IκBα and subsequently binds to and inhibits NF-κB. However, because IκBα remains in MVA/K1L-infected cells (Fig. 3), it seems unlikely that K1L competitively binds to NF-κB, since cytoplasmic IκBα displaced by the African swine fever virus A238L IκBα homolog is degraded (32, 34). Alternatively, K1L may inhibit IκBα degradation by interfering directly with IKK to prevent phosphorylation. There is no evidence that K1L directly acts on IκBα—it is also possible that K1L indirectly inhibits IκBα by hampering kinases that act upstream of IKK.
Inflammation is a crucial mechanism that the innate immune response utilizes to recruit lymphocytes to an area to control an initial infection. It is interesting to speculate that K1L-mediated inhibition of proinflammatory cytokine production would dampen immune responses, resulting in prolonged virus replication and greater disease in the host. Indeed, infection with a vaccinia virus lacking the NF-κB inhibitory A52R gene becomes attenuated when introduced into mice (21). In addition to orthopoxvirus proteins that inhibit NF-κB activation, the more distantly related molluscum contagiosum virus expresses MC159, a product which inhibits double-stranded RNA-dependent protein kinase-induced and death receptor-induced NF-κB activation (12, 20), suggesting that inhibiting NF-κB is an important survival strategy for all poxviruses.
It seems counterintuitive that MVA initially induces NF-κB activation. One hallmark of the Poxviridae family is that viral genome expression occurs exclusively in the host cell cytoplasm. Freed cytoplasmic NF-κB would certainly be available to bind to the poxviral promoters to activate transcription. However, there are no obvious NF-κB consensus binding sites in poxvirus promoters. Regardless, constitutively activated NF-κB would prevent virus survival by inducing immune and inflammatory responses. Thus, expression of viral-encoded NF-κB inhibitors should enhance virus survival. It appears that NF-κB regulation in poxvirus-infected cells is complex, with unique viral gene products inducing and inhibiting NF-κB activation. Using poxviruses as a tool, we have taken a genetic approach to dissect different signal transduction cascades that regulate NF-κB. In determining NF-κB regulators like K1L, it may be possible to obtain a more refined sense of how poxviruses, and other viruses, control the host cell synthesis machinery and the host immune response during infection.
Acknowledgments
We thank Bernard Moss and Gerd Sutter for generously providing the recombinant MVA viruses used in these studies. We also thank Gail Scherba and William M. Schnitzlein of the University of Illinois College of Veterinary Medicine for critical reading of the manuscript.
This work was sponsored by a grant from the Roy J. Carver Charitable Trust.
REFERENCES
- 1.Antoine, G., F. Scheiflinger, F. Dorner, and F. G. Falkner. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244:365-396. [DOI] [PubMed] [Google Scholar]
- 2.Baldi, L., K. Brown, G. Franzoso, and U. Siebenlist. 1996. Critical role for lysines 21 and 22 in signal-induced, ubiquitin-mediated proteolysis of I kappa B-alpha. J. Biol. Chem. 271:376-379. [DOI] [PubMed] [Google Scholar]
- 3.Birbach, A., P. Gold, B. R. Binder, E. Hofer, R. de Martin, and J. A. Schmid. 2002. Signaling molecules of the NF-kappa B pathway shuttle constitutively between cytoplasm and nucleus. J. Biol. Chem. 277:10842-10851. [DOI] [PubMed] [Google Scholar]
- 4.Bowie, A., E. Kiss-Toth, J. A. Symons, G. L. Smith, S. K. Dower, and L. A. O'Neill. 2000. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 97:10162-10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bregeon, F., A. Roch, S. Delpierre, E. Ghigo, A. Autillo-Touati, O. Kajikawa, T. R. Martin, J. Pugin, H. Portugal, J. P. Auffray, and Y. Jammes. 2002. Conventional mechanical ventilation of healthy lungs induced pro-inflammatory cytokine gene transcription. Respir. Physiol. Neurobiol. 132:191-203. [DOI] [PubMed] [Google Scholar]
- 6.Brockman, J. A., D. C. Scherer, T. A. McKinsey, S. M. Hall, X. Qi, W. Y. Lee, and D. W. Ballard. 1995. Coupling of a signal response domain in IκB alpha to multiple pathways for NF-κB activation. Mol. Cell. Biol. 15:2809-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks, M. A., A. N. Ali, P. C. Turner, and R. W. Moyer. 1995. A rabbitpox virus serpin gene controls host range by inhibiting apoptosis in restrictive cells. J. Virol. 69:7688-7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, K., S. Gerstberger, L. Carlson, G. Franzoso, and U. Siebenlist. 1995. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science 267:1485-1488. [DOI] [PubMed] [Google Scholar]
- 9.Carlotti, F., S. K. Dower, and E. E. Qwarnstrom. 2000. Dynamic shuttling of nuclear factor kappa B between the nucleus and cytoplasm as a consequence of inhibitor dissociation. J. Biol. Chem. 275:41028-41034. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter, E. A., J. Ruby, and I. A. Ramshaw. 1994. IFN-gamma, TNF, and IL-6 production by vaccinia virus immune spleen cells. An in vitro study. J. Immunol. 152:2652-2659. [PubMed] [Google Scholar]
- 11.Carroll, M. W., and B. Moss. 1997. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: propagation and generation of recombinant viruses in a nonhuman mammalian cell line. Virology 238:198-211. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhary, P. M., A. Jasmin, M. T. Eby, and L. Hood. 1999. Modulation of the NF-kappa B pathway by virally encoded death effector domains-containing proteins. Oncogene 18:5738-5746. [DOI] [PubMed] [Google Scholar]
- 13.Chen, Z., J. Hagler, V. J. Palombella, F. Melandri, D. Scherer, D. Ballard, and T. Maniatis. 1995. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 9:1586-1597. [DOI] [PubMed] [Google Scholar]
- 14.DiDonato, J., F. Mercurio, C. Rosette, J. Wu-Li, H. Suyang, S. Ghosh, and M. Karin. 1996. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol. Cell. Biol. 16:1295-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiDonato, J. A., M. Hayakawa, D. M. Rothwarf, E. Zandi, and M. Karin. 1997. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 388:548-554. [DOI] [PubMed] [Google Scholar]
- 16.Friedman, J. M., and M. S. Horwitz. 2002. Inhibition of tumor necrosis factor alpha-induced NF-κB activation by the adenovirus E3-10.4/14.5K complex. J. Virol. 76:5515-5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujino, M., X. K. Li, Y. Kitazawa, L. Guo, M. Kawasaki, N. Funeshima, T. Amano, and S. Suzuki. 2002. Distinct pathways of apoptosis triggered by FTY720, etoposide, and anti-fas antibody in human T-lymphoma cell line (Jurkat cells). J. Pharmacol. Exp. Ther. 300:939-945. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-κB puzzle. Cell 109(Suppl.):S81-S96. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 20.Gil, J., J. Rullas, J. Alcami, and M. Esteban. 2001. MC159L protein from the poxvirus molluscum contagiosum virus inhibits NF-κB activation and apoptosis induced by PKR. J. Gen. Virol. 82:3027-3034. [DOI] [PubMed] [Google Scholar]
- 21.Harte, M. T., I. R. Haga, G. Maloney, P. Gray, P. C. Reading, N. W. Bartlett, G. L. Smith, A. Bowie, and L. A. O'Neill. 2003. The poxvirus protein A52R targets Toll-like receptor signaling complexes to suppress host defense. J. Exp. Med. 197:343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haversen, L., B. G. Ohlsson, M. Hahn-Zoric, L. A. Hanson, and I. Mattsby-Baltzer. 2002. Lactoferrin down-regulates the LPS-induced cytokine production in monocytic cells via NF-kappa B. Cell. Immunol. 220:83-95. [DOI] [PubMed] [Google Scholar]
- 23.Hiscott, J., H. Kwon, and P. Genin. 2001. Hostile takeovers: viral appropriation of the NF-κB pathway. J. Clin. Investig. 107:143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karin, M., and B. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 25.Mercurio, F., H. Zhu, B. W. Murray, A. Shevchenko, B. L. Bennett, J. Li, D. B. Young, M. Barbosa, M. Mann, A. Manning, and A. Rao. 1997. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 278:860-866. [DOI] [PubMed] [Google Scholar]
- 26.Meyer, H., G. Sutter, and A. Mayr. 1991. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J. Gen. Virol. 72:1031-1038. [DOI] [PubMed] [Google Scholar]
- 27.Micheau, O., S. Lens, O. Gaide, K. Alevizopoulos, and J. Tschopp. 2001. NF-κB signals induce the expression of c-FLIP. Mol. Cell. Biol. 21:5299-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss, B., and J. L. Shisler. 2001. Immunology 101 at poxvirus U: immune evasion genes. Semin. Immunol. 13:59-66. [DOI] [PubMed] [Google Scholar]
- 29.Moss, B. 2001. Poxviridae: the viruses and their replication, p. 2849-2883. In D. Knipe and P. Howley (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 30.Oie, K. L., and D. J. Pickup. 2001. Cowpox virus and other members of the orthopoxvirus genus interfere with the regulation of NF-κB activation. Virology 288:175-187. [DOI] [PubMed] [Google Scholar]
- 31.Perkus, M. E., S. J. Goebel, S. W. Davis, G. P. Johnson, K. Limbach, E. K. Norton, and E. Paoletti. 1990. Vaccinia virus host range genes. Virology 179:276-286. [DOI] [PubMed] [Google Scholar]
- 32.Powell, P. P., L. K. Dixon, and R. M. Parkhouse. 1996. An IκB homolog encoded by African swine fever virus provides a novel mechanism for downregulation of proinflammatory cytokine responses in host macrophages. J. Virol. 70:8527-8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramsey-Ewing, A. L., and B. Moss. 1996. Complementation of a vaccinia virus host-range K1L gene deletion by the nonhomologous CP77 gene. Virology 222:75-86. [DOI] [PubMed] [Google Scholar]
- 34.Revilla, Y., M. Callejo, J. M. Rodriguez, E. Culebras, M. L. Nogal, M. L. Salas, E. Vinuela, and M. Fresno. 1998. Inhibition of nuclear factor κB activation by a virus-encoded IκB-like protein. J. Biol. Chem. 273:5405-5411. [DOI] [PubMed] [Google Scholar]
- 35.Rokita, H., T. Kupiec, K. Guzik, and A. Koj. 1998. Vaccinia virus-regulated acute phase cytokine production in human fibroblasts, U937 cells and endothelium. Mediators Inflamm. 7:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santoro, M. G., A. Rossi, and C. Amici. 2003. NF-κB and virus infection: who controls whom. EMBO J. 22:2552-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scherer, D. C., J. A. Brockman, Z. Chen, T. Maniatis, and D. W. Ballard. 1995. Signal-induced degradation of I kappa B alpha requires site-specific ubiquitination. Proc. Natl. Acad. Sci. USA 92:11259-11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shisler, J. L., and B. Moss. 2001. Molluscum contagiosum virus inhibitors of apoptosis: the MC159 v-FLIP protein blocks Fas-induced activation of procaspases and degradation of the related MC160 protein. Virology 282:14-25. [DOI] [PubMed] [Google Scholar]
- 39.Slezak, K., K. Guzik, and H. Rokita. 2000. Regulation of interleukin 12 and interleukin 10 expression in vaccinia virus-infected human monocytes and U-937 cell line. Cytokine 12:900-908. [DOI] [PubMed] [Google Scholar]
- 40.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 41.Staib, C., I. Drexler, M. Ohlmann, S. Wintersperger, V. Erfle, and G. Sutter. 2000. Transient host range selection for genetic engineering of modified vaccinia virus Ankara. BioTechniques 28:1137-1142, 1144-. 6:1148. [DOI] [PubMed] [Google Scholar]
- 42.Traenckner, E. B., H. L. Pahl, T. Henkel, K. N. Schmidt, S. Wilk, and P. A. Baeuerle. 1995. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. EMBO J. 14:2876-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willeaume, V., V. Kruys, T. Mijatovic, and G. Huez. 1995. Tumor necrosis factor-alpha production induced by viruses and by lipopolysaccharides in macrophages: similarities and differences. J. Inflamm. 46:1-12. [PubMed] [Google Scholar]
- 44.Wyatt, L. S., M. W. Carroll, C. P. Czerny, M. Merchlinsky, J. R. Sisler, and B. Moss. 1998. Marker rescue of the host range restriction defects of modified vaccinia virus Ankara. Virology 251:334-342. [DOI] [PubMed] [Google Scholar]