Abstract
The assembly of regional biotas and organismal responses to anthropogenic climate change both depend on the capacity of organisms to adapt to novel ecological conditions. Here we demonstrate the concept of evolutionary lag time, the time between when a climatic regime or habitat develops in a region and when it is colonized by a given clade. We analyzed the time of colonization of four clades (three plant genera and one lizard genus) into the Atacama–Sechura Desert of South America, one of Earth’s driest and oldest deserts. We reconstructed time-calibrated phylogenies for each clade and analyzed the timing of shifts in climatic distributions and biogeography and compared these estimates to independent geological estimates of the time of origin of these deserts. Chaetanthera and Malesherbia (plants) and Liolaemus (animal) invaded arid regions of the Atacama–Sechura Desert in the last 10 million years, some 20 million years after the initial onset of aridity in the region. There are also major lag times between when these clades colonized the region and when they invaded arid habitats within the region (typically 4–14 million years). Similarly, hyperarid climates developed ∼8 million years ago, but the most diverse plant clade in these habitats (Nolana) only colonized them ∼2 million years ago. Similar evolutionary lag times may occur in other organisms and habitats, but these results are important in suggesting that many lineages may require very long time scales to adapt to modern desertification and climatic change.
Keywords: niche conservatism, niche evolution, biodiversity, evolutionary biogeography
Natural selection can be a rapid and powerful force and is essential for allowing organisms to invade new habitats and to persist during environmental change (1, 2). However, natural selection is not without limits (e.g., ref. 3), and these limits may have important implications. Many ecological and evolutionary patterns are related to the inability of species to adapt to novel climatic conditions (climatic niche conservatism), including patterns of biogeography, species richness, allopatric speciation, and introduced species invasion (4–7). Despite initial niche conservatism, many clades eventually are able to colonize novel environments. Thus, niche conservatism may also be quantified based on the length of time it takes to adapt to and invade novel ecological conditions. However, little is known about the time scales required for successful colonization in these cases.
Here, we focus on this “evolutionary lag time,” the time lag between the emergence of novel environmental conditions in a region and when that habitat is colonized by a given clade. We illustrate this concept in the contiguous Atacama and Sechura deserts of South America (Atacama–Sechura Desert hereafter). This desert represents one of the harshest and driest environments on Earth (8–10), but nevertheless contains many endemic plant and animal species (11–14). We compare the time of origin of different precipitation regimes within the region (based on geological evidence) to the time of colonization of these environments for three plant clades and one animal clade (based on time-calibrated molecular phylogenies).
The Atacama–Sechura Desert (Fig. 1) encompasses a large region that has been very dry for many millions of years. This desert extends for >3,500 km from 5°S near the Peruvian–Ecuadorean border to 30°S in northern Chile (15). In the Atacama region, arid climates (precipitation of ≤50 mm/y) extend from coastal regions from 5°S to 30°S up to 5,000 m (8). Hyperarid climates (≤5 mm/y) extend from 13°S to 25°S, from coastal areas to 3,000 m (8, 16). The onset of semiarid conditions (≤250 mm/y) in the Atacama–Sechura region can be traced back to the late Jurassic (17), 150 million years ago. Arid conditions (<50 mm/y) have prevailed in this region since the early Oligocene (33 million years ago) (18–20). Current evidence indicates that the minimum age of onset of hyperarid climate in the region is around 8 million years, in the late Miocene (21–23), although some evidence suggests that pulses of hyperaridity could be older (20–26).
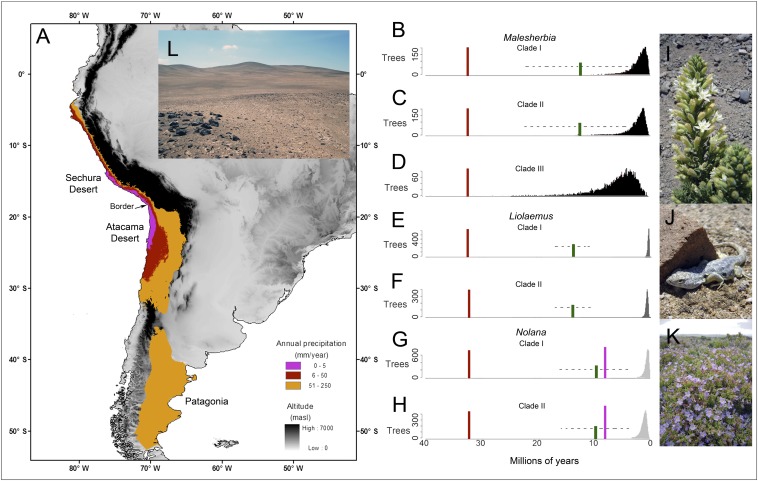
Fig. 1.
(A) Distribution of hyperarid, arid, and semiarid habitats in South America (colors are placed over the elevational distributions). (B–H) Estimation of evolutionary lag times for the colonization of arid and hyperarid habitats for clades of plants and animals in the Atacama–Sechura Desert. The red vertical bar indicates the initial timing of development of arid climates in the region based on geological evidence, whereas the pink vertical bar indicates the timing for hyperarid habitats. The frequency distributions of trees (in black and gray) are for the estimated age of clades in which there is an estimated transition to arid habitats (Malesherbia and Liolaemus) and hyperarid habitats (Nolana). The green vertical bar indicates the estimated age of colonization of the Atacama–Sechura biogeographic region (with the dashed horizontal line indicating the 95% posterior distribution). The evolutionary lag time for colonization of a given habitat by a given clade is the age of the colonization of the region minus the age of colonization of that habitat, where the biogeographic colonization occurs after the development of that habitat in the region. A green bar is not shown for Malesherbia clade III because of uncertainty in the timing of biogeographic colonization of the region. The genus Chaetanthera is not included in the figure because none of the ancestors were distributed in arid regions of South America in terms of mean annual precipitation. Images are Malesherbia densiflora (I), Liolaemus atacamensis (J), Nolana divaricata (K), and hyperarid region of Atacama Desert near Iquique (L).
Given that deserts are relatively harsh environments that may require novel physiological adaptations to allow organisms to invade them, the spread of arid climatic conditions is regarded as an important force in the evolution of plants and animals (27–32). If organisms can adapt rapidly to arid and hyperarid climates, then the ages of the oldest clades that occur under these conditions should correspond to the time when these conditions became prevalent. In contrast, if arid-adapted lineages are much younger than the onset of these climatic conditions, this pattern would suggest an evolutionary lag time during which this lineage lacked the adaptations necessary to invade these habitats.
We reconstructed the climatic and geographical distributions in four clades (three plant clades and one reptile clade) that presently occur in the Atacama–Sechura Desert and include some of the most diverse plant and vertebrate clades in the region. Using time-calibrated phylogenies and Geographic Information System-based climatic data (i.e., annual precipitation), we estimated the timing of shifts from semiarid to arid habitats and from arid to hyperarid habitats. We then tested whether climatic-niche transitions within these clades matched historical climate changes in the region based on geological evidence (33 million years ago, semiarid to arid; 8 million years ago, arid to hyperarid), or whether these clades colonized these habitats long after these climatic changes, suggesting an evolutionary lag time.
We selected three plant genera, Chaetanthera (Asteraceae), Malesherbia (Passifloraceae), and Nolana (Solanaceae) and one animal genus, Liolaemus (Liolaemidae), for which molecular phylogenies are available: Chaetanthera (30 species, with 5 in the Atacama–Sechura Desert) (33); Malesherbia (25/12 species) (34); Nolana (89/83 species) (35); and Liolaemus (223 species/∼20 species) (36, 37). Importantly, these four clades occur primarily in or adjacent to the Atacama–Sechura Desert (SI Appendix, Figs. S1–S4) and thus could colonize the desert based on their geographic proximity. Therefore, a lag time between their colonization and the formation of these deserts is presumably due to their failure to successfully colonize these habitats, and not because they were absent in the general region until recently. We also used biogeographic reconstructions to estimate how long clades were present in the region before colonizing these habitats. If a lineage invaded the geographic region after the formation of the habitat (arid or hyperarid), then the evolutionary lag time was the time between the colonization of the region and the colonization of the specific habitat. Our climatic reconstructions were based on the mean values of annual precipitation among the localities of each species, but we also considered the maximum and minimum values (to address whether species are confined to arid or semiarid habitats or only partially extend their ranges into these climatic regimes).
Results
Our analyses show widespread temporal lags between the onset of arid and hyperarid climates and the colonization of the Atacama–Sechura Desert in these clades. We address hyperarid habitats (≤5 mm/y) first. The genus Nolana originated 12.7 million years ago (Fig. 1 and SI Appendix, Fig. S1). Fifteen Nolana species from six lineages are confined to hyperarid conditions (Fig. 1 and SI Appendix, Fig. S1), and almost all transitions occurred in the last 2 million years (Table 1, Fig. 1, and SI Appendix, Fig. S1). Given that hyperarid conditions originated 8 million years ago, this indicates temporal lags of ∼6 million years for colonization of this habitat (Table 1). Nolana onoana may have colonized hyperarid areas earlier (3.8 million years ago), suggesting a lag time of ∼4.2 million years. Interestingly, the lag time associated with failing to colonize hyperarid regions is much longer than the time needed to generate new species (i.e., many species are only ∼1 million years, SI Appendix, Fig. S1).
Table 1.
Estimates of evolutionary lag time for colonization of hyperarid and arid environments, based on the difference between when a clade colonizes a region and when it colonizes a particular habitat in that region
| Genus | Climate | Nodes/species | Estimated age of colonization of climatic regime based on PGLS reconstruction | Estimated age of colonization of region based on DEC analyses | Estimated evolutionary lag time |
| Nolana | Hyperarid (8 million years ago) | I: N. tomentella | 0.8 (0.2–1.6) | 9.7 (4.0–16.7) [A] | 7.2 |
| N. arequipensis | |||||
| N. pallida | |||||
| II: N. inflata | 0.4 (0.1–1.0) | 9.7 (4.0–16.7) [A] | 7.6 | ||
| N. weissiana | |||||
| N. balsamiflua | 0.7 (0.1–1.8) | ≥12.7 (8.6–20.3) [C] | 7.3 | ||
| N. plicata | 0.8 (0.1–1.7) | 9.7 (4.0–16.7) [A] | 7.2 | ||
| N. chancoana | 1.0 (0.2–2.0) | 9.7 (4.0–16.7) [A] | 7.0 | ||
| N. scaposa | 1.7 (0.3–3.5) | 9.7 (4.0–16.7) [A] | 6.3 | ||
| N. spathulata | 1.7 (0.3–3.5) | 9.7 (4.0–16.7) [A] | 6.3 | ||
| N. spergularoides | 0.9 (0.1–2.0) | 9.7 (4.0–16.7) [A] | 7.1 | ||
| N. lycioides | 1.2 (0.2–2.4) | 9.7 (4.0–16.7) [A] | 6.8 | ||
| N. cerrateana | 1.7 (0.5–2.8) | 9.7 (4.0–16.7) [A] | 6.3 | ||
| N. intonsa | 1.3 (0.4–2.5) | 1.3 (0.3.2.5) [A] | 0 | ||
| N. revoluta | 0.6 (0.1–1.3) | 9.7 (4.0–17.0) [A] | 7.4 | ||
| N. tovariana | 0.6 (0.1–1.3) | 9.7 (4.0–17.0) [A] | 7.4 | ||
| N. johnstonii | 0.8 (0.1–1.7) | ≥12.7 (7.0–20.1) [C] | 7.2 | ||
| N. confinis | 0.8 (0.1–1.7) | ≥12.7 (7.0–20.1) [C] | 7.2 | ||
| N. coronata | 0.9 (0.1–1.9) | ≥12.7 (7.0–20.1) [C] | 7.1 | ||
| N. peruviana | 1.5 (0.4–2.9) | ≥12.7 (7.0–20.1) [C] | 6.5 | ||
| N. tocopillensis | 1.9 (0.8–2.9) | ≥12.7 (7.0–20.1) [C] | 6.1 | ||
| N. lachimbensis | 2.1 (0.9–3.9) | ≥12.7 (7.0–20.1) [C] | 5.9 | ||
| N. onoana | 3.8 (0.6–7.7) | ≥12.7 (7.0–20.1) [C] | 4.2 | ||
| Arid (33 million years ago) | Basal node | 12.7 (8.6–20.3) | ≥12.7 (8.7–20.3) [C] | 0 | |
| Chaetanthera | Hyperarid | — | — | — | — |
| Arid | C. taltalensis | 6.7 (0.8–5.5) | 6.7 (3.2–11.3) [C] | 0 | |
| Malesherbia | Hyperarid | M. tocopillana | 1.9 (0.1–4.9) | 1.9 (0.1–4.9) [C] | * |
| Arid | I: M. tocopillana | 1.9 (0.0–4.9) | 12.2 (4.8–22.3) [B] | 10.3 | |
| M. ardens | 1.9 (0.0–4.9) [C] | * | |||
| II: M. angustisecta | 2.2 (0.1–5.0) | 12.2 (4.8–22.3) [B] | 10.0 | ||
| M. arequipensis | 2.2 (0.3–5.1) [C] | * | |||
| M. tenuifolia | |||||
| III: M. densiflora | 5.6 (1.0–12.4) | 5.6 (1.0–12.4) [D] | * | ||
| M. deserticola | |||||
| M. rugosa | 7.9 (2.3–15.1) | * | * | ||
| M. obtusa | 3.1 (0.3–6.9) | * | * | ||
| Liolaemus | Hyperarid | L. poconchilensis | 7.0 (5.2–8.9) | * | * |
| Arid | I: L. atacamensis | 0.3 (0.1–0.5) | 14.1 (10.5–18.1) [D] | 13.9 | |
| L. nigromaculatus | |||||
| II: L. isabelae | 0.5 (0.2–0.9) | 14.1 (10.5–18.1) [C] | 13.6 | ||
| L. paulinae | |||||
| L. audituvelatus | 2.0 (1.2–2.9) | 8.3 (6.1–10.5) [D] | 6.3 | ||
| L. torresi | 4.0 (2.8–5.3) | 8.3 (6.1–10.5) [D] | 4.3 | ||
| L. josephorum | 3.8 (2.4–5.2) | 14.1 (10.5–18.1) [D] | 10.3 |
Values are median ages given in millions of years. The 95% highest posterior density intervals are given in parentheses. Biogeographical areas are given in brackets: [A] Sechura (4°S–18°S; <1,500 m), [B] Peruvian Andes (4°S–18°S; >1,500 m), [C] coastal Atacama (18°S–29°S; <1,500 m), and [D] high Atacama (18°S–30°S; >1,500 m). DEC, dispersal-extinction-cladogenesis.
The ancestral geographic distribution is ambiguous, making the evolutionary lag time ambiguous also.
The other three groups also recently colonized hyperarid habitats, but on a more limited scale. Many represent single-species colonizations for which exact timing is uncertain. Malesherbia has a single species confined to hyperarid habitats (Malesherbia tocopillana), which originated and colonized these habitats in the last 2 million years, given its age. Two others (Malesherbia arequipensis and Malesherbia tenuifolia) extend into hyperarid habitats (SI Appendix, Fig. S2) and are relatively young (<5 million years). Three Chaetanthera extend into hyperarid habitats (Chaetanthera albiflora, Chaetanthera glabrata, and Chaetanthera taltalensis) (SI Appendix, Table S2 and Fig. S3) and two are relatively young (C. albiflora: ∼2 million years ago and C. glabrata: ∼1 million years ago), suggesting very recent colonization of hyperarid habitat (SI Appendix, Fig. S3). C. taltalensis is older (6.7 million years ago) and may have colonized hyperarid areas earlier. Among sampled Liolaemus, only L. poconchilensis (7.0 million years ago) occurs in hyperarid areas in the region (SI Appendix, Fig. S4), but it is not clear when these habitats were colonized in this lineage.
Arid climates (<50 mm/y) developed at least 33 million years ago in the Atacama–Sechura Desert. Chaetanthera, Malesherbia, and Liolaemus all show dramatic evolutionary lag times for invading these environments (Fig. 1). Considering the age of the habitats and clades alone, no lineages of Chaetanthera, Malesherbia, or Liolaemus presently occurring in arid habitats is older than 10 million years ago (Table 1), indicating a lag time of >20 million years for all three clades.
Incorporating the timing of regional colonization, results for Malesherbia for mean annual precipitation (Fig. 1, Table 1, and SI Appendix, Fig. S2) show lag times of 10.0 million years (lineage I) and 10.3 million years (lineage II). However, considering minimum annual precipitation, lineage III extends into arid habitats with a lag time of >10 million years, although ancestral areas are unclear. Only lineage I is confined to arid habitats based on maximum annual precipitation. For Chaetanthera, arid habitats were colonized <10 million years ago, but the lag time relative to regional colonization is uncertain (Table 1).
Colonization of arid habitats by Liolaemus lizards is even more recent. Considering mean precipitation, only two ancestors of extant species have colonized arid areas (<50 mm/y), and both in the last 1 million years (Fig. 1 and SI Appendix, Fig. S3), indicating lag times of 13.9 and 13.6 million years (Table 1). From these two ancestral species evolved four species currently distributed in arid areas: Liolaemus atacamensis and Liolaemus nigromaculatus (age: 0.3 million years) and Liolaemus isabelae and Liolaemus paulinae (age: 0.5 million years). In addition, three species derived from ancestors distributed in semiarid climates are currently confined to areas with arid climates. Even though the timing of colonization of arid habitats for these species is unknown, it should not be older than the age of these species, allowing us to estimate minimum evolutionary lag times: Liolaemus torresi (age: 4.0 million years; minimum lag: 4.3 million years), Liolaemus josephorum (age: 3.8 million years; minimum lag: 10.3 million years), and Liolaemus audituvelatus (age: 2.0 million years; minimum lag: 6.3 million years). Considering minimum annual precipitation, the ancestor of L. josephorum and Liolaemus platei extended its distribution within arid habitats (age: 3.8) showing a lag of 10.3 million years. Among these Liolaemus species, only L. atacamensis and L. nigromaculatus are confined to arid habitats.
The invasion of arid areas by Nolana is the oldest among these four genera, since the genus apparently originated within arid areas 12.7 million years ago (SI Appendix, Fig. S1). However, the evolutionary lag time is unclear because the colonization of arid habitats might have occurred before the genus originated.
Discussion
We show that there can be significant time intervals between when a climatic regime emerges in a region and when lineages invade that area. Specifically, our results show considerable lag times between when arid and hyperarid habitats developed in western South America and when three plant clades and one animal clade invaded them (>23 million years ago for arid, ∼6 million years ago for hyperarid; Fig. 1 and Table 1). We estimate somewhat shorter lag times when incorporating the timing of biogeographic colonization of the larger geographic region (0–13.9 million years, typically 4–10 million years; Table 1), but this is because of recent colonization of these clades in the region rather than older colonization of arid and hyperarid habitats. Importantly, these lineages may have been unable to invade these biogeographic regions due to their inability to colonize these xeric habitats. Given these results, a major challenge for future research will be to understand the specific functional traits that allowed these clades to invade arid and hyperarid habitats and what factors prevented them from evolving these traits more quickly.
Similar patterns of evolutionary lag times may occur in many other organisms and habitats around the world, and our results provide a baseline to which other ecological transitions can be compared. These estimated lag times also offer an additional approach to quantify levels of niche conservatism (i.e., the greater the lag time, the higher the degree of niche conservatism; for other approaches see ref. 6). However, we note that such lag times can only be estimated when reliable and independent information on the age of the new habitat type is available, and this may be a limitation for some habitat types and regions.
Evolutionary lag times (like those shown here) may have many implications. For example, they may be important in generating differences in biodiversity between habitats. Previous studies have suggested that differences in the timing of colonization of different habitats may explain differences in richness between them (e.g., refs. 38–40), but have not quantified these lag times. Perhaps most importantly, these lag times suggest the time scales that may be required for species to adapt to changing climate conditions and suggest that adaptation to rapid human-driven desertification may occur far too slowly.
We acknowledge that our results could be subject to various sources of error. However, in each case, these issues seem unlikely to overturn our results, especially given that we find concordant patterns of recent colonization both within and between these groups (Fig. 1), and that species from other clades may show similar patterns as well (41). First, extinct or unsampled species may distort these estimates. Species may have invaded arid or hyperarid habitats closer to when these habitats first arose and then subsequently went extinct. However, their extinction would itself suggest that these species did not adapt sufficiently to successfully colonize and persist in these habitats. Our phylogenies include most presently recognized species for the three plant groups. However, only ∼50% of presently recognized Liolaemus species were included (due to limited phylogenetic sampling). In theory, adding unsampled species that occur in arid or hyperarid environments could lead to estimation of earlier colonization dates of these environments in this genus. Unsampled species in other groups might have competitively excluded these lineages from these habitats until very recently, but this seems unlikely (i.e., this hypothesis assumes unseen lineages that left no trace but were nevertheless abundant enough to competitively exclude three different plant clades).
Second, our results are based on ancestral reconstructions that use the present-day climate conditions under which extant species occur. However, our phylogenetic reconstructions support the general pattern of increasing aridity over time inferred from geological evidence. Also, given that most species in these four clades do not presently occur in arid or hyperarid conditions (except Nolana), colonization of these conditions should not be older than suggested by our reconstructions (in other words, if there are reconstruction errors, these errors should underestimate evolutionary lag times, not overestimate them). Furthermore, the results support a model of niche conservatism among species (rather than random variation; see Materials and Methods), so there is no a priori reason to assume our reconstructions will be incorrect.
Third, our estimates of divergence times may be in error. The fossil record for all four groups is weak, and we were typically forced to rely on dates estimated from broader-scale studies to calibrate our trees (Materials and Methods). Nevertheless, the pattern of recent colonization of hyperarid and (for most groups) arid habitats is consistent both within and between groups, and our estimates of divergence dates across groups are independent of each other.
Fourth, even though we discuss this lag in colonization time as being evolutionary, it is possible that nonevolutionary factors explain or contribute to the pattern. For example, the recent colonization of hyperarid environments across groups at similar times may have been favored by some shared, abiotic factor, such as the development of fog banks (42), along with adaptations for using atmospheric water (43). However, if true, this would reinforce the idea that these lineages are otherwise generally unable to invade these environments. Furthermore, this does not explain the lag times for the invasion of arid habitats (i.e., because species in arid habitats are not tied to fog banks). We note that species interactions might also facilitate colonization of arid environments in some cases, with the colonization of some lineages allowing other lineages to invade (44).
Several other factors might also be relevant, but again these seem unlikely to overturn our concordant results between and within clades. For example, biogeographic reconstructions may be imprecise. Nevertheless, these clades in the Atacama–Sechura Desert are clearly much younger than the desert itself, regardless of their distribution in other regions. Our geological estimates of the timing of origin of these habitats is clearly critical, but we used carefully selected and conservative dates, and so errors should only underestimate lag times. There might also be errors in our climatic data (e.g., for species with few localities), but it seems unlikely that these could overturn our major results, given the dramatic differences in climatic distributions among species.
Finally, our results show two contrasts with other recent analyses of the origins of desert biotas. First, a study on the evolution of arid-adapted plants suggests that Cactaceae and other succulent groups underwent extensive radiation ∼10–5 million years ago (45), roughly coinciding with the hypothesized global expansion of arid habitats. In other words, these groups show little evidence of an evolutionary lag in the invasion of arid habitats. In contrast, we find that arid and hyperarid conditions in the Atacama–Sechura Desert developed long before these climatic regimes were invaded by the four groups studied here.
Second, our results suggest that adaptation to arid environments can be reversible. An extensive analysis of plant clades in the Southern Hemisphere showed that transitions from mesic environments to arid environments are relatively common, whereas transitions from arid to mesic environments are rare or nonexistent (5). Their results suggest that deserts are generally biogeographic sinks and not sources of new lineages for other habitats. However, our results show that Nolana species in mesic areas are descended from ancestors that occurred in arid or semiarid habitats, and that this transition occurred relatively recently (1.4 million years ago) and therefore much more rapidly than the long lag time apparently needed to invade arid regions (SI Appendix, Fig. S1).
Overall, our results show that arid and hyperarid climatic conditions have been present in the Atacama–Sechura area much longer than these four clades have been present and diversifying in these habitats. This pattern suggests that a significant evolutionary lag time may be required to allow colonization of these extreme environments in some plant and animal groups. Such evolutionary lag times may be widespread in other systems, and may be important for understanding many patterns, such as differences in biodiversity between habitats and the response of organisms to human desertification and climate change.
Materials and Methods
We estimated a new time-calibrated phylogeny for each clade primarily using published data. For Chaetanthera we used 25 sequences of the nuclear ribosomal DNA internal transcribed spacer (ITS) (46), with outgroups Oriastrum gnaphalioides, Oriastrum acerosum, and Plazia daphnoides. For Malesherbia we used ITS sequences for 24 taxa (47) and one unpublished ITS sequence for Malesherbia corallina obtained following protocols in (48). We included sequences of Turnera cearensis, Turnera sidoides, and Turnera weddelliana as outgroups (Turneraceae) (48).
For Nolana we compiled a dataset of combined plastid and nuclear sequences from 73 species and four outgroups (35, 49–53). We selected Lycium glaucum, Lycium americanum, Lycium deserti, and Lycium bridgesii as outgroups based on their phylogenetic proximity to Nolana (35, 50–53). We used four plastid regions, including one gene NADH dehydrogenase subunit 5, and three intergenic spacers between trnH–tRNA and photosystem II protein D, between ribosomal protein S16 and trnK–tRNA, and between trnC(gca)–tRNA and photosystem II protein M. Also we used two nuclear markers, LEAFY second intron and granule-bound starch synthase I (35, 50–53). GenBank accession numbers are listed in SI Appendix, Table S1.
For Liolaemus, we used a dataset (54) of 1,083 aligned base pairs of the mitochondrial NADH dehydrogenase subunit 2 gene (and adjacent cytochrome c oxidase subunit I and NADH dehydrogenase subunit 1). We included 116 taxa, including all 96 sampled Liolaemus species, representatives of the two other liolaemid genera (one Ctenoblepharys and eight Phymaturus species) and additional outgroups from Leiosauridae (nine species and six genera) and Opluridae (two species and two genera). For all four clades, new alignments were generated using MUSCLE with default parameters (55). Further details of phylogenetic estimation and time calibration are in SI Appendix, SI Materials and Methods).
We characterized aridity of habitats based on annual precipitation (mesic >250 mm/y, semiarid <250 mm/y, arid <50 mm/y, and hyperarid ≤5 mm/y) (56). A species was considered “confined” to a given habitat when both minimum and maximum values of annual precipitation across localities for the species were in the same climatic zone. Data on annual precipitation were extracted from georeferenced localities from the WorldClim database (57) (www.worldclim.org). For plants, localities were obtained from herbaria data bases (Universidad de Concepción, Museo Nacional de Historia Natural, Gray Herbarium, Field Museum, New York Botanical Garden, and Missouri Botanical Garden) and botanical studies (58–62). For Liolaemus, localities were from GBIF and HerpNet. Climatic data and sample sizes are given in SI Appendix, Table S2.
Evolutionary lag times were based on mean annual precipitation for each species obtained by averaging values for all unique localities for each species. We also considered minimum annual precipitation to identify lineages that partially extend their ranges into either arid or hyperarid habitats and maximum annual precipitation to identify lineages completely confined (endemic) to arid or hyperarid habitats. Estimates of the timing of evolutionary transitions from semiarid to arid habitats and arid to hyperarid based on minimum and maximum annual precipitation are show in SI Appendix, Fig. S5.
For each group, we initially analyzed patterns of climatic-niche evolution and biogeography using the highest credible tree and mean branch lengths from BEAST analyses. Patterns of evolution for precipitation variables (mean, minimum, and maximum) were evaluated using GEIGER in R (63). For each clade and variable, we first compared the relative likelihood of the data under Brownian motion (BM), Ornstein-Uhlenbeck (OU), and white noise (lambda = 0) models of evolution (following ref. 39). We compared model fit using the sample-size corrected Akaike Information Criterion (AICc). The OU model consistently had the best fit (SI Appendix, Tables S3–S5), and we used this model. However, reconstructed values using BM and OU models were strongly correlated across clades (SI Appendix, Fig. S10). Indeed, lags calculated for arid categories did not change when using BM for Malesherbia, Chaetanthera, and Liolaemus (SI Appendix, Fig. S12–S14), because habitat transitions occurred at the same nodes under both models. For Nolana, almost all transitions occurred at the same nodes (SI Appendix, Fig. S11), but for two nodes BM increased estimated lag times (node I: 7.2 vs. 9.4 under OU vs. BM and node II: 7.6 vs. 9.3), given more recent invasion of hyperarid habitats under BM. Reconstructions were performed using phylogenetic generalized least squares (PGLS) in COMPARE v 4.6b (64). To implement the OU model, we specified the “exponential model” and input estimated alpha values from GEIGER.
To estimate uncertainty in node ages associated with transitions to arid or hyperarid climate, we reran BEAST analyses to obtain age distributions for these nodes. We focused on minimum ages of clades invading these habitats, but transitions actually occur on branches and could occur at any point along a given branch. Thus, transitions might be somewhat older than the ages of these focal clades. However, our estimates for biogeographic transitions use the same approach (and so underestimate the age of colonization of the region) and the difference between the biogeographic and climatic transition is used to estimate lag times. Thus, these underestimates should generally cancel each other out when estimating lag times.
In addition to reconstructing climatic distributions, we also estimated patterns of regional biogeography and their timing, to compare the timing of colonization of each geographic region and the timing of colonization of arid and hyperarid habitats within that region. The evolutionary lag is the time between the colonization of the region and the colonization of a habitat within the region. Historical biogeography was reconstructed using likelihood analysis of dispersal-extinction-cladogenesis (DEC) in LaGrange (65). Species were assigned to the following broad geographic regions: (A) Sechura (4°S–18°S; <1,500 m), (B) Peruvian Andes (4°S–18°S; >1,500 m), (C) coastal Atacama (18°S–29°S; <1,500 m), (D) high Atacama (18°S–30°S; >1,500 m), (E) coastal Mediterranean Chile (>30°S; <1,500 m), and (F) central Chilean Andes (>30°S; >1,500 m). Most species occur in Chile and Peru, but some species also extend into adjacent countries such as Bolivia and Argentina. We chose 1,500 m as a limit to distinguish species potentially associated with oceanic fog as they belong to a somewhat different habitat type (i.e., “Lomas” formation). Also, 29°S–30°S is considered the border between the northern dry regions (Atacama–Sechura Desert) with summer precipitation and the semiarid Mediterranean-climate area in central Chile with winter precipitation. As these geographic regions are large, they may contain more than one climatic zone, specifically: (A) arid and hyperarid; (B) arid, semiarid, and mesic; (C) arid and hyperarid; (D) arid, hyperarid, and semiarid; (E) semiarid and mesic; and (vi) semiarid and mesic. For Nolana we added the Galapagos Islands (F) to include Nolana galapagensis. For Liolaemus (which is very broadly distributed), we used similar regions, but combined Patagonia, Chaco, and Southern Brazil into a single large geographic unit (G) because of the larger distribution of the clade across southern South America and given that LaGrange only allows for a limited number of regions. All biogeographic analyses were conducted on time-calibrated trees from Bayesian analyses (BEAST; 66).
Supplementary Material
Acknowledgments
We thank C. Woliver and R. A. Pyron for Liolaemus localities, A. Davies for Chaetanthera localities, and C. Peña for laboratory help. This manuscript has been greatly improved thanks to suggestions of B. R. Riddle, M. J. Donoghue and M. Ogburn. P.C.G. is supported by Fondo Nacional de Desarrollo Científico y Tecnológico (3130456), Comisión Nacional de Investigación Científica y Tecnológica (PFB-23), and Iniciativa Científica Milenio Grant (P005-02). Travel by P.C.G. to visit J.J.W. was supported by a Fullbright Fellowship, Becas Chile, and Programa de Mejoramiento de la Calidad y Equidad de la Educación (UCH0803).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308721110/-/DCSupplemental.
References
- 1.Schluter D. The Ecology of Adaptive Radiations. Oxford: Oxford Univ Press; 2000. [Google Scholar]
- 2.Futuyma DJ. 2009. Evolution, 2nd edition (Sinauer, Sunderland, MA) [Google Scholar]
- 3.Barton N, Partridge L. Limits to natural selection. Bioessays. 2000;22(12):1075–1084. doi: 10.1002/1521-1878(200012)22:12<1075::AID-BIES5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 4.Wiens JJ, Graham CH. Niche conservatism: Integrating evolution, ecology, and conservation biology. Annu Rev Ecol Evol Syst. 2005;36:519–539. [Google Scholar]
- 5.Crisp MD, et al. Phylogenetic biome conservatism on a global scale. Nature. 2009;458(7239):754–756. doi: 10.1038/nature07764. [DOI] [PubMed] [Google Scholar]
- 6.Wiens JJ, et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol Lett. 2010;13(10):1310–1324. doi: 10.1111/j.1461-0248.2010.01515.x. [DOI] [PubMed] [Google Scholar]
- 7.Petitpierre B, et al. Climatic niche shifts are rare among terrestrial plant invaders. Science. 2012;335(6074):1344–1348. doi: 10.1126/science.1215933. [DOI] [PubMed] [Google Scholar]
- 8.Arroyo MTK, Squeo FA, Armesto JJ, Villagrán C. Effects of aridity on plant diversity in the northern Chilean Andes: Results of a natural experiment. Ann Mo Bot Gard. 1988;75:55–78. [Google Scholar]
- 9.Houston J. Variability of precipitation in the Atacama desert: Its causes and hydrological impact. Int J Climatol. 2006;26:2181–2198. [Google Scholar]
- 10.Schulz N, Boisier JP, Aceituno P. Climate change along the arid coast of northern Chile. Int J Climatol. 2012;32:1803–1814. [Google Scholar]
- 11.Rundel PW, et al. The phytogeography and ecology of the coastal Atacama and Peruvian deserts. Aliso. 1991;13:1–50. [Google Scholar]
- 12.Arroyo MTK, et al. In: Chilean Winter Rainfall-Valdivian Forests: Hotspots Revisted: Earth’s Biologically Wealthiest and Most Threatened Ecosystems. Mittermeier RA, et al., editors. México, DF: CEMEX; 2004. pp. 99–103. [Google Scholar]
- 13.Vidal MA, Soto ER, Veloso A. Biogeography of Chilean herpetofauna: Distributional patterns of species richness and endemism. Amphib-reptil. 2009;30:151–171. [Google Scholar]
- 14.Guerrero PC, Duran P, Walter HE. Latitudinal and altitudinal patterns of the endemic cacti from the Atacama Desert to Mediterranean Chile. J Arid Environ. 2011;75:991–997. [Google Scholar]
- 15.Peel MC, Finlayson BL, McMahon TA. Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sci. 2007;11:16331644. [Google Scholar]
- 16.Garreaud RD, Vuille M, Campagnucci R, Marengo J. Present-day South American climate. Palaeogeogr Palaeoclimatol Palaeoecol. 2009;281:180–195. [Google Scholar]
- 17.Hartley AJ, Chong G, Houston J, Mather AE. 150 million years of climate stability: Evidence from the Atacama Desert, northern Chile. J Geol Soc London. 2005;162:421–441. [Google Scholar]
- 18.Hartley AJ. Andean uplift and climate change. J Geol Soc London. 2003;160:7–10. [Google Scholar]
- 19.Lamb S, Davis P. Cenozoic climate change as a possible cause for the rise of the Andes. Nature. 2003;425(6960):792–797. doi: 10.1038/nature02049. [DOI] [PubMed] [Google Scholar]
- 20.Dunai TJ, González López GA, Juez-Larré J. Oligocene/Miocene age of aridity in the Atacama Desert revealed by exposure dating of erosion sensitive landforms. Geology. 2005;33:321–324. [Google Scholar]
- 21.Kiefer E, Dörr MJ, Ibbeken H, Götze HJ. Gravity-based mass balance of an alluvial fan giant: The Arcas fan, Pampa del Tamarugal, northern Chile. Revista Geológica de Chile. 1997;24:165–185. [Google Scholar]
- 22.Reich M, et al. Supergene enrichment of copper deposits since the onset of modern hyperaridity in the Atacama Desert, Chile. Miner Depos. 2009;44:497–504. [Google Scholar]
- 23.Schlunegger F, Kober F, Zeilinger G, von Rotz R. Sedimentology-based reconstructions of paleoclimate changes in the Central Andes in response to the uplift of the Andes, Arica region between 19 and 21°S latitude, northern Chile. Int J Earth Sci. 2010;99(Suppl 1):S123–S137. [Google Scholar]
- 24.Rech JA, Currie BS, Michalski G, Cowan AM. Neogene climate change and uplift in the Atacama Desert, Chile. Geology. 2006;34:761–764. [Google Scholar]
- 25.Nishiizumi K, Caffee MW, Finkel RC, Brimhall G, Monte T. Remnants of a fossil alluvial fan landscape of Miocene age in the Atacama Desert of northern Chile using cosmogenic nuclide exposure age dating. Earth Planet Sci Lett. 2005;237:499–507. [Google Scholar]
- 26.Evenstar LA, et al. Multiphase development of the Atacama planation surface recorded by cosmogenic 3He exposure ages: Implications for uplift and Cenzoic climate change in western South America. Geology. 2009;37:27–30. [Google Scholar]
- 27.Stebbins G. Aridity as a stimulus to plant evolution. Am Nat. 1952;86:33–44. [Google Scholar]
- 28.Axelrod D. Drought, diastrophism, and quantum evolution. Evolution. 1967;21:201–209. doi: 10.1111/j.1558-5646.1967.tb00149.x. [DOI] [PubMed] [Google Scholar]
- 29.Axelrod D. Age and origin of Sonoran Desert vegetation. Occ Pap Cal Acad Sci. 1979;132:1–74. [Google Scholar]
- 30.Pianka E. Habitat specificity, speciation and species density in Australian desert lizards. Ecology. 1969;50:498–502. [Google Scholar]
- 31.Evans ME, Smith SA, Flynn RS, Donoghue MJ. Climate, niche evolution, and diversification of the “bird-cage” evening primroses (Oenothera, sections Anogra and Kleinia) Am Nat. 2009;173(2):225–240. doi: 10.1086/595757. [DOI] [PubMed] [Google Scholar]
- 32.Guerrero PC, et al. Phylogenetics and predictive distribution modeling provide insights into infrageneric relationships and the evolution of the Eriosyce subgen. Neoporteria (Cactaceae) Plant Syst Evol. 2011;297:11–128. [Google Scholar]
- 33.Davies AMR. 2009. A systematic revision of Chaetanthera Ruiz & Pav., and the reinstatement of Oriastrum Poepp. & Endl. (Asteraceae: Mutisieae). PhD dissertation (Ludwig Maximilian University, Munich)
- 34.Gengler-Nowak K. Reconstruction of the biogeographical history of Malesherbiaceae. Bot Rev. 2002;68:171–188. [Google Scholar]
- 35.Dillon MO, Tu T, Xie L, Quipuscoa–Silvestre V, Wen J. Biogeographic diversification in Nolana (Solanaceae), a ubiquitous member of the Atacama and Peruvian Deserts along the western coast of South America. J Syst Evol. 2009;47:457–476. [Google Scholar]
- 36.Uetz P, Goll J, Hallermann J. 2012. The Reptile Database. Available at www.reptile-database.org. Accessed February 20, 2012.
- 37.Pincheira-Donoso D, Scolaro JA, Sura P. A monographic catalogue on the systematics and phylogeny of the South American iguanian lizard family Liolaemidae (Squamata, Iguania) Zootaxa. 2008;1800:1–85. [Google Scholar]
- 38.Wiens JJ, Parra-Olea G, Garcia-Paris M, Wake DB. Phylogenetic history underlies elevational patterns of biodiversity in tropical salamanders. Proc Biol Sci. 2007;274:919–928. doi: 10.1098/rspb.2006.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kozak KH, Wiens JJ. Niche conservatism drives elevational diversity patterns in Appalachian salamanders. Am Nat. 2010;176(1):40–54. doi: 10.1086/653031. [DOI] [PubMed] [Google Scholar]
- 40.Kozak KH, Wiens JJ. Phylogeny, ecology, and the origins of climate-richness relationships. Ecology. 2012;93:S167–S181. [Google Scholar]
- 41.Heibl C, Renner SS. Distribution models and a dated phylogeny for Chilean Oxalis species reveal occupation of new habitats by different lineages, not rapid adaptive radiation. Syst Biol. 2012;61(5):823–834. doi: 10.1093/sysbio/sys034. [DOI] [PubMed] [Google Scholar]
- 42.Cereceda P, Larraín H, Osses P, Farías M, Egaña I. The spatial and temporal variability of fog and its relation to fog oasis in the Atacama Desert, Chile. Atmos Res. 2008;87:312–323. [Google Scholar]
- 43.Mooney HA, Gulmon SL, Ehleringer J, Rundel PW. Atmospheric water uptake by an Atacama Desert shrub. Science. 1980;209(4457):693–694. doi: 10.1126/science.209.4457.693. [DOI] [PubMed] [Google Scholar]
- 44.Valiente-Banuet A, Rumebe AV, Verdú M, Callaway RM. Modern Quaternary plant lineages promote diversity through facilitation of ancient Tertiary lineages. Proc Natl Acad Sci USA. 2006;103(45):16812–16817. doi: 10.1073/pnas.0604933103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arakaki M, et al. Contemporaneous and recent radiations of the world’s major succulent plant lineages. Proc Natl Acad Sci USA. 2011;108(20):8379–8384. doi: 10.1073/pnas.1100628108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hershkovitz MA, Arroyo MTK, Bell C, Hinojosa LF. Phylogeny of Chaetanthera (Asteraceae: Mutisieae) reveals both ancient and recent origins of the high elevation lineages. Mol Phylogenet Evol. 2006;41(3):594–605. doi: 10.1016/j.ympev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Gengler-Nowak K. Molecular phylogeny and taxonomy of Malesherbiaceae. Syst Bot. 2003;28:333–344. [Google Scholar]
- 48.Truyens S, Arbo MM, Shore JS. Phylogenetic relationships, chromosome and breeding system evolution in Turnera (Turneraceae): Inferences from ITS sequence data. Am J Bot. 2005;92(10):1749–1758. doi: 10.3732/ajb.92.10.1749. [DOI] [PubMed] [Google Scholar]
- 49.Dillon MO, et al. Phylogeny of Nolana (Nolaneae, Solanoideae, Solanaceae) as inferred from granule-bound starch synthase I (GBSSI) sequences. Taxon. 2007;56:1000–1011. [Google Scholar]
- 50.Tu T, Dillon MO, Sun H, Wen J. Phylogeny of Nolana (Solanaceae) of the Atacama and Peruvian deserts inferred from sequences of four plastid markers and the nuclear LEAFY second intron. Mol Phylogenet Evol. 2008;49(2):561–573. doi: 10.1016/j.ympev.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 51.Olmstead RG, et al. A molecular phylogeny of the Solanaceae. Taxon. 2008;57:1159–1181. [Google Scholar]
- 52.Levin RA, Bernardello G, Whiting C, Miller JS. A new generic circumscription in tribe Lycieae (Solanaceae) Taxon. 2011;60:681–690. [Google Scholar]
- 53.Miller JS, Kamath A, Damashek J, Levin RA. Out of America to Africa or Asia: Inference of dispersal histories using nuclear and plastid DNA and the S-RNase self-incompatibility locus. Mol Biol Evol. 2011;28(1):793–801. doi: 10.1093/molbev/msq253. [DOI] [PubMed] [Google Scholar]
- 54.Schulte JA, 2nd, Moreno-Roark F. Live birth among iguanian lizards predates Pliocene–Pleistocene glaciations. Biol Lett. 2010;6(2):216–218. doi: 10.1098/rsbl.2009.0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edgar RC. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garreaud RD, Molina A, Farías M. Andean uplift, ocean cooling and Atacama hyperaridity: A climate modeling perspective. Earth Planet Sci Lett. 2010;292:39–50. [Google Scholar]
- 57.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. The WorldClim Interpolated Global Terrestrial Climate Surfaces. Version 1.3. Available at www.worldclim.org. Accessed April 22, 2011.
- 58.Ricardi M. Revisión taxonómica de las Malesherbiaceas. Gayana Botánica. 1967;16:3–139. [Google Scholar]
- 59.Gengler KM, Crawford DJ. Genetic diversities of four little-known species of Malesherbia (Malesherbiaceae) endemic to the arid inter-Andean valleys of Peru. Brittonia. 2000;52:303–310. [Google Scholar]
- 60.Dillon MO, Leiva-González S, Quipuscoa-Silvestre V. Five new species of Nolana (Solanaceae-Nolaneae) from Peru and notes on the classification of additional taxa. Arnaldoa. 2007;14:171–190. [Google Scholar]
- 61.Dillon MO, Arancio G, Luebert F. Five new species of Nolana (Solanaceae-Nolaneae) from Chile. Arnaldoa. 2007;14:191–212. [Google Scholar]
- 62.Douglas AC, Freyre R. Floral development, stigma receptivity and pollen viability in eight Nolana (Solanaceae) species. Euphytica. 2010;174:105–117. [Google Scholar]
- 63.Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. GEIGER: Investigating evolutionary radiations. Bioinformatics. 2008;24(1):129–131. doi: 10.1093/bioinformatics/btm538. [DOI] [PubMed] [Google Scholar]
- 64.Martins EP. 2004. COMPARE, ver. 4.6b: Computer programs for the statistical analysis of comparative data. Distributed by the author. (Department of Biology, Indiana University, Bloomington, IN). Available at http://compare.bio.indiana.edu/
- 65.Ree RH, Smith SA. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Syst Biol. 2008;57(1):4–14. doi: 10.1080/10635150701883881. [DOI] [PubMed] [Google Scholar]
- 66.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.