Abstract
Full-length tissue factor (flTF), the coagulation initiator, is overexpressed in breast cancer (BrCa), but associations between flTF expression and clinical outcome remain controversial. It is currently not known whether the soluble alternatively spliced TF form (asTF) is expressed in BrCa or impacts BrCa progression. We are unique in reporting that asTF, but not flTF, strongly associates with both tumor size and grade, and induces BrCa cell proliferation by binding to β1 integrins. asTF promotes oncogenic gene expression, anchorage-independent growth, and strongly up-regulates tumor expansion in a luminal BrCa model. In basal BrCa cells that constitutively express both TF isoforms, asTF blockade reduces tumor growth and proliferation in vivo. We propose that asTF plays a major role in BrCa progression acting as an autocrine factor that promotes tumor progression. Targeting asTF may comprise a previously unexplored therapeutic strategy in BrCa that stems tumor growth, yet does not impair normal hemostasis.
Keywords: regulated pre-mRNA processing, outside-in signaling
In breast cancer (BrCa), proteins that modulate splicing events such as ASF/SF2 and SR(Serine/Arginine-rich)p55, are frequently up-regulated and contribute to cell transformation (1, 2). BrCa cells exhibit specific alternative splicing signatures that were proposed as potential prognostic factors in BrCa (3). Alternative splicing of proteins, such as spleen tyrosine kinase (Syk), p53, phosphatase and tensin homolog (PTEN), chemokine (C-X-C motif) receptor 3 (CXCR3), and ras-related C3 botulinum toxin substrate 1 (Rac1) impacts BrCa cell behavior and therefore, disease progression (4–8).
Full-length tissue factor (flTF) is the initiator of blood coagulation (9). Following vascular damage, flTF binds its ligand FVII(a), which triggers clot formation. Aside from subendothelial tissues, flTF is also abundant on cancer cells (9) and fuels tumor progression by modulating integrin α3β1 function, cell migration (10), and FVIIa-dependent protease activated receptor (PAR)2 activation, but flTF-β1 integrin complexation enhances PAR2 activation (11). flTF-dependent PAR2 activation results in the production of VEGF, CXCL1, and IL-8, thus promoting the angiogenic switch and consequently, tumor growth in vivo (10, 11).
Alternative splicing of TF pre-mRNA results in the deletion of exon 5 and thus a frameshift in exon 6, yielding a transmembrane domain-lacking isoform that can be secreted (12). Human and murine alternatively spliced TF (asTF) contain novel C termini with poor homology to one another or any other protein, and the murine asTF C terminus is longer than that of human asTF (12, 13). High expression of asTF in tumor cell lines suggests a role in tumor progression (10, 14). Subcutaneous growth of pancreatic cancer cells overexpressing asTF results in larger and more vascularized tumors (15). We recently discovered that asTF induces angiogenesis, independent of PAR2 activation, by acting as an integrin ligand (16). Thus, flTF and asTF facilitate cellular signaling via distinct mechanisms critical to tumor cell behavior.
Currently, nothing is known about asTF expression and function in breast cancer. Regulated splicing of TF pre-mRNA in human monocytes is controlled by several serine-rich (SR) proteins, including ASF/SF2 and SRp55 (17, 18). Expression of SR proteins is frequently perturbed in BrCa tumors (2), and it is thus plausible that the relative abundance of flTF and asTF is also altered in BrCa.
Prior studies that attempted to correlate “TF expression” in BrCa specimens with clinical parameters, such as tumor grade and disease outcome, did not discriminate between flTF and asTF (19, 20). Thus, there is uncertainty as to how the two TF isoforms associate with/contribute to BrCa progression. We set out to characterize flTF and asTF expression in a large set of BrCa tissue specimens. Identified associations were tested in vitro using innovative cell models, and in vivo with recapitulation of distinct BrCa subtypes.
Results
asTF Expression Positively Correlates with BrCa Grade and Stage.
Prior studies pointed to a role for TF in tumor progression (19–21). To explore whether asTF and flTF differentially contribute to BrCa progression, we analyzed asTF and flTF protein expression in a BrCa tissue array comprising specimens from 574 BrCa patients (22). asTF and flTF were detectable in >95% of the BrCa specimens with various degree of tumor-cell positivity. Healthy mammary tissue showed limited expression of asTF compared with flTF (asTF: 4% of all specimens; flTF: 38%) (Fig. S1A). Specificity of previously validated flTF- and asTF-specific antibodies (23) was reconfirmed (Fig. S1B). Confocal analysis revealed flTF localization on the cell membrane, but asTF was mostly intracellular (Fig. S1C).
Expression of asTF positively correlated with histological grade as well as tumor size (Table 1), but flTF expression only correlated with grade. asTF expression also correlated with age. No significant associations were found between asTF or flTF levels and estrogen receptor (ER), progesterone receptor (PgR), or human epidermal growth factor receptor 2 (HER2) status. This finding raises the possibility that asTF impacts BrCa progression in a manner qualitatively distinct from that of flTF.
Table 1.
Association of asTF and flTF with patient and tumor characteristics
| Characteristic | Total N (%) | asTF- N (%)* | asTF+ N (%)* | P | flTF- N (%)* | flTF+ N (%)* | P |
| Total | 574 (100) | 119 (100) | 328 (100) | 157 (100) | 351 (100) | ||
| Age (y) | |||||||
| <40 | 48 (8.4) | 5 (4.2) | 26 (7.9) | 15 (9.6) | 26 (7.4) | ||
| 40–60 | 277 (48.3) | 72 (60.5) | 153 (46.6) | 0.03 | 75 (47.8) | 171 (48.7) | 0.71 |
| ≥60 | 249(43.4) | 42 (35.3) | 149 (45.4) | 67 (42.7) | 154 (43.9) | ||
| Missing* | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Grade | |||||||
| I | 81 (14.1) | 26 (21.8) | 28 (8.5) | 30 (19.1) | 41 (11.7) | ||
| II | 282 (49.1) | 58 (48.7) | 159 (48.5) | <0.001 | 88 (56.1) | 157 (44.7) | <0.001 |
| III | 203 (35.4) | 33 (27.7) | 138 (42.1) | 35 (22.3) | 149 (42.5) | ||
| Missing* | 8 (1.4) | 2 (1.7) | 3 (0.9) | 4 (2.5) | 4 (1.1) | ||
| Histotype | |||||||
| Ductal | 514 (89.5) | 104 (87.4) | 300 (91.5) | 133 (84.7) | 320 (91.2) | ||
| Lobular | 53 (9.2) | 13 (10.9) | 25 (7.6) | 0.39 | 20(12.7) | 27 (7.7) | 0.065 |
| Missing* | 7 (1.2) | 2 (1.7) | 3 (0.9) | 4 (2.5) | 4 (1.1) | ||
| T status | |||||||
| T1 | 211 (36.8) | 56 (47.1) | 97 (29.6) | 64 (40.8) | 120 (34.2) | ||
| T2 | 272 (47.4) | 46 (38.7) | 173 (52.7) | 0.002 | 71 (45.2) | 171 (48.7) | 0.26 |
| T3–4 | 72 (12.5) | 13 (10.9) | 49 (14.9) | 17 (10.8) | 51 (14.5) | ||
| Missing* | 19 (3.3) | 4 (3.4) | 9 (2.7) | 5 (3.2) | 9 (2.6) | ||
| N status | |||||||
| N0 | 307 (53.5) | 64 (53.8) | 166 (50.6) | 93 (59.2) | 182 (51.9) | ||
| N1–3 | 250 (43.6) | 53 (44.5) | 153 (46.7) | 0.62 | 62 (39.5) | 158 (45.0) | 0.18 |
| Missing* | 17 (3.0) | 2 (1.7) | 9 (2.7) | 2 (1.3) | 11 (3.1) | ||
| ER status | |||||||
| Negative | 208 (36.2) | 37 (31.1) | 124 (37.8) | 54 (34.4) | 123 (35.0) | ||
| Positive | 344 (59.9) | 79 (66.4) | 193 (58.8) | 0.17 | 93 (59.2) | 214 (61.0) | 0.96 |
| Missing* | 22 (3.8) | 3 (2.5) | 11 (3.4) | 10 (6.4) | 14 (4.0) | ||
| PgR status | |||||||
| Negative | 234(40.8) | 40 (33.6) | 136 (41.4) | 50 (31.8) | 144 (41.0) | ||
| Positive | 311(54.2) | 77 (64.7) | 181 (55.2) | 0.10 | 97 (61.8) | 191 (54.4) | 0.06 |
| Missing* | 29 (5.1) | 2 (1.7) | 11 (3.4) | 10 (6.4) | 16 (4.6) | ||
| HER2† | |||||||
| Negative | 406 (70.7) | 86 (72.3) | 236 (71.9) | 105 (66.9) | 244 (69.5) | ||
| Positive | 50 (8.7) | 6 (5.0) | 32 (9.8) | 0.14 | 9 (5.7) | 32 (9.1) | 0.28 |
| Missing* | 118 (20.6) | 27 (22.7) | 60(18.3) | 43 (27.4) | 75 (21.4) |
N, axillary lymph node; T, tumor.
During specimen processing, some tumor punches were lost leading to a smaller patient number per staining.
HER2 status was not known for all patients.
asTF Expression Enhances Proliferation of BrCa Cells.
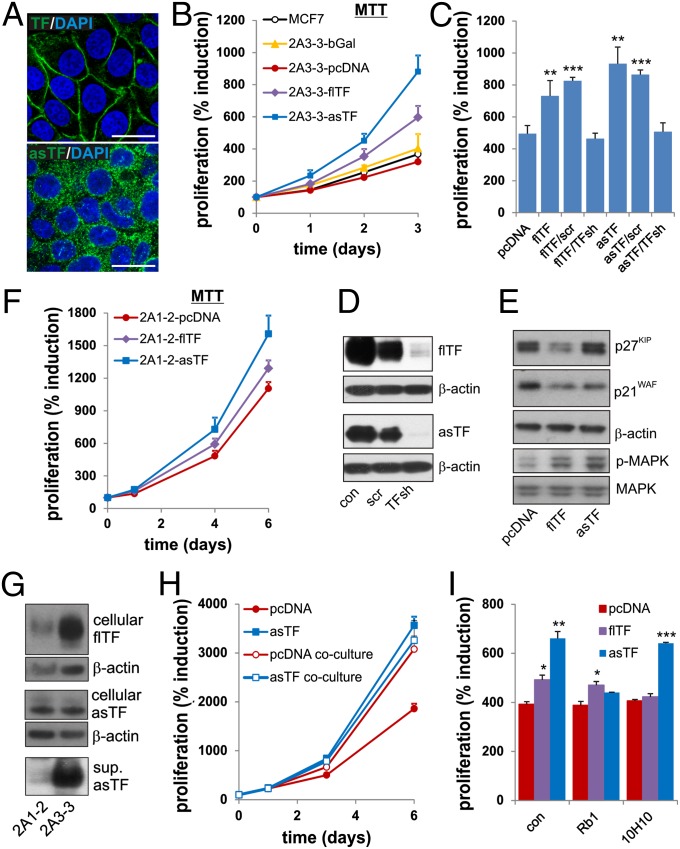
To explore the mechanistic link between the unique correlation of asTF expression and BrCa clinical parameters, we constructed MCF-7 cells harboring a genomic flippase recognition target (FRT) insertion, thus containing a unique locus-specific DNA acceptor site and established several FRT lines, selecting clone 2A3-3 for further studies. Insertion of asTF and flTF cDNA resulted in similar mRNA expression levels, but intracellular protein levels of asTF were lower (Fig. S2 A and B), likely because of asTF secretion (see below and Fig. 1G). Confocal analysis revealed that flTF was localized on the plasma membrane, confirmed by FACS analysis (Fig. S2D), but asTF was present in vesicular structures (Fig. 1A). Only 2A3-3-flTF cells exhibited significant coagulant activity (Fig. S2C). asTF protein levels were ∼fivefold higher in 2A3-3-asTF cell lysates than in lysates of tumor tissue-array specimens with high asTF positivity, likely because of the constitutive asTF overexpression of 2A3-3 cells and the appreciable presence of asTF-negative stroma (up to 50%) in our tumor specimens (Fig. S2E). The 2A3-3 cells expressing flTF, asTF, an aspecific protein (β-Galactosidase), or empty vector control cells (pcDNA) were tested in an MTT proliferation assay and compared with parental MCF-7 cells. The 2A3-3-pcDNA and 2A3-3-β-Gal proliferation rates were identical to those of MCF-7 (Fig. 1B), but proliferation rates of 2A3-3-asTF cells were increased by ≥twofold; those of 2A3-3-flTF cells were only modestly increased. Cell counting and genomic DNA measurements confirmed these results (Fig. S3 A and B). An independently established second 2A3-3-asTF line showed similar proliferation rates (Fig. S3C) and TF-specific shRNA eliminated enhanced proliferation (Fig. 1 C and D), confirming that the effect was asTF-dependent. Because asTF contains an unusual C terminus as a result of a frameshift, a possibility exists that asTF expression may increase cell proliferation through activation of the unfolded protein response. However, we found no evidence for unfolded protein response activation or protein aggregation, neither in flTF- nor in asTF-expressing cells (Fig. S3 D and E). Increased cell numbers were also not a result of increased cell survival, as all cell lines exhibited similar viability levels (Fig. S3F). asTF and flTF expression resulted in down-regulation of cell cycle inhibitors and enhanced phosphorylation of the promitogenic p42/p44 MAP kinase (Fig. 1E). Effects of flTF and asTF expression on proliferation were somewhat less pronounced in an MCF-7 FRT clone (2A1-2) with lower flTF/asTF expression (Fig. 1 F and G, and Fig. S3G). The 2A1-2 cells exhibited decreased asTF secretion, but intracellular asTF levels were equal to those in 2A3-3 cells (Fig. 1G), suggesting that asTF secretion is important to the enhancement of proliferation. Indeed, coculture of control cells with asTF-expressing cells increased the proliferation of control cells (Fig. 1H). Culturing control cells in 2A3-3-asTF–conditioned medium enhanced proliferation, and asTF depletion from the medium reversed this effect (Fig. S4A). Addition of recombinant asTF to pcDNA cells increased proliferation at concentrations as low as 1 nM, well below asTF concentrations detectable in the plasma of metastatic breast cancer patients (Fig. S4 B and C). Incubation of asTF-expressing 2A3-3 cells with an asTF-blocking antibody, but not with an flTF-blocking antibody, reduced asTF-dependent cell proliferation (Fig. 1I). Limited flTF-elicited proliferation was not dependent on PAR2, as incubation with PAR2- and FVII-blocking antibodies was without effect (Fig. S4D). These results demonstrate that secreted asTF enhances BrCa cell proliferation in an autocrine fashion.
Fig. 1.
asTF expression induces cancer cell proliferation. (A) FRT cells (clone 2A3-3) received flTF or asTF cDNA by homologous recombination. Localization of flTF (Upper) and asTF (Lower) was assessed by confocal microscopy using specific antibodies [monoclonal flTF-specific Ab 4509 and polyclonal asTF-specific Ab, as described previously (23)]. (Scale bars, 25 μm.) (B) Proliferation of 2A3-3 cells containing the cDNAs indicated was monitored using MTT assay. (C) 2A3-3-flTF or 2A3-3-asTF were transduced with TF-specific or control shRNA. Cells were subjected to MTT assay 3 d after the start of the experiment. (D) flTF and asTF expression in 2A3-3 cells transduced with TF-specific or control shRNA constructs. (E) Expression of cell cycle inhibitors and MAP kinase phosphorylation was assessed in 2A3-3 cell lines. (F) Cell line (2A1-2) harboring an FRT site at a transcriptionally less active region received empty vector, flTF or asTF cDNA and proliferation was monitored using MTT assay. (G) Cell-associated and secreted levels of asTF and flTF in 2A3-3 and 2A1-2 cells. (H) pcDNA cells and asTF-expressing cells were grown separately, or in adjoining 12-well plates containing an open port, allowing asTF diffusion to control cells. Proliferation was monitored using MTT assay. (I) 2A3-3-pcDNA, 2A3-3-flTF, or 2A3-3-asTF cells were treated with 50 μg/mL flTF-signaling blocking antibody (10H10) or asTF-blocking antibody (RabMab1, Rb1), proliferation was assessed 3 d later using MTT assay. *P < 0.05, **P = 0.01, and ***P = 0.001.
asTF Augments Pro-Oncogenic Gene Expression.
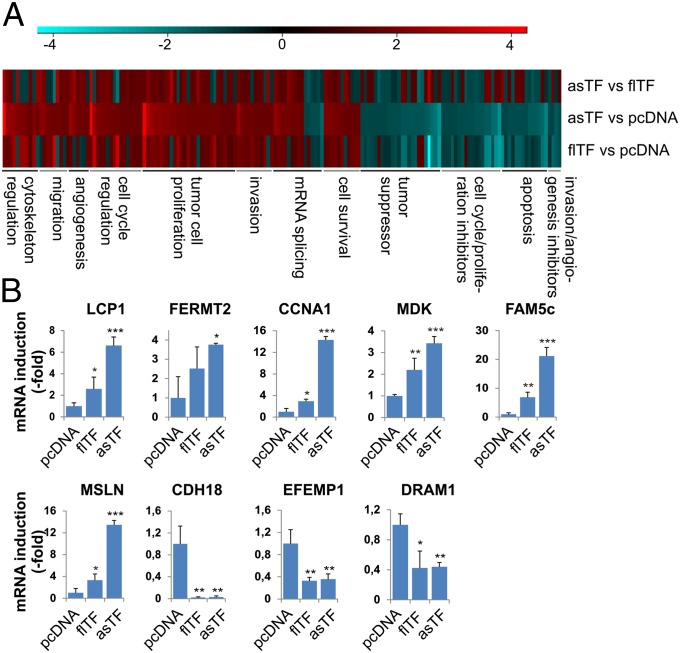
We compared gene expression profiles in asTF-expressing 2A3-3 cells with those in 2A3-3-pcDNA– or flTF-expressing 2A3-3 cells. Compared with 2A3-3-pcDNA or flTF-expressing cells, asTF expression up-regulated genes involved in cell cycle progression [e.g., cyclin A1 (CCNA1)], tumor proliferation [e.g., midkine (MDK)], cytoskeletal reorganization/motility [e.g., fermitin family homologue 2 (FERMT2)], invasion [e.g., family with sequence similarity 5, member C (FAM5c)], and cell survival [e.g., mesothelin (MSLN)] (Fig. 2 A and B). Moreover, expression of asTF down-regulated several tumor suppressors [e.g., cadherin 18 (CDH18)], and genes involved in cell cycle arrest [e.g., EGF containing fibulin-like extracellular matrix protein 1 (EFEMP1)] and apoptosis [DNA-damage regulated autophagy modulator 1 (DRAM1)] (Fig. 2 A and B, and Table S1). Expression of SRSF protein kinase 2 (SRPK2), which activates the TF pre-mRNA splicing regulator ASF/SF2 (24), was altered by asTF and flTF, suggesting that TF splice variants may regulate their own expression. These results indicate that asTF enhances BrCa cell proliferation via modulation of cell cycle regulators, proliferation inducers, and tumor suppressors/proapoptotic proteins.
Fig. 2.
Microarray analysis of flTF and asTF expressing cells. (A) Heat map representation of relevant genes in flTF and asTF expressing 2A3-3 cells, compared with control. The heat map comprises the genes whose expression was significantly up- or down-regulated (P < 0.05) by at least 1.33-fold. Results of four independent experiments are shown; red and blue denote up- and down-regulation, respectively. (B) Up-regulation of selected genes was verified using real-time PCR.
asTF Enhances Proliferation by Binding to β1 Integrins.
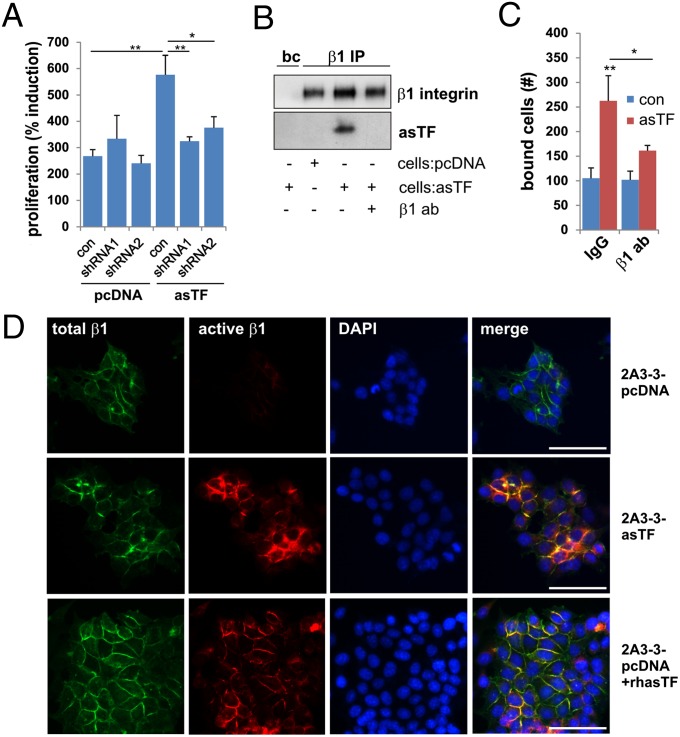
Because asTF induces angiogenesis via binding integrins on endothelial cells (16), we reasoned that asTF-dependent BrCa cell proliferation may also be integrin-dependent. Silencing of the β1 integrin subunit resulted in diminished proliferation of 2A3-3-asTF cells (Fig. 3A and Fig S5A) and flTF cells (Fig. S5 A and B), but not 2A3-3-pcDNA cells; shRNA silencing of the β3 integrin subunit, which is not expressed in these cells, was without effect (Fig. S5C).
Fig. 3.
TF variant-induced proliferation is integrin dependent. (A) pcDNA control cells or asTF-expressing 2A3-3 cells were transduced with shRNA to silence β1 integrins, and proliferation of these cells was compared with control after 3 d. (B) 2A3-3-pcDNA or -asTF cells were mock-treated or incubated with a β1 (epitope 579–799)-reactive antibody. The cells were lysed in Brij78-containing lysis buffer and β1 integrin was precipitated using AIIB2. asTF coimmunoprecipitation was assessed by Western Blot. (C) 2A3-3-pcDNA cells were preincubated with 10 nM recombinant asTF in the presence or absence of an antibody against β1 integrin epitope 579–799 and seeded on collagen I. After washing, remaining cells were counted. (D) 2A3-3-asTF cells or 2A3-3-pcDNA cells, untreated or incubated with 10 nM recombinant asTF, were fixed and stained with AIIB2 (total β1 integrin; green) and HUTS-21 (activated β1 integrin; red). (Scale bars, 50 μm.) *P < 0.05 and **P = 0.01.
Artificially truncated recombinant flTF (“sTF”) was recently found to induce endothelial cell proliferation by binding to the integrin β1 region between amino acid residues 579 and 799 (25). Because sTF contains the entire N-terminal region of asTF, we tested whether asTF binds to this integrin region. A β1 579–799 aa domain-specific antibody and a peptide mimicking this domain inhibited proliferation of 2A3-3-asTF, but not that of control cells or 2A3-3-flTF cells, even after prolonged incubation (Fig. S5 D and E). Coimmunoprecipitation (IP) demonstrated that asTF directly binds to β1 integrin and binding was lost after preincubation with the β1 579–799 aa antibody (Fig. 3B). Binding of asTF to β1 integrins was confirmed using a modified ELISA, showing that asTF binds well to recombinant α6β1 (Fig. S5F), which is consistent with our earlier findings (16). Preincubation of control cells in suspension with recombinant asTF enhanced cell binding to collagen and fibronectin, but not vitronectin (Fig. S5G), indicating that asTF binding may modulate integrin activation; functional blockade of β1 reversed this effect (Fig. 3C). In support of an activating effect of asTF on β1 integrins, reactivity of HUTS-21, an antibody that recognizes the active conformation of β1 integrins, was increased when 2A3-3 cells expressed asTF or were exposed to recombinant asTF (Fig. 3D). To ascertain asTF’s colocalization with β1 integrins, we preincubated pcDNA cells with fluorescently tagged asTF; partial colocalization with β1 integrins on the cell surface was observed (Fig. S5H). Because β1 integrin blockade in 2A3-3 cells only partially inhibited asTF-dependent proliferation, these results suggest that, aside from β1 integrins, asTF likely binds to other membrane-associated proteins.
asTF Induces Anchorage-Independence and Tumor Growth in Vivo.
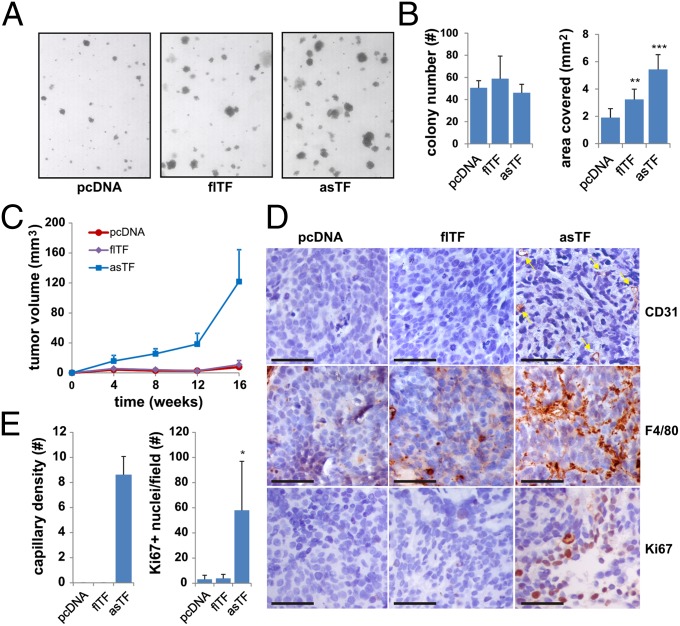
We next investigated the effects of asTF expression on oncogenic potential using soft-agar assays. Although asTF did not affect the number of colonies, it caused a threefold increase in colony size; the impact of flTF expression on colony size was marginal (Fig. 4 A and B).
Fig. 4.
TF variant-induced transformation and tumor growth. (A) 2A3-3-pcDNA, flTF or asTF cells were seeded in soft agar and allowed to grow for 14 d. Images were captured and colony number per area covered determined using ImageJ (B). (C) 2A3-3-pcDNA, flTF or asTF cells were injected into the mammary fat pad of NOD-SCID mice, and tumor growth assessed for 16 wk. Mean and SEM are shown. (D) Tumors were analyzed by immunohistochemistry to assess vascular density (CD31), macrophage infiltration (Mac3), and proliferation rate (Ki67). (Scale bars, 50 μm.) (E) Quantification of vascular structures per view field. *P < 0.05, **P = 0.01, and ***P = 0.001.
asTF-dependent tumor growth was then assessed orthotopically. asTF expression increased tumor expansion (Fig. 4C), but flTF-expressing 2A3-3 cells yielded tumors that were similar in size or smaller than those formed by control cells. asTF-expressing cells yielded large tumors with little stroma, whereas control and flTF cells gave rise to small tumor islands surrounded by stroma (Fig. S6). flTF and asTF protein expression was confirmed in tumors in vivo, ruling out that poor growth of flTF-expressing cells was because of loss of flTF expression (Fig. S6). asTF-expressing tumors had more CD31+ capillaries and macrophage infiltrate, and contained more proliferating tumor cells, specifically at the tumor periphery (Fig. 4 D and E). These data demonstrate that asTF promotes BrCa cell proliferation in vitro and in vivo.
asTF Blockade Reduces Growth of BrCa Cells Expressing Endogenous asTF.
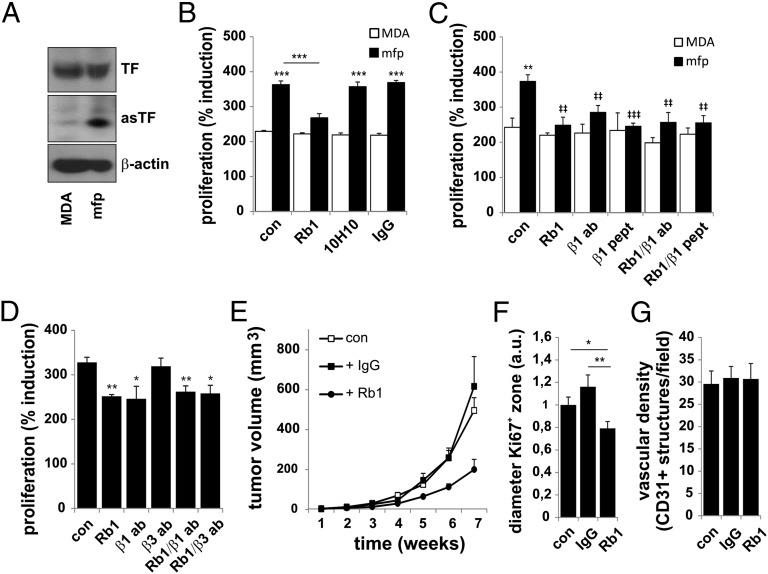
Although we dissected the role of asTF in tumor growth using MCF-7 cells constructed to express either asTF or flTF, native asTF is coexpressed with flTF. Moreover, MCF-7 cells express low levels of PAR2, which may mechanistically explain the lack of 2A3-3-flTF-dependent tumor expansion in vivo. Because MDA-MB-231 BrCa cells express high levels of flTF and PAR2 (11), we used them to assess the role of asTF in an flTF/PAR2+ setting. Although asTF levels in MDA-MB-231 cells were low (Fig. 5A), a more aggressive MDA-MB-231 subline that had been isolated from the mammary fat pad following orthotopic implantation (MDA-MB-231-mfp) (26) had significantly higher asTF levels, but flTF levels were unchanged. In agreement, spliceosomal proteins that promote biosynthesis of asTF mRNA were up-regulated in MDA-MB-231-mfp cells (Fig. S7A). Importantly, asTF levels in these cells were lower or equal to the asTF levels in BrCa specimens (Fig. S2E). asTF-specific antibody blockade significantly inhibited proliferation of MDA-MB-231-mfp cells (Fig. 5B), but had no effect on proliferation of the parental MDA-MB-231 line, demonstrating functional specificity. Selective anti-flTF antibody 10H10 did not inhibit proliferation of either cell type in vitro, which is consistent with the notion that flTF potentiates angiogenesis—but not proliferation—in these cells (11) (Fig. 5B). A β1 integrin-blocking antibody and the 579–799 aa integrin peptide also inhibited proliferation of MDA-MB-231-mfp cells, but did not further reduce proliferation in the presence of the anti-asTF antibody (Fig. 5C), confirming that asTF augments proliferation in MDA-MB-231-mfp via β1 integrins. The β3 integrin blockade was without effect (Fig. 5D).
Fig. 5.
asTF induces tumor cell proliferation of BrCa cells endogenously expressing flTF/asTF/PAR2. (A) flTF and asTF expression in MDA-MB-231 and MDA-MB-231-mfp cells. β-Actin is used as a loading control. (B) MDA-MB-231 (MDA) and MDA-MB-231-mfp (mfp) cells were grown in the presence of asTF-specific antibody (Rb1), flTF-specific antibody 10H10, or IgG control. Proliferation was assessed after 3 d using MTT assay. (C) As in B, but blocking antibodies against β1 integrins or the β1 peptide was added separately or in combination with Rb1. Asterisks indicate significant differences compared with control (MDA-MB-231) cells (*P < 0.05, **P = 0.01, ***P = 0.001); ‡indicates significant differences compared with MDA-MB-231-mfp cells. (D) As in B, a β3-blocking antibody was added. (E) MDA-MB-231-mfp cells were injected in the mammary fat pad of NSG mice in the presence or absence of 100 μg Rb1 or IgG control. Mean tumor volume and SEM are shown. (F) Tumor specimens were stained with anti-Ki67, and the proliferation zone diameter (Ki67+ area at the periphery of the tumor) was determined. Values are expressed as arbitrary units, with the control set at 1. (G) Quantification of CD31+ structures in the tumor specimens shown in F.
We then implanted MDA-MB-231-mfp cells orthotopically in nonobese diabetic (NOD)-SCIDγ (NSG) mice in the presence or absence of asTF-blocking antibody. asTF blockade with as little as 100 μg antibody significantly inhibited tumor growth (Fig. 5E) and resulted in a reduction of the proliferation zone at the tumor periphery (Fig. 5F and Fig. S7B). Notably, asTF blockade did not reduce vascular density, suggesting that asTF does not impact angiogenesis in a model featuring a proangiogenic flTF-PAR2 axis (Fig. 5G and Fig. S7B). Thus, asTF blockade decreases the tumorigenic potential of BrCa cells expressing native asTF, flTF, and PAR2.
Discussion
Until now, contributions of asTF to tumor progression have remained unclear. This study is unique in reporting: (i) asTF’s selective abundance in BrCa tissue; (ii) asTF-dependent, autocrine augmentation of BrCa cell proliferation; and (iii) the efficacy of anti-asTF monoclonal antibodies in stemming BrCa growth. Analysis of BrCa specimens from 574 patients revealed that asTF positively correlates with both grade and the T-status of cancer lesions, as well as the patients’ age, but flTF correlates solely with tumor grade and is detectable in ∼40% of normal breast tissue, compared with ∼4% for asTF. We demonstrate herein that asTF up-regulates BrCa cell proliferation irrespective of its impact on angiogenesis in an asTF overexpression model, as well as in BrCa cells that express native asTF. In contrast to asTF-triggered proliferation, flTF-triggered proliferation rates were low in our TF overexpressing cells; furthermore, flTF-dependent proliferation was not observed in an aggressive MDA-MB-231 cell line that expresses asTF. Thus, asTF appears to be the major TF variant that promotes BrCa cell proliferation.
asTF up-regulated genes that play pivotal roles in cell cycle progression and proliferation. CNNA1 and CNNA2, important regulators of cyclin-dependent kinases during S phase, and anaphase promoting complex 10 (ANAPC10) were significantly up-regulated in 2A3-3-asTF cells compared with control or flTF expressing cells. Growth factors [MDK, GAL, and tissue inhibitor of metalloproteinases 1 (TIMP1)] were also up-regulated. Although we observed up-regulation of some cell survival genes, we found no evidence for altered cell survival in asTF-expressing cells. Still, it cannot be ruled out that these genes contribute to the cumulative impact of asTF expression on tumor xenografts.
Our studies further revealed that asTF-integrin interactions were responsible for increased proliferation of BrCa cells. asTF colocalized with and bound to β1 integrins and β1 integrin silencing reversed asTF-dependent proliferation. An antibody against the β1 region encompassing residues 579–799 or a peptide mimicking this domain, reversed asTF-dependent—but not flTF-dependent—proliferation, indicating that asTF binds to this distinct β1 region. We hypothesize that asTF induces a conformational change in β1 integrins that render them prone to activation, as we used a β1 integrin-blocking antibody that is reactive with the membrane-proximal β-tail domain (βTD) of the β1 integrin subunit, and the 579–799 integrin peptide features this domain. The βTD contains a CD-loop that contacts the ligand-binding integrin βA domain and the hybrid domain, and this contact is lost upon integrin activation (27, 28). It has been postulated that the CD-loop acts as a deadbolt, preventing integrin activation by locking βA in an inactive state. Indeed, a number of antibodies that activate β1 integrins bind to the βTD or the βA interface, and the βTD has been shown to regulate ligand binding (27, 29). We propose that asTF induces the removal of the CD-loop deadbolt. However, direct conformational effects of asTF on β1 integrin function may not be the sole means by which asTF modulates the “integrin profile” of BrCa cells: asTF expression also up-regulates FERMT2, a positive regulator of integrin activation (30), and suppresses tensin 3, a negative regulator of integrin function (31).
The in vitro phenotype of asTF-expressing 2A3-3 cells (enhanced proliferation and soft agar colony growth), was recapitulated in vivo but flTF-expressing cells that proliferated only moderately faster than control cells in vitro produced tumors of the same size as control cells. It is not clear why flTF overexpression in MCF7 cells did not enhance tumor growth in vivo, but the paucity of PAR2 expression may be a contributing factor. PAR2 is instrumental in flTF-dependent tumor angiogenesis (11), and poor expansion of these cells may result from a lack of PAR2-dependent angiogenesis. This finding is in agreement with the results of in vivo experiments using PAR2-expressing MDA-MB-231-mfp cells: asTF blockade did not affect vascular development in tumor xenografts, although we did not directly test the influence of asTF-induced angiogenesis on BrCa growth. Our results indicate that asTF does not significantly influence angiogenesis in the MDA-MB-231-mfp xenograft model, but up-regulation of CD31+ vessels in asTF-expressing 2A3-3 tumors suggests that asTF-dependent angiogenesis may be a contributing factor in 2A3-3 xenografts. Differences in secreted asTF levels and presence of a functional flTF-PAR2 axis in MDA-MB-231-mfp cells may explain why asTF differently affects vascular density in these two xenograft models.
Alternative splicing has been deemed critical to the proliferation of BrCa cells: overexpression of ASF/SF2, a major SR protein and regulator of the flTF/asTF mRNA ratio in monocytes (18), leads to enhanced proliferation, transformation, and BrCa growth in vivo (1). Furthermore, the expression of SRp40—the spliceosomal protein that promotes asTF synthesis (17), the levels of which are up-regulated in MDA-MB-231-mfp cells—is increased in human BrCa and associates with lymph node metastasis (32). It may be of interest to investigate whether the effects of heightened SRp40 expression in BrCa are in part dependent on asTF production.
In conclusion, autocrine asTF expression induces integrin-mediated BrCa cell proliferation that contributes to tumor growth, rendering asTF a unique target for anticancer strategies that modulate the biological activity of this minimally coagulant TF form, thereby avoiding adverse impacts on hemostasis.
Experimental Procedures
See SI Text for reagents, cell culture, proliferation assays, Western blotting, microarray analysis, and soft agar experiments. See Table S2 for primer sequences used.
Immunofluorescence Studies.
For HUTS-21 staining, cells were fixed in methanol for 5 min. In all other experiments, cells were fixed in 2% (wt/vol) formaldehyde, permeabilized with 0.1% Triton-X100 when appropriate. Cells were incubated overnight with primary antibodies followed by incubation with secondary antibodies conjugated to Alexa-488 or Alexa-594. Coverslips were mounted using Vectashield containing DAPI (Vector Laboratories). In some experiments, cells were incubated with fluorescently-conjugated asTF for 20 min, before fixation. Images were acquired using a Leica SP5 confocal microscope and a Leica DMI6000B.
Orthotopic Breast Cancer Models and Immunohistochemistry.
Animal experiments were approved by the animal welfare committee of the Leiden University Medical Center (LUMC). Five animals per experimental group were used. The 2A3-3 cells (2 × 106 in 50 μL) were injected into the inguinal fat pad of NOD-SCID mice (Charles River). Tumor growth was measured using calipers and the formula volume = (length × width × width)/2. For MDA-MB-231-mfp growth in vivo, 0.5 × 106 cells were injected in fat pads of NSG mice (Charles River). After completion of the experiment, mice were killed and tumors were extracted and fixed in 4% formalin. Sections were deparaffinized, rehydrated, and endogenous peroxidase activity was blocked with 0.3% H2O2. Antigen retrieval was done in sodium citrate buffer for 10 min at 100 °C. Sections were blocked with 10% normal goat serum in PBS and incubated overnight at 4 °C with primary antibody. Sections were incubated for 30 min with Envision (Dako), visualized using DAB, and counterstained with hematoxylin.
Tissue Microarray Analysis.
A tissue array containing tumor material from 574 nonmetastasized breast cancer patients that mostly underwent tumor resection at the LUMC between 1985 and 1994 (22). Approval was obtained from the LUMC Medical Ethics Committee. Age, tumor grade, histological type, tumor-node-metastasis status, median follow-up (17.9 y), locoregional or distant tumor recurrence, and expression of ER, PgR, and HER2 were known. Tumors were graded according to the current pathological standards. Normal mammary tissue of 266 patients (46%) was available for analysis. Sections were cut and stained for flTF and asTF as described above. The percentage of asTF and flTF positive tumor cells was scored by two blinded observers. Patients in the first quartile were deemed negative.
Statistical Analyses.
Assessment of the associations between flTF/asTF expression and clinical variables were performed using SPSS and Stata. Cohen’s κ coefficient for interobserver agreement was 0.85 and 0.88 for asTF and flTF, respectively. The χ2 test was used to evaluate associations between clinicopathological parameters and asTF/flTF expression. Analysis of in vitro and in vivo experiments was carried out using t tests. Mean and SD are shown in figures, unless stated otherwise. Significant differences in bar graphs are indicated by *P < 0.05, **P = 0.01, and ***P = 0.001.
Supplementary Material
Acknowledgments
We thank Aat van Wijngaarden and Annemarie van Oeveren-Rietdijk for their assistance in ELISA and FACS experiments. This work is supported in part by the Netherlands Organization for Scientific Research VIDI Grant 91710329 (to H.H.V.) and National Institutes of Health/National Cancer Institute Grant CA160293-01A1 (to V.Y.B.)
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE41872).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307100110/-/DCSupplemental.
References
- 1.Anczuków O, et al. The splicing factor SRSF1 regulates apoptosis and proliferation to promote mammary epithelial cell transformation. Nat Struct Mol Biol. 2012;19(2):220–228. doi: 10.1038/nsmb.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karni R, et al. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat Struct Mol Biol. 2007;14(3):185–193. doi: 10.1038/nsmb1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venables JP, et al. Identification of alternative splicing markers for breast cancer. Cancer Res. 2008;68(22):9525–9531. doi: 10.1158/0008-5472.CAN-08-1769. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, et al. Alternative splicing disrupts a nuclear localization signal in spleen tyrosine kinase that is required for invasion suppression in breast cancer. Cancer Res. 2003;63(15):4724–4730. [PubMed] [Google Scholar]
- 5.Courtois S, et al. DeltaN-p53, a natural isoform of p53 lacking the first transactivation domain, counteracts growth suppression by wild-type p53. Oncogene. 2002;21(44):6722–6728. doi: 10.1038/sj.onc.1205874. [DOI] [PubMed] [Google Scholar]
- 6.Okumura N, Yoshida H, Kitagishi Y, Nishimura Y, Matsuda S. Alternative splicings on p53, BRCA1 and PTEN genes involved in breast cancer. Biochem Biophys Res Commun. 2011;413(3):395–399. doi: 10.1016/j.bbrc.2011.08.098. [DOI] [PubMed] [Google Scholar]
- 7.Datta D, et al. Ras-induced modulation of CXCL10 and its receptor splice variant CXCR3-B in MDA-MB-435 and MCF-7 cells: Relevance for the development of human breast cancer. Cancer Res. 2006;66(19):9509–9518. doi: 10.1158/0008-5472.CAN-05-4345. [DOI] [PubMed] [Google Scholar]
- 8.Radisky DC, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436(7047):123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Berg YW, Osanto S, Reitsma PH, Versteeg HH. The relationship between tissue factor and cancer progression: insights from bench and bedside. Blood. 2012;119(4):924–932. doi: 10.1182/blood-2011-06-317685. [DOI] [PubMed] [Google Scholar]
- 10.Dorfleutner A, Hintermann E, Tarui T, Takada Y, Ruf W. Cross-talk of integrin alpha3beta1 and tissue factor in cell migration. Mol Biol Cell. 2004;15(10):4416–4425. doi: 10.1091/mbc.E03-09-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Versteeg HH, et al. Inhibition of tissue factor signaling suppresses tumor growth. Blood. 2008;111(1):190–199. doi: 10.1182/blood-2007-07-101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogdanov VY, et al. Alternatively spliced human tissue factor: A circulating, soluble, thrombogenic protein. Nat Med. 2003;9(4):458–462. doi: 10.1038/nm841. [DOI] [PubMed] [Google Scholar]
- 13.Bogdanov VY, et al. Identification and characterization of murine alternatively spliced tissue factor. J Thromb Haemost. 2006;4(1):158–167. doi: 10.1111/j.1538-7836.2005.01680.x. [DOI] [PubMed] [Google Scholar]
- 14.Haas SL, et al. Expression of tissue factor in pancreatic adenocarcinoma is associated with activation of coagulation. World J Gastroenterol. 2006;12(30):4843–4849. doi: 10.3748/wjg.v12.i30.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobbs JE, et al. Alternatively spliced human tissue factor promotes tumor growth and angiogenesis in a pancreatic cancer tumor model. Thromb Res. 2007;120(Suppl 2):S13–S21. doi: 10.1016/S0049-3848(07)70126-3. [DOI] [PubMed] [Google Scholar]
- 16.van den Berg YW, et al. Alternatively spliced tissue factor induces angiogenesis through integrin ligation. Proc Natl Acad Sci USA. 2009;106(46):19497–19502. doi: 10.1073/pnas.0905325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandradas S, Deikus G, Tardos JG, Bogdanov VY. Antagonistic roles of four SR proteins in the biosynthesis of alternatively spliced tissue factor transcripts in monocytic cells. J Leukoc Biol. 2010;87(1):147–152. doi: 10.1189/jlb.0409252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tardos JG, et al. SR proteins ASF/SF2 and SRp55 participate in tissue factor biosynthesis in human monocytic cells. J Thromb Haemost. 2008;6(5):877–884. doi: 10.1111/j.1538-7836.2008.02946.x. [DOI] [PubMed] [Google Scholar]
- 19.Contrino J, Hair G, Kreutzer DL, Rickles FR. In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease. Nat Med. 1996;2(2):209–215. doi: 10.1038/nm0296-209. [DOI] [PubMed] [Google Scholar]
- 20.Rydén L, et al. Evidence for tissue factor phosphorylation and its correlation with protease-activated receptor expression and the prognosis of primary breast cancer. Int J Cancer. 2010;126(10):2330–2340. doi: 10.1002/ijc.24921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bluff JE, et al. Anti-tissue factor short hairpin RNA inhibits breast cancer growth in vivo. Breast Cancer Res Treat. 2011;128(3):691–701. doi: 10.1007/s10549-010-1149-8. [DOI] [PubMed] [Google Scholar]
- 22.van Nes JG, et al. COX2 expression in prognosis and in prediction to endocrine therapy in early breast cancer patients. Breast Cancer Res Treat. 2011;125(3):671–685. doi: 10.1007/s10549-010-0854-7. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasan R, et al. Splice variants of tissue factor promote monocyte-endothelial interactions by triggering the expression of cell adhesion molecules via integrin-mediated signaling. J Thromb Haemost. 2011;9(10):2087–2096. doi: 10.1111/j.1538-7836.2011.04454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang HY, et al. SRPK2: A differentially expressed SR protein-specific kinase involved in mediating the interaction and localization of pre-mRNA splicing factors in mammalian cells. J Cell Biol. 1998;140(4):737–750. doi: 10.1083/jcb.140.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collier ME, Ettelaie C. Induction of endothelial cell proliferation by recombinant and microparticle-tissue factor involves beta1-integrin and extracellular signal regulated kinase activation. Arterioscler Thromb Vasc Biol. 2010;30(9):1810–1817. doi: 10.1161/ATVBAHA.110.211854. [DOI] [PubMed] [Google Scholar]
- 26.Jessani N, et al. Carcinoma and stromal enzyme activity profiles associated with breast tumor growth in vivo. Proc Natl Acad Sci USA. 2004;101(38):13756–13761. doi: 10.1073/pnas.0404727101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta V, et al. The beta-tail domain (betaTD) regulates physiologic ligand binding to integrin CD11b/CD18. Blood. 2007;109(8):3513–3520. doi: 10.1182/blood-2005-11-056689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong JP, Stehle T, Goodman SL, Arnaout MA. New insights into the structural basis of integrin activation. Blood. 2003;102(4):1155–1159. doi: 10.1182/blood-2003-01-0334. [DOI] [PubMed] [Google Scholar]
- 29.Luque A, et al. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355-425) of the common beta 1 chain. J Biol Chem. 1996;271(19):11067–11075. doi: 10.1074/jbc.271.19.11067. [DOI] [PubMed] [Google Scholar]
- 30.Montanez E, et al. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 2008;22(10):1325–1330. doi: 10.1101/gad.469408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martuszewska D, et al. Tensin3 is a negative regulator of cell migration and all four Tensin family members are downregulated in human kidney cancer. PLoS ONE. 2009;4(2):e4350. doi: 10.1371/journal.pone.0004350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang CS, Shen CY, Wang HW, Wu PE, Cheng CW. Increased expression of SRp40 affecting CD44 splicing is associated with the clinical outcome of lymph node metastasis in human breast cancer. Clin Chim Acta. 2007;384(1-2):69–74. doi: 10.1016/j.cca.2007.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.