Abstract
Mutualisms between species are interactions in which reciprocal exploitation results in outcomes that are mutually beneficial. This reciprocal exploitation is evident in the more than a thousand plant species that are pollinated exclusively by insects specialized to lay their eggs in the flowers they pollinate. By pollinating each flower in which she lays eggs, an insect guarantees that her larval offspring have developing seeds on which to feed, whereas the plant gains a specialized pollinator at the cost of some seeds. These mutualisms are often reciprocally obligate, potentially driving not only ongoing coadaptation but also diversification. The lack of known intermediate stages in most of these mutualisms, however, makes it difficult to understand whether these interactions could have begun to diversify even before they became reciprocally obligate. Experimental studies of the incompletely obligate interactions between woodland star (Lithophragma; Saxifragaceae) plants and their pollinating floral parasites in the moth genus Greya (Prodoxidae) show that, as these lineages have diversified, the moths and plants have evolved in ways that maintain effective oviposition and pollination. Experimental assessment of pollination in divergent species and quantitative evaluation of time-lapse photographic sequences of pollination viewed on surgically manipulated flowers show that various combinations of traits are possible for maintaining the mutualism. The results suggest that at least some forms of mutualism can persist and even diversify when the interaction is not reciprocally obligate.
Keywords: coevolution, geographic divergence, correlated traits, trait matching
Coevolving mutualisms among free-living species often favor the formation of networks of interacting species rather than reciprocally specialized pairs of species (1–3). In contrast, mutualisms between species living in intimate (i.e., symbiotic) association often favor the evolution of greater degrees of reciprocal specialization (3). This contrast is evident in pollination mutualisms: plants and pollinators generally form complex networks, but the most consistent deviations from this pattern are the interactions between plants and the insects that lay eggs in the flowers they pollinate. Pollination by these floral parasites, sometimes called nursery pollination, involves reciprocally obligate interactions between a plant species and either one insect species or a small group of insect species within a lineage, potentially driven by selection imposed on the parasitic component of the interaction. Pollination by floral parasites has arisen and diversified repeatedly among plant lineages that are common components of terrestrial communities worldwide (4–9). Examples include yuccas in North America, figs in tropical environments, globeflowers (Trollius; Ranunculaceae) in boreal ecosystems, and leafflower (Glochidion; Phyllanthaceae) trees in Asia and the Pacific islands (7, 10–12). The pollinators have coradiated with the plants, but often in ways that reflect the complex historical biogeography of these interactions (13, 14).
Intermediate stages in the evolution of these obligate interactions are lacking among living species in most of these mutualisms. That makes it difficult to assess whether these interactions could have begun to diversify even before the interactions became reciprocally obligate for the partners. The interactions between Greya moths (Prodoxidae) and their woodland star (Lithophragma; Saxifragaceae) host plants, however, provide clues to how these mutualisms can diversify even in the absence of, or before, the evolution of reciprocally obligate mutualism. Greya moths are closely related to yucca moths (15), and both of these prodoxid lineages include pollinating floral parasites (16). Yucca moths are the sole pollinators of yucca, and Greya moths are generally the most important pollinators of woodland stars. In some communities, however, insects other than Greya moths, especially solitary bees or bombyliid flies, contribute importantly to the pollination of woodland stars (17–20). Nevertheless, the interaction between woodland stars and Greya moths has persisted for millions of years as the plants and moths have diversified (18, 21).
We studied how the traits involved in the interaction and the potential for mutualism have changed as woodland stars and their Greya moth pollinators have diverged geographically in western North America. Greya moths pollinate at least five woodland star species within two lineages as they oviposit into the flowers (Fig. 1). The woodland star species have diverged in many floral traits, including major changes in the position of the ovary within flowers (22, 23). The species also differ in stigma structure, style length and fusion, petal size and shape, and other aspects of overall floral shape.
Fig. 1.
Divergence in morphology and the interaction between woodland star (Lithophragma spp.) species and their Greya moth pollinators. A window has been surgically cut into the side of each flower to show how pollination occurs during oviposition. As she punctures the floral ovary with her ovipositor, or slides through an opening in the side of the style to lay eggs, a moth simultaneously pollinates the flower with pollen adhering to her abdomen. Flowers vary among species from those having a completely inferior ovary, short style, and a stigma receptive only along the sides (Upper Left) to those having a completely superior ovary, a long style, and a stigma receptive over the upper stigmatic surface (Lower Right). For scale, length of a moth wing averages 6.6–7.7 mm among these populations, and floral width averages 3.0–4.0 mm. (Upper, Left to Right) Plants in the L. parviflorum clade: L. parviflorum North, L. parviflorum South, and L. affine. (Lower, Left to Right) Plants in the L. heterophyllum clade: L. bolanderi, L. cymbalaria, and L. heterophyllum. Moths are all members of the G. politella species complex.
We chose six sites that encompassed the latitudinal range of the interactions between woodland stars and Greya moths. Together the sites included all of the major Lithophragma species involved in the interaction (Table S1). Lithophragma is a well-defined genus of seven to nine species restricted to western North America (24). The species pollinated by Greya moths occur mostly within two clades. The Lithophragma parviflorum clade includes two species with indistinct boundaries, L. parviflorum and Lithophragma affine (24). L. parviflorum is widespread in western North America, whereas L. affine occurs mostly in the Coast Ranges of California and some areas of the central Sierra Nevada. The Lithophragma heterophyllum clade is distributed as a ring around the Central Valley of California, with L. heterophyllum in habitats north, northeast, and west of the valley, Lithophragma cymbalaria southwest of the valley in the Coast Ranges and Transverse Ranges, and Lithophragma bolanderi east and southeast of the valley in the southern Sierra Nevada (Table S1).
Woodland star species are pollinated by moths in the Greya politella species complex, which includes up to four geographically separated, and molecularly divergent, cryptic species (21). The study sites included all four lineages, hereafter labeled G. politella PNW, OR, CA, and SN for Pacific Northwest, southern Oregon, California, and southern Sierra Nevada, respectively. These divergent lineages are separated geographically and generally differ in the Lithophragma species they use as hosts. Most G. politella populations, including all of the populations in this study, visit, pollinate, and lay eggs only in Lithophragma (25).
As in other prodoxid moths that are the major pollinators of their host plants (i.e., yucca moths), the entire life history of G. politella moths is closely associated with the local host plant population. Adults mate on the host plants and take nectar only from flowers of the host plants. When a female inserts her abdomen through the corolla to oviposit, pollen adhering to one or more parts of her abdomen rubs off onto the stigma, thereby pollinating the flower (Fig. 1). Larvae complete their development on a single host individual. Movie S1 shows examples of moths ovipositing into two Lithophragma species.
At three of the study sites (Turnbull, Taylor, and Marble) G. politella was the only Greya species. At the other three sites an inefficient pollinator of Lithophragma, Greya obscura, cooccurred with G. politella. The inefficient pollinator contributes to pollination only during nectaring, because it does not oviposit directly into the flowers (18). G. obscura is normally found only in populations that also have G. politella and may depend on the interaction between G. politella and woodland stars for its local persistence. The study therefore encompassed a broad range of the ecological contexts in which Lithophragma interacts with its major pollinator, G. politella.
For each of the six sites, we assessed morphological divergence and codivergence in the local woodland star and G. politella populations. We then used time-lapse photography on experimentally manipulated flowers to determine how the mechanisms of oviposition and pollination have changed as populations have diverged in traits. In addition, we experimentally evaluated the efficacy of the moths as pollinators in each population.
Results
Multitrait Divergence.
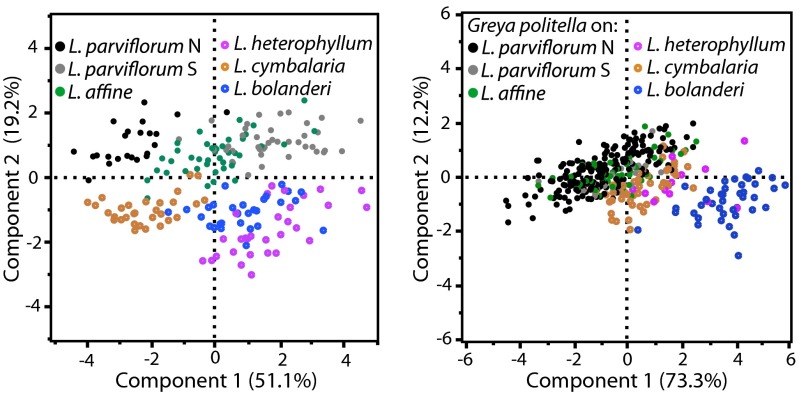
The two Lithophragma lineages differed in traits associated with overall floral length, the size and shape of the stigma and style, and petal width (principal component 2), whereas species within each lineage differed in a highly correlated combination of traits associated with overall size and shape (principal component 1) (Fig. 2). Divergence within the G. politella species complex occurred through a suite of correlated traits affecting overall shape and size along a single principal component axis (Fig. 2). Discriminant analyses indicated that mouthpart (i.e., proboscis) length and ovipositor length showed especially strong divergence among populations, as would be expected from traits associated directly with nectaring and oviposition in flowers (Table S2).
Fig. 2.
Divergence in size and shape among Lithophragma plant populations (Left) and G. politella moth populations (Right) analyzed using principal component analysis. Values in parentheses after the axis labels are the percentage of variance attributable to that principal component axis. n = (plants; moths): L. parviflorum North (36; 200), L. parviflorum South (40; 20), L. affine (42; 48), L. heterophyllum (30; 12), L. cymbalaria (30; 48), L. bolanderi (30; 40).
Codivergence of Traits.
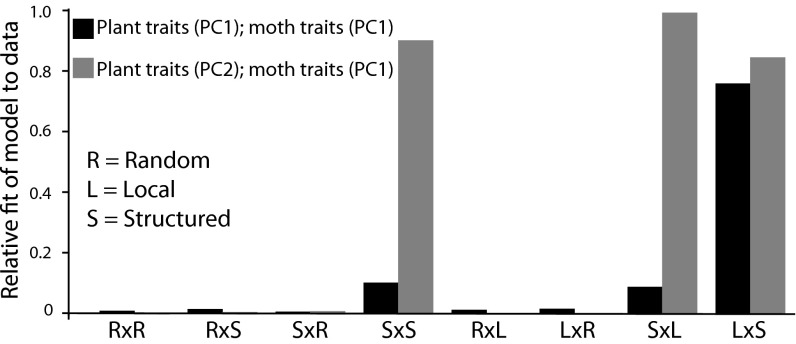
Hierarchical assessment of divergence in plant and moth traits suggested that the codivergence of plant and moth traits is structured geographically and phylogenetically. The models providing the best fit to the data were those taking into account the identity of the local population and the genetic relationships among populations (Fig. 3). Hence, contrasting combinations of trait values have arisen in the two plant lineages and their associated moth lineages, and additional codivergence of plants and moths has arisen among populations within these lineages.
Fig. 3.
Relative accuracy of fit of models to the observed data for geographic covariation of woodland star floral traits and Greya moth traits. The observed data are the pattern of geographic covariation in local plant traits compared with local moth traits (plant traits vs. moth traits). The alternative models include either random assignment of individuals to populations or random assignment of individuals only to populations within the larger clade from which they were drawn (e.g., assignment of L. bolanderi moths to plants within the L. heterophyllum, L. cymbalaria, and L. bolanderi clade). Models based on these assignments are called random, local, and structured. Accuracy is defined here as A = 1 − |(observed − expected)/observed|, in which observed = observed value, expected = mean value of a given model, and |x| is the absolute value of the number. The higher the value of A, the higher the accuracy, scaled to a maximum value of 1.
Functional Morphology.
When visiting a flower, a female first takes nectar, then turns around and oviposits through the corolla. After she has completed the sequence, she usually rests for minutes to hours on that flower. We called each such a sequence a “bout,” and we removed the female after she had completed one bout on a flower. The time-lapse digital sequences of bouts showed that G. politella PNW, OR, and CA moths (n = 28) pierce the ovary through or just above the nectary disk. In contrast, G. politella SN moths (n = 10), which oviposit only into flowers of L. bolanderi, slide the ovipositor through a slit between the uniquely elongated unfused styles of this species (Fig. 1).
We used additional time-lapse sequences to determine how moths probe when presented with a woodland star population with a different floral morphology. We were specifically interested in testing how G. politella SN moths would probe flowers during a bout when presented with the closely related L. cymbalaria, which lacks the wide slit found in its normal host. All of the SN moths (n = 5) attempted to oviposit by searching along the style for a slit between the styles, as they would do when probing their normal host. In the inverse experiment, G. politella CA moths (n = 4) locally adapted to L. cymbalaria were offered L. bolanderi. All of the CA moths oviposited directly through the nectary disk rather than through the slits between the styles.
Populations differed in the moth body parts that touched an anther or the stigma during a bout (Table 1). For example, moths visiting L. bolanderi always touched either the anther or the stigma with the abdomen, whereas moths visiting L. parviflorum N did so only 27% of the time. The probability of a moth part touching the stigma or an anther did not depend on bout duration (i.e., number of photos) (length effect: P = 0.414, χ2 = 0.67, df = 1, logistic regression including effects of population and duration). Despite the differences among populations in plant morphology and in moth morphology and behavior, moths visiting plants from their local population touched either an anther or the stigma during 82–100% of bouts in all populations. The percentages did not differ significantly among populations (Table S3). Hence, different combinations of plant and moth traits all resulted in a high probability that a moth would contact the floral reproductive parts.
Table 1.
Differences among sites in the probability of contact between moth body parts and either the stigma or an anther during nectaring and oviposition obtained through time-lapse photography of surgically modified flowers
| Parameter | Percentage of bouts resulting in contact |
|||
| Proboscis | Head | Abdomen | Membrane | |
| Lithophragma | ||||
| parviflorum N | 45.5 | 0.0 | 27.3 | 72.7 |
| parviflorum S | 66.7 | 11.1 | 88.9 | 66.7 |
| affine | 100.0 | 0.0 | 70.0 | 90.0 |
| bolanderi | 69.2 | 0.0 | 100.0 | 84.6 |
| cymbalaria | 27.3 | 0.0 | 54.6 | 54.6 |
| χ2 (df = 4) | 17.03 | 6.32 | 17.55 | 5.80 |
| P | 0.002 | 0.18 | 0.002 | 0.208 |
“Membrane” refers to the membrane by which the ovipositor is attached to the terminal segment of the abdomen. Trials with L. heterophyllum were insufficient to include in the analysis. Number of scored images and bouts: L. parviflorum N (1056, 11), L. parviflorum S (886, 9), L. affine (1523, 10), L. bolanderi (1344, 13), L. cymbalaria (2782, 22). Statistical differences among populations were evaluated using χ2 contingency analyses.
Pollination Efficacy.
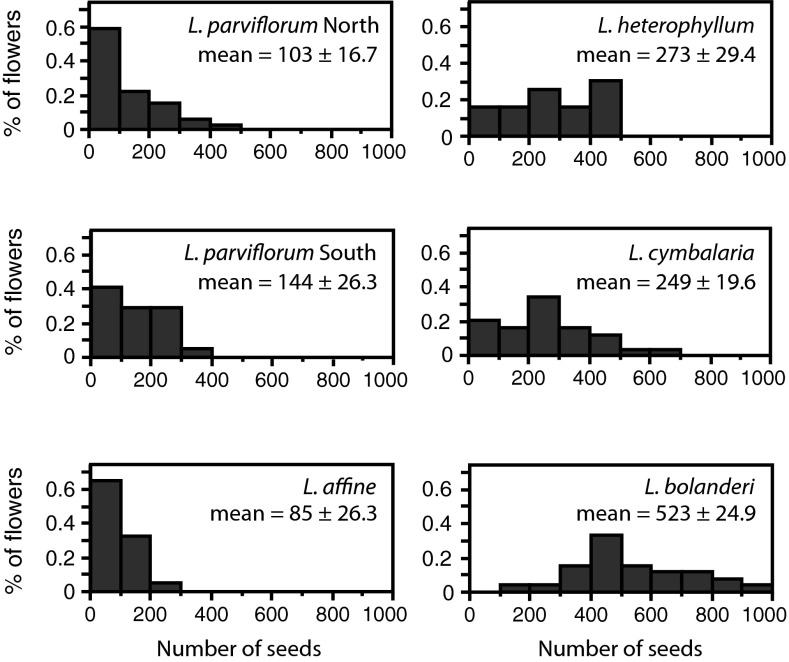
Even with these different oviposition behaviors and interactions between plant and moth body parts, every moth population was an effective pollinator of its local Lithophragma population. The mean number of developing seeds resulting from a single bout, however, differed significantly among populations, ranging from 85 seeds for L. affine to 523 for L. bolanderi (Fig. 4). Moreover, a pollination bout resulted in significantly more developing seeds in plants from the L. heterophyllum clade than in plants from the L. parviflorum clade (Fig. 4; P < 0.001 for clade effect, generalized linear model; Table S4).
Fig. 4.
Distribution of the number of developing seeds after a single pollination/oviposition bout by a moth in the G. politella species complex. (Left) Three populations in the L. parviflorum clade. (Right) Three populations in the L. heterophyllum clade. Values are means ± 1 SE. n = 62 flowers for L. parviflorum N, 25 for L. parviflorum S, 25 for L. affine, 20 for L. heterophyllum, 45 for L. cymbalaria, and 28 for L. bolanderi.
The distribution of the number of seeds resulting from a bout also differed among populations and clades (Fig. 4). These distributions are, in effect, the distributions of ecological outcome of the interaction from the perspective of plant fitness. The distributions were strongly skewed toward a smaller number of seeds in the L. parviflorum/affine lineage, but not in the other lineage. To the extent that the number of seeds is an index of fitness, then, the potential strength of the mutualism differs between these lineages, but the moths are effective pollinators of both lineages.
Analysis of generalized linear models suggested that the differences among populations in the number of developing seeds were due primarily to the interaction of plant and moth traits (i.e., significant clade and site effects) rather than to other factors (Table S4). The total number of ovules available for pollination also contributed a significant, but smaller, effect. In all populations, however, a single bout resulted in pollination of less than two-thirds of ovules in all populations. Hence, in no population was seed set in the moth pollination trials limited by the number of available ovules. The duration of a bout, pollen load on a moth, and the number of eggs deposited during oviposition did not significantly affect the number of developing seeds. There were no significant interaction effects among the factors (Table S4).
In separate experiments, hand pollination of flowers reinforced the conclusion that the number of seeds produced per flower in the moth pollination trials was determined primarily by pollination efficacy rather than by factors extrinsic to the plant–moth interaction (Table S5). Selfed flowers produced on average five or fewer seeds in all populations (n = 165 crosses). Flowers outcrossed by hand to other individuals within the same population produced on average more than 100 more seeds than moth-pollinated flowers (n = 166 crosses). Hence, flowers of all populations were capable of developing more seeds on average than the number produced during a single moth visit. The ratio of the number of seeds resulting from a moth pollination bout compared with hand pollination of outcrossed plants was 38% in L. parviflorum North, 56% in L. parviflorum South, 27% in L. affine, 76% in L. bolanderi, 48% in L. cymbalaria, and 37% in L. heterophyllum (Table S5).
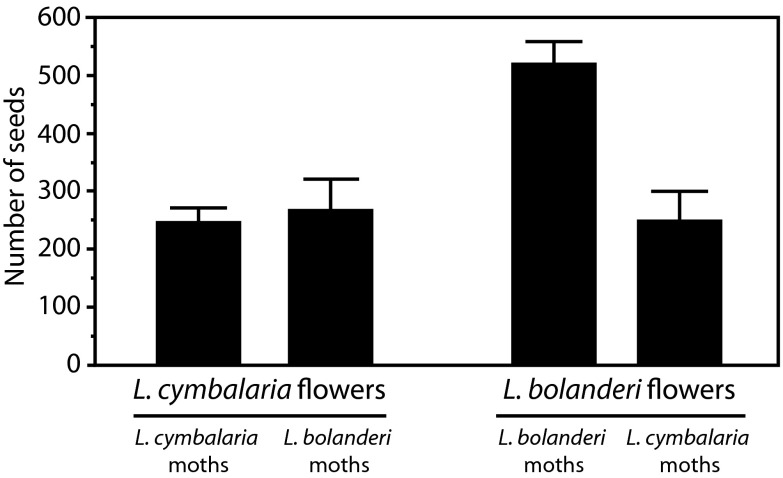
We further analyzed how moth and plant traits affect pollination efficacy by allowing moths from the L. cymbalaria-feeding population and the L. bolanderi-feeding population to oviposit into flowers from the other population. Although these two plant species are phylogenetically close relatives, the functional morphology trials had shown that moths associated with these two species differ in where they insert the ovipositor within a flower, and the pollination efficacy experiments had shown that these two species differ in pollination efficacy. G. politella SN moths from the L. bolanderi population pollinated L. cymbalaria plants as effectively as moths from the L. cymbalaria population, but moths from the L. cymbalaria population were poorer pollinators of L. bolanderi than L. bolanderi moths (Fig. 5). Hence, the evolution of an unusual oviposition behavior in the G. politella SN population has not resulted in specialization so strong that it would prevent it from being an effective pollinator of a Lithophragma population with a different combination of floral traits.
Fig. 5.
Pollination efficacy of G. politella moths pollinating their local woodland star species compared with a woodland star species from a different locality. Values show a significant interaction effect of moth plant × plant population (Likelihood ratio χ21,99 = 6.01 P = 0.014, generalized linear model). Values are means ± 1 SE. Number of trials = 45 L. cymbalaria moths on L. cymbalaria plants; 11 L. cymbalaria moths on L. bolanderi plants; 28 L. bolanderi moths on L. bolanderi plants; 19 L. bolanderi moths on L. cymbalaria plants.
Discussion
The combined analyses of morphology, time-lapse photographic sequences, and pollination efficacy experiments suggest that the interactions between woodland star plants and Greya moths have retained the potential for mutualism even as populations have diverged in morphology, the moths have diverged in behavior, and the taxa have undergone speciation. This complex pollination mutualism has persisted and diversified over millions of years even without the interaction becoming reciprocally obligate in these two genera.
The view that these interactions are robust is further reinforced by ecological studies showing that Greya moths are the major, and sometimes almost the sole, pollinator in most populations that have been studied in detail, even though the interaction is swamped in some localities by unrelated copollinators (18, 20, 26). The results therefore provide insight into how the early stages of mutualisms between plants and pollinating floral parasites can evolve and persist. These mutualisms can remain robust even as populations diverge in suites of correlated traits and as some plant populations continue to be pollinated at least sometimes by other taxa.
Prodoxid moths have evolved relationships with plants that range in ecological outcome from parasitism to mutualism (25). Most species are parasitic as larvae on floral, leaf, or stem tissues. The two lineages that have evolved pollination mutualisms with plants, Greya moths and yucca moths, include a combination of parasitic and mutualistic species. Mutualistic species have evolved from parasitic species, and parasitic species have evolved from mutualistic species. Locally parasitic or weakly mutualistic species, however, do not necessarily compromise these mutualisms. Within Greya, relatively inefficient G. obscura moths cooccur and contribute to pollination at some sites along with the more efficient G. politella moths (18). Within the yucca moths, some nonpollinating cheater species have evolved from mutualistic species (27, 28), but the cheater moths persist only where the mutualistic moth species are present to pollinate the flowers. Hence, although some local interactions between these plant and moth lineages may include species that are poor mutualists or even parasites, the pollination mutualism has persisted as the lineages have continued to diversify.
There is, however, nothing inevitable about the evolution of mutualism once plants have been colonized by floral parasites. Parasitism of flowers or seeds is common among insects, but many plant linages have flowers poorly suited to pollination by ovipositing insects (25). Interactions between some plant and insect lineages, such as Silene plants and Hadena moths, seem to remain at the interface between parasitism and mutualism; however, like Lithophragma and Greya, they may possibly vary in ecological outcome among populations (4, 29). It is likely, though, that more plants are pollinated by floral parasites than is currently known. For example, only within the past decade has pollination by insects during oviposition been discovered in the Phyllanthaceae in the tropical Pacific, involving possibly hundreds of plant species, and discovery of similar pollination mutualisms within this and other plant families continues (6, 11, 30, 31).
The matching of floral and pollinator traits has long been considered evidence for coevolution and illustrative of the intricate, and potentially fragile, mutualistic relationships between species (32–36). Plant species often have pollinators whose body parts closely complement the flowers they visit, resulting in effective pollination (37–40). Evolution of the presence, size, or shape of some characters can alter pollination efficacy by creating mismatches in traits (41–44). The results for woodland stars and Greya moths, however, indicate that there is a wide range of trait combinations that can sustain mutualisms in codiverging lineages. No simple pattern of matching of plant and moth traits is required to maintain the mutualism, even though some local interactions result in greater pollination efficiency than others. In addition to the morphological traits assessed here, woodland stars also produce a complex array of floral volatiles that vary among species and populations (45). Hence, the number of potentially coevolving and geographically varying traits is even greater than those assessed here.
More generally, these results suggest that mutualisms between species may have become so pervasive worldwide because they often show considerable evolutionary flexibility rather than fragility. Divergence of all coevolving species is undoubtedly driven by multiple selection pressures and also by other evolutionary processes, such as genetic drift acting from time to time on traits in some small populations. The interplay of many evolutionary processes during population divergence and speciation, however, does not necessarily undermine the ability of coevolutionary selection to maintain mutualisms between pairs of species even when those interactions are not reciprocally obligate. Once formed, these mutualisms may often be malleable in the trait combinations that allow the maintenance of the mutualism. Any fitness advantage gained through interacting with another species can be favored by selection, even if the traits do not match optimally. In that sense, it is no wonder that all eukaryotic species have evolved mutualisms with other taxa.
Materials and Methods
Multitrait Divergence.
Floral measurements included petal length, petal width, overall flower length, floral width, internal floral depth, floral flair, stigma diameter, and pistil height above the nectary disk (Fig. S1). Moth measurements included wingspan, proboscis length, sixth abdominal segment length, seventh abdominal segment length, and ovipositor length. On the basis of preliminary studies, these were the traits that, directly or indirectly, were likely to affect pollination. The abdominal segments were included because, in G. politella females, the seventh segment is two to three times longer than other abdominal segments. This unusual abdominal elongation is not found in G. politella males or in other Greya species. Floral and moth measurements were made on individuals collected at the study sites. Floral measurements were on the second flower produced on each plant. Morphology was analyzed using principal component analysis on the correlations among traits, with principal component 1 capturing primarily variation in size and overall shape and principal component 2 capturing primarily variation in components of shape orthogonal to principal component 1.
Codivergence of Traits.
We assessed codivergence of traits initially by comparing plant and moth principal component values. We then grouped plant and moth individuals hierarchically according to different assumptions to assess the combined contributions of local trait matching and larger phylogenetic effects on the observed patterns. We began by combining all individuals into one plant population and one moth population. We then hierarchically grouped individuals according to the known patterns of genetic divergence among the sampled populations: three plant species in each of the two lineages, and four cryptic moth species within the G. politella species complex. Using randomization and model selection procedures based on likelihood approximations, we assessed whether a grouping of plant and/or moth populations based on patterns of genetic divergence explained the data better than a completely randomized distribution of traits among populations. The full randomization sampled all individuals without replacement and randomly assigned them among populations. The structured randomization constrained assignments based on genetic relatedness among populations. The models evaluated variation in the values of the first principal component of the moth traits relative to either the first or second principal component of the plant traits. Collectively, the models analyzed how much genetic structuring of populations beyond a random model was needed to approach the observed pattern of plant and moth trait combinations in the data (further details in SI Materials and Methods).
Functional Morphology.
Assessment of how pollination occurs during oviposition was made using time-lapse photography on flowers into which a window had been cut into the side of the calyx (Fig. 1). Females, which are unusually docile for moths, were gently placed on a flower and allowed to nectar and oviposit. Nectaring almost invariably precedes oviposition, and a single sequence of nectaring and oviposition (hereafter bout) by a moth takes up to an hour. Each digital image was analyzed to determine which moth part (proboscis, head, side of the abdomen, terminal abdominal membrane) touched which plant part (stigma, anthers) during the entire bout. Terminal abdominal membrane refers to the membrane by which the ovipositor is attached to the terminal abdominal segment. This membrane expands beyond the terminal abdominal segment when a moth extends its ovipositor. The final analysis included 7,591 scorable frames distributed over 65 bouts. Scorable frames were those in which it was possible to determine with certainty whether a female touched an anther or the stigma.
Pollination Efficacy.
Pollination efficacy was assessed in the laboratory by placing a female moth directly on a previously unvisited flower in a mesh cage (1 m in height and width and 0.5 m in depth). After she contacted the petals, the moth would either rest for some time or immediately lower her head to begin nectaring. Each female was observed throughout the nectaring and oviposition bout (hereafter bout) to make certain that the trial assessed the effect on seed set of a single time that a female inserted her abdomen into the flower. As soon as she withdrew her abdomen from the flower, the trial ended. The entire procedure was then repeated using the same female but on a new flower of a plant from a different maternal lineage of the same plant population. Only virgin flowers receiving newly collected pollen were used in the assessment of pollination efficacy. Separate experiments used hand-pollination of flowers to test for self-compatibility and maximum potential seed set. Emasculated flowers were pollinated either with anthers from a different flower on the same plant or anthers from three different plants of the same population. More details in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Catherine Fernandez, Sarah Dwiggins, Aliya Ingersoll, Anna-Liisa Laine, Anna Nordén, Galen Pelzmann, Bridget Piculell, Katherine Rich, Lindsey Roark, Jill Thompson, and Jim Velzy for help. This work was supported by National Science Foundation Grant DEB-0839853.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307451110/-/DCSupplemental.
References
- 1.Bascompte J, Jordano P. Plant-animal mutualistic networks: The architecture of biodiversity. Annu Rev Ecol Evol Syst. 2007;38:567–593. [Google Scholar]
- 2.Jordano P, Bascompte J, Olesen JM. Invariant properties in coevolutionary networks of plant-animal interactions. Ecol Lett. 2003;6(1):69–81. [Google Scholar]
- 3.Thompson JN. The Geographic Mosaic of Coevolution. Chicago: Univ of Chicago Press; 2005. [Google Scholar]
- 4.Reynolds RJ, Kula AAR, Fenster CB, Dudash MR. Variable nursery pollinator importance and its effect on plant reproductive success. Oecologia. 2012;168(2):439–448. doi: 10.1007/s00442-011-2095-9. [DOI] [PubMed] [Google Scholar]
- 5.Godsoe W, Yoder JB, Smith CI, Pellmyr O. Coevolution and divergence in the Joshua tree/yucca moth mutualism. Am Nat. 2008;171(6):816–823. doi: 10.1086/587757. [DOI] [PubMed] [Google Scholar]
- 6.Kawakita A. Evolution of obligate pollination mutualism in the tribe Phyllantheae (Phyllanthaceae) Plant Sp Biol. 2010;25(1):3–19. [Google Scholar]
- 7.Ibanez S, Dujardin G, Després L. Stability of floral specialization in Trollius europaeus in contrasting ecological environments. J Evol Biol. 2009;22(6):1183–1192. doi: 10.1111/j.1420-9101.2009.01731.x. [DOI] [PubMed] [Google Scholar]
- 8.Horn K, Holland JN. Discrimination among floral resources by an obligately pollinating seed-eating moth: Host-marking signals and pollination and florivory cues. Evol Ecol Res. 2010;12(1):119–129. [Google Scholar]
- 9.Compton SG, et al. Ancient fig wasps indicate at least 34 Myr of stasis in their mutualism with fig trees. Biol Lett. 2010;6(6):838–842. doi: 10.1098/rsbl.2010.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herre EA, Jander KC, Machado CA. Evolutionary ecology of figs and their associates: Recent progress and outstanding puzzles. Annu Rev Ecol Evol Syst. 2008;39:439–458. [Google Scholar]
- 11.Hembry DH, Okamoto T, Gillespie RG. Repeated colonization of remote islands by specialized mutualists. Biol Lett. 2012;8(2):258–261. doi: 10.1098/rsbl.2011.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pellmyr O, Segraves KA, Althoff DM, Balcázar-Lara M, Leebens-Mack J. The phylogeny of yuccas. Mol Phylogenet Evol. 2007;43(2):493–501. doi: 10.1016/j.ympev.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Althoff DM, Segraves KA, Smith CI, Leebens-Mack J, Pellmyr O. Geographic isolation trumps coevolution as a driver of yucca and yucca moth diversification. Mol Phylogenet Evol. 2012;62(3):898–906. doi: 10.1016/j.ympev.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Compton SG, Liu M, Chen X-Y. Fig trees at the northern limit of their range: The distributions of cryptic pollinators indicate multiple glacial refugia. Mol Ecol. 2012;21(7):1687–1701. doi: 10.1111/j.1365-294X.2012.05491.x. [DOI] [PubMed] [Google Scholar]
- 15.Davis DR, Pellmyr O, Thompson JN. Biology and systematics of Greya Busck and Tetragma, new genus (Lepidoptera: Prodoxidae) Smithson Contrib Zool. 1992;524:1–88. [Google Scholar]
- 16.Pellmyr O, Thompson JN. Multiple occurrences of mutualism in the yucca moth lineage. Proc Natl Acad Sci USA. 1992;89(7):2927–2929. doi: 10.1073/pnas.89.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson JN, Pellmyr O. Mutualism with pollinating seed parasites amid co-pollinators: constraints on specialization. Ecology. 1992;73(5):1780–1791. [Google Scholar]
- 18.Thompson JN, Laine AL, Thompson JF. Retention of mutualism in a geographically diverging interaction. Ecol Lett. 2010;13(11):1368–1377. doi: 10.1111/j.1461-0248.2010.01529.x. [DOI] [PubMed] [Google Scholar]
- 19.Cuautle M, Thompson JN. Evaluating the co-pollinator network structure of two sympatric Lithophragma species with different morphology. Oecologia. 2010;162(1):71–80. doi: 10.1007/s00442-009-1424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson JN, Cunningham BM. Geographic structure and dynamics of coevolutionary selection. Nature. 2002;417(6890):735–738. doi: 10.1038/nature00810. [DOI] [PubMed] [Google Scholar]
- 21.Rich KA, Thompson JN, Fernandez CC. Diverse historical processes shape deep phylogeographic divergence in a pollinating seed parasite. Mol Ecol. 2008;17(10):2430–2448. doi: 10.1111/j.1365-294X.2008.03754.x. [DOI] [PubMed] [Google Scholar]
- 22.Soltis DE, Hufford L. Ovary position diversity in Saxifragaceae: Clarifying the homology of epigyny. Int J Plant Sci. 2002;163(2):277–293. [Google Scholar]
- 23.Kuzoff RK, Hufford L, Soltis DE. Structural homology and developmental transformations associated with ovary diversification in Lithophragma (Saxifragaceae) Am J Bot. 2001;88(2):196–205. [PubMed] [Google Scholar]
- 24.Kuzoff RK, Soltis DE, Hufford L, Soltis PS. Phylogenetic relationships within Lithophragma (Saxifragaceae): Hybridization, allopolyploidy and ovary diversification. Syst Bot. 1999;24(4):598–615. [Google Scholar]
- 25.Thompson JN. In: In Search of the Causes of Evolution: From Field Observations to Mechanisms. Grant PR, Grant BR, editors. Princeton: Princeton Univ Press; 2010. pp. 228–245. [Google Scholar]
- 26.Thompson JN, Fernandez CC. Temporal dynamics of antagonism and mutualism in a geographically variable plant-insect interaction. Ecology. 2006;87(1):103–112. doi: 10.1890/05-0123. [DOI] [PubMed] [Google Scholar]
- 27.Segraves KA, Althoff DM, Pellmyr O. Limiting cheaters in mutualism: Evidence from hybridization between mutualist and cheater yucca moths. Proc Biol Sci. 2005;272(1577):2195–2201. doi: 10.1098/rspb.2005.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellmyr O, Balcázar-Lara M, Segraves KA, Althoff DM, Littlefleld RJ. Phylogeny of the pollinating yucca moths, with revision of Mexican species (Tegeticula and Parategeticula; Lepidoptera, Prodoxidae) Zool J Linn Soc. 2008;152(2):297–314. [Google Scholar]
- 29.Labouche A-M, Bernasconi G. Cost limitation through constained oviposition in a plant-pollinator/seed predator mutualism. Funct Ecol. 2013;27(2):509–521. [Google Scholar]
- 30.Kato M, Takimura A, Kawakita A. An obligate pollination mutualism and reciprocal diversification in the tree genus Glochidion (Euphorbiaceae) Proc Natl Acad Sci USA. 2003;100(9):5264–5267. doi: 10.1073/pnas.0837153100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishida C, Kono M, Sakai S. A new pollination system: Brood-site pollination by flower bugs in Macaranga (Euphorbiaceae) Ann Bot (Lond) 2009;103(1):39–44. doi: 10.1093/aob/mcn212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darwin C. On the Various Contrivances by Which British and Foreign Orchids Are Fertilised by Insects, and the Good Effects of Intercrossing. London: John Murray; 1862. [PMC free article] [PubMed] [Google Scholar]
- 33.Herrera CM. Multiplicity in Unity: Plant Subindividual Variation and Interactions with Animals. Chicago: Univ of Chicago Press; 2009. [Google Scholar]
- 34.Gómez JM, Perfectti F, Bosch J, Camacho JPM. A geographic selection mosaic in a generalized plant–pollinator–herbivore system. Ecol Monogr. 2009;79(2):245–263. [Google Scholar]
- 35.Anderson B, Terblanche JS, Ellis AG. Predictable patterns of trait mismatches between interacting plants and insects. BMC Evol Biol. 2010;10:204. doi: 10.1186/1471-2148-10-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pauw A, Stofberg J, Waterman RJ. Flies and flowers in Darwin’s race. Evolution. 2009;63(1):268–279. doi: 10.1111/j.1558-5646.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- 37.Agosta SJ, Janzen DH. Body size distributions of large Costa Rican dry forest moths and the underlying relationship between plant and pollinator morphology. Oikos. 2005;108(1):183–193. [Google Scholar]
- 38.Temeles EJ, Kress WJ. Adaptation in a plant-hummingbird association. Science. 2003;300(5619):630–633. doi: 10.1126/science.1080003. [DOI] [PubMed] [Google Scholar]
- 39.Anders Nilsson L. Deep flowers for long tongues. Trends Ecol Evol. 1998;13(7):259–260. doi: 10.1016/s0169-5347(98)01359-7. [DOI] [PubMed] [Google Scholar]
- 40.Hargreaves AL, Harder LD, Johnson SD. Floral traits mediate the vulnerability of aloes to pollen theft and inefficient pollination by bees. Ann Bot (Lond) 2012;109(4):761–772. doi: 10.1093/aob/mcr324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muchhala N. Adaptive trade-off in floral morphology mediates specialization for flowers pollinated by bats and hummingbirds. Am Nat. 2007;169(4):494–504. doi: 10.1086/512047. [DOI] [PubMed] [Google Scholar]
- 42.Irwin RE, Adler LS. Correlations among traits associated with herbivore resistance and pollination: implications for pollination and nectar robbing in a distylous plant. Am J Bot. 2006;93(1):64–72. [Google Scholar]
- 43.Gómez JM, Perfectti F, Camacho JPM. Natural selection on Erysimum mediohispanicum flower shape: Insights into the evolution of zygomorphy. Am Nat. 2006;168(4):531–545. doi: 10.1086/507048. [DOI] [PubMed] [Google Scholar]
- 44.Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. Pollination syndromes and floral specialization. Annu Rev Ecol Syst. 2004;35:375–403. [Google Scholar]
- 45.Friberg M, Schwind C, Raguso RA, Thompson JN. Extreme divergence in floral scent among woodland star species (Lithophragma spp.) pollinated by floral parasites. Ann Bot (Lond) 2013;111(4):539–550. doi: 10.1093/aob/mct007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.