Abstract
Toll-like receptors (TLRs) play key roles in detecting pathogens and initiating inflammatory responses that, subsequently, prime specific adaptive responses. Several mechanisms control TLR activity to avoid excessive inflammation and consequent immunopathology, including the anti-inflammatory cytokine IL-10. Recently, several TLR-responsive microRNAs (miRs) have also been proposed as potential regulators of this signaling pathway, but their functional role during the inflammatory response still is incompletely understood. In this study, we report that, after LPS engagement, monocytes up-regulate miR-146b via an IL-10–mediated STAT3-dependent loop. We show evidence that miR-146b modulates the TLR4 signaling pathway by direct targeting of multiple elements, including the LPS receptor TLR4 and the key adaptor/signaling proteins myeloid differentiation primary response (MyD88), interleukin-1 receptor-associated kinase 1 (IRAK-1), and TNF receptor-associated factor 6 (TRAF6). Furthermore, we demonstrate that the enforced expression of miR-146b in human monocytes led to a significant reduction in the LPS-dependent production of several proinflammatory cytokines and chemokines, including IL-6, TNF-α, IL-8, CCL3, CCL2, CCL7, and CXCL10. Our results thus identify miR-146b as an IL-10–responsive miR with an anti-inflammatory activity based on multiple targeting of components of the TLR4 pathway in monocytes and candidate miR-146b as a molecular effector of the IL-10 anti-inflammatory activity.
Toll-like receptors (TLRs) have important roles in detecting pathogens and initiating inflammatory responses that, subsequently, prime specific adaptive immune responses during infection (1). It is therefore important that TLR signaling pathways are tightly regulated. One of the most effective suppressor of TLR-induced inflammatory cytokine production is IL-10, which displays powerful inhibitory actions on innate immune cells (2,3), not only by direct inhibition of cytokine transcription (4,5) but also by destabilizing their coding RNA (6) and blocking their translation (7). MicroRNAs (miRs) are small (22–24 nt) noncoding RNA sequences acting primarily as translational repressors of gene transcripts by interacting with their 3′ UTRs (8,9). In the field of inflammation, miRs are attracting increasingly interest for their ability to regulate strength and timing of TLR responses (10). Although their role in the resolution of inflammation is just beginning to be explored, their emerging importance in the modulation of TLR signaling strongly suggests a possible regulation of their expression by anti-inflammatory stimuli. In this respect, IL-10 has been recently shown to inhibit miR-155 induction by TLRs (11), thus increasing the expression of the miR-155 target gene SH2 domain-containing inositol-5′-phosphatase 1 (SHIP1) and promoting expression of anti-inflammatory genes (12). Moreover, a direct role of miR-187 in IL-10–mediated suppression of proinflammatory cytokines has been recently demonstrated (13), and miR-21 has been reported to promote an anti-inflammatory response by increasing IL-10 production through the down-regulation of programmed cell death 4 (PDCD4) (14). We here report that LPS induces expression of miR-146b via an IL-10–dependent loop, and demonstrate that miR-146b play an anti-inflammatory role in monocytes by direct targeting multiple elements involved in the TLR4 signaling pathway, thus making this miR a candidate feedback modulator of the LPS response potentially involved in inflammation resolution.
Results
MiR-146b Expression Is Induced by IL-10 in Human Monocytes.
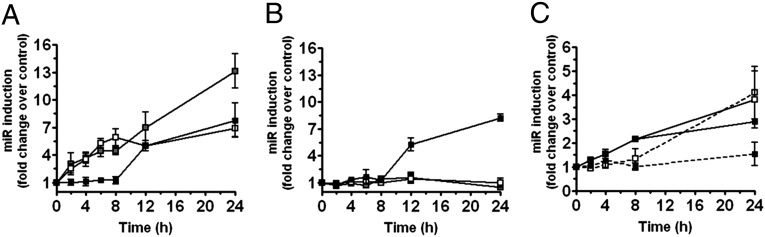
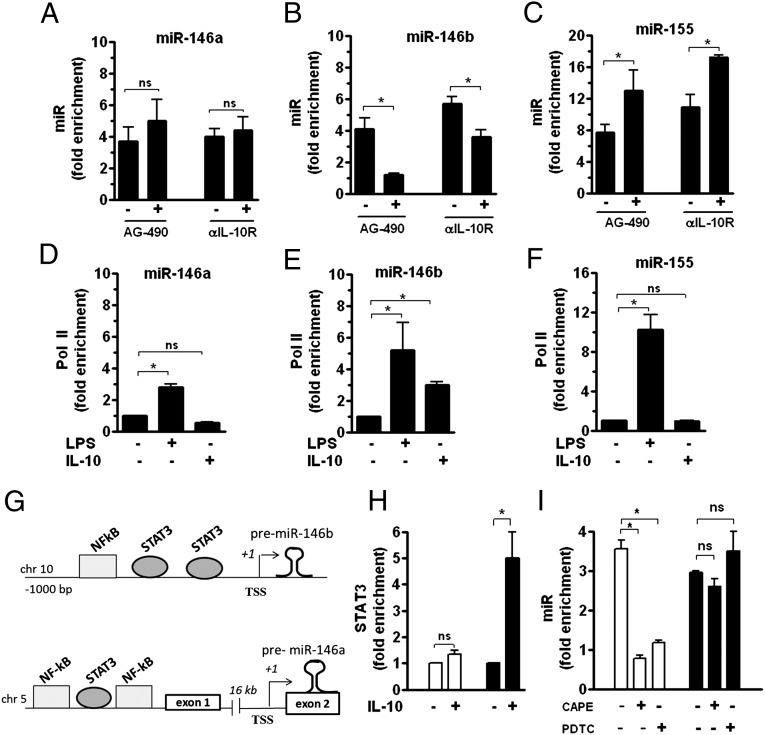
TLRs have been shown to regulate a distinct panel of miR in monocytes, including miR-155 and miR-146a (15–17). We have here identified miR-146b, a second member of the miR-146 family located within an intergenic region on chromosome 10, as an LPS-responsive miR induced at a later time point compared with miR-146a and miR-155 (Fig. 1A). MiR-146b induction by LPS was also mirrored by its enrichment in the RNA-induced silencing complex (RISC), suggesting its functional role in human primary monocytes (Fig. S1A). Analysis of miR-146b expression in monocytes stimulated with IL-1β and different TLR agonists, including the TLR2 agonist palmitoyl-3-cysteine-serine-lysine 4 (Pam3CsK4), the TLR3 agonist poly(I:C), the TLR7 agonist imiquimod, and the TLR9 agonist synthetic CpG oligonucleotides (ODN), showed that miR-146b induction is restricted to the signaling pathway activated by IL-1β and TLR2/TLR4 (Fig. S2A). As the ability of different stimuli to induce miR-146b directly correlates with their ability to induce IL-10 production (Fig. S2B), we asked whether IL-10 could be involved in the induction of miR-146b by LPS. As shown in Fig. 1B, IL-10 stimulation induced miR-146b but was unable to induce expression of miR-146a and miR-155, and suppressed the LPS-dependent induction of miR-155, as previously reported (11). Consistent with these results, the inhibition of the LPS-induced endogenous IL-10 by using an anti–IL-10 receptor blocking monoclonal antibody or the JAK/STAT inhibitor AG-490 (18) resulted in a significant reduction of miR-146b induction by LPS, whereas miR-146a expression was not affected and miR-155 levels were further increased (Fig. 2 A–C). Finally, the LPS-dependent induction of miR-146b observed in murine bone marrow-derived macrophages was severely reduced when macrophages were obtained from IL-10−/− animals, indicating that, also in the murine system, miR-146b is induced by LPS via an IL-10–dependent feedback loop. Conversely, miR-146a induction by LPS was not significantly different in WT and IL-10−/− macrophages, further demonstrating the IL-10 dependency of miR-146b and not miR-146a (Fig. 1C).
Fig. 1.
IL-10 induces miR-146b expression. Expression levels of miR-146a (white symbols), miR-146b (black symbols), and miR-155 (gray symbols) were measured by qPCR in triplicate samples of human monocytes cultured for the indicated times with 100 ng/mL LPS (A) or with 20 ng/mL IL-10 (B). Results are expressed as fold change vs. untreated cells (mean ± SEM; n = 3). (C) Bone marrow-derived macrophages from WT (solid line) or IL-10−/− mice (dashed line) were stimulated or not stimulated for the indicated time with 100 ng/mL LPS, and expression of miR-146b (closed symbols) and miR-146a (open symbols) was quantified by qPCR in triplicate samples. Results expressed as fold change vs. untreated cells (mean ± SEM; n = 3).
Fig. 2.
MiR-146b induction after LPS challenge is driven by IL-10. (A–C) Human monocytes were pretreated or not pretreated for 30 min with 5 µM of the JAK/STAT inhibitor AG-490 and then stimulated for 12 h with 100 ng/mL LPS. Alternatively, monocytes stimulated for 12 h with 100 ng/mL LPS were cultured in the presence of 10 µg/mL anti-IL-10 receptor (-αIL-10R) or isotype control mAb. MiR levels were measured by qPCR in triplicate samples and results expressed as fold change vs. control (mean ± SEM; n = 3). ChIP assays were carried out by using anti-Pol II Ab and analyzed by qPCR with specific primers binding to the miR-146b, miR-146a, and miR-155 promoters (D, E, and F, respectively). Data from qPCR have been normalized to input DNA and displayed as fold change vs. untreated cells (mean ± SEM; n = 3). (G) Graphical representation of predicted promoter regions reporting binding sites of transcription factors of potential interest. (H) Monocytes were stimulated or not stimulated for 4 h with 20 ng/mL IL-10. ChIP assays were carried out by using anti-STAT3 Ab and analyzed by qPCR with specific primers binding to miR-146a (white columns) and miR-146b (black columns) promoters. (I) Cells were pretreated for 1 h with the NF-κB inhibitors PDTC (1 µM) or CAPE (2 µM) and then stimulated for 12 h with 100 ng/mL LPS. MiR-146a (white columns) and miR-146b (black columns) expression levels were measured by qPCR in triplicate samples and results expressed as fold change vs. control (mean ± SEM; n = 3).
The human mature miR-146b is generated by processing of the premiR-146b molecule transcribed from an intergenic region on chromosome 10, with the predicted transcription start site located 700 bp upstream of the mature miR-146b sequence (15). To gain additional insight into the role of IL-10 in the transcriptional regulation of miR-146b in monocytes, the recruitment of polymerase II (Pol II) to the miR-146b promoter region in the presence of LPS or IL-10 was investigated. As expected, ChIP analysis indicated that, in LPS-stimulated monocytes, Pol II was recruited to the miR-146b promoter, as well as to the miR-146a and miR-155 promoters (Fig. 2 D–F). Conversely, in monocytes stimulated with IL-10, Pol II recruitment was observed only on the promoter region of miR-146b and not miR-146a or miR-155, consistent with the selective IL-10–mediated up-regulation of miR-146b expression (Fig. 2 D–F). To identify putative cis-regulatory elements critical for IL-10–dependent gene transcription, a comparative bioinformatic analysis covering 1,000 bp upstream of the premiR-146b coding region was performed. Conserved putative binding sites for STAT3, the main transcription factor mediating the IL-10 anti-inflammatory action in monocytes (19), were predicted on both miR-146a and miR-146b promoter regions (Fig. 2G); however, ChIP analysis on monocytes stimulated with IL-10 showed an IL-10–dependent significant recruitment of STAT3 protein on the region encompassing the two predicted STAT3 binding sites exclusively in the miR-146b promoter region, whereas no STAT3 recruitment was found on the miR-146a promoter region (Fig. 2H). In the miR-146b promoter region, two putative NF-κB binding sites were also predicted. Interestingly, the NF-κB chemical inhibitors caffeic acid phenetyl ester (CAPE) and pyrrolidine dithiocarbamate (PDTC) significantly inhibited miR-146a expression levels, consistent with previous reports of NF-κB driving the expression of miR-146a in LPS-stimulated monocytes (15), but had no role on miR-146b induction by LPS in monocytes (Fig. 2I). These data indicate that miR-146a and miR-146b undergo a profound different regulation in monocytes exposed to pro- and anti-inflammatory stimuli and identify miR-146b, but not miR-146a, as an IL-10–dependent miR, suggesting that miR-146b may play a role in mediating the anti-inflammatory activity of IL-10.
The TLR/IL-1 Receptor Signaling Pathway as a miR-146b Target.
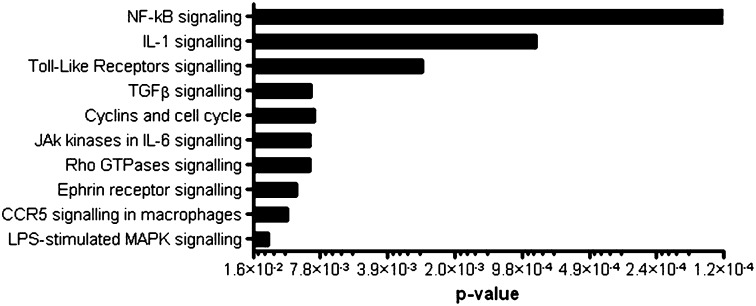
To gain insight into the functional role of miR-146b in the context of LPS-mediated inflammation, we chose an in silico approach to identify potential miR-146b targets. As algorithms based on seed pairing and evolutionary conservation typically have low specificity predictive value, we combined miRanda (20) predictions with pathways analysis based on the Ingenuity Pathway Analysis database (www.ingenuity.com), mapping biomolecular networks based on known pathways, Gene Ontology, and interactions. Interestingly, miR-146b targets showed significant enrichment in “TLR signaling,” “NF-κB signaling,” and “IL-1β signaling” pathways (Fig. 3), leading us to investigate the hypothesis that miR-146b may contribute to the IL-10-dependent feedback inhibitory loop fine tuning the inflammatory response induced in monocytes by TLR/IL-1 receptor (IL-1R) activation. In particular, miR-146b was predicted to directly target both receptors and key signal transducers of the TLR/IL-1 signaling pathway but not effector molecules, with the remarkable exception of IL-6 (Fig. S3).
Fig. 3.
miR-146b targets the TLR signaling pathway. Canonical pathways significantly enriched for miR-146b predicted target genes as identified by the Ingenuity Pathways Analysis library.
TLR4 Is a Direct Target of miR-146b.
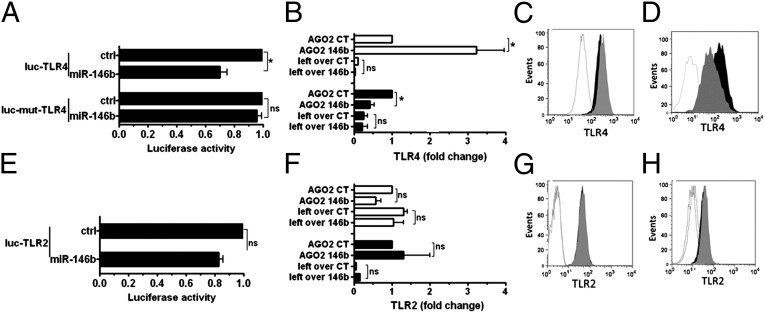
To validate predictions, the direct targeting of TLR2 and TLR4 by miR-146b was investigated. In 293T cells, miR-146b significantly decreased luciferase activity of a reporter gene containing the TLR4 3′UTR, and the deletion of 5 nt in the 3′UTR seed match sequence abolished the inhibitory effect of miR-146b on luciferase levels, indicating that the observed down-regulation was dependent on the predicted miR-146b target site (Fig. 4A). The significant enrichment of TRL4 mRNA in the RISC complexes immunoprecipitated from human monocytic THP-1 cells transduced with the lentiviral-based expression vector pRRL-miR146b, compared with THP-1 cells transduced with the control lentiviral construct pRRL-ctrl, provide direct evidence that miR-146b directly targets TLR4 (Fig. 4B). Consistent with this, pRRL-146b–transduced THP-1 cells showed a significant decrease of TLR4 protein levels compared with THP-1 cells transduced with pRRL-ctrl [mean fluorescence intensity (MFI), pRRL-ctrl, 1,117 ± 120; pRRL-146b, 790 ± 98; P < 0.05; Fig. 4C]. Protein reduction was not mirrored by a corresponding reduction at the transcript level (pRRL-ctrl, 0.040 ± 0.25; pRRL-146b, 0.044 ± 0.19, relative to GAPDH), indicating that miR-146b acts by blocking translation of TLR4 mRNA. As a complementary approach, we used lentiviral expression vectors to obtain a permanent miR-146b inhibition (miRzip-146b), and we found a significant decrease of TLR4 transcript in the RISC complex of LPS-stimulated THP-1 cells (Fig. 4B), also mirrored by a corresponding increase of TLR4 protein in miRzip-146b cells compared with miRzip-ctrl THP-1 cells (MFI, miRzip-ctrl, 3,616 ± 98; miRzip-146b, 5,323 ± 111; P < 0.05; Fig. 4D). Conversely, miR-146b overexpression did not induce any impairment of the TLR2 transcript stability as assessed by luciferase assay (Fig. 4E), did not enrich TLR2 transcript in the RISC complex (Fig. 4F), and did not reduce TLR2 protein expression (MFI, pRRL-ctrl, 1,671 ± 59; pRRL-146b, 1,530 ± 61; P value not significant; Fig. 4G). In parallel experiments, THP-1 cells stimulated with LPS in the presence of miRzip-146b did not show any increase of the TLR2 transcript in the RISC complex (Fig. 4F), nor a decrease in TLR2 protein expression compared with miRzip-ctrl THP-1 cells (MFI, miRzip-ctrl, 1,001 ± 20; miRzip-146b, 1,186.5 ± 157; P value not significant; Fig. 4H). Taken together, these results indicate that, contrary to predictions, TLR2 is not a direct target of miR-146b.
Fig. 4.
TLR4 is a direct target of miR-146b. (A and E) Luciferase constructs with the entire 3′UTR of TLR4 (luc-TLR4) or the corresponding construct mutated in the miR-146b seed region (luc-mut-TLR4) or TLR2 (luc-TLR2) were cotransfected in 293T cells with miR-146b mimic or a negative control mimic (ctrl). Results are expressed as the ratio between renilla and firefly luciferase activities (mean percent variation ± SEM; n = 3). (B and F) Cell extracts from THP-1 cells transduced with pRRL-ctrl (CT; closed columns) or pRRL-146b (146b; closed columns) or transduced with miRzip-ctrl (CT; open columns) or miRzip-146b (146b; closed columns) were subjected to RIP assay by using anti-Ago2 or IgG control Abs, and levels of TLR4 and TLR2 transcripts (B and F, respectively) were assayed in triplicate by qPCR in RIP (IP AGO2) and leftover samples. Results are expressed as normalized fold enrichment (mean percent variation ± SEM; n = 3). (C, D, G, and H) Protein levels were measured by flow cytometry on THP-1 cells transduced with pRRL-ctrl (gray histogram) or pRRL-146b (black histogram) (C and G, TLR4 and TRL2, respectively) or with miRzip-ctrl (gray) or miRzip-146b (black; D and H, TLR4 and TRL2, respectively). The isotype control staining is shown by the white histogram. One experiment representative of four performed with similar results is shown.
Multitargeting of TLR Signaling Pathway by miR-146b.
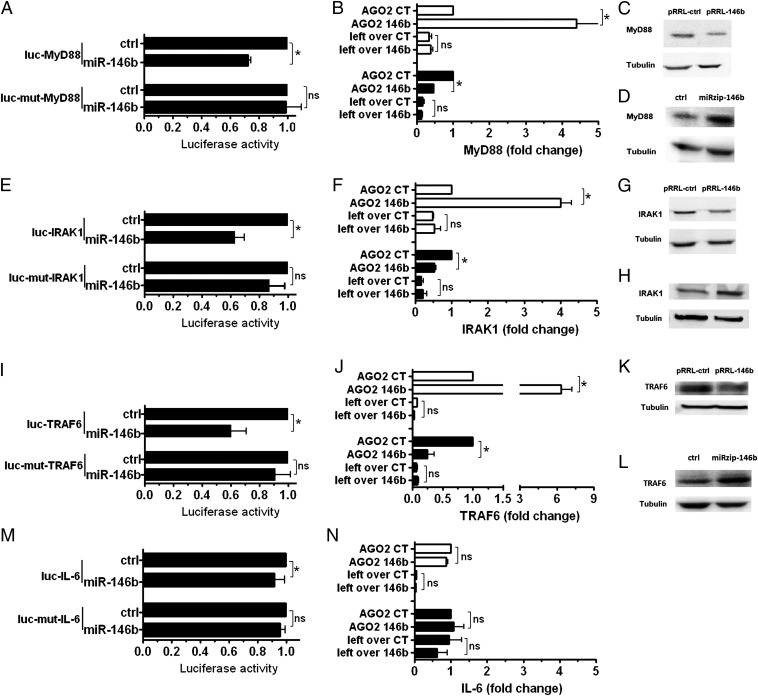
As the TLR/IL-1R signaling pathway scored as a major target of miR-146b, signaling adaptors involved in this pathway were investigated. Luciferase assays validated myeloid differentiation primary response 88 (MyD88), interleukin-1 recpetor-associated kinase 1 (IRAK-1), and TNF receptor-associated factor 6 (TRAF6) as direct targets of miR-146b, and, in all cases, abrogation of miR-146b effects by mutagenesis of its seed match regions in target 3′UTR demonstrated the specificity of its action (Fig. 5 A, E, and I, respectively). Consistent with this, RISC immunoprecipitation (RIP) analysis revealed a significant enrichment of MyD88, IRAK-1, and TRAF6 transcripts in pRRL-146b–transduced THP-1 cells and a corresponding reduction in THP-1 cells transduced with miRzip-146b but not miRzip-ctrl (Fig. 5 B, F, and L, respectively). Western blot analysis confirmed that enforced expression of miR-146b reduced MyD88, IRAK-1, and TRAF6 protein levels (Fig. 5 C, G, and M, respectively), whereas miR-146b inhibition enhanced their protein expression levels (Fig. 5 D, H, and N). Conversely, even though IL-6 was also predicted as a direct target of miR-146b, luciferase assay on the IL-6 3′UTR and RIP assay did not confirm this prediction (Fig. 5 O and P, respectively). Quantitative real-time PCR (qPCR) experiments revealed that miR-146b targeting affected stability of MyD88 and TRAF6 but not IRAK-1 transcripts, suggesting that, in this latter case, the miR-146b effect is likely mediated by translation repression. Conversely, miR-146a significantly destabilized IRAK-1 and TRAF6 but not MyD88 transcript (Fig. S4 A–C). The predicted energy interactions of miR-146a and miR-146b on their corresponding seeds on these targets did not correlate with their effect on the transcripts’ stability (Fig. S4 A–C), indicating the involvement of other still unknown parameters. Taken together, these results demonstrate that miR-146b targets multiple elements involved in the TLR signaling system, as previously described for miR-146a (15), but also indicate that the two miR-146 isoforms adopt different mechanisms to regulate TLR adaptors, suggesting they may exert different functions on the TLR signaling pathway.
Fig. 5.
Multitargeting of the TLR4 signaling pathway by miR-146b. 3′UTR luciferase constructs were cotransfected in 293T cells with miR-146b mimic or a negative control mimic (ctrl). MyD88 (A), IRAK-1 (E), TRAF6 (I), and IL-6 (M). Results are expressed as the ratio between renilla and firefly luciferase activities (mean percent variation ± SEM; n = 3). Cell extracts from pRRL-ctrl–transduced (CT) or pRRL-146b–transduced (146b) THP-1 cells (white histograms); miRzip-ctrl–transduced or miRzip-146b–transduced THP-1 cells (black histograms) were subjected to RIP assay by using anti-Ago2 or IgG control Abs. Levels of MyD88 (B), IRAK-1 (F), TRAF6 (J), and IL-6 (N) mRNAs were assayed in triplicate by qPCR in RIP (IP AGO2) and leftover samples and expressed as normalized fold enrichment. (mean percent variation ± SEM; n = 3). Protein levels were analyzed by Western blot and normalized on tubulin, used as loading controls. MyD88 (C and D), IRAK-1 (G and H), and TRAF6 (K and L). One experiment representative of four is shown.
MiR-146b Controls Induction of Proinflammatory Cytokines by TLR Agonists.
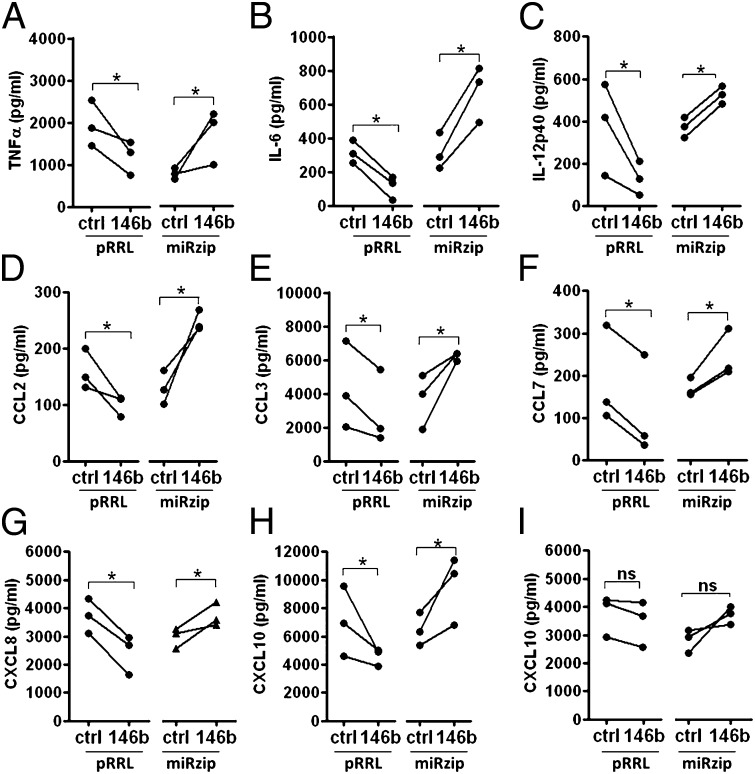
As we demonstrated a direct targeting of multiple elements involved in the TLR/IL-1R signaling pathway by miR-146b, we investigated its biological impact on the TLR-dependent production of proinflammatory cytokines. In THP-1 cells exposed to LPS, we observed a significant reduction of proinflammatory cytokine and chemokines when miR-146b expression was enhanced by cell transduction with pRRL-146b and a significant enhancement when miR-146b expression was inhibited by cell transduction with miRzip-146b (Fig. 6 A–H). Similar results were obtained when the TLR2 agonist Pam3CsK4 was used (Fig. S5). Finally, we measured the effect of miR-146b on the production of the IFN-inducible CXCL10 induced by LPS, mainly a secondary consequence of TRIF-dependent IFN-β production, or IFN-γ, which operates through the activation of a MyD88/IRAK-1–independent STAT1-dependent pathway (21). Consistent with the notion that miR-146b specifically operates on the TLR/IL-1R signaling pathway, the induction of CXCL10 production by LPS was significantly impaired in pRRL-146b–transduced THP-1 cells and enhanced in miRzip-146b–transduced THP1 cells (Fig. 6H), whereas its induction by IFN-γ was unaffected (Fig. 6I). Taken together, these data identify miR-146b as an anti-inflammatory miR able to reduce the inflammatory signal transmitted through the engagement of TLR4 by a multiple targeting mechanisms directed to the receptor and its adaptor proteins.
Fig. 6.
MiR-146b down-regulates TLR4-dependent production of proinflammatory cytokines. (A–H) Proinflammatory cytokine levels measured by ELISA in cell-free supernatants of THP-1 cells transduced with lentiviral vectors overexpressing miR-146b (pRRL-146b and its respective control) or a miR-146b sponge (miRzip-146b and its respective control) after stimulation with 1 µg/mL LPS. (I) CXCL10 levels measured by ELISA in cell-free supernatants collected under the same experimental conditions after stimulation with 10 ng/mL IFN-γ.
Discussion
Negative regulation of the immune response plays an important role in controlling homeostasis of the immune system and in preventing development of autoimmune diseases. Multiple regulatory mechanisms, including a complex network of receptors, transcription factors, adaptors, and effector molecules, have evolved to keep activation of the immune system in check. Evidence is now emerging indicating that miR might constitute an additional negative feedback mechanism operating in the innate immune system (22–24). MiR-146a has been the first miR associated to the inflammatory response, described as an miR rapidly induced by proinflammatory stimuli in phagocytes (15), and experimental evidence has clearly defined its role of negative regulator of inflammation (10, 25, 26) and its involvement in endotoxin tolerance (27, 28). The miR-146 family also includes miR-146b, which is encoded by a distinct gene on a separate chromosome and only differs from miR-146a by 2 nt at the 3′ end in its mature sequence. Despite their sequence similarity, it is still unclear whether these two miRs fulfill redundant or distinct functions. Unlike miR-146a, little information is available on the biological role in the context of the immune response of miR-146b, which has been mostly associated with tumor biology, being expressed at lower levels in many human solid tumors compared with normal tissues (29–32).
Here we report that LPS stimulation induces miR-146b expression in human monocytes, with delayed kinetics with respect to miR-146a. Most relevant, our study provides evidence for a link between miR-146b and IL-10, demonstrating that miR-146b induction depends on the activity of IL-10 produced after LPS challenging, whereas, to the contrary, IL-10 does not influence miR-146a expression. This is consistent with ChIP data showing a central role of STAT3 in the induction of miR-146b but not miR-146a, which instead depends upon NF-κB. Bioinformatic analysis revealed a significant enrichment of miR-146b potential targets in the TLR signaling, IL-1 signaling, and NF-kB signaling pathways, which play an essential role in the innate immune response driving transcriptional activation of genes encoding for proinflammatory cytokines and costimulatory molecules, which subsequently control the activation of antigen-specific adaptive immune responses. Luciferase and RIP assays validated the LPS receptor TLR4 and the proximal adaptor molecules MyD88, IRAK-1, and TRAF6 as true miR-146b targets. As these molecules sustain the TLR4 signaling pathway and showed a relatively mild down-regulation at the protein level, these findings are consistent with the present understanding that miRs might conceivably exert their major activities through the subtle individual regulation of multiple targets involved in a common signaling pathway rather then operating a strong repression of isolated targets (29, 30). In keeping with this mechanism of regulation, enforced expression of miR-146b resulted in the marked reduction of proinflammatory cytokines highly expressed upon TLR triggering. These findings suggest that miR-146b mediates some of the anti-inflammatory activities of IL-10 repressing the inflammatory response in monocytes by direct targeting of transcripts encoding TLR4 and key adaptors/signaling molecules.
Although our results leave open the question whether the two miR-146 isoforms are functionally equivalent, their divergent and asynchronous transcriptional regulation suggests they play differential roles during distinct temporal windows of the inflammatory process. During the development of the inflammatory process miR-146a and b may represent the components of a relay team in which one isoform succeeds to other to control expression of pro-inflammatory genes, first through the activity of the LPS-depend induction of miR-146a, and subsequently during the resolution phase maintaining transcripts repression through the IL-10 dependent induction of miR-146b. In this respect it is worth noticing that miR-146a–deficient mice are indeed overresponsive to bacterial challenge and produce excessive amount of proinflammatory cytokines (31), but they show an autoimmune phenotype with late onset and incomplete penetrance and lack a global change in the expression of putative miR-146 targets (32). This phenotype is suggestive of the existence of a compensatory mechanism operating in miR-146a–deficient animals. Finally, similar to miR-146b, miR-146a has been shown to target IRAK-1 and TRAF6, although in different cellular contexts (15, 29), but its ability to also directly target MyD88 and TLR4 has not been investigated.
Further research is required to understand whether miR-146b truly serves as a molecular switch involved in the resolution of inflammation, but it is interesting to note that this miR has been reported to be expressed during the resolution phase in a murine model of acute inflammation (33). A broader and deeper understanding of how miR-146b acts in concert with the growing number of negative regulators, particularly in the context of complex inflammatory processes involving signaling by multiple receptors, may well lead to novel therapeutic approaches for the rapidly expanding number of diseases driven by dysregulated inflammatory responses.
Materials and Methods
Materials.
A detailed list of materials is provided in SI Materials and Methods.
Cell Purification and Culture.
Human monocytes were obtained from healthy donor buffy coats by two-step gradient centrifugation using Ficoll (Biochrom) and Percoll (Amersham). Human studies were approved by the ethical committee of Istituto Clinico Humanitas, Milan, Italy. Monocytes and THP-1 cell line (American Type Culture Collection) were resuspended in RPMI 1640 (Lonza) supplemented with 10% (vol/vol) heat-inactivated FBS (Lonza), 100 U/mL penicillin/streptomycin (Lonza), and 25 mMl-glutamine (Lonza). The HEK-293T cell line (American Type Culture Collection) was grown in Dulbecco's modified Eagle medium (DMEM) (Cambrex) supplemented with 10% (vol/vol) FBS, 100 U/mL penicillin/streptomycin, and 25 mM l-glutamine. Murine bone marrow-derived macrophages were obtained as described in SI Materials and Methods.
ChIP Assay.
ChIP experiments were performed as described elsewhere (34) and in SI Materials and Methods.
ELISA.
Antibodies and detection reagents for ELISAs were purchased from R&D Systems and used according to the manufacturer’s instructions. Samples were diluted so that the optical density fell within the optimal portion of a log standard curve.
Quantification of miR and mRNA.
Total RNA was purified by using TRIzol Reagent (Ambion), and qPCR was conducted by using a 7900HT Real-Time PCR System. One hundred nanograms of total RNA were reverse-transcribed for quantification of miR expression by using TaqMan MiRNA Reverse Transcription Kit (Applied Biosystems), according to the manufacturer’s instructions and as described in detail in SI Materials and Methods.
Constructs.
The 3′UTR of TLR4, TLR2, MyD88, IRAK-1, TRAF6, and IL-6 were amplified from genomic DNA and cloned in the biosensor psiCHECK-2 vector (Promega). PremiR-146b and premiR-146a were amplified from genomic DNA and cloned in the pcDNA3 expression vector as described in SI Materials and Methods. To knock down miR-146b expression, the miRzip lentivector-based construct anti–miR-146b and the relative control were purchased from System Biosciences. The list of oligonucleotides used is reported in Table S1.
Luciferase Reporter Assay.
HEK-293T cells were transfected after 24 h with 100 ng psiCHECK-2–3′UTR reporter construct and 700 ng pcDNA3-miR or pcDNA3 as control, by using Lipofectamine 2000 (Invitrogen), according to the manufacturer’s protocol, as described in detail in SI Materials and Methods.
FACS Analysis.
Cells were washed twice with PBS solution containing 1% BSA. Aspecific binding was blocked by using Fc-block (BD Biosciences). Washed cells were resuspended in a 1:200 dilution of APC-conjugated anti-human TLR4 (clone HTA125; eBioscience) or anti-human TLR2 antibody (clone 383936; R&D Systems) or the mouse IgG2a isotype control APC (eBioscience). Stained cells were washed twice with PBS solution containing 1% BSA and analyzed by flow cytometry (FACSCanto; BD Biosciences).
Immunoprecipitation of Ago2-Bound RNAs.
Immunoprecipitation of Ago2-bound RNAs (RIP), which contains miRs and their target mRNA, was performed as previously described (35), with minor modifications and as described in detail in SI Materials and Methods. Briefly, 30 × 106 pRRL-ctrl and pRRL-146b THP-1 cells were stimulated for 2 h with 1 µg/mL LPS, whereas miRzip-ctrl and miRzip-146b THP-1 cells were stimulated for 12 h with 1 µg/mL LPS. In all experiments, an aliquot of immunoprecipitation supernatants, corresponding to 0.5 × 106 cell equivalent, was removed after immunoprecipitation (indicated as “left over”) and used as control for the specificity of the assay. Results were expressed as fold enrichment relative to Ago2-immunoprecipitation control samples.
Statistical Analysis.
Statistical evaluation was performed with use of the Student t test or one-way ANOVA and reported in figures. P values less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
This study was conducted in the context of Fondazione Humanitas per la Ricerca and was supported by Ministero dell’Istruzione dell’Università e della Ricerca PRIN (Progetto di Rilevante Interesse Nazionale) Research Grants 2002061255 and FIRB (Fondo per Investimenti in Ricerca di Base) Research Grant RBFR08CW8G, a University of Verona Joint Project Grant, the Italian Association for Cancer Research, Regione Lombardia LIIN (Lombardian Innate Immunity Network) Project, Fondazione Cariplo, and the European Community Seventh Framework Programme TIMER Project.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219852110/-/DCSupplemental.
References
- 1.O’Neill LA. How Toll-like receptors signal: What we know and what we don’t know. Curr Opin Immunol. 2006;18(1):3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Murthy PK, Dennis VA, Lasater BL, Philipp MT. Interleukin-10 modulates proinflammatory cytokines in the human monocytic cell line THP-1 stimulated with Borrelia burgdorferi lipoproteins. Infect Immun. 2000;68(12):6663–6669. doi: 10.1128/iai.68.12.6663-6669.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siewe L, et al. Interleukin-10 derived from macrophages and/or neutrophils regulates the inflammatory response to LPS but not the response to CpG DNA. Eur J Immunol. 2006;36(12):3248–3255. doi: 10.1002/eji.200636012. [DOI] [PubMed] [Google Scholar]
- 4.Tebo JM, Kim HS, Gao J, Armstrong DA, Hamilton TA. Interleukin-10 suppresses IP-10 gene transcription by inhibiting the production of class I interferon. Blood. 1998;9(12):4742–4749. [PubMed] [Google Scholar]
- 5.Zhou L, Nazarian AA, Smale ST. Interleukin-10 inhibits interleukin-12 p40 gene transcription by targeting a late event in the activation pathway. Mol Cell Biol. 2004;24(6):2385–2396. doi: 10.1128/MCB.24.6.2385-2396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kishore R, Tebo JM, Kolosov M, Hamilton TA. Cutting edge: clustered AU-rich elements are the target of IL-10-mediated mRNA destabilization in mouse macrophages. J Immunol. 1999;162(5):2457–2461. [PubMed] [Google Scholar]
- 7.Knödler A, et al. Post-transcriptional regulation of adapter molecules by IL-10 inhibits TLR-mediated activation of antigen-presenting cells. Leukemia. 2009;23(3):535–544. doi: 10.1038/leu.2008.301. [DOI] [PubMed] [Google Scholar]
- 8.Selbach M, et al. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455(7209):58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 9.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Neill LA, Sheedy FJ, McCoy CE. MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nat Rev Immunol. 2011;11(3):163–175. doi: 10.1038/nri2957. [DOI] [PubMed] [Google Scholar]
- 11.McCoy CE, et al. IL-10 inhibits miR-155 induction by toll-like receptors. J Biol Chem. 2010;285(27):20492–20498. doi: 10.1074/jbc.M110.102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connell RM, Chaudhuri AA, Rao DS, Baltimore D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci USA. 2009;106(17):7113–7118. doi: 10.1073/pnas.0902636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossato M, et al. IL-10-induced microRNA-187 negatively regulates TNF-α, IL-6, and IL-12p40 production in TLR4-stimulated monocytes. Proc Natl Acad Sci USA. 2012;109(45):E3101–E3110. doi: 10.1073/pnas.1209100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheedy FJ, et al. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat Immunol. 2010;11(2):141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 15.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103(33):12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci USA. 2007;104(5):1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bazzoni F, et al. Induction and regulatory function of miR-9 in human monocytes and neutrophils exposed to proinflammatory signals. Proc Natl Acad Sci USA. 2009;106(13):5282–5287. doi: 10.1073/pnas.0810909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alas S, Bonavida B. Inhibition of constitutive STAT3 activity sensitizes resistant non-Hodgkin’s lymphoma and multiple myeloma to chemotherapeutic drug-mediated apoptosis. Clin Cancer Res. 2003;9(1):316–326. [PubMed] [Google Scholar]
- 19.Benkhart EM, Siedlar M, Wedel A, Werner T, Ziegler-Heitbrock HW. Role of Stat3 in lipopolysaccharide-induced IL-10 gene expression. J Immunol. 2000;165(3):1612–1617. doi: 10.4049/jimmunol.165.3.1612. [DOI] [PubMed] [Google Scholar]
- 20.Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010;11(8):R90. doi: 10.1186/gb-2010-11-8-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi XF, et al. Essential involvement of cross-talk between IFN-gamma and TNF-alpha in CXCL10 production in human THP-1 monocytes. J Cell Physiol. 2009;220(3):690–697. doi: 10.1002/jcp.21815. [DOI] [PubMed] [Google Scholar]
- 22.Zhou R, O’Hara SP, Chen XM. MicroRNA regulation of innate immune responses in epithelial cells. Cell Mol Immunol. 2011;8(5):371–379. doi: 10.1038/cmi.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry MM, et al. Rapid changes in microRNA-146a expression negatively regulate the IL-1beta-induced inflammatory response in human lung alveolar epithelial cells. J Immunol. 2008;180(8):5689–5698. doi: 10.4049/jimmunol.180.8.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10(2):111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 25.Etzrodt M, et al. Regulation of monocyte functional heterogeneity by miR-146a and Relb. Cell Rep. 2012;1(4):317–324. doi: 10.1016/j.celrep.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jurkin J, et al. miR-146a is differentially expressed by myeloid dendritic cell subsets and desensitizes cells to TLR2-dependent activation. J Immunol. 2010;184(9):4955–4965. doi: 10.4049/jimmunol.0903021. [DOI] [PubMed] [Google Scholar]
- 27.Nahid MA, Pauley KM, Satoh M, Chan EK. miR-146a is critical for endotoxin-induced tolerance: IMPLICATION IN INNATE IMMUNITY. J Biol Chem. 2009;284(50):34590–34599. doi: 10.1074/jbc.M109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quinn EM, Wang J, Redmond HP. The emerging role of microRNA in regulation of endotoxin tolerance. J Leukoc Biol. 2012;91(5):721–727. doi: 10.1189/jlb.1111571. [DOI] [PubMed] [Google Scholar]
- 29.Bhaumik D, et al. Expression of microRNA-146 suppresses NF-kappaB activity with reduction of metastatic potential in breast cancer cells. Oncogene. 2008;27(42):5643–5647. doi: 10.1038/onc.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baek D, et al. The impact of microRNAs on protein output. Nature. 2008;455(7209):64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boldin MP, et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med. 2011;208(6):1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao JL, et al. NF-kappaB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. Proc Natl Acad Sci USA. 2011;108(22):9184–9189. doi: 10.1073/pnas.1105398108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Recchiuti A, Krishnamoorthy S, Fredman G, Chiang N, Serhan CN. MicroRNAs in resolution of acute inflammation: Identification of novel resolvin D1-miRNA circuits. FASEB J. 2011;25(2):544–560. doi: 10.1096/fj.10-169599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamassia N, et al. Uncovering an IL-10-dependent NF-kappaB recruitment to the IL-1ra promoter that is impaired in STAT3 functionally defective patients. FASEB J. 2010;24(5):1365–1375. doi: 10.1096/fj.09-145573. [DOI] [PubMed] [Google Scholar]
- 35.Hendrickson DG, Hogan DJ, Herschlag D, Ferrell JE, Brown PO. Systematic identification of mRNAs recruited to argonaute 2 by specific microRNAs and corresponding changes in transcript abundance. PLoS ONE. 2008;3(5):e2126. doi: 10.1371/journal.pone.0002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.