Abstract
INTRODUCTION
Fistuloclysis is an alternative method for enteral nutrition infusion, and has been successfully employed for the management of patients with high output small bowel fistula. However it has some deficiencies also.
PRESENTATION OF CASE
A 42-year-old woman with multiple high output enterocutaneous fistula was submitted to fistuloclysis with reinfusion of chyme, after a period of several complications due to parenteral nutrition.
DISCUSSION
Enteral nutrition provide better nutrition and fewer complications than parenteral nutrition. The enterocutaneous fistula usually does not allow enteral nutrition, however the use of fystuloclysis can fix this issue. The reinfusion of chyme provide the possibility of oral intake and better control of hydroeletrolitics disorders.
CONCLUSION
More studies on the physiological effects of the chyme recirculation could add more data contributing to the clarification of this complex issue, but we believe that patients with high output and very proximal enterocutaneous fistula can be sucessfully treated with fistuloclysis and recirculation of chyme.
Keywords: Enterocutaneous fistulae, Fistuloclysis, Enteral nutrition
1. Introduction
Enterocutaneous fistulae (ECF) are common postoperative entities in patients submitted to repeated laparotomies for severe intra-abdominal infections, mainly when the abdomen is left open. These fistulae cause large volumes of fluid loss, leading to severe dehydration, electrolyte imbalances, and malnutrition usually resulting from intestinal failure, contributing to morbidity and mortality.1–3 Until the corrective surgery, parenteral nutrition (PN) provides nutritional support; however, it is complex, expensive, highly demanding of expert nursing care providers,1 and has catheter-related disadvantages. Alternatively, enteral nutrition (EN) is advantageous for gastrointestinal tract (GIT) protection, presenting lower incidence of infection, maintaining gut mucosa and pulmonary immunity, and limiting intestinal atrophy, which is associated with prolonged PN.4,5 However, EN has the disadvantage of usually requiring a nasoenteral tube for diet infusion.6,7 Fistuloclysis is an alternative method for EN infusion, and has been successfully employed for the management of patients with high output small bowel fistulae that have at least 75 cm of intact distal small bowel.2,3
Usually the management of ECF begins with PN for two or more weeks, after which it may either be continued or substituted with EN. Fistuloclysis may be an alternative if PN causes severe adverse effects, if oral feeding increases fistula output, or if prolonged nutritional support is required.1,8 Others have reported successful use of the succus entericus collected from the proximal enteric stoma using an appropriate pump system for reinfusion through a rubber catheter with a balloon into the distal fistula or stoma, aiming to provide adequate nutrients.9,10
2. Case report
A 42-year-old woman was admitted to our service in February 2009, with abdominal pain, malaise, weakness, and an enterocutaneous fistula at the site of a previous ileostomy that resulted from a left colectomy in 2006 for a bleeding sigmoid adenocarcinoma, followed by an additional six laparotomies for acute abdominal obstructions, and a final laparotomy for the ileostomy closure. She reported permanent use of vasopressin, prednisone, and thyroid hormone since a hypophysectomy in 2004 for an astrocytoma. She presented with severe electrolyte imbalances, malnutrition (anemia, hypoalbuminemia, and muscular atrophy), and sepsis. A CT scan showed intra-abdominal fluid collections. Two days later, a laparotomy was performed with six segmental small bowel resections. A mesh onlay was employed on the fascia to strengthen the abdominal wall. A skin dehiscence occurred on the 7th postoperative (PO) day, followed by a proximal jejunal high output (1.5 L/day) ECF, confirmed on the 20th PO day by a contrast X-ray evaluation. Five fistula orifices on the dehiscent abdominal wall were observed after the removal of the onlay mesh, with the proximal fistula at 20 cm from the Treitz angle. Total PN was initiated on the 21st PO day, but two septic shock episodes from catheter infection and frequent hypokalemia occurred, requiring catheter replacement. A fistulogram on the 47th PO day showed no contrast leakage through the distal EC fistulae. A Foley catheter (FC) was then inserted into the distal fistula opening for fistuloclysis EN starting with an infusion of 10 mL/h of a 1.3 kcal/mL semielemental formula, progressively increasing to 60 mL/h, while PN was decreased and interrupted 2 weeks later. At that time, a regular Foley catheter was introduced in the proximal fistula and a triple-lumen Foley catheter in the distal fistula, replacing the initial regular one. These catheters were anchored on the abdominal wall with insufflated cuffs, and the proximal and distal Foley catheters were connected through a diet infusion pump (Fig. 1). In order to prevent bubbles from the intestinal tract from damaging the pump, a water seal system was developed (Fig. 1). The triple-lumen Foley in the distal fistula allowed simultaneous infusion of fistuloclysis EN and chyme from the proximal into the distal fistula. The chyme was collected from the proximal fistula into the distal in order to enable the introduction of a low residue diet. In order to prevent small leaks around the FCs, maintain cleanliness of the abdominal wound, and reduce the size of the dehiscent area, a vacuum-assisted closure therapy (VACT) was employed.
Fig. 1.
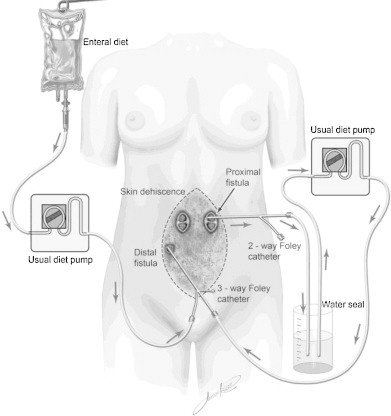
A combination of chyme recirculation and fistuloclysis was possible using simple diet pumps, Foley catheters, and a water seal system.
Two months later, the albumin level had increased from 2 to 3.5 and the body weight from 55 to 57 kg. The muscular trophism also increased while the body edema decreased and the patient's physiological conditions improved. After 112 days from our initial surgery, 64 days with fistuloclysis and 50 days with recirculation of chyme, an elective relaparotomy was performed: almost 1.5 m of small bowel was removed, followed by one entero-entero anastomosis with an estimated 2 m of remaining small bowel. A two-layered mesh bridging technique was employed to close the abdomen without tension. The patient was extubated on the 3rd PO day, left the ICU on the 5th PO day, started receiving an oral diet on 15th PO day, and was discharged from the hospital on the 30th PO day. A skin dehiscence occurred, exposing the mesh on the 7th PO day, but did not delay the patient's discharge. Six months later the patient was well, with no enteric fistula.
3. Discussion
In the present case, owing to hypokalemia and septic catheter-related complications, as well as the short (20 cm) jejunal segment proximal to the fistula, a fistuloclysis was started on the 48th PO day. This was associated with an early increase in the serum albumin level and body weight, as well as the decreased body edema, allowing PN interruption within 2 weeks (63rd PO day).
Chyme reinfusion reversed the hypokalemia, and, associated with fistuloclysis, prevented further disturbances, allowing the patient to maintain a satisfactory nutritional status.
The increase of the small bowel caliber and wall thickness reported by other studies after receiving nutrients via fistuloclysis1 seems to be corroborated by our findings as shown in Fig. 2a and b. Although the exposed external recirculation of chyme is considered anti-esthetic, the addition of the VACT allowed for easy wound care, without the anti-esthetic implications reported by others.1 Although based on the same principle of extracorporeal circulation of chyme, the technique employed here differs from French studies11,12 in that while they used only a chyme aspiration–reinfusion system connected to a karaya gum on the abdominal wall, the dehiscent abdominal skin forced us to use an additional Foley catheter. Furthermore, we also employed the fistuloclysis for nutritional support.
Fig. 2.
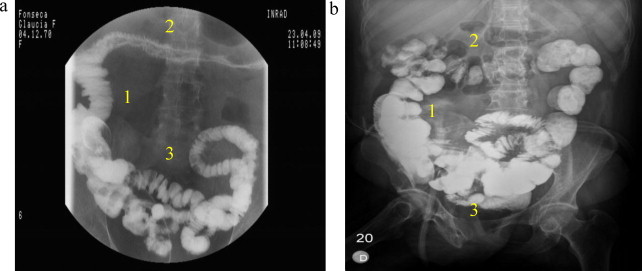
(a) The initial fistulography. The transverse colon is atrophic as well as the intestinal loops and (b) after 50 days with fistuloclysis and reinfusion of chyme. The length and number of intestinal loops have increased.
4. Conclusion
The use of a simplified system with a double-lumen Foley catheter for chyme collection from the proximal (after 20 cm jejunum) fistula and a triple-lumen one for chyme infusion into the distal fistula simultaneously with fistuloclysis allowed for successful management of a patient with multiple jejunal fistulae exiting at a large abdominal wound that resulted from dehiscence and skin loss after multiple laparotomies.
Further studies on the physiological effects of chyme recirculation would add more data to contribute to the clarification of this complex issue, but we believe that patients with high output and very proximal enterocutaneous fistulae can be successfully treated with fistuloclysis and recirculation of chyme. The improvement in the patient's nutrition, as observed in this study, is probably a major contributing factor of a positive postoperative outcome.
Conflict of interest statement
None.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report. The identity was preserved. No photos were used.
Author contributions
Adriano Pflug contributed to the study design, data collection, data analysis, and writing of the article; Belchor Fontes contributed to the data analysis and writing of the article; Mario Faro and Samir Rasslan contributed to the data analysis; and Edivaldo Utiyama contributed to the study design and data analysis.
References
- 1.Teubner A., Morrison K., Ravishankar H.R., Anderson I.D., Scott N.A., Carlson G.L. Fistuloclysis can successfully replace parenteral feeding in the nutritional support of patients with enterocutaneous fistula. British Journal of Surgery. 2004;91:625–631. doi: 10.1002/bjs.4520. [DOI] [PubMed] [Google Scholar]
- 2.Scripcariu V., Carlson G., Bancewick K., Irving M.H., Scott N.A. Reconstructive abdominal operations after laparostomy and multiple repeat laparotomies for severe intra-abdominal infection. British Journal of Surgery. 1994;81:1475–1478. doi: 10.1002/bjs.1800811024. [DOI] [PubMed] [Google Scholar]
- 3.Evenson A.R., Fischer J.E. Current management of enterocutaneous fistula. Journal of Gastrointestinal Surgery. 2006;10:455–464. doi: 10.1016/j.gassur.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Ludsk K.A., Croce M.A., Fabian T.C. Enteral versus parenteral feeding: effects on septic morbidity after blunt and penetrating abdominal trauma. Annals of Surgery. 1992;215:503–511. doi: 10.1097/00000658-199205000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kudsk K.A. Current aspects of mucosal immunology and its influence by nutrition. American Journal of Surgery. 2002;183:390–398. doi: 10.1016/s0002-9610(02)00821-8. [DOI] [PubMed] [Google Scholar]
- 6.Ham M., Horton K., Kaunitz J. Fistuloclysis: a case report and literature review. Nutrition in Clinical Practice. 2007;22:553–557. doi: 10.1177/0115426507022005553. [DOI] [PubMed] [Google Scholar]
- 7.Slater R. Nutritional management of enterocutaneous fistulas. British Journal of Nursing. 2009;18(February–March (4)):225–230. doi: 10.12968/bjon.2009.18.4.39619. [DOI] [PubMed] [Google Scholar]
- 8.Sathyanarayana N., Shenoy K.R., Alvares J.F., Pai S.B. Enteral feeding by fistuloclysis in a midjejunal fistula. Indian Journal of Gastroenterology. 2005;24(May–June (3)):124–125. [PubMed] [Google Scholar]
- 9.Calicis B., Parc Y., Caplin S., Frileux P., Dehni N., Ollivier J.M. Treatment of postoperative peritonitis of small-bowel origin with continuous enteral nutrition and succus entericus reinfusion. Archives of Surgery. 2002;137:296–300. doi: 10.1001/archsurg.137.3.296. [DOI] [PubMed] [Google Scholar]
- 10.Lévy E., Cosnes J., Bloch P., Parc R., Huguet C., Loygue J. Reinfusion of the upper digestive secretions into the lower part of the intestine decreases stomal flow from temporary enterostomies. Gastroenterologie Clinique et Biologique. 1979;3:447–451. [PubMed] [Google Scholar]
- 11.Levy E., Palmer D.L., Frileux P., Parc R., Huguet C., Loygue J. Inhibition of upper gastrointestinal secretions by reinfusion of succus entericus into the distal small bowel. A clinical study of 30 patients with peritonitis and temporary enterostomy. Annals of Surgery. 1983;198(November (5)):596–600. doi: 10.1097/00000658-198311000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levy E., Parc R., Loygue L., Loygue J. Stomies terminales, jéjunales ou iléales temporaires de sauvetage avec réinstillation autorégulée. Nouvelle Presse Medicale. 1977;6:461–462. [PubMed] [Google Scholar]


