Abstract
INTRODUCTION
The rates of pancreatic cancer development in the early stages of growth remain unclear; but it is generally believed that they demonstrate a rapid degree of progression. There is evidence to suggest that pancreatic cancers measuring less than 1 cm demonstrate better survival rates, hence it is clear that detecting pancreatic cancers less than 1 cm in size is of paramount importance. However, to date, there has been no scientifically adequate research to show the growth rate of small pancreatic cancers less than 1 cm in the early stages.
PRESENTATION OF CASE
We present the case of a 65-year-old woman whose small pancreatic cancer possibly demonstrated a slow progressive rate as it grew to an invasive carcinoma measuring 1 cm diameter from over the 29 months.
DISCUSSION
It is reasonable to assume that the progression of some pancreatic cancers until 1 cm size, can take up to 29 months. During this silent period, it is crucial to detect such a small pancreatic cancer by means of the initial US and subsequent EUS and ERCP. It is clear, therefore, that clinicians have to be aware of the growth rate of small pancreatic cancers and in particular high risk patients should be encouraged to monitor size of the main pancreatic duct by means of US on regular basis.
CONCLUSION
This could give better outcomes for pancreatic cancer patients. Hopefully, by detecting these lethal, pancreatic cancers in their early stages, it will give us an extension of time to perform effective therapies.
Keywords: Small pancreatic cancer, Invasive ductal carcinoma, Pancreas, Slow progression, Main pancreatic duct dilatation
1. Introduction
Pancreatic cancer is one of the major causes of malignant tumour deaths worldwide. The incidence of pancreatic cancer has been gradually rising over the last two decades.1 There is no doubt that the morbidity and mortality rates of pancreatic cancer patients are significantly high. Generally, it can be argued that the early stage of other cancers such as stomach and colon have demonstrated better prognosis than the advanced stage. However, it has been alluded to that the prognosis of even a small pancreatic cancer (T1, less than 2 cm) does not have a better prognosis than the advanced pancreatic cancer.2
In case of that T1 pancreatic cancers are divided into two categories: less than 1 cm tumour size (TSz) and tumour between 1 and 2 cm. Consequently, TSz < 1 group indicates a relative better prognosis.3–5 Under the present circumstances, however, it might be difficult to detect such a small pancreas cancer, mainly due to the absence of conspicuous symptoms and effective screening strategies. In particular, more than 90% of pancreatic cancer measuring 1 cm or less in diameter do not demonstrate any specific symptoms. Although there are limitations in detecting all small pancreatic cancer, it is of vital importance to spare no effort to find small pancreatic cancer. Therefore, it is important to investigate the natural progression of the cancer, but it still remains unclear. Given this case, it is reasonable to assume that the progression of some pancreatic cancers until 1 cm size, can take up to 29 months.
2. Presentation of case
A 65-year-old female patient presented to our hospital without any symptoms, after a private practitioner identified a rise of her serum carcinoembryonic antigen 19-9 (CA19-9) level; which was examined upon her request, albeit without any original symptoms. There was a moderate increase in tumour markers: CA19-9 52 U/ml (normal range: 0–37 U/ml). No signs of pancreatitis or cholangitis were detected. A transition of CA19-9 was demonstrated with other critical events in Fig. 1. Enhanced CT, US, EGD and colonoscopy (CS) were performed. However, ERCP was not performed, because she simply denied an invasive procedure. Although, at the time, the main pancreatic duct dilatation of 3.0 mm was observed by CT scan, no method of imaging was able to detect any malignant lesion (Fig. 2a). After the clinical examinations and diagnostic imaging, she returned to the private practitioner for her CA19-9 levels to be monitored on a regular basis.
Fig. 1.
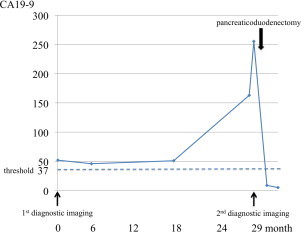
Her CA19-9 levels steadily kept above the threshold over the 18 months, and then sharply increased to 255.3 U/ml. After her surgery, the CA19-9 levels plummeted to within a normal limit.
Fig. 2.
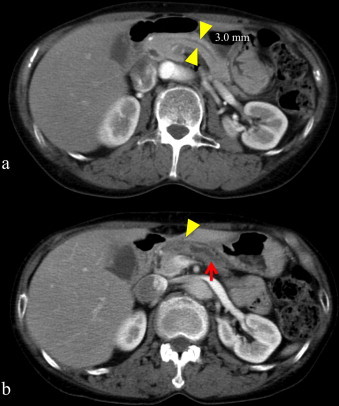
(a) CT scan demonstrated the main pancreatic duct dilatation (3.0 mm) two years before resection of the pancreatic cancer. Yellow arrow head: the main pancreatic duct. (b) The enhanced CT scan revealed one solitary lesion measuring 10 mm at the pancreatic body. Yellow arrow head: poor enhanced lesion, pancreatic cancer. Red arrow: the dilated main pancreatic duct. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The patients consulted at our hospital, because her serum CA19-9 rapidly increased during the last 11 months over a 29 months-period observation. Upon presentation, her current CA19-9 levels were raised to 255.3 U/ml. Her other serum examination results were as follows: haemoglobin A1c (HbA1c) 6.6% (normal range: 3.5–5.7%), fast blood sugar 144 mg/dl (normal range: 80–110 mg/dl), and normal range of serum amylase 46 U/ml (normal range: 25–160 U/ml). CT and EUS demonstrated one solitary lesion measuring 9.5 mm at the pancreatic body superior to the portal vein (Figs. 2b and 3). Subsequent ERCP demonstrated an irregular, abrupt stricture of the main pancreatic duct, accompanied by a dilated main pancreatic duct in the body and tail of the pancreas. Consequently, the mass was diagnosed as an adenocarcinoma of the pancreas using brush cytology from the ERCP.
Fig. 3.
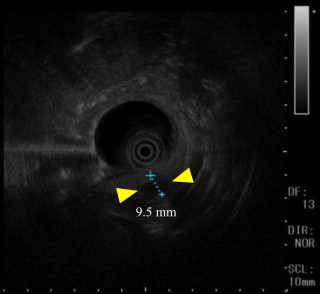
EUS detected a low echoic lesion (Yellow arrow head) measuring 9.5 mm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The tumour had not infiltrated the portal vein, the superior mesenteric artery or vein, or the coeliac plexus of nerves, and was without metastases. Therefore, a normal pancreaticoduodenectomy was performed without vesselplasty. Her clinical course was stable without complications, and she was discharged after 14 days of hospitalisation. The patient is currently systemically well, without any signs of tumour recurrence 12 months post-operatively.
According to the International Union Against Cancer (UICC) and Japan Pancreas Society (JPS) classification system, pathological examination for the pancreas revealed an invasive ductal carcinoma, well differentiated, T1N0M0, Stage IA (UICC), tub1, Ph 1.1 × 1.0 cm, pT1, pCH(−), pDU(−), pS(−), pRP(−), pPV(−), pA(−), pPL(−), pN0/21, pPCM(−), pDPM(−), intermediate type, INFβ, ly0, v0, ne0, mpd(−) (JPS) (Fig. 4). There was no evidence of IPMN or other type of pancreatic malignant tumour. Ki 67 labelling index was 35–40%. CA19-9 immunohistological examination also confirmed that the tumour firmly expressed CA19-9.
Fig. 4.
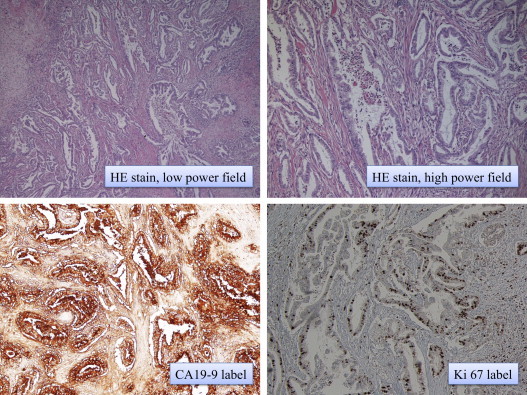
Pathological examination for the pancreas revealed an invasive ductal carcinoma measuring 1.1 × 1.0 cm. Immunohistological examination confirmed strong expression of CA19-9. Ki 67 labelling index was 35–40%.
3. Discussion
Malignant pancreatic tumours can be divided into the following categories: pancreatic cancer (invasive ductal carcinoma), IPMN, mucinous cystic tumour (MCT), neuroendocrine tumours (NET), and islet cell tumours. The most typical pancreatic cancer, being invasive ductal carcinoma, is one of the worst malignant tumours in terms of the patient outcomes mainly due to rapid growth rate, difficulty in early diagnosis, and high resistance against the existing therapies. These factors lead to a median survival among all patients of up to 1 year.6 However, these severe prognoses could be improved by the early detection of pancreatic cancer. Less than TSz 1 in diameter pancreatic cancer can indicate a possible better prognosis. Generally, it has been pointed out that US and CT reveal the dilatation of pancreatic duct in 57% of patients, while a tumour mass is depicted in only 9% patients.7 CA19-9 levels indicate a low sensitivity and specificity for TSz < 1 pancreatic cancers (threshold value; 37 U/ml). However, ERCP, EUS, and EUS-FNA are considered as the most sensitive procedures to detect small pancreatic cancers: these procedures potentially break the limit of clinical detection. Yasuda et al. have shown that ERCP and EUS can detect such lesions, even those cancers less than TSz < 1.8
Considering our case, CA19-9 monitoring was clearly important to diagnose a small pancreatic cancer. Difficulties remain in the full utilisation of ERCP/EUS as they can lead to severe complications, such as a severe pancreatitis. Given the poor prognosis of pancreatic cancer, however, it can be argued that an aggressive application of ERCP/EUS might be beneficial in a case where CT or US detect minor dilatation of the main pancreatic duct or cystic lesion in pancreas. Furthermore, risk factors of pancreatic cancer, such as family cancer history, hereditary pancreatitis, smoking etc., already have been established. Among these high risk patients, it might be useful to monitor CA19-9 and size of the main pancreatic duct by means of US on regular basis.
From a point of cell kinetics, this tumour growth pattern might be compatible with the Gompertzian view where the initial small tumours grow slower, the following middle stage tumours grow exponentially and reach the limit of clinical detection.9,10 During the initial 18 months period, when CA19-9 level kept just above the threshold, the pancreatic cancer had not appeared invasive. Following these at least 18 months, the tumour substantially had gained invasive ability. By means of CT scan reports, the doubling time of pancreatic cancer, measuring from the initial range 13–47 mm to the final range 15–47 mm, was estimated as 159 ± 67 (median, 144) days.11 However, the growth rate of small pancreatic cancers has not become clear as a reliable study. Under the existing condition, Hisa et al.,12 have reported a case of a small pancreatic cancer which demonstrated a slow progression rate, with the tumour volume doubling time of 252 days, which is even longer than that shown in the above study. Considering our case, it is reasonable to believe that the pancreatic cancer already existed 29 months ago, because CA19-9 levels clearly decreased to within a normal limit (8.9 IU/ml) after her surgery and subsequent immunohistological examination revealed strong expression of CA19-9. The Ki 67 labelling index in this case was 35–40% which correspond to the average of another previous report (28 ± 15%).13 It is important to realise that the pancreatic cancer provides curable 29 months period, albeit with normal pathological findings. Needless to say, therefore, it is crucial to detect such a small pancreatic cancer during this relatively long, asymptomatic silent period.
4. Conclusion
This report implicates that the development of pancreatic cancer until TSz 1 could take up to 29 months in some cases. Slight dilatation of the main pancreatic duct or cystic lesion in pancreas, albeit without tumour detection in US or enhanced CT scan, needs further investigation using ERCP and EUS which could potentially reveal pancreatic cancer in the less than TSz 1 period. This case report is therefore vitally important as it increases clinical awareness of the fact that early identification of these lethal cancers might give physicians an extension of time to intervene and perform possibly curative therapies which could lead to improved survival rates in these patients. The present case may shed light on the natural history of the early pancreatic cancer.
Conflict of interest statement
None.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Authors’ contributions
Tsukasa Nakamura is the surgical fellow in charge of patient care. He is also the primary surgeon who performed the whole operations (pancreaticoduodenectomy), obtained consent, wrote, reviewed and proofread the entire case. In addition to this, he also performed literature review. Koji Masuda is the Registrar responsible for taking intra operative photo, proof-reading the draft and assisting with literature search. Shumpei Harada is the registrar and second surgeon who jointly performed the operation. He also assisted in writing the case, proof-reading it and reviewing the draft several times. Kiyokazu Akioka is a consultant of the unit, providing overall supervision, direction and suggestion for the case report. Hirotaka Sako is a consultant of the unit, responsible overall for the care of patient. He also reviewed and proofread the draft and offered critical views.
References
- 1.Parkin D.M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA: A Cancer Journal for Clinicians. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Tsuchiya R., Noda T., Harada N., Miyamoto T., Tomioka T., Yamamoto K. Collective review of small carcinomas of the pancreas. Annals of Surgery. 1986;203(1):77–81. doi: 10.1097/00000658-198601000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung K.W., Kim M.H., Lee T.Y., Kwon S., Oh H.C., Lee S.S. Clinicopathological aspects of 542 cases of pancreatic cancer: a special emphasis on small pancreatic cancer. Journal of Korean Medical Science. 2007;22(Suppl.):S79–S85. doi: 10.3346/jkms.2007.22.S.S79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egawa S., Takeda K., Fukuyama S., Motoi F., Sunamura M., Matsuno S. Clinicopathological aspects of small pancreatic cancer. Pancreas. 2004;28(3):235–240. doi: 10.1097/00006676-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Ariyama J., Suyama M., Satoh K., Sai J. Imaging of small pancreatic ductal adenocarcinoma. Pancreas. 1998;16(3):396–401. doi: 10.1097/00006676-199804000-00030. [DOI] [PubMed] [Google Scholar]
- 6.Carpelan-Holmstrom M., Nordling S., Pukkala E., Sankila R., Luttges J., Kloppel G. Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re-evaluating the data of the Finnish Cancer Registry. Gut. 2005;54:385–387. doi: 10.1136/gut.2004.047191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mertz H.R., Sechopoulos P., Delbeke D., Leach S.D. EUS, PET, and CT scanning for evaluation of pancreatic adeno-carcinoma. Gastrointestinal Endoscopy. 2000;52:367–371. doi: 10.1067/mge.2000.107727. [DOI] [PubMed] [Google Scholar]
- 8.Yasuda I., Iwashita T., Doi S., Nakashima M., Moriwaki H. Role of EUS in the early detection of small pancreatic cancer. Digestive Endoscopy. 2011;23(Suppl. 1):22–25. doi: 10.1111/j.1443-1661.2011.01113.x. [DOI] [PubMed] [Google Scholar]
- 9.Norton L. A Gompertzian model of human breast cancer growth. Cancer Research. 1988;48(December (24, Pt 1)):7067–7071. [PubMed] [Google Scholar]
- 10.Retsky M.W., Swartzendruber D.E., Wardwell R.H., Bame P.D. Is Gompertzian or exponential kinetics a valid description of individual human cancer growth? Medical Hypotheses. 1990;33(October (2)):95–106. doi: 10.1016/0306-9877(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 11.Furukawa H., Iwata R., Moriyama N. Growth rate of pancreatic adenocarcinoma: initial clinical experience. Pancreas. 2001;22(May (4)):366–369. doi: 10.1097/00006676-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Hisa T., Ohkubo H., Shiozawa S., Ishigame H., Takamatsu M., Furutake M. Growth process of small pancreatic carcinoma: a case report with imaging observation for 22 months. World Journal of Gastroenterology. 2008;14(March (12)):1958–1960. doi: 10.3748/wjg.14.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanton K.J., Sidner R.A., Miller G.A., Cummings O.W., Schmidt C.M., Howard T.J. Analysis of Ki-67 antigen expression, DNA proliferative fraction, and survival in resected cancer of the pancreas. American Journal of Surgery. 2003;186(November (5)):486–492. doi: 10.1016/j.amjsurg.2003.07.002. [DOI] [PubMed] [Google Scholar]


