Abstract
INTRODUCTION
A phyllodes tumor is a neoplasm of mixed mesenchymal and epithelial origin affecting the breast. It may pursue a benign or malignant evolution with distant metastases in the latter case. Sites most commonly affected by metastases are the lungs and bones. Simple mastectomy is the mainstay of treatment. This article presents the first described case of metastasis to the adrenal gland after sarcomatous transformation of a phyllodes tumor. A review of the literature is presented afterwards.
PRESENTATION OF CASE
A 57-year old female patient presented with a voluminous breast mass which was completely resected. Unfortunately she presented with malignant recurrence in the breast which was also resected. A later recurrence within the lung presented and was completely resected but showed aspects of sarcomatous changes. Finally a recurrence was pathologically documented within the adrenal gland. Unfortunately, disease later progressed and the patient refused further treatment at that point.
DISCUSSION
While malignant transformation of breast phyllodes tumors and metastasis is relatively common, the prognosis for initially benign lesion that are completely excised is usually good. This case represents the first documented metastasis to the adrenal gland of a breast phyllodes tumor.
CONCLUSION
We presented the first case of adrenal metastasis of a phyllodes tumor after sarcomatous degeneration. This is an unusual presentation of a relatively uncommon but well-recognized disease of variable malignant potential.
Abbreviation: CT, Computerized tomodensitometry
Keywords: Phyllodes tumor, Breast tumor, Breast surgery
1. Introduction
Phyllodes tumors of the breast are relatively uncommon. They originate from both mesenchymal and epithelial cells of the breast tissue.1 The majority of these tumors are benign but some show malignant potential and tend to metastasize. Recurrences may happen and tend to be increasing in aggressiveness until a frank sarcoma is present. The commonest sites of metastases are the lungs and bones, but other sites have also been described.2 We present the case of a patient with recurrent phyllodes tumor with adrenal gland metastasis. To the authors’ knowledge, this is the first case described in the literature.
2. Case report
A 57-year old female patient presented in early 2001 with a large (20 cm) tumor of the left breast. She was not known for any genetic syndrome and had received a bilateral salpingo-ovariectomy and hysterectomy for benign disease in the past. A biopsy of the breast mass revealed a low-grade phyllodes tumor. The patient had a large breast (cup size DD) so a breast-conserving surgery was performed. The pathology report confirmed the presence of a 23 cm × 18 cm low-grade benign phyllodes tumor.
One year later, the patient presented two new lesions in the left breast. A core-needle biopsy confirmed the recurrence of phyllodes tumor. A total mastectomy was then performed, with negative macroscopic margins. At pathology, three lesions were identified, two of high-grade morphology and one of intermediate grade. Fig. 1 shows an increase in cellularity, pleomorphism and mitoses, all suggesting a malignant disease. Margins were clear of disease and there was no lympho-vascular invasion. Histopathologic assessment of two nodes yielded negative results.
Fig. 1.
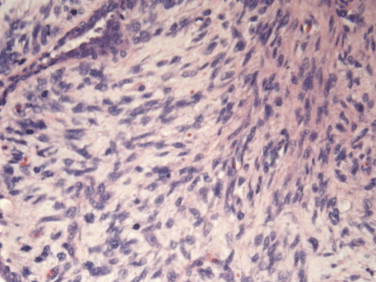
High power field of the modified radical mastectomy. Histology shows hypercellularity, pleomorphism, and cellular condensation around the epithelial structures with numerous mitoses. A diagnosis of malignant phyllodes tumor was established.
After 5 years of uneventful evolution, the patient started to present shortness of breath. A chest X-ray was performed and a new nodule was present on the right lung. A thoraco-abdominal CT scan showed the presence of a 16 mm nodule in the apical segment of the right lower lobe, and no other suspicious lesion. A PET-scan confirmed the presence of this lesion with no other suspicious lesion. A trans-thoracic biopsy of the nodule revealed mesenchymal tumor of unknown origin with marked pleomorphism and frequent mitoses. The patient was then operated for a right superior and middle bilobectomy. Pathology reported a sarcomatous tumor with glandular structures surrounded by fusiform cells with increased pleomorphism, atypia and many mitoses (more than 10 per high power field). Immunohistochemistry showed positivity for CD10 and vimentine. Pathology was compatible with metastasis originating from the initial breast phyllodes tumor. The bronchial margins were at least 3 cm but there was invasion of the visceral pleura.
One year later, the patient presented weight loss and fatigue. A CT-scan showed two new lesions: a left adrenal tumor measuring 8.2 cm × 9.3 cm with heterogeneous composition (Fig. 2) and a 3.4 cm × 3.6 cm pelvic tumor of the same radiologic appearance as the adrenal tumor. There was a plane between the adrenal and the left kidney. The PET scan revealed moderate hyperactivity in the adrenal lesion and no activity in the pelvic lesion. The patient underwent left adrenalectomy and the pathology report showed hypercellular mesenchymal proliferation with marked nuclear pleomorphism and numerous mitoses without glandular proliferation (Fig. 3). After comparison, the adrenal tumor displayed the same histologic pattern as the lung tumor. Hence, pathologic analysis suggested an high-grade adrenal metastasis of a phyllodes tumor of the breast, with an intact capsule. During the same operation, it was found that the pelvic tumor was in fact retro-rectal and was fixed. A biopsy of the mass revealed a carcinomatous architecture (glandular). Immunohistochemistry on the retro-rectal tumor showed positivity for cytokeratin 7, negativity for CD10, and diffuse positivity for estrogen receptors, suggesting a gynecologic origin. A diagnosis of retro-rectal clear-cell adenocarcinoma was established, and it was clear that this tumor was totally different from the adrenal tumor.
Fig. 2.
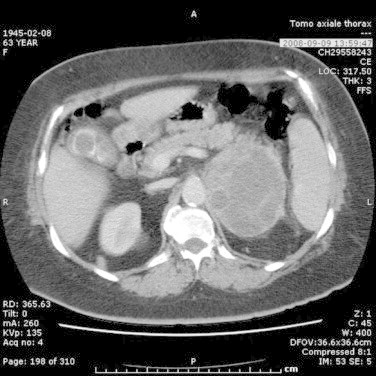
Abdominal CT scan showing heterogeneous left adrenal mass.
Fig. 3.
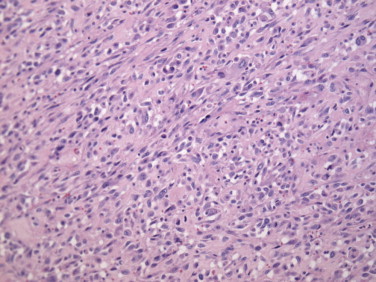
Histology of the adrenal tumor shows hypercellular mesenchymal proliferation. Comparison with the lung tumor histology suggested another metastasis of the phyllodes tumor.
A consultation in medical genetics yielded no conclusion with regards to the etiology of this patient's disease. Her case was presented at multiple tumor boards and she received five cycles of systemic chemotherapy (gemcitabine and docetaxel) for the retro-rectal clear cell adenocarcinoma with complete response confirmed by PET scan in March 2009.
A few months later, the patient complained of generalized fatigue. An abdominal ultrasound revealed a 22 cm mass in the region of the left adrenal area pushing and possibly involving the left kidney. A new PET-CT in August 2009 revealed hypermetabolic lesions in the left adrenal region, in both lungs, and possible diffuse bone marrow invasion. The patient refused further investigation and treatment and opted for palliative care. She unfortunately died about 8 months later, so around 9 years after the initial diagnosis.
3. Discussion
Phyllodes tumors of the breast (cystosarcoma phyllodes) are relatively uncommon but well-recognized tumors. They represent less than 1% of all breast tumors and they occur mostly in women aged 35–55 of Latin or East Asian descent.3 They can be caused by genetic syndromes, notably Li–Fraumeni syndrome, which is related to breast cancers, brain tumors, soft tissue sarcomas, bone sarcomas and rarely other types of tumors. Histologically, phyllodes tumors are described as a mixed epithelial and mesenchymal tumor with the mesenchymal aspect usually being the only malignant one. Most phyllodes tumors are benign and do not metastasize, but recurrence rates are not negligible. In around 10–20% of all phyllodes tumors, a malignant potential is identified, with recurrence rates quite high (30–40%). Metastases may occur in 25–40% of cases.4 When metastasis does occur, it happens on average 2 years after the initial diagnosis.5 The most common sites of distant metastasis are lungs and bones. Some cases of metastases to the mediastinum or central nervous system have been reported, but none for adrenal metastasis.6
Interestingly, recurrent phyllodes tumors are known to be more aggressive, with a higher rate of another recurrence or metastasis. This seemed to be the case with our patient. Diagnosis may be suggested by mammography (large well defined oval or lobulated tumor with coarse microcalcifications) or by breast ultrasound (heterogeneous, solid mass sometimes associated with cystic components) but unfortunately, an out of doubt distinction with fibroadenoma is not possible. Excisional biopsy is thus required since diagnosis is based on histopathological elements and architecture. Also, immuno-histological pathological assays represent important ancillary techniques.7 For instance, significant markers related to the malignant potential of the tumor are Ki67, CD10, CD117 and p53. Vimentin is expressed by the mesenchymal component whereas cytokeratines, normally associated with epithelial tumors, are usually absent in the mesenchymal tissue of the tumor. It is expressed exclusively in the epithelial component.7,8 The recommended treatment of primary phyllodes tumors, local recurrence and distant metastasis is surgery with clear margins. In fact, clear margins reduce recurrence by about 50% and seem to be the most important prognostic factor.9,10 Another prognostic factor influencing recurrence and disease-free survival is the initial grade of the phyllodes tumor.11 Even if our patient underwent lymph node sampling and total mastectomy, the indication to perform lymph node dissection has been studied, and as with other types of sarcomas, does not seem to provide any survival benefit. In fact, phyllodes tumors do not seem to metastasize to lymph nodes. Radiotherapy is currently not recommended; neither are chemotherapy or hormonotherapy.12,13
4. Conclusion
Phyllodes tumors are rare tumors of mixed mesenchymal and epithelial origin affecting the breast. Although they are most often benign, they can be malignant and metastasize. In cases of metastases, the lungs and bones are the most common sites affected. To the authors’ knowledge, this is the first case of adrenal metastasis of a phyllodes tumor of the breast. It brings a new entity in the differential diagnosis of adrenal tumors. The case also shows that phyllodes tumors must be treated aggressively since they do have a potential for high malignancy and fatality.
Conflict of interest statement
None
Funding
No funding was used for this paper.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
Yves Collin reviewed the chart and wrote the paper. François Chagnon reviewed the literature, counseled the theoretic aspects of the text, wrote parts of the text and reviewed the paper. These two are the main authors. Charles Mongeau was a pathologist and acted as the consultant for the pathological aspects. Gonzalo L. Gonzalez-Amaya was the patient's surgeon and counseled the writing process too. Lucas Sideris reviewed the paper and counseled the oncological parts of it.
Contributor Information
Yves Collin, Email: yves.collin@usherbrooke.ca.
François Chagnon, Email: chagnonf@yahoo.com.
Charles J. Mongeau, Email: cjmongeau@videotron.ca.
Lucas Sideris, Email: lucas.sideris@umontreal.ca.
References
- 1.Rosen P.P. 3rd ed. Lippincott Williams & Wilkins; Philadelphia: 2009. Rosen's breast pathology. [Google Scholar]
- 2.Reinfuss M., Mituś J., Smolak K., Stelmach A. Malignant phyllodestumours of the breast. A clinical and pathological analysis of 55 cases. European Journal of Cancer. 1993;29A(9):1252–1256. doi: 10.1016/0959-8049(93)90067-p. [DOI] [PubMed] [Google Scholar]
- 3.Abeel A., Mangi M., Barbara L., Smith M.P., Michele A., Gadd M. Surgical management of phyllodes tumors. Archives of Surgery. 1999;134:487–491. doi: 10.1001/archsurg.134.5.487. [DOI] [PubMed] [Google Scholar]
- 4.Feig B.W., Berger D.H., Fuhrman G.M. 4th ed. Lippincott Williams & Wilkins; Houston, TX: 2006. The M.D. Anderson surgical oncology book. p. 57 [Chapter 2] [Google Scholar]
- 5.Beverley A., Carter M., David L., Page M. Phyllodes tumor of the breast: local recurrence versus metastatic capacity. Human Pathology. 2004;35(9):1051–1052. doi: 10.1016/j.humpath.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Edna K., Valdes M., Susan K., Boolbol M., Jean-Marc Cohen M., Sheldon M. Malignant transformation of a breast fibroadenoma to cystosarcoma phyllodes: case report and review of the literature. American Surgeon. 2005;71(4):348–352. [PubMed] [Google Scholar]
- 7.Schnitt S.J., Collin L.C. Lippincott Williams & Wilkins; Philadelphia: 2009. Biopsy interpretation of the breast; pp. 166–178. [Google Scholar]
- 8.Jacklin R.K.P.F. Optimising preoperative diagnosis in phyllodes tumour of the breast. Journal of Clinical Pathology. 2006;59:454–459. doi: 10.1136/jcp.2005.025866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan P.H., Jayabaskar T., Chuah K.L., Lee H.Y., Tan Y., Hilmy M. Phyllodes tumors of the breast: the role of pathologic parameters. American Journal Clinical Pathology. 2005;123:529–540. doi: 10.1309/U6DV-BFM8-1MLJ-C1FN. [DOI] [PubMed] [Google Scholar]
- 10.Confavreux C., Lurkin A., Mitton N., Blondet R., Saba C., Ranchère D. Sarcomas and malignant phyllodes tumours of the breast – a retrospective study. European Journal of Cancer. 2006;42:2715–2721. doi: 10.1016/j.ejca.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 11.Mohsen Shabahang M.P., Dido Franceschi M., Magesh Sundaram M., Manuel H., Castillo M., Frederick L. Surgical management of primary breast sarcoma. American Surgeon. 2002;68:673–674. [PubMed] [Google Scholar]
- 12.Parker S.J., Harries S.A. Phyllodes tumours. Postgrad Medical Journal. 2001;77:428–435. doi: 10.1136/pmj.77.909.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feig B., Ching C.D. 5th ed. Lippincott Williams & Wilkins; Houston, TX: 2012. The MD Anderson surgical oncology handbook; pp. 79–80. [Google Scholar]


