Abstract
The human immunodeficiency virus Tat regulatory protein is essential for virus replication and pathogenesis. From human peripheral blood mononuclear cells of three Tat toxoid-immunized volunteers, we isolated five Tat-specific human monoclonal antibodies (HMAbs): two full-length immunoglobulin G (IgG) antibodies and three single-chain fragment-variable (scFv) antibodies. The two IgGs were mapped to distinct epitopes within the basic region of Tat, and the three scFvs were mapped to the N-terminal domain of Tat. The three scFvs were highly reactive with recombinant Tat in Western blotting or immunoprecipitation, but results were in contrast to those for the two IgGs, which are sensitive to a particular folding of the protein. In transactivation assays, scFvs were able to inhibit both active recombinant Tat and native Tat secreted by a transfected CEM cell line while IgGs neutralized only native Tat. These HMAbs were able to reduce viral p24 production in human immunodeficiency virus type 1 strain IIIB chronically infected cell lines in a dose-dependent manner.
Human immunodeficiency virus type 1 (HIV-1) encodes both structural and regulatory proteins important in the pathogenesis of AIDS. Among the regulatory proteins, Tat is a small (14 kDa) 86- to 101-amino-acid nuclear protein secreted early after infection and is absolutely required for efficient transcription of HIV-1 provirus and viral replication (4). Despite its nuclear localization and function and the lack of any secretory signal sequence, Tat is released in vitro by infected cells and can bind and translocate to the cell membrane of different bystander uninfected cells (8). Extracellular Tat exerts many immunosuppressive functions, such as inhibition of interleukin-12 production by human peripheral blood mononuclear cells (PBMCs) (14), production of alpha interferon (34), inhibition of T-cell proliferation with mitogens or antigens (32), and induction of HIV-1 coreceptor expression (27), as well as many other deleterious biological effects (9). Low levels of extracellular Tat were detected in vivo in the serum of HIV-infected patients (33), but at these concentrations Tat is physiologically active in vitro. High anti-Tat antibody titers in asymptomatic patients who progress slowly to the disease have been reported and decrease with AIDS symptoms (21, 35). The natural innate immunoglobulin M (IgM) antibodies directed against two defined sequences of Tat may also provide initial defense against the pathological effects of extracellular Tat after HIV infection (24).
In the Tat protein, four B-cell linear epitopes were identified but only two regions (amino acids [aa] 1 to 12 and 41 to 50) have limited antigenic polymorphism among HIV-1 strains (10) and may be of potential value in developing a universal Tat immunogen or reactive human anti-Tat antibody preparation for passive immunotherapy. Some murine monoclonal antibodies (MAbs) to Tat protein block exogenous Tat-mediated transactivation (31) or attenuate primary HIV-1 infection and replication in chronically infected cell lines (20, 28). These antibodies can also abolish the intercellular traffic of the extracellular Tat and the corresponding biological responses (5). Suitable therapeutic agents such as human monoclonally specific antibodies able to bind strongly to the extracellular Tat can conceivably be capable of inhibiting the deleterious functions of Tat. Only two previous reports described human MAbs (HMAbs) against Tat (19, 24). Here we describe the generation of five new HMAbs directed against the two key epitopes of Tat, two complete IgGs and three single-chain fragment-variable (scFv) antibodies, and we assess their abilities to block Tat-induced transactivation and viral replication.
PBMCs were purified from blood obtained from two healthy HIV-negative volunteers (J and G) and from one HIV-1-seropositive patient (B) who were all immunized with Tat toxoid (11, 12). The three sera presented high antibody titers to Tat (1/16,000 and 1/32,000 for subjects J and G, respectively, and 1/500 for patient B) and inhibited Tat-mediated transactivation (17). The PBMCs of the three individuals were used for Epstein-Barr virus B-cell immortalization as previously described (6) and also for mRNA extraction to generate cDNA libraries. After immortalization, only two lymphoblastoid cell lines (J and B) produced Tat-specific antibodies, and two stable clones, J3B2 (IgG1λ) and B1E3 (IgG1κ), reactive in enzyme-linked immunosorbent assay (ELISA) with recombinant Tat (rTat), were established. A glutathione S-transferase-Tat expression vector was provided by Andrew Rice, and rTat was purified following the instructions of the AIDS Research and Reference Reagents Program Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (22) and used for ELISA. Two distinct IgG(κ+λ) scFv libraries were generated from J and G cDNA libraries by using an IgG VH- and VL-specific primer set (25) and were selectively enriched for rTat-binding phages in immunotubes as previously described (26). Nineteen of 96 picked-up clones of the fourth panning of library J showed high reactivity to rTat in ELISA, but all shared the same restriction pattern in DNA fingerprinting (scFv J1). Thirty phage scFvs out of 92 were positive in ELISA for library G, and two distinct clones were isolated (scFv G1 and scFv G2). scFvs were analyzed for their DNA sequences with an ABI PRISM 3100 Genetic Analyzer (Perkin-Elmer/Applied Biosystem Division, Foster City, Calif.) using the BigDye Terminator Cycle sequencing kit (Perkin-Elmer/Applied Biosystem Division). scFv G1- and G2-deduced amino acid sequences share the same Vκ light chain but show differences in the VH3 heavy chain (G1 CDR3, RGSTGKALDYCSPRTL; G2 CDR3, ERSQQHCNPSLLHSNGKNYAE). The clone J1 sequence (VH3/Vλ) is unrelated to those of G1 and G2 (J1 CDR3, RDRYCSSPGCYKGADGGRLKDY). These three Tat ELISA-reactive phage scFv clones were propagated in Escherichia coli HB2151 for production of soluble scFv bearing a Pk tag for immunodetection and a 6× His tag for purification, using an Ni-nitrilotriacetic acid column (Amersham Biosciences, Saclay, France).
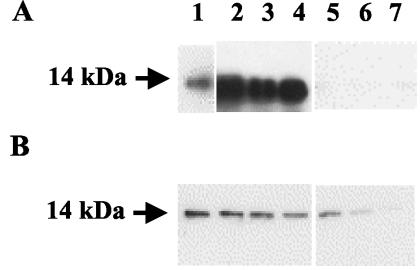
To determine the nature of the epitopes recognized by the different HMAbs, polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate (SDS-PAGE) was carried out (15) with 0.5 μg of denatured rTat protein per well. After transfer to a nitrocellulose membrane (Schleicher & Schuell, Ecquevilly, France) the three scFvs (J1, G1, and G2) stained a 14-kDa band detected with anti-pK tag antibody (Serotec, Oxford, United Kingdom) followed by horseradish peroxidase-conjugated anti-mouse antibody for scFvs, but no protein band was visualized with the IgGs J3B2 and B1E3 with horseradish peroxidase-conjugated rabbit anti-human IgG (Dakopatts A/S, Glostrup, Denmark) (Fig. 1A). The epitope recognized by scFvs is most likely linear, as they bind rTat under denaturing conditions in Western blotting. Soluble rTat was immunoprecipitated with the different HMAbs in the presence of protein G-Sepharose beads (Sigma, Saint-Quentin Fallavier, France) for IgG or by Ni-nitrilotriacetic acid-Superflow agarose beads (Qiagen, Courtaboeuf, France) for scFv. The immunocomplexes were dissociated by boiling in Laemmli sample buffer, and then SDS-PAGE and membrane transfer were performed as described above. The presence of Tat was detected in Western blotting with a rabbit antiserum to HIV-1 Tat (Bryan Cullen, National Institutes of Health) (13). The three anti-Tat scFvs and IgG J3B2 demonstrate a strong binding to soluble rTat protein, while IgG B1E3 seems to weakly react with its target in solution (Fig. 1B). This could suggest that the two IgGs bind Tat only when it displays a particular conformation and not when the protein is totally unfolded under denaturing conditions.
FIG. 1.
Immunoreactivity of HMAbs to rTat. (A) rTat submitted to SDS-PAGE and Western blotting was detected by antiserum from a Tat-immunized human volunteer (lane 1), scFv G1 (lane 2), scFv G2 (lane 3), scFv J1 (lane 4), IgG J3B2 (lane 5), IgG B1E3 (lane 6), and control IgG (lane 7). (B) rTat was first immunoprecipitated with the antibodies indicated for panel A, SDS-PAGE and Western blotting were carried out, and immunodetection was performed using antiserum from a Tat-immunized rabbit.
To map precisely the epitopes recognized by the HMAbs, ELISA was carried out as described previously (1) using 100 ng of different overlapping peptides spanning the entire consensus sequence of the HIV-1 clade B Tat protein. The long Tat fragments aa 8 to 53, 19 to 53, 1 to 20, and 44 to 61 were synthesized according to published methods (3, 16). A complete set of HIV-1 clade B consensus short overlapping Tat peptides (15 aa) was obtained through the AIDS Research and Reference Reagent Program. As seen in Table 1, the three scFvs (G1, G2, and J1) recognized only the N-terminal peptide 1-20 (aa 1 to 20) while IgG J3B2 reacted with peptide 44-61 and IgG B1E3 reacted with two peptides, 19-53 and 44-61. With short overlapping peptides covering the Tat N-terminal domain the three scFvs reacted with peptide 1-15 but not with peptide 5-19. IgG B1E3 and J3B2 reacted with peptide 33-47 and peptide 37-51 (Table 1). The two IgGs are thus directed to the basic domain of Tat, but as their ELISA reaction patterns differ slightly, they may recognize distinct epitopes within this region. This site is also considered an immunodominant Tat epitope, and no polymorphism among the different HIV-1 clades in this sequence has been observed (10).
TABLE 1.
ELISA reaction patterns of anti-Tat HMAbs
| HMAb |
A405 valuea
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synthetic peptides
|
Overlapping peptides
|
rTat 1-86 | ||||||||||
| 1-20 | 8-53 | 19-53 | 44-61 | 1-15 | 5-19 | 9-23 | 33-47 | 37-51 | 41-55 | 45-59 | ||
| scFv G1 | 0.70 | 0.06 | 0.07 | 0.06 | 0.94 | 0.07 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 1.10 |
| scFv G2 | 0.49 | 0.04 | 0.08 | 0.04 | 0.57 | 0.09 | 0.08 | 0.05 | 0.05 | 0.05 | 0.05 | 0.95 |
| scFv J1 | 0.57 | 0.07 | 0.08 | 0.05 | 0.64 | 0.06 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.94 |
| IgG B1E3 | 0.10 | 0.07 | 0.23 | 0.65 | 0.05 | 0.05 | 0.05 | 0.92 | 0.41 | 0.02 | 0.03 | 0.45 |
| IgG J3B2 | 0.11 | 0.04 | 0.06 | 0.98 | 0.05 | 0.05 | 0.05 | 0.65 | 0.66 | 0.02 | 0.05 | 0.48 |
Results are presented as arithmetic means of absorbance values of three independent experiments.
The HMAbs were tested in ELISA with synthetic Tat proteins from different HIV strains, kindly provided by E. Loret (18), corresponding to clade B, predominant in Europe and the Americas (HXB2), clade E, predominant in Thailand (CM240), clade C, predominant in Brazil (92Br), clade D, predominant in Zaïre (Eli), and clade A, predominant in Uganda (Ug11RP). All scFvs recognized the clade B Tat proteins Bru and HXB2 but did not bind to the others. Tat sequences from all clades except clade B used in this study show a mutation of R to N at position 7 (18). As amino acid polymorphism at this site has been shown to confer distinct antigenic specificities (10), it is very likely that binding of the N-terminus-specific scFvs is affected by a mutation at this position. IgG HMAbs J3B2 and B1E3 bound only to the rTat protein but did not react with any of the synthetic Tat proteins, regardless of the HIV-1 clade, even with synthetic Tat HXB2, which has the same sequence as rTat used as a positive control under the same experimental conditions. Valvatne et al. (31) observed the same discrepancy with four mouse MAbs to different Tat proteins, depending on the origin of the recombinant antigens. These data confirm that binding of the HMAbs directed to the basic region may be highly dependent on a particular folding of the Tat protein. Fully synthetic Tat proteins may not display the specific structure that allows IgGs B1E3 and J3B2 to bind. This feature was also observed when determining the affinity constants of the HMAbs for synthetic Tat peptides. The kinetic and equilibrium constants for biotinylated peptides corresponding to aa 1 to 20 and 39 to 86 of Tat were calculated according to a previously described method (23) using the Biacore 3000 system (Biacore, Uppsala, Sweden). Affinity constants of the three scFvs for aa 1 to 20 were very high (Kd of 0.37 nM for G1, 0.086 nM for G2, and 0.44 nM for J1). IgG B1E3 showed a high affinity (Kd of 0.2 nM) to aa 39 to 86, but J3B2 failed to interact with this synthetic peptide.
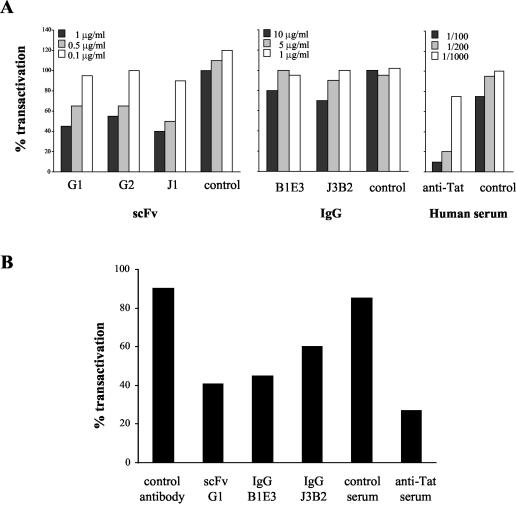
HMAbs were then assessed for their biological properties relative to Tat activity. The ability of HMAbs to interfere with Tat-driven transactivation was assayed by inducing long terminal repeat (LTR)-dependent chloramphenicol acetyltransferase (CAT) production in HL3T1 cells containing an integrated HIV-1 LTR-CAT gene construct (7). To inhibit transactivation, serial dilutions of anti-Tat HMAbs were preincubated for 60 min with 250 ng of exogenous active rTat protein from HIV-1 strain IIIB per ml (kindly provided by Aventis Pasteur, Lyon, France) and put in contact for 90 min with HL3T1 cells (80% confluence) in HL-1 serum-free medium (Invitrogen, Cergy Pontoise, France) in the presence of 100 μM chloroquine (Sigma) (8). Tat medium was replaced by fresh HL-1 medium with 100 μM chloroquine, and cells were cultured for 24 h. HL3T1 cells were washed and lysed, and CAT production was monitored by using a CAT ELISA kit (Roche Diagnostics, Meylan, France). Dose-dependent neutralization of Tat transactivation by the three scFvs (G1, G2, and J1) was observed (Fig. 2A), reaching nearly 70% of inhibition when 1 μg of J1/ml was used. When assayed at 10 μg/ml, IgG J3B2 and IgG B1E3 showed, respectively, 30 and 20% inhibition of transactivation. Control serum or antibodies did not show any relevant effect, but serum from a Tat toxoid-vaccinated donor clearly inhibited Tat transactivation in a dose-dependent manner.
FIG. 2.
Neutralization of Tat transactivation by HMAbs. LTR-dependent CAT production by HL3T1 cells was measured after preincubation of active rTat with increasing concentrations of antibodies (A) or after coculture with CEM cells transiently expressing native Tat 1-72 in the presence of fixed amounts of antibodies (5 μg/ml for G1 and 10 μg/ml for B1E3, J3B2, and control antibody) (B). Results are expressed as percentages of transactivation, considering 100% as the value obtained with Tat alone. Data provided are the mean values from three independent experiments (standard deviation, <10% in all experiments).
Previous reports stated that antibodies directed to the Tat N-terminal domain or to the basic region could equally inhibit exogenous Tat-induced transactivation by preventing Tat uptake by the cell (29, 30). The limited ability of B1E3 and J3B2 to neutralize transactivation in this assay could be linked to their poor reactivity to rTat, as observed in Western blotting, immunoprecipitation, or Biacore experiments, while scFvs seem to bind strongly to the rTat N-terminal epitope under these experimental conditions. To mimic the in vivo situation, we submitted the five HMAbs to a similar LTR-CAT transactivation assay by cocultivating HL3T1 cells with CEM cells, producing a native Tat protein. CEM cells were washed with phosphate-buffered saline and electroporated with pSV2tat72 plasmid DNA (1 μg/106 cells), allowing a transient expression of a 72-aa active Tat (8). CEM transformed cells (106/well) were transferred to a 12-well plate containing HL3T1 cells (80% confluence), and HMAbs and control antibodies were added to the wells. The coculture was carried out at 37°C with 5% CO2 for 48 h, and CAT production by HL3T1 cells was assayed as described above. Transiently transfected CEM cells were able to release Tat extracellularly in its active form at a concentration sufficient to induce CAT production in HL3T1 cells. As shown in Fig. 2B, this Tat-dependent transactivation was inhibited by all the anti-Tat HMAbs tested in this assay, ranging from 60% inhibition of Tat activation by scFv G1 (representative of N-terminus-specific scFvs) to 55% for IgG B1E3 and 40% for IgG J3B2. Irrelevant control antibody showed a weak nonspecific effect on Tat transactivation (below 15% inhibition). This inhibition of intercellular traffic of extracellular native Tat by a mouse MAb directed to the N terminus of Tat has also been reported by Demirhan et al. (5). These results highlight the fact that IgGs B1E3 and J3B2 are able to bind and neutralize Tat protein produced by human cells in its native form. These HMAbs’ characteristics could contribute to resolution of the three-dimensional structure of Tat.
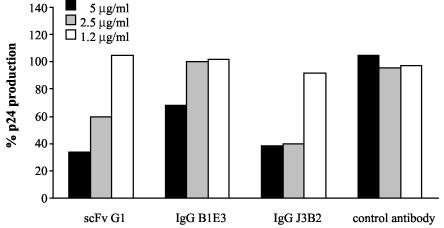
We then studied the neutralizing potential of these HMAbs against viral replication. H9 cells chronically infected with HIV-1 (strain IIIB) were cultured in RPMI medium supplemented with 10% fetal calf serum and seeded (2 × 105 cells/well) in a 48-well plate. Dilutions of HMAbs and irrelevant control antibody were added to the wells, and infected-cell culture was carried on for 72 h. Supernatants were harvested and measured for HIV antigen p24 by using an ELISA kit (Murex HIV Antigen MAb; Laboratoires Abbott, Rungis, France). scFv G1 and IgG J3B2 displayed more than 50% inhibition of p24 production when used at concentrations of 5 and 2.5 μg/ml, and IgG B1E3 at 5 μg/ml showed a 30% inhibition (Fig. 3). These results indicate that inhibition of secreted Tat contributes significantly to hampering viral replication in vitro. Such involvement of an autocrine-paracrine loop sustained by actively released Tat protein has been previously documented (20, 28).
FIG. 3.
Inhibition of HIV-1 IIIB replication by HMAbs. Production of p24 HIV antigen by H9 chronically infected cells in the presence of HMAbs or control antibody was measured after 3 days of culture. Results are expressed as percentages of p24 production, considering 100% as the value obtained without antibodies in the culture medium. Data provided come from a typical experiment.
Extracellular Tat plays an important role in the course of HIV-1 infection, and Tat-specific antibodies could be effective in minimizing chronic viremia and progression to AIDS. Active or passive immunizations with Tat have been proposed, assuming that the Tat sequence would be sufficiently conserved among the different virus strains. However, a study of mouse MAb binding specificities showed clearly some variability in the Tat protein (2). The anti-Tat HMAbs obtained in this study are directed to two B-cell epitopes which overlap with functionally active domains of Tat and show limited (N-terminal region) or no (basic region) antigenic polymorphism in the geographically diverse strains (10). These HMAbs could be studied for their potential role in passive immunotherapy in macaque models to abolish Tat extracellular pleiotropic effects and to prevent progression to AIDS when combined with drugs directed to other regulatory and structural viral proteins.
Acknowledgments
We thank Aventis Pasteur (Lyon, France) for providing the active recombinant Tat, E. Loret for the synthetic Tat proteins of different HIV-1 strains, and D. Sblattero and A. Bradbury for the pDAN phagemid vector. We also thank A. Blayac and M. Juan for excellent technical assistance.
This study was supported by grants from the French National AIDS Research Agency (ANRS) and Ensemble contre le SIDA (Sidaction). E.M. was supported by a fellowship from ProMetic Biosciences Inc, Montréal, Canada.
REFERENCES
- 1.Belliard, G., A. Romieu, J. Zagury, H. Dali, O. Chaloin, R. Le Grand, E. Loret, J. P. Briand, B. Roques, C. Desgranges, and S. Muller. 2003. Specificity and effect on apoptosis of Tat antibodies from vaccinated and SHIV-infected rhesus macaques and HIV-infected individuals. Vaccine 21:3186-3199. [DOI] [PubMed] [Google Scholar]
- 2.Brake, D. A., J. Goudsmit, W. J. A. Krone, P. Schammel, N. Appleby, R. H. Meloen, and C. Debrouck. 1990. Characterization of murine monoclonal antibodies to the Tat protein from human immunodeficiency virus type 1. J. Virol. 64:962-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornille, F., K. Wecker, A. Loffet, R. Genet, and B. Roques. 1999. Efficient solid-phase synthesis of Vpr from HIV-1 using low quantities of uniformly 13C-, 15N-labeled amino acids for NMR structural studies. J. Pept. Res. 54:427-435. [DOI] [PubMed] [Google Scholar]
- 4.Dayton, A. I., J. G. Sodroski, C. A. Rosen, W. C. Goh, and W. A. Haseltine. 1986. The trans-activator gene of human T cell lymphotropic virus type III is required for replication. Cell 44:941-947. [DOI] [PubMed] [Google Scholar]
- 5.Demirhan, I., A. Chandra, O. Hasselmayer, and P. Chandra. 1999. Intercellular traffic of human immunodeficiency virus type 1 transactivator protein defined by monoclonal antibodies. FEBS Lett. 445:53-56. [DOI] [PubMed] [Google Scholar]
- 6.Desgranges, C., J. Paire, C. Pichoud, S. Souche, D. Frommel, and C. Trepo. 1987. High affinity human monoclonal antibodies directed against hepatitis B surface antigen. J. Virol. Methods 16:281-292. [DOI] [PubMed] [Google Scholar]
- 7.Felber, B. K., and G. N. Pavlakis. 1988. A quantitative bioassay for HIV-1 based on trans-activation. Science 239:184-187. [DOI] [PubMed] [Google Scholar]
- 8.Frankel, A. D., and C. O. Pabo. 1988. Cellular uptake of the Tat protein from human immunodeficiency virus. Cell 55:1189-1193. [DOI] [PubMed] [Google Scholar]
- 9.Gallo, R. C. 1999. Tat as one key to HIV-induced immune pathogenesis and Pat toxoid as an important component of a vaccine. Proc. Natl. Acad. Sci. USA 96:8324-8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein, G., G. Tribbick, and K. Manson. 2001. Two B cell epitopes of HIV-1 Tat protein have limited antigenic polymorphism in geographically diverse HIV-1 strains. Vaccine 19:1738-1746. [DOI] [PubMed] [Google Scholar]
- 11.Gringeri, A., E. Santagostino, M. Muça-Perja, H. Le Buanec, B. Bizzini, A. Lachgar, J. F. Zagury, J. Rappaport, A. Burny, R. C. Gallo, and D. Zagury. 1999. Tat toxoïd as a component of a preventive vaccine in seronegative subjects. J. Acquir. Immune Defic. Syndr. 20:371-375. [DOI] [PubMed] [Google Scholar]
- 12.Gringeri, A., E. Santagostino, M. Muca-Perja, P. M. Mannucci, J. F. Zagury, B. Bizzini, A. Lachgar, M. Carcagno, J. Rappaport, M. Criscuolo, W. Blattner, A. Burny, R. C. Gallo, and D. Zagury. 1998. Safety and immunogenicity of HIV-1 Tat toxoid in immunocompromised HIV-1-infected patients. J. Hum. Virol. 1:293-298. [PubMed] [Google Scholar]
- 13.Hauber, J., A. Perkins, E. Heimer, and B. Cullen. 1987. Trans-activation of human immunodeficiency virus gene expression is mediated by nuclear events. Proc. Natl. Acad. Sci. USA 84:6364-6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito, M., T. Ishida, L. He, F. Tanabe, Y. Rongge, Y. Miyakada, and H. Terunuma. 1998. HIV type 1 Tat protein inhibits interleukin 12 production by human peripheral blood mononuclear cells. AIDS Res. Hum. Retrovir. 14:845-849. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 16.Neimark, J., and J. P. Briand. 1993. Development of a fully automated multichannel peptide synthesizer with integrated TFA cleavage capability. Pept. Res. 6:219-228. [PubMed] [Google Scholar]
- 17.Noonan, D. M., A. Gringeri, R. Meazza, O. Rosso, S. Mazza, M. Muça-Perja, H. Le Buanec, R. S. Accolla, A. Albini, and S. Ferrini. 2003. Identification of immunodominant epitopes in inactivated Tat vaccinated healthy and HIV-1 infected volunteers. J. Acquir. Immune Defic. Syndr. 33:47-55. [DOI] [PubMed] [Google Scholar]
- 18.Opi, S., J. M. J. Peloponese, D. Esquieu, G. Campbell, J. de Mareuil, A. Walburger, M. Solomiac, C. Gregoire, E. Bouveret, D. L. Yirrell, and E. P. Loret. 2002. Tat HIV-1 primary and tertiary structures critical to immune response against non-homologous variants. J. Biol. Chem. 27:35915-35919. [DOI] [PubMed] [Google Scholar]
- 19.Pilkington, G. R., L. Duan, M. Zhu, W. Keil, and R. J. Pomerantz. 1996. Recombinant human Fab antibody fragments to HIV-1 Rev and Tat regulatory proteins: direct selection from a combinatorial phage display library. Mol. Immunol. 33:439-450. [DOI] [PubMed] [Google Scholar]
- 20.Re, M. C., G. Furlini, M. Vignoli, E. Ramazzotti, G. Roderigo, V. De Rosa, G. Zauli, S. Lolli, S. Capitani, and M. La Placa. 1995. Effect of antibody to HIV-1 Tat protein on viral replication in vitro and progression of HIV-1 disease in vivo. J. Acquir. Immune Defic. Syndr. 10:408-416. [DOI] [PubMed] [Google Scholar]
- 21.Re, M. C., M. Vignoli, G. Furlini, D. Gibellini, V. Colangeli, F. Vitone, and M. La Placa. 2001. Antibodies against full-length Tat protein and some low-molecular-weight Tat-peptides correlate with low or undetectable viral load in HIV-1 seropositive patients. J. Clin. Virol. 21:81-89. [DOI] [PubMed] [Google Scholar]
- 22.Rhim, H., C. O. Echetebu, C. H. Herrmann, and A. P. Rice. 1994. Wild type and mutant HIV-1 and HIV-2 Tat proteins expressed in Escherichia coli as fusions with glutathione S-transferase. J. Acquir. Immune Defic. Syndr. 7:1116-1121. [PubMed] [Google Scholar]
- 23.Richalet-Secordel, P., N. Rauffer-Bruyere, L. L. Christensen, B. Ofenloch-Haehnle, C. Seidel, and M. Van Regenmortel. 1997. Concentration measurement of unpurified proteins using biosensor technology under conditions of partial mass transport limitation. Anal. Biochem. 249:165-173. [DOI] [PubMed] [Google Scholar]
- 24.Rodman, T. C., J. D. Lutton, S. Jiang, H. B. Al-Kouatly, and R. Winston. 2001. Circulating natural IgM antibodies and their corresponding human cord blood cell-derived Mabs specifically combat the Tat protein of HIV. Exp. Hematol. 29:1004-1009. [DOI] [PubMed] [Google Scholar]
- 25.Sblattero, D., and A. Bradbury. 1998. A definitive set of oligonucleotide primers for amplifying human V regions. Immunotechnology 3:271-278. [DOI] [PubMed] [Google Scholar]
- 26.Sblattero, D., and A. Bradbury. 2000. Exploiting recombination in single bacteria to make large phage antibody libraries. Nat. Biotechnol. 8:75-80. [DOI] [PubMed] [Google Scholar]
- 27.Secchiero, P., D. Zella, S. Capitani, R. C. Gallo, and G. Zauli. 1999. Extracellular HIV-1 Tat protein up-regulates the expression of surface CXC-chemokine receptor 4 in resting CD4+ T cells. J. Immunol. 162:2427-2431. [PubMed] [Google Scholar]
- 28.Steinaa, L., A. M. Sorenson, J. O. Nielsen, and J. E. S. Hansen. 1994. Antibody to HIV-1 Tat proteins inhibits the replication of virus in culture. Arch. Virol. 139:263-271. [DOI] [PubMed] [Google Scholar]
- 29.Tikhonov, I., T. Ruckwardt, G. Hatfield, and C. Pauza. 2003. Tat-neutralizing antibodies in vaccinated macaques. J. Virol. 77:3157-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tosi, G., R. Meazza, A. De Lerma Barbaro, A. D'Agostino, S. Mazza, G. Corradin, A. Albini, D. M. Noonan, S. Ferrini, and R. S. Accolla. 2000. Highly stable oligomerization forms of HIV-1 Tat detected by monoclonal antibodies and requirement of monomeric forms for the transactivating function on the HIV-1 LTR. Eur. J. Immunol. 30:1120-1126. [DOI] [PubMed] [Google Scholar]
- 31.Valvatne, H., A. M. Szilvay, and D. E. Helland. 1996. A monoclonal antibody defines a novel HIV type 1 Tat domain involved in trans-cellular trans-activation. AIDS Res. Hum. Retrovir. 12:611-619. [DOI] [PubMed] [Google Scholar]
- 32.Viscidi, R. P., K. Mayor, H. M. Lederman, and A. D. Frankel. 1989. Inhibition of antigen-induced lymphcyte proliferation by Tat protein from HIV-1. Science 246:1606-1608. [DOI] [PubMed] [Google Scholar]
- 33.Xiao, H., C. Neuveut, H. L. Tiffany, M. Benkirane, E. A. Rich, P. M. Murphy, and K. T. Jeang. 2000. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of co-receptor use by HIV-1. Proc. Natl. Acad. Sci. USA 97:11466-11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zagury, D., A. Lachgar, V. Chams, L. S. Fall, J. Bernard, J. F. Zagury, B. Bizzini, A. Gringeri, E. Santagostino, J. Rappaport, M. Feldman, A. Burny, and R. C. Gallo. 1998. Interferon alpha and Tat involvement in the immunosuppression of uninfected T cells and C-C chemokine decline in AIDS. Proc. Natl. Acad. Sci. USA 95:3851-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zagury, J., A. Sill, W. Blattner, A. Lachgar, H. Le Buanec, M. Richardson, J. Rappaport, H. Hendel, B. Bizzini, A. Gringeri, M. Carcagno, M. Criscuolo, A. Burny, R. C. Gallo, and D. Zagury. 1998. Antibodies to the HIV-1 Tat protein correlated with nonprogression to AIDS : a rationale for the use of Tat toxoid as an HIV-1 vaccine. J. Hum. Virol. 1:282-292. [PubMed] [Google Scholar]