Abstract
Purpose
Porcine proliferative enteropathy (PPE) is known as one of the most important risk factors causing economic losses in swine industry worldwide. This study was conducted to evaluate the efficacy of a commercial oral attenuated Lawsonia intracellularis vaccine (Enterisol Ileitis) against PPE under a commercial pig farm condition in Korea.
Materials and Methods
Thirty two-day-old 672 piglets were randomly allocated into vaccinated and control groups. All piglets in the vaccinated group were inoculated with a commercial attenuated L. intracellularis vaccine as following the manufacturer's instruction. Body weights of all pigs in both groups were measured on the vaccination day and 6, 14, and 20 weeks post vaccination and an average daily weight gain (ADWG) was calculated. Health status was observed biweekly during the whole trial.
Results
The vaccinated group showed significantly higher body weight (p<0.05) and ADWG (p<0.05) than those of the control group. The vaccinated group had significantly reduced impairments in activity, growth, defecation frequency, and stool hardness (p<0.05). Additional health benefits and improved weight gain by the vaccination produced a 4.2:1 return of investment, and the higher gross margin was $4.80 per pig.
Conclusion
Our finding suggests that the L. intracellularis vaccine program has effects on the substantial health and economic benefits in the Korean swine industry.
Keywords: Lawsonia intracellularis, Intestinal diseases, Ileitis, Vaccines, Swine
Introduction
Porcine proliferative enteropathy (PPE), also called ileitis, is a common enteric disease in pigs caused by a gram-negative, obligate intracellular bacterium, Lawsonia intracellularis. The disease is responsible for economic losses, which has been estimated at more than $1.63 ($1=€ 0.8) per infected pig, due to sudden death, bloody diarrhea, stunted growth, increased body weight variation and increased costs of feeding and medication worldwide [1-3].
The disease has been categorized into two forms, as proliferative hemorrhagic enteropathy (PHE) in mature animals (>4 months old) and porcine intestinal adenomatosis (PIA) in weaners or growing pigs (1.5 to 4 months old) [4]. PHE is characterized by the acute symptom of intestinal blood loss and sudden death without premonitory signs. PIA is characterized by decreased weight gain, increased weight variation, and reduced feed conversion rate with low mortality [5].
Several methods, such as hygienic improvement, use of antibiotics and vaccination, have been suggested to control the disease and vaccination has been known as the most effective prophylactic tool [1,6-8]. A commercial oral attenuated L. intracellularis vaccine-Enterisol Ileitis (Boehringer Ingelheim, Ingelheim, Germany) developed by serial in vitro passage in tissue culture has been used in several countries including Korea. Previous studies demonstrated that the vaccine application reduced secondary viral/bacterial infections and mortality/culling rates [2,6,9]. L. intracellularis was detected from more than 50% of Korean commercial pig herds [10]. Although the vaccine has been used intensively in Korea since 2009, the efficacy of the vaccine has not been evaluated under a commercial pig farm condition.
In the present study, we assessed the efficacy of the Enterisol Ileitis vaccine in a Korean commercial swine farm with endemic PPE.
Materials and Methods
Experimental farm
A 550-sow, farrow-to-finish farm was recruited for this study. Endemic PPE has been confirmed using L. intracellularis specific polymerase chain reaction (PCR) [11] and enzyme-linked immunosorbent assay (ELISA) kit (Enterisol Ileitis-ELISA, Boehringer Ingelheim) in whole age groups of pigs from the farm. In the farm, pigs were usually moved from a nursery unit to a growing-finishing unit when they were around 10 weeks of age. All pens used in this study had half-slatted and half-solid concrete floors. Each nursery pen contained 30 pigs and was equipped with 1 self-feeder and 1 nipple drinker. Each growing-finishing pen also contained 30 pigs and was equipped with 1 self-feeder and 2 nipple drinkers. PPE, coccidiosis, salmonellosis, porcine reproductive and respiratory syndrome (PRRS), Mycoplasma pneumonia infection, Actinobacillus pleuropneumoniae infection, Glasser's disease, atrophic rhinitis, postweaning multisystemic wasting syndrome, porcine dermatitis and nephropathy syndrome, and Streptococcus suis infection have been diagnosed in the farm. However, the farm was free from transmissible gastroenteritis, swine dysentery, and swine influenza. The average mortality of the farm was approximately 6%.
Experimental animals
Thirty two-day-old pigs born in the same house were randomly allocated into two groups (control group, 336 pigs [male, 183; female, 123; F1, 30]; vaccinated group, 336 pigs [male, 183; female, 123; F1, 30]). The mean±standard deviation (SD) of body weight in each group was 7.8±1.4 kg. The vaccinated group was inoculated with a 2 mL of attenuated L. intracellularis vaccine (Enterisol Ileitis, Boehringer Ingelheim) orally according to the manufacturer's instruction. The control group orally received a 2 mL of normal saline. The pigs in both groups were separately raised in different pens in the same house. All pigs were maintained under identical feeding and management practices. No antibiotic was administered for 3 days prior to and post vaccination. All other vaccinations (Briefly, PRRS, swine erysipelas, Japanese encephalitis, and hog cholera vaccines) were applied to all pigs used in this study according to the established vaccination program of the farm.
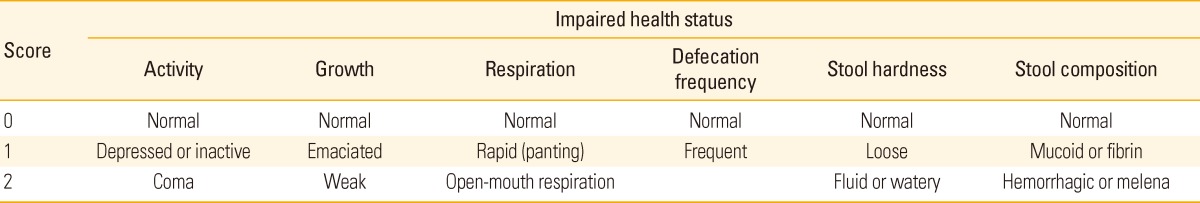
All pigs were weighed on the vaccination day and 6, 14, and 20 weeks post vaccination. Average daily weight gain (ADWG) was determined by dividing weight gain (g) with the number of days between weighing. Health status of every single pig in both groups was evaluated using six criteria (Table 1). Health status of pigs was scored on the vaccination day and 1 day, 2, 4, 6, 8, 10, 12, 14, 16, 18, and 20 weeks post vaccination. Rectal swabs and serum samples were collected from randomly selected 36 pigs in each group on the vaccination day and 4, 6, 10, 14, and 20 (only sera) weeks post vaccination. Presence of L. intracellularis in rectal swabs were analyzed using L. intracellularis specific PCR. Serum samples were screened using the Enterisol Ileitis-ELISA kit.
Table 1.
Criteria for scoring of health status of pigs
For a cost-benefit analysis, several assumptions have been applied; $3.20 ($1=₩1,000)/carcass weight Korean grading grid; 3.2 kg/kg feed conversion rate of the farm; $0.50/kg mean cost of feed; 368 kg feed meal amount until slaughter; 115 kg aiming slaughter weight.
Statistical analyses
Statistical analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). Body weight and ADWG data were analyzed as a repeated measurement design, assuming the model contained group (vaccinated or control group), weighing date (weeks 0, 6, 14, 20), and group×weighing date as fixed effects, sex as a covariate, and pigs as a random effect. The differences of body weight and ADWG between weighing date were tested using a t-test adjusted through the Tukey-Kramer method. The impaired health status during the study period was analyzed using the generalized estimating equation approach following a multinomial distribution. Effects of vaccination on time to death and culling were evaluated using a survival analysis technique, the Kaplan-Meier analysis. For all analyses, p<0.05 were considered statistically significant.
Results
Body weight was not significantly different between two groups at the first three measuring points (0, 6, and 14 weeks post vaccination), while the vaccinated group showed significantly higher body weight than that of the control group at 20 weeks post vaccination. The standard deviation of body weight, indicating weight variation between pigs, gradually increased with age in both groups but was not different between two groups (Table 2). The vaccinated group showed significantly higher ADWG than that of the control group in whole experimental period and the fattening periods, between 14 and 20 weeks post vaccination (Table 3).
Table 2.
Mean of bodyweight of pigs after vaccination
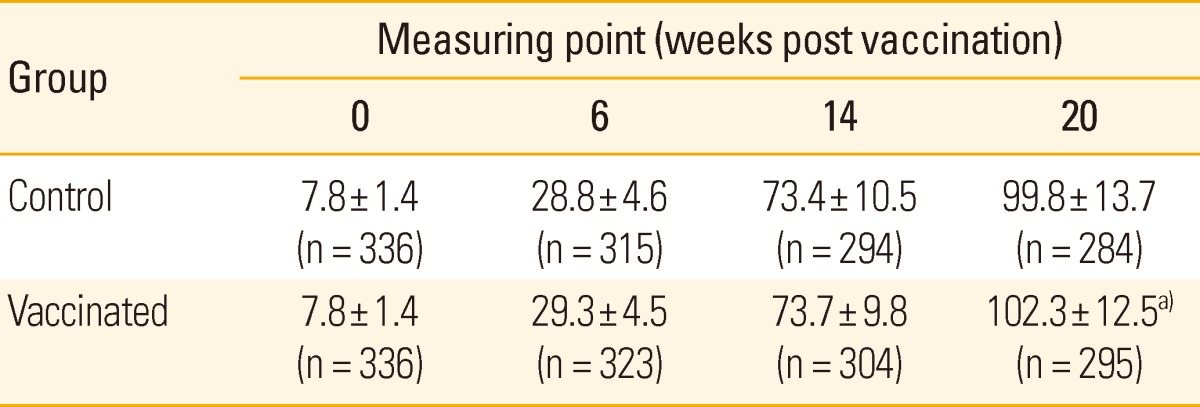
Values are presented as mean±standard deviation (kg).
n, number of pigs.
a)p < 0.05 for the difference between two groups.
Table 3.
Average daily weight gain after vaccination
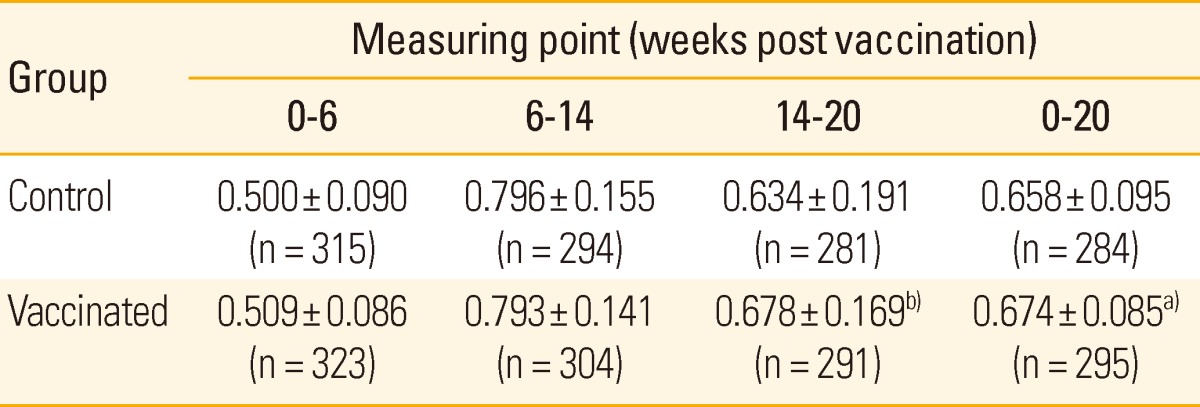
Values are presented as mean±standard deviation (kg).
n, number of pigs.
a)p < 0.05, b)p < 0.01 for the differences between two groups.
The vaccinated group showed significantly better health status in activity, growth, defecation frequency and stool hardness, compared with the control group (Table 4). Although the vaccinated group showed lower proportions of death and culling (8.3% and 3.6%, respectively) than the control groups (11.3% and 4.2%, respectively), there were no significant differences between the two groups in the time to death or culling (data not shown). In a cost-benefit analysis, we estimated a 4.2:1 return-of-investment.
Table 4.
Odds ratio and 95% confidence interval for the impaired health status
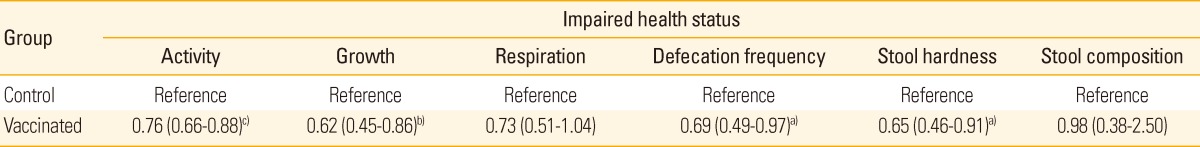
a)p < 0.05, b)p < 0.01, c)p < 0.001 for the differences between two groups.
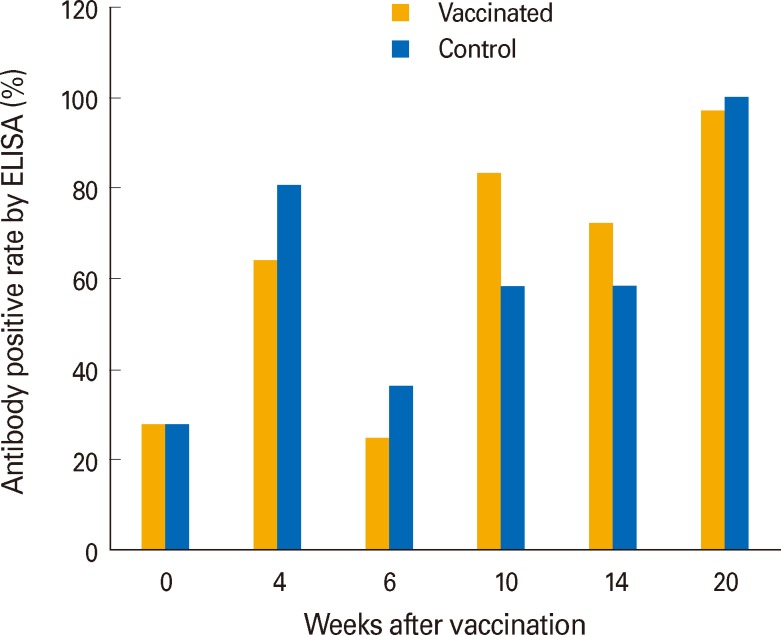
L. intracellularis was detected from only one sample among 360 fecal swab samples collected through the whole experiment (data not shown). However, ELISA results indicated that the L. intracellularis specific serum antibody level was prone to increase with age in both groups (Fig. 1).
Fig. 1.
Antibody levels against Lawsonia intracellularis determined by enzyme-linked immunosorbent assay (ELISA) after vaccination.
Discussion
Our present study showed that the vaccinated pigs against L. intracellularis achieved better health status and higher body weight gain and ADWG than unvaccinated pigs. These results did not contrast with previous results in other studies [2,6,9].
ELISA results indicated that sero-conversion against L. intracellularis occurred between 12 and 16 weeks of age in both groups (Fig. 1). This suggests that pigs were exposed to the infective environment approximately between 9 and 13 weeks of age. We assumed that PPE infection of pigs was associated with their move into the growing-finishing unit. All pigs were moved from the nursery to the growing-finishing unit around 10 weeks of age, and they were mixed together within a group and put in a pen together in the growing-finishing unit. Although both groups were raised under similar PPE endemic condition, their health statuses were significantly different from each other (Table 4). From this, we assumed that the pigs might be mobilized to be more resistant against various infections as well as PPE. In line with the health status, a treatment record showed consistent benefits of vaccination (data not shown). The vaccinated group required 10 individual antibiotic injections during experiment, while 55 treatments were applied to the control group. The one PCR-positive result suggested that there were a few pigs that had active lesions caused by L. intracellularis when we collected fecal samples, because only fecal sample obtained from pigs having active lesions can be PCR positive [12].
The vaccinated pigs showed improved body weight gain (2.5 kg) and ADWG (16 g) than unvaccinated pigs. A meta-analysis study, based on the data of Denmark, Germany, Philippines, and Switzerland, showed that L. intracellularis vaccination resulted in significantly increased body weight gain (3.0 kg) and ADWG (26 g) during fattening period [9]. Another study, conducted from three farms of Australia, also showed significantly increased ADWG of approximately 50, 30, and 150 g in the vaccinated group between 8 and 22 weeks of age, compared with the control group in the same period [2].
The cost-benefit analysis also explained that the vaccinated pigs produced a higher gross margin of $4.80 than the control pigs, and this was almost consistent with meta-analysis based on the European data ($5.36) [9]. Moreover, better economic benefit from vaccination can be achieved by other factors, such as reduced antibiotic use.
This study firstly evaluated the efficacy and return-of-invest of L. intracellularis vaccination in Korea, but there were some limitations. We did not assess whether L. intracellularis was the cause of death, culling and impaired health status in pigs. This trial was not conducted in the various pig farm conditions, so caution is needed to generalize our results to the total of South Korean swine industry.
In conclusion, L. intracellularis vaccination had effects on increasing body weight gain and preventing enteric disease. Additionally, this vaccination provided the substantial health and economic benefits.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Guedes RM, Gebhart CJ, Armbruster GA, Roggow BD. Serologic follow-up of a repopulated swine herd after an outbreak of proliferative hemorrhagic enteropathy. Can J Vet Res. 2002;66:258–263. [PMC free article] [PubMed] [Google Scholar]
- 2.McOrist S, Smits RJ. Field evaluation of an oral attenuated Lawsonia intracellularis vaccine for porcine proliferative enteropathy (ileitis) Vet Rec. 2007;161:26–28. doi: 10.1136/vr.161.1.26. [DOI] [PubMed] [Google Scholar]
- 3.McOrist S, Smith SH, Green LE. Estimate of direct financial losses due to porcine proliferative enteropathy. Vet Rec. 1997;140:579–581. doi: 10.1136/vr.140.22.579. [DOI] [PubMed] [Google Scholar]
- 4.Rowland AC, Lawson GH. Porcine proliferative enteropathies. In: Leman AD, Straw BE, Mengeling WL, D'Allaire S, Taylor DJ, editors. Diseases of swine. 7th ed. Ames: Iowa State University Press; 1992. pp. 560–569. [Google Scholar]
- 5.Lawson GH, Gebhart CJ. Proliferative enteropathy. J Comp Pathol. 2000;122:77–100. doi: 10.1053/jcpa.1999.0347. [DOI] [PubMed] [Google Scholar]
- 6.Almond PK, Bilkei G. Effects of oral vaccination against Lawsonia intracellularis on growing-finishing pig's performance in a pig production unit with endemic porcine proliferative enteropathy (PPE) Dtsch Tierarztl Wochenschr. 2006;113:232–235. [PubMed] [Google Scholar]
- 7.Kroll JJ, Roof MB, McOrist S. Evaluation of protective immunity in pigs following oral administration of an avirulent live vaccine of Lawsonia intracellularis. Am J Vet Res. 2004;65:559–565. doi: 10.2460/ajvr.2004.65.559. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz K, Knittel J, Walter D, Roof M, Anderson M. Effect of oral tiamulin on the development of porcine proliferative enteropathy in a pure-culture challenge model. Swine Health Prod. 1999;7:5–11. [Google Scholar]
- 9.Voets H. Global efficacy and economics of Enterisol® Ileitis: a meta-analysis; European Enterisol® Ileitis Symposium; 2005 Oct 13-15; Barcelona. Ingelheim am Rhein: Boehringer Ingelheim; 2005. pp. 2–4. [Google Scholar]
- 10.Lee SW, Kim TJ, Park SY, et al. Prevalence of porcine proliferative enteropathy and its control with tylosin in Korea. J Vet Sci. 2001;2:209–212. [PubMed] [Google Scholar]
- 11.Jones GF, Ward GE, Murtaugh MP, Lin G, Gebhart CJ. Enhanced detection of intracellular organism of swine proliferative enteritis, ileal symbiont intracellularis, in feces by polymerase chain reaction. J Clin Microbiol. 1993;31:2611–2615. doi: 10.1128/jcm.31.10.2611-2615.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guedes RM, Gebhart CJ. Onset and duration of fecal shedding, cell-mediated and humoral immune responses in pigs after challenge with a pathogenic isolate or attenuated vaccine strain of Lawsonia intracellularis. Vet Microbiol. 2003;91:135–145. doi: 10.1016/s0378-1135(02)00301-2. [DOI] [PubMed] [Google Scholar]