Abstract
Background
We investigated the effects of pre-emptive administration of ketamine and norBNI on pain behavior and the expression of DREAM, c-Fos, and prodynorphin proteins on the ipsilateral side of the rat spinal cord at 2 and 4 hours after formalin injection.
Methods
Eighty-four male Sprague Dawley rats were divided into 4 major groups consisting of control rats (C) (n = 12), rats given only formalin injections (F) (n = 24), and rats treated with pre-emptive administration of either ketamine (K+F) (n = 24) or norBNI (N+F) (n = 24). The non-control groups were further divided into subgroups consisting of rats that were sacrificed at 2 and 4 hours (n = 12 for each group) after formalin injection. Pain behavior was recorded for 1 hour. After 2 and 4 hours, the rats were sacrificed and the spinal cords (L4-L5 sections) were removed for immunohistochemistry and Western blot analysis.
Results
The pain behavior response was reduced in the K+F group compared to the other groups during the second phase of the formalin pain response. We detected an increase in the nuclear DREAM protein level in the K+F group at 2 and 4 hours and a transient decrease in the N+F group at 2 hours; however, it increased at 4 hours after injection. Fos-like immunoreactivity (FLI) and Prodynorphin-like immunoreactivity (PLI) neurons decreased in the K+F group but increased in the N+F group at 2 hours after injection. While FLI decreased, PLI increased in all groups at 4 hours after injection.
Conclusions
We suggest that NMDA and kappa opioid receptors can modulate DREAM protein expression, which can affect pain behavior and protein transcriptional processes at 2 hours and bring about either harmful or protective effects at 4 hours after formalin injection.
Keywords: DREAM protein, formalin test, kappa opioid receptor, NMDA receptor, rat spinal cord
INTRODUCTION
The Downstream Regulatory Element Antagonist Modulator (DREAM) protein has been identified in in-vitro studies as a putative calcium-dependent transcriptional repressor for the c-Fos and prodynorphin genes. The prodynorphin gene contains a consensus DNA sequence known as the Downstream Regulatory Element (DRE) for direct association with the DREAM protein. The DREAM protein suppresses the genetic machinery that reads the DNA code for prodynorphin and, thus, reduces dynorphin peptide production [1].
Transgenic knockout DREAM protein mice have shown a reduced pain behavior response (analgesia) across a spectrum of pain models involving multiple modalities (thermal, mechanical, chemical), tissue types (somatic versus visceral), and acute (nociceptive) versus chronic (inflammatory or neuropathic) pain [2,3]. The DREAM-knockout mice were also found to have elevated levels of mRNA for prodynorphin in the spinal cord. The elevated levels of prodynorphin mRNA expression and of dynorphin peptides in the spinal cord are thought to account for the reduced pain behavior in these mice compared with wild-type mice [2,3]. It has been proposed that these effects act through kappa opioid receptor activation and do not involve the NMDA receptor [2]. Administration of both naloxone (a non-selective opioid receptor antagonist) and norBNI (a kappa opioid receptor antagonist) but not MK-801 (an NMDA receptor antagonist) restored the pain behavior of DREAM-knockout mice to that of wild-type mice [2]. This finding involving DREAM-knockout mice strengthens the hypothesis that reduced pain behavior is mediated through the activation of both dynorphin peptide and the kappa opioid receptor.
The NMDA and kappa opioid receptors, however, have been shown to modulate pain responses in normal rats [4-6]. It would be interesting to determine how the NMDA and kappa opioid receptors modulate the DREAM protein in normal rats, particularly with respect to changes in the expressions of the Fos and prodynorphin proteins and in pain behavior responses during acute pain. Therefore, this study was conducted to determine the effect on the pain behavior response and changes in DREAM, Fos, and prodynorphin proteins after pre-emptive administration of ketamine (NMDA receptor antagonist) or norBNI (kappa opioid receptor antagonist) in the normal rat spinal cord during acute pain.
MATERIALS AND METHODS
1. Animal preparation
Eighty-four male Sprague Dawley rats, 10 weeks old and weighing 250-300 g each, were used in this study. The animals were obtained from the Animal Research and Service Centre, Universiti Sains Malaysia. The rats were maintained in a 12-h light-dark cycle and allowed access to food and water ad libitum. They were allowed to adapt to their surroundings for at least 4 days in the ARASC prior to the experiments. Animal experiments were approved by the Animal Ethics Committee of Universiti Sains Malaysia.
The rats were divided into 4 groups as follows. For Group 1, the control group, rats were not given any treatment (C group) (n = 12). For Group 2, rats were injected with 50 µl of dilute (5%) formalin into the plantar aspect of the left hindpaw using a 26-gauge needle (F group) (n = 24). Five percent formalin was chosen because of its maximum effect on the pain behavior response [7-9]. For Group 3, rats were treated with pre-emptive administration of ketamine (KETAVA, Atlantic Labs, Thailand) (5 mg/kg body weight) intraperitoneally (i.p.) and given formalin injections (K+F group) (n = 24). For Group 4, rats were treated with pre-emptive administration of nor-binaltorphimine dihydrochloride (norBNI) (Sigma, USA) (2 mg/kg body weight) i.p. and given formalin injections (N+F group) (n = 24). The F, K+F, and N+F groups were further divided into subgroups consisting of rats that were sacrificed at 2 and 4 hours after formalin injection (n = 12 for each group). Ketamine and norBNI were given i.p at 30 minutes and 24 hours prior to the experimental procedure, respectively. Ketamine was given 30 minutes before formalin injection based on our previous study [10]. Previous studies have shown that acute norBNI administration results in long-lasting (≥ 3 weeks) blockade of kappa opioid receptors [11-13]. Because µ-opioid receptor-mediated actions of norBNI have been reported during the first 4 h after administration, a 24-h pretreatment interval was used [14,15].
2. Behavioral response scoring
Each rat was placed in a perspex-testing chamber measuring 26 cm × 20 cm × 20 cm. A mirror was placed below the floor of the chamber at a 45° angle to allow an unobstructed view of the rat's paws. Pain behavior or nociceptive responses were recorded beginning from the in jection of formalin, tabulated every minute, and averaged at 5-minute intervals for 1 hour [16]. The scores were as follows:
0 = The injected paw is not favored (i.e., foot flat on the floor with toes splayed), indicating insignificant or no pain
1 = The injected paw has little or no weight on it, with no toe splaying, indicating mild pain
2 = The injected paw is elevated and the heel is not in contact with any surface, indicating moderate pain
3 = The injected paw is licked, bitten, or shaken, indicating severe pain
3. Immunohistochemistry analysis
At 2 and 4 hours after formalin injection, the rats were sacrificed with an overdose of sodium pentobarbitone (Ceva Sante Animale, France) via intraperitoneal injection. This method was used to avoid damage to the spinal cord [17]. Thoracotomy was performed to expose the heart. An 18 G needle (branula) was inserted into the left ventricle, and a snip was made to the right atrium for an outlet. Perfusion was performed using the gravity method, with phosphate buffered saline (PBS) followed by 500 ml of cold 4% paraformaldehyde in phosphate buffer (PB) 0.1 M (pH 7.4). Segments L4 and L5 (which innervate the hindpaw) of the spinal cord were then dissected from the rats. Following overnight cryoprotection in 20% sucrose in PB 0.1 M, the L4 and L5 segments were cut into coronal sections (30 µm thick) using a cryostat, and every third section was collected as a free-floating section in PBS. Sections were then rinsed with Tris-buffered saline (TBS) twice for 5 minutes and incubated with primary rabbit polyclonal antibodies for Fos (Calbiochem, USA) diluted 1:20,000 or polyclonal anti-prodynorphin antiserum raised from a guinea pig (Genetex, USA) diluted 1:500 in buffer (2% normal goat serum, 0.2% triton-X, TBS) for 48 hours. Sections were rinsed with TBS in triton-X (TBS/TX) 3 times for 10 minutes each and then incubated with biotinylated goat anti-rabbit IgG (Santa Cruz, USA) or biotinylated goat anti-guinea pig IgG (Genetex, USA) diluted 1:200 in buffer (2% normal goat serum, 0.2% triton-X, TBS) for 1 hour. After additional rinses in TBS/TX, all sections were incubated with avidin-biotin-HRP (Santa Cruz; diluted 1:50 in TBS) for 1 hour at room temperature. Sections were again rinsed 3 times with TBS/TX and treated with diaminobenzidine (Sigma, UK) (0.02% in TBS, 0.2% hydrogen peroxide) as the chromogen, until a brown coloration in the solution was observed. Finally, sections were rinsed 4 times and mounted on slides, air-dried, dehydrated, and covered with a coverslip. Sections were examined using an image analyzer (Leica MPS 60) at magnifications using 40× and 100× objective lenses. The data for the total number of Fos-like immunoreactivity (FLI) and prodynorphin-like immunoreactivity (PLI) neurons on the ipsilateral sides were measured manually in the specific laminar regions of the spinal grey matter landmark, in accordance with Molander et al. [18].
4. Western blot analysis
The DREAM protein level in the spinal cord was determined by Western blot analysis. At 2 and 4 hours after formalin injection, the rats were sacrificed by decapitation using a guillotine. The spinal cord tissue in the lumbar enlargement was removed directly from the rats, without a fixation process, and separated into ipsilateral and contralateral sides by a cut in the spinal cord at the midline. The tissue was immediately deep frozen by liquid nitrogen and kept at -80℃ until further analysis. Protein was extracted from the spinal cord tissue using NE-PER extraction reagents (Pierce, USA). Before use, the NE-PER extraction reagents were mixed with the concentrated Halt™ Protease Inhibitor cocktail kit, EDTA-free (Pierce, USA) in a volume of 10 µl/ml per reagent. The protein concentration of the extracted samples was measured with the Bicinchoninic Acid (BCA) protein assay kit. Protein samples containing 40-50 µg of total proteins (after optimization) were denatured and subjected to SDS-PAGE using a 12% resolving gel. The proteins from the polyacrylamide gels were transferred to a nitrocellulose membrane (Bio-Rad, USA) using a modified technique [19]. The nitrocellulose membrane was washed with deionized water and then incubated in blocking solution (5% BSA in PBS) for 1 hour at room temperature. Following that, the nitrocellulose membrane was washed 3 times for 10 minutes in Tris-buffered saline-Tween-20 (TBS-T20). The nitrocellulose was then incubated with rabbit polyclonal DREAM antibody (dilution 1:500 in TBST) or mouse monoclonal β-actin antibody (dilution 1:2,000 in TBST) overnight at 4℃. The nitrocellulose membrane was then incubated with HRP-conjugated goat anti-rabbit antibody (dilution 1:5,000 in TBST) or mouse secondary antibody (dilution 1:5,000 in TBST) for 1 hour at room temperature. In between incubations, the nitrocellulose membrane was washed 3 times in TBS-T20 for 10 minutes each. Finally, the blot was examined using the Immobilon Western chemiluminescent HRP substrate, and an image was taken using an image analyzer. The integrated density values (IDV) of the DREAM and β-actin proteins were measured using the Spot Denso AlphaView™ software programmed in the image analyzer. The mean relative intensity or fold change was determined by the following formula:
Mean Relative Intensity = (IDV DREAM protein- IDV endogenous control) target group - (IDV DREAM protein- IDV endogenous control) calibrator group.
5. Statistical analysis
Statistical analyses were performed using the Statistical Package of Social Sciences software (SPSS), version 18. Pain behavior responses were divided into 2 phases consisting of phase 1 (mean score at 5 minutes) and phase 2 (mean scores from 15 to 60 minutes). Pain behavior responses in phases 1 and 2 were analyzed by a non-parametric Kruskal-Wallis test. When a significant value was detected, it was further analyzed by the Mann Whitney test, which was conducted for comparison between treatment groups in each phase. The total number of FLI and PLI neurons expressed and the mean relative DREAM protein level were analyzed using one-way analysis of variance (ANOVA). Significant values detected were further analyzed by a post hoc least significance different (LSD) test for comparison between treatment groups. All data are presented as the mean ± SEM, and the level of significance was set at P < 0.05.
RESULTS
1. Pain behavior response
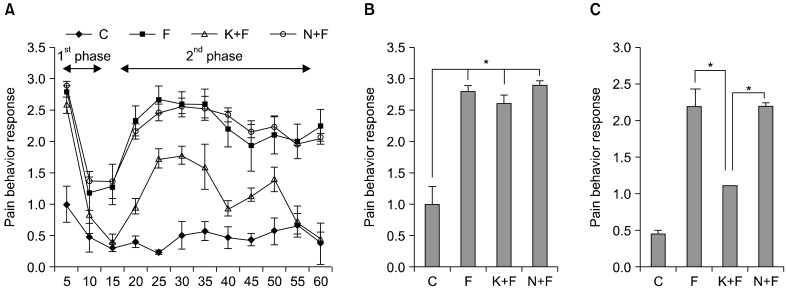
In general, the pain behavior response in the C group was significantly lower than in all other groups at almost every minute. At 15 minutes, the pain behavior response was significantly lower in the K+F group compared to the F and N+F groups. Furthermore, the pain behavior response in the K+F group was significantly attenuated at 20 minutes until 60 minutes post-formalin injection compared to the F and N+F groups (Fig. 1A). For the specific phases, the pain behavior responses were not significantly different between the F, K+F, and N+F groups during phase 1 (Fig. 1B). However, pre-emptive administration of ketamine in the K+F group significantly lowered the pain behavior response when compared to the F (P < 0.001) and N+F (P < 0.001) groups during phase 2 (Fig. 1C). In addition, the difference in pain behavior response between the F and N+F groups during phase 2 was not statistically significant (Fig. 1C).
Fig. 1.
(A) Pain behavior response for all groups in 1-hour periods. Control group (C), formalin injected group (F), ketamine and formalin injected group (K+F), norBNI and formalin injected group (N+F). Values are the means ± S.E.M. n = 6 for all groups. (B) Pain behavior response during phase 1. *P < 0.001 compared to C group (C) Pain behavior response during phase 2. *P < 0.001 compared to K+F group.
2. Mean relative DREAM protein level
We tried to use the immunohistochemistry technique to measure DREAM protein expression. Interestingly, no obvious cell profile was seen, and the staining revealed a punctuate pattern, making it difficult to count the number of DREAM protein neurons in each laminae (data not shown). Thus, Western blot analysis was then performed to determine the DREAM protein levels on the ipsilateral side of the spinal cord after formalin injection.
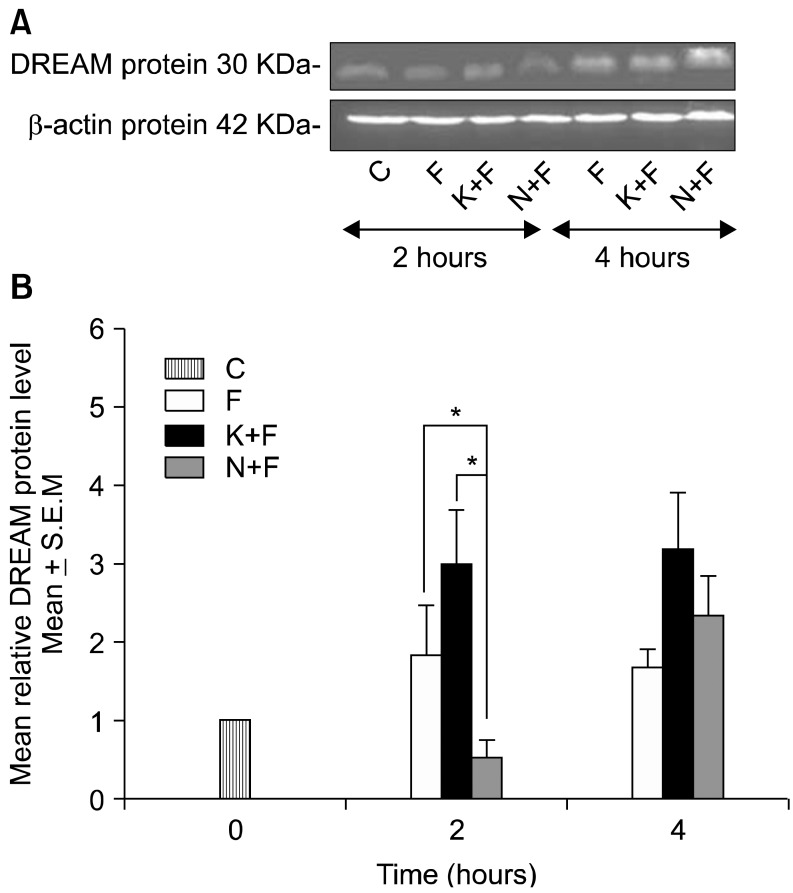
The mean relative DREAM protein level was significantly increased in the K+F group when compared to the N+F group (P < 0.01) at 2 hours after injection. In contrast, pre-emptive administration of norBNI (N+F group) significantly decreased the mean relative DREAM protein level when compared to the F (P < 0.01) and K+F (P < 0.01) groups at 2 hours after injection. However, all groups showed a similarly increased pattern in the mean relative DREAM protein level at 4 hours after injection (Fig. 2).
Fig. 2.
(A) A representative example of Western blot results for nuclear extracts on the ipsilateral side between all groups with quantification analysis of the integrated density value. (B) Columns represent the mean relative DREAM protein level ± S.E.M for 6 separate experiments. The mean relative DREAM protein level (fold change) represents comparative levels of the DREAM protein in the experimental groups (formalin injected group, F, ketamine and formalin injected group, K+F, norBNI and formalin injected group, N+F) over the calibrator group (control group, C) after normalization by its loading control (housekeeping protein, β-actin protein) at 2 and 4 hours after formalin injection. n = 6 for each group. *P < 0.01 compared between F and N+F groups, compared between K+F and N+F groups.
3. Total number of FLI neurons
Formalin injection significantly increased the total number of FLI neurons compared to the control group (Table 1, Fig. 3). However, the total number of FLI neurons was significantly decreased in the K+F group when compared to the F (P < 0.01) and N+F (P < 0.05) groups at 2 hours after injection. The total number of FLI neurons was increased in the N+F group, which was similar to the increased pattern in the F group at 2 hours after injection. At 4 hours after injection, the total number of FLI neurons was decreased in all groups, and there were no statistically significant differences between all groups (Table 1).
Table 1.
Total Number of FLI Neurons on the Ipsilateral Side for All Groups at 2 and 4 Hours after Formalin Injection
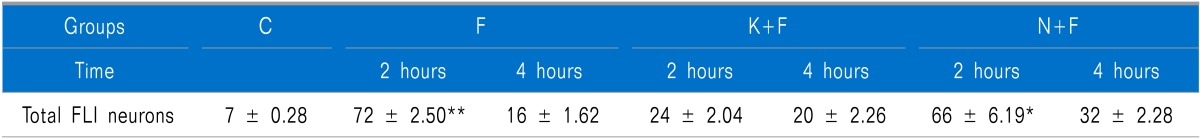
Values are the means ± S.E.M. n = 6 for each group. C: control group, F: formalin injected group, K+F: ketamine and formalin injected group, N+F: norBNI and formalin injected group. **P < 0.01 compared between F and K+F group at 2 hours after formalin injection, *P < 0.05 compared between N+F and K+F group at 2 hours after formalin injection.
Fig. 3.
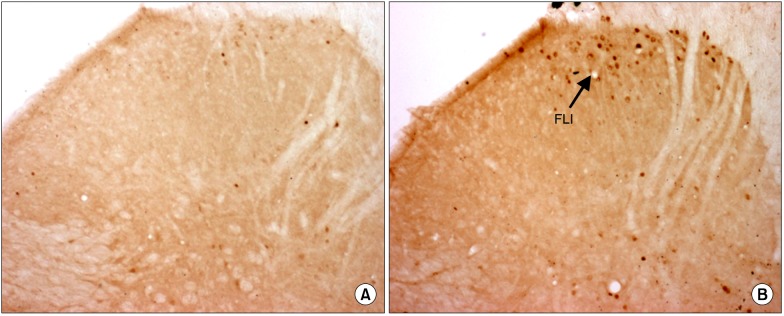
Photomicrographs (original magnification 40× objective lens) showing Fos-like immunoreactivity (FLI) expression on the ipsilateral side of spinal cord sections of control group (C) (A) and formalin injected group (F) (B). Arrow indicates the dark staining FLI.
4. Total number of PLI neurons
Formalin injection was found to significantly increase the total number of PLI neurons compared to the control group (Table 2, Fig. 4). The total number of PLI neurons was significantly decreased in the K+F group when compared to the F (P < 0.01) and N+F (P < 0.01) groups at 2 hours after injection. The total number of PLI neurons was higher in the N+F group when compared to the F group at 2 hours after injection but did not reach statistical significance. However, at 4 hours after injection, the total number of PLI neurons was increased in all groups, and there were no statistically significant differences between all groups (Table 2).
Table 2.
Total Number of PLI Neurons on the Ipsilateral Side for all Groups at 2 and 4 Hours after Formalin Injection

Values are the means ± S.E.M. n = 6 for each group. C: control group, F: formalin injected group, K+F: ketamine and formalin injected group, N+F: norBNI and formalin injected group. **P < 0.01 compared between F and K+F group, compared between the N+F and K+F group at 2 hours after formalin injection.
Fig. 4.
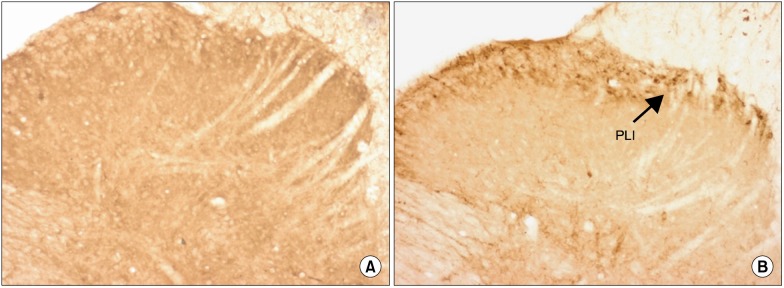
Photomicrographs (original magnification 40× objective lens) showing Prodynorphin-like immunoreactivity (PLI) expression on the ipsilateral side of spinal cord sections of the control group (C) (A) and formalin injected group (F) (B). Arrow indicates the dark staining PLI.
DISCUSSION
In this study, pre-emptive administration of ketamine (K+F group), which is an NMDA receptor antagonist, clearly prevented the pain behavior response during phase 2 (Fig. 1C). Previous studies have also reported similar effects with pre-emptive administration of an NMDA receptor antagonist on pain behavior response, spinal Fos protein, and prodynorphin protein expression [10,20]. These findings can be extended to the present study, in which antagonism of the NMDA receptor by ketamine (K+F group) affected central sensitization processes, which have a role in the modulation of prodynorphin and Fos protein expression and pain behavior responses in the formalin test. However, it cannot be denied that these effects could also be secondary to tonic inhibition by the kappa opioid receptor. Antagonism of the NMDA receptor by ketamine results in the inhibitory effect of the kappa opioid receptor becoming more dominant and apparent.
However, the inhibitory effects of the kappa opioid receptor on pain behavior responses and Fos and prodynorphin expression were not clearly seen in this study. The administration of norBNI intraperitoneally has been reported to increase flinching behavior after formalin injection when compared to a control group that received only formalin injections [21]. However, we must consider the fact that in Ossipov et al. [21], the quantification of pain behavior was performed by counting the incidence of flinching. Unlike Ossipov et al. [21], the present study assessed pain behavior responses based on the weighted scores technique [16]. The advantage of the weighted scores technique over single parameter methods is that it takes into account more than one behavior, and it is more likely to reflect the pain experience of the animal being tested [22]. As another contributory effect, the pain behavior in this study may be related to the percentage of formalin used. Two percent of formalin was used by Ossipov et al. [21] compared to 5% of formalin in the present study. It has been shown that different percentages of formalin can influence pain behavior in rats in a dose-dependent manner [7-9]. In addition, the effects of pre- emptive administration of norBNI in acute pain are still contradictory. Pre-emptive administration of norBNI has also been reported to have no effect [23] and an antinociceptive effect [24] in the acute pain model.
The presence of noxious stimuli such as peripheral in flammation activates the spinal NMDA receptor, the neurokinin 1 (NK1) receptor, a T-type voltage-gated calcium channel that synergistically triggers a rise in the cytosolic free calcium concentration of spinal projection neurons [25]. The binding of the DREAM protein to the DRE site was released by the direct binding of the DREAM protein and intracellular calcium or phosphorylated cAMP-responsive element modulator (CREM) [26]. An increase in the calcium influx has also been found in the translocation of the DREAM protein into the nucleus [27], with a resultant upregulation of DREAM protein levels in the nucleus. These effects are consistent with the findings of this study. The mean relative DREAM protein level in the nuclear extract was increased in the F group on the ipsilateral side. This finding is also similar to a previous study showing that the DREAM protein in nuclear extracts and its mRNA level increased in the rat spinal cord on the ipsilateral side after noxious stimulation [28,29]. Therefore, the release of the DREAM protein from the DRE site can lead to its upregulation in the nucleus, driven by an autoregulatory feedback mechanism to regulate the expression of the c-Fos and prodynorphin genes and proteins, which are upregulated during this period.
Interestingly, in this study, pre-emptive administration of ketamine (K+F group) significantly increased the mean relative DREAM protein level; in contrast, pre-emptive administration of norBNI (N+F group) decreased the mean relative DREAM protein level in the nuclear extract on the ipsilateral side at 2 hours after formalin injection. During basal conditions or in the absence of intracellular Ca2+ in brain astrocytes, the DREAM protein is bound to the DRE site of the c-Fos gene and assumes a nuclear localization [30]. Administration of glutamate, the neurotransmitter for the NMDA receptor, caused decreased nuclear localization of the DREAM protein, translocated it into the cytoplasm, and restored the distribution of the DREAM protein in brain astrocytes [30] as well as in retinal Muller glial cells [31]. Blockade of the NMDA receptor by the non-competitive antagonist MK801 reverses this effect [32]. This suggests that the transcription of the c-Fos gene is activated when the DREAM protein translocates out of the nucleus. It is known that c-Fos and prodynorphin gene transcription requires a high level of intracellular Ca2+, which is achieved through an NMDA receptor-mediated mechanism [32]. In the present study, pre-emptive administration of ketamine possibly prevented translocation of the DREAM protein out of the nucleus, causing it to accumulate in the nucleus, thus repressing c-Fos and prodynorphin gene transcriptional processes. Other glutamate receptors, such as the metabotropic glutamate receptor (mGluR), also became dominant after inhibition of the NMDA receptor in this study, contributing to the upregulation of the DREAM protein level in the nuclear compartment. The group 1 mGluR has been reported to regulate DREAM protein activity in neurons [33].
In contrast, inhibition of the kappa opioid receptor allows the NMDA receptor function to become dominant, resulting in increased levels of intracellular Ca2+. In this study, the release of the DREAM protein from the DRE site permitted the transcription of the c-Fos and prodynorphin genes and thus increased Fos and prodynorphin protein expression in the N+F group. Before transcription can occur, however, we believe that the DREAM protein must be translocated out of the nucleus. As mentioned earlier, activation of the NMDA receptor resulted in decreased nuclear localization of the DREAM protein in retinal Muller glial cells [31] and brain astrocytes [30]. Therefore, we assume that the dominant function of the NMDA receptor in the N+F group is to mediate the mechanisms for the translocation of the DREAM protein out of the nucleus. This permits the upregulation of c-Fos and prodynorphin gene transcription, which in this study resulted in increased Fos and prodynorphin expression in the N+F group at 2 hours after formalin injection.
Furthermore, the DREAM protein has been identified in in-vitro studies as a putative calcium-dependent transcriptional repressor for the c-Fos and prodynorphin genes [1]. Thus, we wished to determine whether changes in the DREAM protein after pre-emptive administration of ketamine or norBNI could also affect this protein's role as a repressor for c-Fos and prodynorphin gene expression. In this study, we found that the effect of pre-emptive administration of ketamine (K+F group) decreased the total numbers of FLI and PLI neurons expressed on the ipsilateral side at 2 hours after formalin injection. In contrast, pre-emptive administration of norBNI (N+F group) increased the total numbers of FLI and PLI neurons expressed on the ipsilateral side in a similar pattern to the F group at 2 hours after formalin injection. In this study, the role of the DREAM protein as a repressor for c-Fos and prodynorphin gene expression is reflected in changes to the expression of FLI and PLI neurons when compared to changes in the DREAM protein level at 2 hours after formalin injection. The FLI and PLI neuron expressions at 2 hours after formalin injection in the F, K+F, and N+F groups are perhaps the consequences of the pain behavior responses in these groups.
Interestingly, at 4 hours after formalin injection, FLI neuron expression decreased in all groups. This effect was expected because the c-Fos gene is an immediate early gene, and its expression is transient [34]. The maximum expression of the FLI neurons was observed at 2 hours after formalin injection, and then it decreased at 4 hours after injection. However, PLI neuron expression increased for all groups at 4 hours after formalin injection. The increased pattern of PLI neurons at 4 hours is probably due to the important role of the prodynorphin protein in the mechanism for persistent pain [35]. In this study, this effect seems more pronounced in the DREAM protein level than in changes to the c-Fos gene expression at 4 hours after injection. The DREAM protein level increased in all groups at 4 hours after formalin injection. The upregulation of the DREAM protein level likely contributed to the elevated prodynorphin protein expression at 4 hours after injection, which could be either harmful or protective in the modulation of pain. However, the DREAM protein has been reported to act as a neuroprotective agent during excitotoxicity-related diseases [36]. The explanation for these effects is not clear, and further study is needed to elucidate the role of the DREAM protein.
In conclusion, we suggest that the NMDA and kappa opioid receptors can modulate DREAM protein expression, which can affect c-Fos and prodynorphin gene transcription at 2 hours after formalin injection and, therefore, the pain behavior response. In addition, we suggest that the upregulation of the DREAM protein seen at 4 hours after formalin injection could be either harmful or protective in the modulation of pain in response to elevated prodynorphin protein expression.
ACKNOWLEDGEMENTS
This study was supported by the Universiti Sains Malaysia (USM) Research University Grant Scheme [1001/PPSK/812022] and the USM Postgraduate Research Grant Scheme [1001/PPSK/8142001].
References
- 1.Carrión AM, Link WA, Ledo F, Mellström B, Naranjo JR. DREAM is a Ca2+-regulated transcriptional repressor. Nature. 1999;398:80–84. doi: 10.1038/18044. [DOI] [PubMed] [Google Scholar]
- 2.Cheng HY, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, Kockeritz LK, et al. DREAM is a critical transcriptional repressor for pain modulation. Cell. 2002;108:31–43. doi: 10.1016/s0092-8674(01)00629-8. [DOI] [PubMed] [Google Scholar]
- 3.Lilliehook C, Bozdagi O, Yao J, Gomez-Ramirez M, Zaidi NF, Wasco W, et al. Altered Abeta formation and long-term potentiation in a calsenilin knock-out. J Neurosci. 2003;23:9097–9106. doi: 10.1523/JNEUROSCI.23-27-09097.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Obara I, Mika J, Schafer MK, Przewlocka B. Antagonists of the kappa-opioid receptor enhance allodynia in rats and mice after sciatic nerve ligation. Br J Pharmacol. 2003;140:538–546. doi: 10.1038/sj.bjp.0705427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sevostianova N, Danysz W, Bespalov AY. Analgesic effects of morphine and loperamide in the rat formalin test: interactions with NMDA receptor antagonists. Eur J Pharmacol. 2005;525:83–90. doi: 10.1016/j.ejphar.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Snijdelaar DG, van Rijn CM, Vinken P, Meert TF. Effects of pre-treatment with amantadine on morphine induced antinociception during second phase formalin responses in rats. Pain. 2005;119:159–167. doi: 10.1016/j.pain.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda T, Nishimoto C, Shiga Y, Toyooka H. The formalin test: effects of formalin concentration and short-term halothane inhalation. Reg Anesth Pain Med. 2001;26:407–413. doi: 10.1053/rapm.2001.25926. [DOI] [PubMed] [Google Scholar]
- 8.Lee IO, Jeong YS. Effects of different concentrations of formalin on paw edema and pain behaviors in rats. J Korean Med Sci. 2002;17:81–85. doi: 10.3346/jkms.2002.17.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim MJ, Hong BH, Zhang EJ, Ko YK, Lee WH. Antinociceptive effects of intraperitoneal and intrathecal vitamin E in the rat formalin test. Korean J Pain. 2012;25:238–244. doi: 10.3344/kjp.2012.25.4.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayati AA, Zalina I, Myo T, Badariah AA, Azhar A, Idris L. Modulation of formalin-induced fos-like immunoreactivity in the spinal cord by swim stress-induced analgesia, morphine and ketamine. Ger Med Sci. 2008;6:Doc05. [PMC free article] [PubMed] [Google Scholar]
- 11.Grossman A, Clement-Jones V. Opiate receptors: enkephalins and endorphins. Clin Endocrinol Metab. 1983;12:31–56. doi: 10.1016/s0300-595x(83)80028-0. [DOI] [PubMed] [Google Scholar]
- 12.Horan P, Taylor J, Yamamura HI, Porreca F. Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J Pharmacol Exp Ther. 1992;260:1237–1243. [PubMed] [Google Scholar]
- 13.Spanagel R, Almeida OF, Shippenberg TS. Evidence thatnor-binaltorphimine can function as an antagonist at multiple opioid receptor subtypes. Eur J Pharmacol. 1994;264:157–162. doi: 10.1016/0014-2999(94)00449-8. [DOI] [PubMed] [Google Scholar]
- 14.Endoh T, Matsuura H, Tanaka C, Nagase H. Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch Int Pharmacodyn Ther. 1992;316:30–42. [PubMed] [Google Scholar]
- 15.Broadbear JH, Negus SS, Butelman ER, de Costa BR, Woods JH. Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on kappa-opioid agonists in the mouse writhing assay. Psychopharmacology (Berl) 1994;115:311–319. doi: 10.1007/BF02245071. [DOI] [PubMed] [Google Scholar]
- 16.Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- 17.Hao S, Takahata O, Mamiya K, Iwasaki H. Sevoflurane suppresses noxious stimulus-evoked expression of Fos-like immunoreactivity in the rat spinal cord via activation of endogenous opioid systems. Life Sci. 2002;71:571–580. doi: 10.1016/s0024-3205(02)01704-6. [DOI] [PubMed] [Google Scholar]
- 18.Molander C, Xu Q, Grant G. The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. J Comp Neurol. 1984;230:133–141. doi: 10.1002/cne.902300112. [DOI] [PubMed] [Google Scholar]
- 19.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berrino L, Oliva P, Massimo F, Aurilio C, Maione S, Grella A, et al. Antinociceptive effect in mice of intraperitoneal N-methyl-D-aspartate receptor antagonists in the formalin test. Eur J Pain. 2003;7:131–137. doi: 10.1016/S1090-3801(02)00086-1. [DOI] [PubMed] [Google Scholar]
- 21.Ossipov MH, Kovelowski CJ, Wheeler-Aceto H, Cowan A, Hunter JC, Lai J, et al. Opioid antagonists and antisera to endogenous opioids increase the nociceptive response to formalin: demonstration of an opioid kappa and delta inhibitory tone. J Pharmacol Exp Ther. 1996;277:784–788. [PubMed] [Google Scholar]
- 22.Coderre TJ, Fundytus ME, McKenna JE, Dalal S, Melzack R. The formalin test: a validation of the weighted-scores method of behavioural pain rating. Pain. 1993;54:43–50. doi: 10.1016/0304-3959(93)90098-A. [DOI] [PubMed] [Google Scholar]
- 23.Wu HE, Hung KC, Mizoguchi H, Nagase H, Tseng LF. Roles of endogenous opioid peptides in modulation of nocifensive response to formalin. J Pharmacol Exp Ther. 2002;300:647–654. doi: 10.1124/jpet.300.2.647. [DOI] [PubMed] [Google Scholar]
- 24.Choi SS, Han KJ, Lee HK, Han EJ, Suh HW. Possible antinociceptive mechanisms of opioid receptor antagonists in the mouse formalin test. Pharmacol Biochem Behav. 2003;75:447–457. doi: 10.1016/s0091-3057(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jäger T, et al. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science. 2006;312:1659–1662. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- 26.Ledo F, Carrión AM, Link WA, Mellström B, Naranjo JR. DREAM-alphaCREM interaction via leucine-charged domains derepresses downstream regulatory element-dependent transcription. Mol Cell Biol. 2000;20:9120–9126. doi: 10.1128/mcb.20.24.9120-9126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaidi NF, Thomson EE, Choi EK, Buxbaum JD, Wasco W. Intracellular calcium modulates the nuclear translocation of calsenilin. J Neurochem. 2004;89:593–601. doi: 10.1046/j.1471-4159.2004.02362.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Li Y, Yang YR, Zhu HH, Han JS, Wang Y. Distribution of downstream regulatory element antagonist modulator (DREAM) in rat spinal cord and upregulation of its expression during inflammatory pain. Neurochem Res. 2007;32:1592–1599. doi: 10.1007/s11064-007-9364-3. [DOI] [PubMed] [Google Scholar]
- 29.Long I, Suppian R, Ismail Z. Increases in mRNA and DREAM protein expression in the rat spinal cord after formalin induced pain. Neurochem Res. 2011;36:533–539. doi: 10.1007/s11064-010-0375-0. [DOI] [PubMed] [Google Scholar]
- 30.Edling Y, Ingelman-Sundberg M, Simi A. Glutamate activates c-fos in glial cells via a novel mechanism involving the glutamate receptor subtype mGlu5 and the transcriptional repressor DREAM. Glia. 2007;55:328–340. doi: 10.1002/glia.20464. [DOI] [PubMed] [Google Scholar]
- 31.Chavira-Suárez E, Ramírez M, Lamas M. D-Serine/N-methyl-D-aspartate receptor signaling decreases DNA-binding activity of the transcriptional repressor DREAM in Müller glia from the retina. Neurosci Lett. 2008;432:121–126. doi: 10.1016/j.neulet.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 32.Coderre TJ, Melzack R. The role of NMDA receptor-operated calcium channels in persistent nociception after formalin-induced tissue injury. J Neurosci. 1992;12:3671–3675. doi: 10.1523/JNEUROSCI.12-09-03671.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J, Kim I, Oh SR, Ko SJ, Lim MK, Kim DG, et al. Regulation of DREAM expression by group I mGluR. Korean J Physiol Pharmacol. 2011;15:95–100. doi: 10.4196/kjpp.2011.15.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 35.Ji RR, Befort K, Brenner GJ, Woolf CJ. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J Neurosci. 2002;22:478–485. doi: 10.1523/JNEUROSCI.22-02-00478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Su P, Liang P, Liu T, Liu X, Liu XY, et al. The DREAM protein negatively regulates the NMDA receptor through interaction with the NR1 subunit. J Neurosci. 2010;30:7575–7586. doi: 10.1523/JNEUROSCI.1312-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]