Abstract
Postural orthostatic tachycardia syndrome (POTS) refers to the presence of orthostatic intolerance with a heart rate (HR) increment of 30 beats per minute (bpm) or an absolute HR of 120 bpm or more. There are sporadic reports of the autonomic nervous system dysfunction in migraine and fibromyalgia. We report a case of POTS associated with migraine and fibromyalgia. The patient was managed with multidisciplinary therapies involving medication, education, and exercise which resulted in symptomatic improvement. We also review the literature on the association between POTS, migraine, and fibromyalgia.
Keywords: fibromyalgia, migraine, postural orthostatic tachycardia syndrome
Postural orthostatic tachycarda syndrome (POTS) has been documented in migraine [1] and fibromyalgia [2], which is defined when the orthostatic symptoms are associated with a heart rate (HR) increment of more than 30 beats per minute (bpm) or a standing HR of 120 bpm or more in the absence of postural hypotension [3]. Fibromyalgia is a debilitating psychosomatic illness with widespread pain characterized by various combinations of neurologic and autonomic symptoms [4]. Sympathetic hyperactivation has been postulated in the central sensitization which may take part in the augmented pain experience [5]. The diagnosis is based on the subjective report by the patient of widespread pain and symptom severity according to the 2010 American College of Rheumatology (ACR) preliminary diagnostic criteria [6], which can be applied in fibromyalgia patients in Korea [7]. Fibromyalgia was diagnosed in migraine patients with frequencies between 22% and 40% [8]. There are sporadic reports of the autonomic nervous system dysfunction in migraine [1] and fibromyalgia [2,9]. We present a case with POTS associated with migraine and fibromyalgia.
CASE REPORT
A 51-year-old woman presented with a 4-month history of presyncope and sometimes syncope on standing. These episodes occurred with premonitory signs of nausea, lightheadedness, orthostatic visual blurring, and tachycardia. They were all relieved in recumbency. She also complained of severe throbbing headache in the right occipitotemporal area preceded by photopsia, which was accompanied by vomiting, unilateral tinnitus, photophobia and phonophobia. Headache impact test-6 score was 78 [10]. She had widespread body pain, chronic fatigue, muscle tenderness, and difficulty sleeping. The diagnosis of fibromyalgia was made according to the ACR 2010 criteria [6]. Widespread pain index and symptom severity scale score were 9 and 12 respectively. Fibromyalgia impact questionnaire score was 87 [11]. Beck depression inventory score [12] was 47 which reflected a state of severe depression.
She smoked for 6 years. Her medical history including recent viral infections was not significant except for migraine. She denied the use of medications. She had laparoscopic assisted vaginal hysterectomy for a uterine myoma 2 years ago.
Examination between the attacks revealed a blood pressure (BP) of 120/70 mmHg, regular pulse at 76 bpm, no carotid or supraclavicular bruits, and no cardiac murmurs. Her physical examination was normal except for 13 specific points of tenderness on palpation; left and right occiput, trapezius, supraspinatus muscles, lower cervical spaces, second ribs, knees, and left lateral epicondyle. Cranial nerve examination was normal and there was no motor or sensory deficit. Cerebellar function and gait were normal.
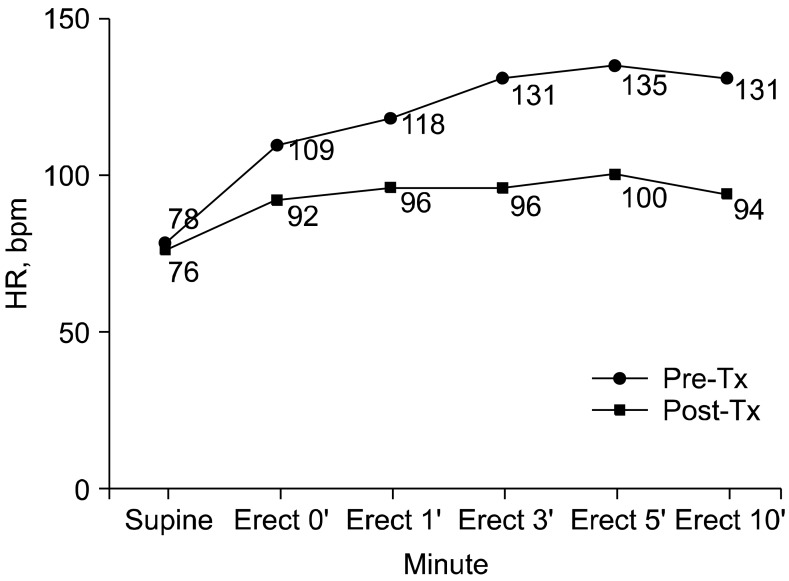
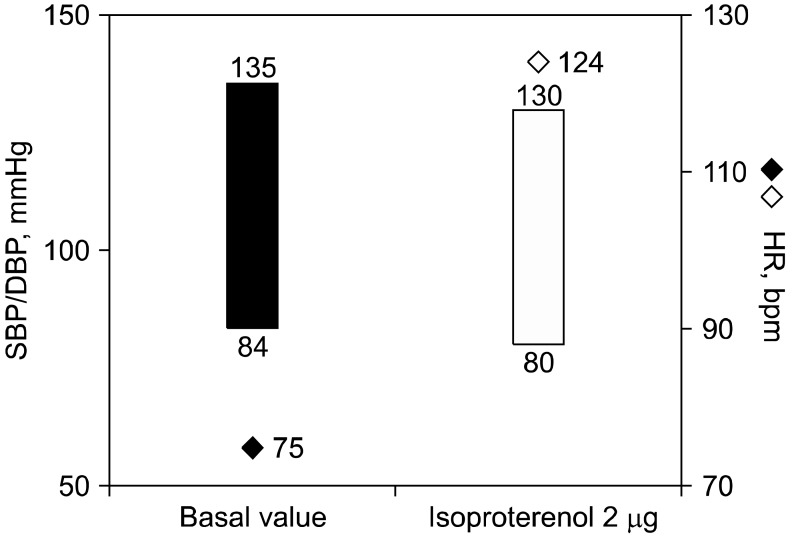
Blood tests including electrolyte, epinephrine and norepinephrine, thyroid function test, Venereal Disease Research Laboratory (VDRL) test, HIV, rheumatoid factor, lupus anticoagulant, antiphospholipid antibody, antinuclear antibody, antineutrophil cytoplasmic antibody, and anti-Ro/La antibody were unremarkable. 24-hour Holter monitoring, an echocardiogram, an electroencephalogram, and brain CT angiogram were normal. BP and HR responses during lying to standing were characterized by increase in HR from 78 to 135 bpm without a significant decrease of BP (Fig. 1). On the autonomic function test, HR variance at deep breathing was decreased to 5.04 bpm (normal > 12 bpm) and the expiration-to-inspiration (E:I) ratio was within normal range. During Valsalva maneuver, the ratio between HR in phases II and IV was normal. The sympathetic skin response (SSR) was unremarkable. On the head-up tilt table test (HUTT) at 70° for 30 minutes, changes in systolic BP, diastolic BP, and HR were calculated with respect to basal values. Very low dose isoproterenol (2 µg) infusion test demonstrated excessive tachycardia of HR increment of 49 bpm without a significant decrease of BP (Fig. 2). HUTT was interrupted because of the patient's intense malaise, lightheadedness, and palpitations as she recognized these symptoms as prodromes of her episodes of transient loss of consciousness.
Fig. 1.
Pre-and post-treatment heart rate responses to active standing test. HR: heart rate, bpm: beats per minute, Tx: treatment.
Fig. 2.
The head-up tilt table test with an isoproterenol challenge. SBP/DBP: systolic blood pressure/diastolic blood pressure, HR: heart rate, bpm: beats per minute.
The patient has been treated with topiramate, antidepressants and antiepileptic GABAergic drugs with gradual improvement of the headache and widespread pain over one month. She was commenced on propranolol with no symptomatic improvement. She was subsequently treated with midodrine and fludrocortisone and advised to increase salt and fluid intake. Application of an abdominal binder, aerobic exercise and physical counter-maneuvers [13] were recommended to help with autonomic conditioning. They resulted in a reduction of supine and erect HR to 76 and 92 bpm, respectively, along with symptomatic improvement over 2 months (Fig. 1).
DISCUSSION
The active standing test and HUTT with an isoproterenol challenge of this patient identified POTS and evidence of sympathetic β-adrenergic supersensitivity. The HR variance analysis also showed that the patient had a reduced vagally mediated variability in HR.
Pathogenesis of POTS is heterogenous and may include sympathetic hyperactivity with peripheral denervation, hypovolemia, and physical deconditioning [3]. The evaluation should include an electrocardiogram or 24-hour Holter monitoring to exclude cardiac conduction abnormalities. Both active standing tests and HUTT are sensitive for the diagnosis of POTS [3]. Supine plasma norepinephrine concentrations are often elevated, suggesting hyperadrenergic state [2]. However, our patient had normal range of norepinephrine and epinephrine level. We suppose that plasma norepinephrine may not reflect the central sympathetic activity. Pharmacologic agents including β-blockers, midodrine, fludrocortisones, and octreotide have been used for the relief of symptoms [3]. Increased sodium intake, elastic support hose, aerobic and resistance training exercise have been shown to be beneficial and should be encouraged [3].
Fibromyalgia was diagnosed in between 22 and 40% of migraine [8]. Patients who suffered from both migraine and fibromyalgia were older, and reported incapacitating headaches, more insomnia and mental stress, and lower quality of life [8]. The causative relationship between fibromyalgia and migraine is not yet clear.
The association between migraine, POTS and vasovagal syncope was found in 0.18% of migraine [1]. The link between migraine and autonomic dysfunction is not clear, which may be explained by an imbalance between the sympathetic and parasympathetic systems [1].
Autonomic abnormality in fibromyalgia explains the diverse clinical manifestations such as irritable bowel syndrome, sleep disorders, chronic fatigue, and sicca symptoms [3]. Deconditioning of the muscles, consequence of pain related physical inactivity, is assumed to lead to autonomic dysfunction in fibromyalgia [3]. Our patient was additionally diagnosed with POTS and fibromyalgia following admission to our hospital. The coexistence of fibromyalgia and autonomic intolerance in migraine patients should be considered when choosing appropriate therapeutic measures. Beck depression inventory score in this patient showed severe depression. We could not conclude whether the mental distress was caused by the fibromyalgia or migraine, or was an independent exacerbating factor for her symptoms.
We recognize several limitations to our observations that could limit the clinical interpretation. We were unable to perform quantitative assessment of sudomotor function such as thermoregulatory sweat testing (TST), quantitative sudomotor axon reflex testing (QSART) [3]. Although these tests are very useful, they are not available except in highly specialized centers. It should also be noted that transcranial Doppler monitoring is valuable for evaluating changes in cerebral blood flow during HUTT, although data in POTS are inconsistent [14]. More precise information with quantitative sudomotor function and transcranial Doppler analysis may be helpful for assessing and treating this patient.
In conclusion, we report a case with severe disabling manifestations of POTS in migraine and fibromyalgia. Autonomic dysfunction is often associated with the diverse clinical manifestations of fibromyalgia and migraine. Further studies are needed to demonstrate autonomic dysregulation in migraine with fibromyalgia, which may lead to diagnostic precision and effective clinical management.
References
- 1.Piovesan EJ, Sobreira CF, Scola RH, Lorenzoni PJ, Lange MC, Werneck LC, et al. Episodic migraine associated with postural orthostatic tachycardia syndrome and vasovagal syncope: migraine triggers neuromediated syncope. Arq Neuropsiquiatr. 2008;66:77–79. doi: 10.1590/s0004-282x2008000100018. [DOI] [PubMed] [Google Scholar]
- 2.Staud R. Autonomic dysfunction in fibromyalgia syndrome: postural orthostatic tachycardia. Curr Rheumatol Rep. 2008;10:463–466. doi: 10.1007/s11926-008-0076-8. [DOI] [PubMed] [Google Scholar]
- 3.Low PA, Sandroni P, Joyner MJ, Shen WK. Postural tachycardia syndrom. In: Low PA, Benarroch EE, editors. Clinical autonomic disorders. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 515–533. [Google Scholar]
- 4.Shim JC. Fibromyalgia syndrome. J Korean Pain Soc. 2004;17:80–87. [Google Scholar]
- 5.Yunus MB. Towards a model of pathophysiology of fibromyalgia: aberrant central pain mechanisms with peripheral modulation. J Rheumatol. 1992;19:846–850. [PubMed] [Google Scholar]
- 6.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 7.Kim SM, Lee SH, Kim HR. Applying the ACR preliminary diagnostic criteria in the diagnosis and assessment of fibromyalgia. Korean J Pain. 2012;25:173–182. doi: 10.3344/kjp.2012.25.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Tommaso M, Sardaro M, Serpino C, Costantini F, Vecchio E, Prudenzano MP, et al. Fibromyalgia comorbidity in primary headaches. Cephalalgia. 2009;29:453–464. doi: 10.1111/j.1468-2982.2008.01754.x. [DOI] [PubMed] [Google Scholar]
- 9.Bou-Holaigah I, Calkins H, Flynn JA, Tunin C, Chang HC, Kan JS, et al. Provocation of hypotension and pain during upright tilt table testing in adults with fibromyalgia. Clin Exp Rheumatol. 1997;15:239–246. [PubMed] [Google Scholar]
- 10.Kosinski M, Bayliss MS, Bjorner JB, Ware JE, Jr, Garber WH, Batenhorst A, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. 2003;12:963–974. doi: 10.1023/a:1026119331193. [DOI] [PubMed] [Google Scholar]
- 11.Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18:728–733. [PubMed] [Google Scholar]
- 12.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 13.Wieling W, Colman N, Krediet CT, Freeman R. Nonpharmacological treatment of reflex syncope. Clin Auton Res. 2004;14(Suppl 1):62–70. doi: 10.1007/s10286-004-1009-x. [DOI] [PubMed] [Google Scholar]
- 14.Hermosillo AG, Jordan JL, Vallejo M, Kostine A, Márquez MF, Cárdenas M. Cerebrovascular blood flow during the near syncopal phase of head-up tilt test: a comparative study in different types of neurally mediated syncope. Europace. 2006;8:199–203. doi: 10.1093/europace/eul001. [DOI] [PubMed] [Google Scholar]