Abstract
Osteoblasts and adipocytes may develop from common bone marrow mesenchymal precursors. Transgenic mice overexpressing ΔFosB, an AP-1 transcription factor, under the control of the neuron-specific enolase (NSE) promoter show both markedly increased bone formation and decreased adipogenesis. To determine whether the two phenotypes were linked, we targeted overexpression of ΔFosB in mice to the osteoblast by using the osteocalcin (OG2) promoter. OG2-ΔFosB mice demonstrated increased osteoblast numbers and an osteosclerotic phenotype but normal adipocyte differentiation. This result firmly establishes that the skeletal phenotype is cell autonomous to the osteoblast lineage and independent of adipocyte formation. It also strongly suggests that the decreased fat phenotype of NSE-ΔFosB mice is independent of the changes in the osteoblast lineage. In vitro, overexpression of ΔFosB in the preadipocytic 3T3-L1 cell line had little effect on adipocyte differentiation, whereas it prevented the induction of adipogenic transcription factors in the multipotential stromal cell line ST2. Also, ΔFosB isoforms bound to and altered the DNA-binding capacity of C/EBPβ. Thus, the inhibitory effect of ΔFosB on adipocyte differentiation appears to occur at early stages of stem cell commitment, affecting C/EBPβ functions. It is concluded that the changes in osteoblast and adipocyte differentiation in ΔFosB transgenic mice result from independent cell-autonomous mechanisms.
Although osteoblasts and adipocytes represent two morphologically and functionally distinct cell types, it has been proposed that they may develop from a common mesenchymal precursor in the bone marrow (36, 40, 43). Indeed, an inverse relationship between adipocyte and osteoblast differentiation has been suggested, as exemplified by increased bone marrow adipocytes in age-related bone loss (4, 6, 29, 32, 60) or after treatment with glucocorticoids (57). Several regulatory factors involved in osteoblast and adipocyte differentiation have been identified (27, 42, 45). However, the identities of the factors that control commitment at the branching point between the osteoblast and adipocyte lineages and the degree of plasticity between the two cell types are still uncertain (33, 38).
We have recently reported that transgenic mice overexpressing ΔFosB, a member of the activator protein 1 (AP-1) family of transcription factors, under the control of the neuron-specific enolase (NSE) promoter develop not only a severe and progressive osteosclerotic phenotype, characterized as increased bone formation, but also a pronounced decrease in adipogenesis and fat levels (25, 47, 53). The AP-1 family of basic leucine zipper transcription factors comprises various combinations of Jun (c-Jun, JunB, and JunD) and Fos (c-Fos, FosB, Fra-1, and Fra-2) proteins, which upon dimer formation regulate gene transcription by binding to consensus response elements present in the promoter region of target genes (23). Several studies have demonstrated an important regulatory role of AP-1 factors, especially the Fos-related proteins, in bone formation and osteoblast function (16, 19, 20, 26, 59). ΔFosB is a C-terminally truncated splice variant of FosB that lacks the proline-rich transactivation domain but has maintained the ability to bind DNA and heterodimerize with other AP-1 factors (30, 31, 64).
Although the increased bone formation in the NSE-ΔFosB mice was shown to be due, at least in part, to a cell-autonomous effect on cells of the osteoblast lineage, the dramatic decrease in adipogenesis observed in these mice, as revealed by decreased abdominal fat, low leptin levels in serum, and reduced number of adipocytes in the bone marrow, could be independently cell autonomous to the adipocyte lineage or secondary to the alteration in osteoblast differentiation. This uncertainty is due to the fact that the NSE promoter directs ΔFosB transgene expression in several tissues in addition to the brain, including bone and white adipose tissue. We therefore generated transgenic mice overexpressing ΔFosB under the control of the mouse osteocalcin promoter, OG2, thereby directing the transgene specifically to cells of the osteoblastic lineage, and determined their bone and fat phenotypes. OG2-ΔFosB mice demonstrated increased osteoblast function and an osteosclerotic phenotype, firmly establishing that the skeletal phenotype is cell autonomous to the osteoblast lineage, independent of the fate of adipocytes. In contrast, no change in adipocyte formation was observed, indicating that the decreased adipogenesis phenotype of the NSE-ΔFosB mice is independent of the osteosclerotic phenotype. Furthermore, in vitro studies with cells transfected with ΔFosB indicated that the inhibitory effects of ΔFosB on adipocyte differentiation occur mainly at early stages of stem cell commitment, possibly by affecting C/EBPβ functions. It is concluded that the bone and fat phenotypes of ΔFosB transgenic mice result from independent and cell-autonomous mechanisms.
MATERIALS AND METHODS
Construction of plasmids.
The pII1.3Luc construct containing 1.3 kb of the murine osteocalcin promoter OG2 (15) was provided by G. Karsenty, the promoter was subcloned into pBluescript SK (Stratagene) for subsequent sequencing, and the construct was designated pBS-OG2. The 1.3-kb OG2 promoter was released from pBS-OG2 by digestion with KpnI and HindIII restriction endonucleases and subcloned into the ptTAk vector (51), provided by Eric Nestler (8), to create pOG2-tTA. ΔFosB cDNA in the pTetOp vector was provided by Eric Nestler (8), cut out by digestion with KpnI and XbaI, and subcloned into the pcDNA3.1 expression vector (Invitrogen). A construct encoding only the truncated isoform of ΔFosB, termed Δ2ΔFosB, which arises from translational initiation from the methionine at position 79 was generated by replacing the EcoRV-BstEII fragment with the HindIII-BamHI fragment encoding Δ2ΔFosB. A mutant form of ΔFosB that expresses only the full-length isoform, named 2i3i, was generated by mutating the second and third methionines at positions 50 and 79 to isoleucines by two rounds of PCR-induced mutation of ΔFosB with the Quickchange kit (Stratagene), with primers M2F, M2R, M3F, and M3R (Table 1).
TABLE 1.
Sequences of oligonucleotides used for PCR and electrophoretic mobility shift assay
| Primer | Sequence |
|---|---|
| M2F | 5′-GAGTGCGCCGGTCTCGGGGAAATACCCGGCTCCTTCGTGCCAACG-3′ |
| M2R | 5′-CGTTGGCACGAAGGAGCCGGGTATTTCCCCGAGACCGGCGCACTC-3′ |
| M3F | 5′-CAACCCACCCTCATCTCTTCCATAGCCCAGTCCCAGGGGCAGCCA-3′ |
| M3R | 5′-TGGCTGCCCCTGGGACTGGGCTATGGAAGAGATGAGGGTGGGTTG-3′ |
| DFGSTF | 5′-GTCCCGAATTCGGGAAATGTTTCAAGCT-3′ |
| D2GSTF | 5′-TAATGAATTCCTTCCATGGCCCAGTCC-3′ |
| DFGSTR | 5′-CTGAACTCGAGTCCTCCTCATCTTCC-3′ |
| TTAF | 5′-CAT ATG CGG ATT AGA AAA ACA ACT-3′ |
| TTAR | 5′-ATC GGT AAA CAT CTG CTC AAA CTC-3′ |
| FosBF2 | 5′-GAG TCT CAG TAC CTG TCT TC-3′ |
| FosBB2 | 5′-GTC CAC TGG TGC TTG TGC T-3′ |
| ΔFosBF | 5′-GTG GGC CTT CAA CCA GCA CAA-3′ |
| ΔFosBB | 5′-GCA CTT AGC TGC AGC CAG CTT GT-3′ |
| ACTINF | 5′-AGG CCC CTC TGA ACC CTA AG-3′ |
| ACTINR | 5′-CAA CAC AGC CTG GAT GGC TA-3′ |
| D-site | 5′-TGCAGATTGCGCAAT-3′ |
Glutathione S-transferase (GST) fusion constructs were generated by PCR either from Δ2ΔFosB cDNA with primers D2GSTF and DFGSTR (Table 1) or from 2i3i cDNA with primers DFGSTF and DFGSTR (Table 1), containing EcoRI and XhoI restriction sites. PCR products were digested and cloned into pGEX4T2 (Amersham). EcoRI/XhoI fragments were excised from 2i3i-pGEX4T2 and Δ2-pGEX4T2 and subcloned into pCMV-Myc (Clontech). C/EBPα, C/EBPβ, peroxisomal proliferator-activated receptor γ (PPARγ), adipsin, and lipoprotein lipase cDNA, used as probes in Northern blot analysis, were kindly provided by J. Gimble, and C/EBPβ cDNA in pBlueskript SK, used for transfections, was received from D. P. Ramji.
Transgenic mice.
A DNA fragment containing the promoter, open reading frame, simian virus 40 intron, and polyadenylation signal in pOG2-tTA was gel purified and microinjected into the pronuclei of oocytes from (SJL × C57BL6)F2 mouse ova. Viable embryos were implanted into pseudopregnant recipients and allowed to develop to term. Founders were identified by isolating tail DNA with the Tissue DNeasy kit (Qiagen) and analyzing for the transgene by PCR with primers TTAF and TTAR (Table 1), which were also subsequently used for routine genotyping of the transgenic mice. The ΔFosB transgene was identified with primers FosBF2 and FosBB2 (Table 1).
As previously described (8, 24, 25, 47, 53), NSE-ΔFosB mice were generated by crossing mice expressing the tetracycline transactivator (tTA) under the control of the NSE promoter with mice carrying the ΔFosB gene under the control of the tetracycline-responsive promoter TetOp. Similarly, OG2-ΔFosB mice were generated by cross-breeding the OG2-tTA transgenic mice with the TetOp-ΔFosB mice. For all experiments, the bitransgenic (NSE-ΔFosB or OG2-ΔFosB) mice were bred as heterozygotes, and monotransgenic (NSE-tTA or OG2-tTA) littermates were used as controls. All animal protocols were approved by the Yale University Institutional Animal Care and Use Committee.
Histomorphometric analysis.
All mice used for analysis were injected with calcein (20 mg/kg; Sigma) and demeclocycline (20 mg/ml; Sigma) at 10 and 3 days before sacrifice, respectively, to label bone mineralization fronts (52). Bone sections were fixed in 3.7% formaldehyde-phosphate buffered saline and embedded by standard procedures in methylmethacrylate resin (52). Five-micrometer toluidine blue-stained and 10-μm unstained sections were analyzed for bone histomorphometry by standard procedures (37) with the Osteomeasure system (Osteometrics, Atlanta, Ga.). All measurements were performed in a blinded fashion. Von Kossa staining of proximal tibia to illustrate increased trabecular bone volume was done according to standard procedures (2).
Quantification of abdominal fat and serum leptin levels.
Ten-week-old OG2-ΔFosB mice and control littermates were anesthetized with metofane (Medical Developments) and bled by cardiac puncture, and serum samples were used immediately for measurement of leptin levels with the QuantikineM radioimmunoassay (R&D Systems, Inc.) Abdominal adipose tissue was removed and weighed.
Cell cultures.
Primary calvarial cell cultures were prepared from 10-week-old control, OG2-ΔFosB, and NSE-ΔFosB mice by standard techniques (3) and used immediately for expression analysis. Primary bone marrow cells were established from 10-week-old control, OG2-ΔFosB, and NSE-ΔFosB mice by plating the marrow flushed from tibia and femur in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 1% penicillin-streptomycin (complete medium). Nonadherent cells were removed after 5 days, and the adherent cells were trypsinized, replated, and allowed to reach confluency. Complete medium supplemented with 50 μg of ascorbic acid per ml, 5 mM β-glycerophosphate, and 10 nM dexamethasone (all from Sigma) was added (day 0) to induce differentiation, and the cultures were maintained under these conditions for the indicated number of days.
3T3-L1 cells, obtained from the American Type Culture Collection, were cultured in complete medium. Differentiation was induced in confluent preadipocytes (designated day 0) by adding 10 μg of insulin per ml, 1 μM dexamethasone, and 0.5 mM 3-isobutyl-1-methylxanthine (all from Sigma) in complete medium. After 48 h, the medium was replaced with complete medium containing 1 μg of insulin per ml for an additional 48 h. The cells were fully differentiated by day 8, and the adipocyte phenotype was examined at the time points indicated. The murine stromal cell-line ST2, provided by Aventis Pharma, Inc., was cultured in RPMI 1640 medium supplemented with 10% sodium bicarbonate, 10% fetal bovine serum, and 1% penicillin-streptomycin. Adipocyte differentiation was induced by culturing the cells for 2 days in medium supplemented with 0.5 μM hydrocortisone (Sigma), 500 μM 3-isobutyl-1-methylxanthine, and 60 μM indomethacin (Sigma). 3T3-L1 and ST2 cells were transfected with the FuGene6 transfection reagent as recommended by the manufacturer (Roche), and lines stably expressing the transgene were established by geneticin (G-418) (Invitrogen) selection.
Staining.
Bone marrow cultures were fixed in 10% formalin, and osteoblastic cells were visualized by the alkaline phosphatase leukocyte staining kit (Sigma). To stain lipid-filled vacuoles, fixed cells were incubated with Oil Red O (Sigma) for 30 min, followed by three washes with phosphate-buffered saline, as described (50). The degree of adipocyte differentiation was estimated by eluting the stain in isopropanol and measuring the absorbance at 540 nm.
RNA purification and real-time RT-PCR.
Total RNA was extracted from various tissues isolated from 10-week-old OG2-ΔFosB mice and control littermates with the Trizol (Gibco) method (9). Quantitative ΔFosB mRNA expression in tissues was determined by real-time reverse transcription-PCR (RT-PCR) analysis on the iCycler (Bio-Rad), with the one-step QuantiTect SYBR Green RT-PCR kit (Qiagen) as described by the manufacturer. Primers ΔFosBF, ΔFosBB, ACTINF, and ACTINR (Table 1) were designed with the Primer Express V1.0 software (Perkin-Elmer Applied Biosystems Inc.).
Northern blotting.
Twenty micrograms of total RNA was resolved in 1% denaturing agarose-formaldehyde gels and transferred onto Hybond-N nylon membranes (Amersham) as described (48). Randomly 32P-labeled cDNA probes were purified on Wizard minicolumns (Promega), and membranes were incubated at 42°C overnight in hybridization buffer (50% formamide, 5× SSPE [1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA, pH 7.7], 5× Denhardt's solution, 0.1% sodium dodecyl sulfate [SDS]). Membranes were washed for 1 h at 42°C in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% SDS and 1 h at 42°C in 1× SSC-0.1% SDS before being exposed to X-ray film. 18S rRNA was used as an internal control.
Immunoprecipitation and Western blot analysis.
Cells were lysed in modified radioimmunoprecipitation assay (mRIPA) buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.1% NP-40, 0.25% sodium deoxycholate, 1 mM EGTA) supplemented with 1 mM NaF, 1 mM Na3VO4, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 10 μg of pepstatin per ml, and 1 mM phenylmethylsulfonyl fluoride. The lysates were cleared by centrifugation, and the protein concentration was determined by the BCA assay (Pierce); 500 μg of total cell lysate protein was incubated with 3 to 5 μg of polyclonal anti-C/EBPβ antibody (Santa Cruz) in 500 μl of mRIPA buffer at 4°C with shaking for 2 h. The samples were then incubated with 50 μl of a 50% protein G-Sepharose slurry (Amersham Pharmacia Biotech) for 1 h at 4°C with shaking. The beads were then washed three times in mRIPA buffer and boiled in 30 μl of loading buffer. Proteins were electrophoresed through an SDS-12% polyacrylamide gel and transferred at 35 V overnight to Protran nitrocellulose membranes (Schleicher & Schleicher Inc.). Total cell lysates (50 μg) were electrophoresed on 10 to 12% gradient SDS-PAGE gels and transferred to nitrocellulose membranes. Western blotting was performed with specific antibodies by standard techniques. Immunoreactive bands were detected by the ECL method (Amersham Pharmacia Biotech). All antibodies were purchased from Santa Cruz Biotechnology.
GST pull-down.
GST fusion proteins were expressed and purified with the B-PER bacterial protein extraction reagent (Pierce) according to the manufacturer's instructions. In short, transformed Escherichia coli BL21 cells expressing the GST fusion proteins were harvested and lysed in B-PER reagent. The cellular extracts were cleared by centrifugation and incubated with glutathione-Sepharose 4B beads (Amersham Pharmacia Biotech) for 20 min at room temperature with gentle agitation. The suspensions were washed and resuspended in mRIPA buffer to make a 50% slurry. The purified GST fusion proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie blue staining. For interaction studies, GST fusion proteins were incubated with 500 μg of total cell lysate protein in mRIPA buffer for 2 h at 4°C. The samples were washed three times in mRIPA buffer, the beads were sedimented and boiled in 10 μl of loading buffer, and the complexes were analyzed by Western blotting.
Electrophoretic mobility shift assay.
Nuclear extracts from cells were prepared as described previously (49). The electrophoretic mobility shift assay and supershift analysis were performed as described (46), with a 32P-labeled double-stranded oligonucleotide corresponding to a consensus C/EBPβ binding site (D-site, Table 1) (56). For supershift analysis, anti-C/EBPβ antibody (Santa Cruz) was incubated with nuclear extracts for 30 min on ice before addition of the labeled probe. Following electrophoresis, gels were dried, and the protein-DNA complexes were visualized by autoradiography.
Statistical analysis.
The data are represented as means ± standard error of the mean, and statistical analysis was performed with Student's t test. A P value of less than 0.05 was considered statistically significant.
RESULTS
Bone-specific overexpression of ΔFosB.
With the same tetracycline-regulated system as used for the previously described NSE-ΔFosB transgenic mice (8, 47), bone-specific overexpression of the ΔFosB transgene was obtained by driving transgene expression with the osteoblast-specific osteocalcin promoter OG2. Transgenic mice expressing the tetracycline transactivator (tTA) under the control of the OG2 promoter were generated and crossed with transgenic mice carrying the ΔFosB cDNA under the control of the TetOp promoter, which requires the binding of tTA in order to drive transcription. In the absence of tetracycline, tTA binds to the promoter and activates transcription of ΔFosB cDNA, whereas in the presence of tetracycline, tTA undergoes a conformational change and cannot bind to the TetOp promoter, preventing transcription of the transgene.
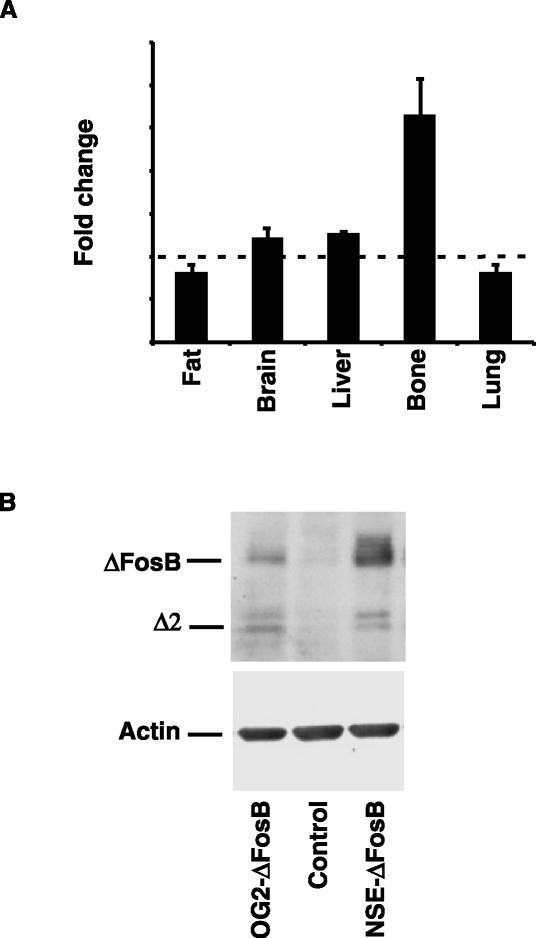
As shown in Fig. 1, the use of the OG2 promoter resulted in bone-specific overexpression of ΔFosB. Real-time RT-PCR analysis of the ΔFosB transcript in a number of tissues revealed an approximately threefold overexpression of ΔFosB in bone, whereas no statistically significant differences in ΔFosB expression levels were observed in liver, lung, brain, or adipose tissues (Fig. 1A). In addition, Western blot analysis of ΔFosB expression in osteoblasts isolated from OG2-ΔFosB transgenic mice further demonstrated overexpression of the transgene, although at lower levels than in the NSE-ΔFosB transgenic animals (Fig. 1B). Since osteocalcin is known to be upregulated towards the end of osteoblast differentiation, it is, however, likely that transgene expression would be higher in more mature cells.
FIG. 1.
Bone-specific overexpression of the ΔFosB transgene in OG2-ΔFosB mice. (A) Real time RT-PCR analysis of ΔFosB mRNA levels in fat, brain, liver, bone, and lung tissues in 10-week-old OG2-ΔFosB transgenic mice compared to control littermates. (B) Western blot analysis of ΔFosB protein levels in primary calvarial osteoblasts established from 10-week-old OG2-ΔFosB transgenic mice compared to control littermates and a low-expressing founder line of the NSE-ΔFosB transgenic mice. The full-length ΔFosB and truncated Δ2ΔFosB isoforms are indicated. Actin levels were used as an internal control.
OG2-ΔFosB transgenic mice are osteosclerotic.
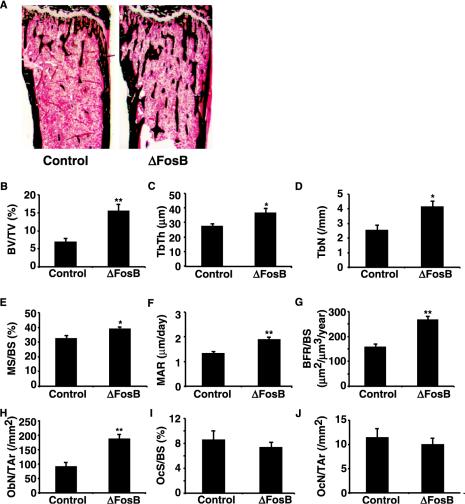
Having established that bone-specific overexpression of ΔFosB occurs in the OG2-ΔFosB transgenic mice, the bone phenotype was examined. As in the NSE-ΔFosB transgenic mice, Von Kossa staining of mineralized tissue in the proximal tibia from 10-week-old animals demonstrated an increased trabecular bone volume in the bone marrow of OG2-ΔFosB mice compared to control littermates (Fig. 2A). Detailed histomorphometric analysis showed a more than twofold increase in trabecular bone volume by this age (Fig. 2B). The increased trabecular bone volume was associated with statistically significant increases in trabecular thickness and trabecular number (Fig. 2C and D). The dynamic bone formation parameters (mineralizing surface, mineral apposition rate, and bone formation rate) were all significantly higher in the OG2-ΔFosB mice (Fig. 2E, F, and G), and the number of osteoblasts per total area of bone tissue was more than doubled (Fig. 2H). However, the number of osteoblasts relative to the bone perimeter was unchanged (not shown), indicating that osteoblast differentiation was increased while the amount of bone formed per osteoblast was unchanged. Also, neither the osteoclast surface nor osteoclast number was significantly different between the OG2-ΔFosB transgenic animals and control littermates (Fig. 2I and J), suggesting that, as in the NSE-ΔFosB mice, bone resorption was unchanged.
FIG. 2.
Osteosclerotic phenotype in OG2-ΔFosB transgenic mice. (A) Von Kossa-stained proximal tibia from 10-week-old control and OG2-ΔFosB transgenic mice. (B to J) Histomorphometric analysis of toluidine blue-stained sections from OG2-ΔFosB transgenic mice and control littermates at 10 weeks of age. (B) Trabecular bone volume (BV/TV); (C) trabecular thickness (TbTh); (D) trabecular number (TbN); (E) mineralizing surface (MS/BS); (F) mineral apposition rate (MAR); (G) bone formation rate (BFR/BS); (H) osteoblast number (ObN/TAr); (I) osteoclast surface (OcS/BS); (J) osteoclast number (OcN/TAr). Data presented are means ± standard error of the mean; *, P < 0.05; **, P < 0.005, Student's t test.
Unchanged adipogenesis in OG2-ΔFosB transgenic mice.
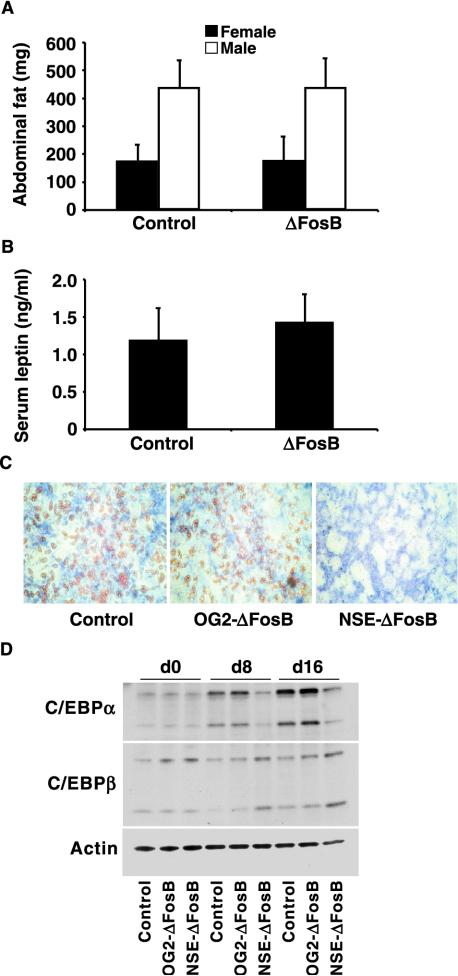
In contrast to the recurrence of the osteosclerotic phenotype, there was no apparent change in adipogenesis when ΔFosB expression was targeted exclusively to cells of the osteoblast lineage. Similar amounts of abdominal fat were found in the transgenic mice and control littermates (Fig. 3A). Consistent with this finding, and in contrast to the NSE-ΔFosB mice, the level of the adipocyte-secreted hormone leptin in serum was unaltered (Fig. 3B).
FIG. 3.
Unchanged adipogenesis in OG2-ΔFosB transgenic mice. (A) Abdominal fat weight in 10-week-old OG2-ΔFosB and control female and male animals. Data are shown as means ± standard error of the mean. There was no significant difference between gender-matched OG2-ΔFosB and control animals. (B) Immunoassay quantification of leptin levels in serum in 10-week-old OG2-ΔFosB and control mice. Data are shown as means ± standard error of the mean. There was no significant difference between gender-matched OG2-ΔFosB and control animals. (C) Long-term primary bone marrow mesenchymal cell cultures established from control, OG2-ΔFosB, and NSE-ΔFosB transgenic mice and maintained under osteogenic conditions were stained for alkaline phosphatase to visualize osteoblasts and with Oil Red O to visualize lipid-filled vacuoles in mature adipocytes, as described in Materials and Methods. (D) Western blot analysis of C/EBPα and C/EBPβ protein levels in total cell extracts from cultures of primary bone marrow cells from control, OG2-ΔFosB, and NSE-ΔFosB transgenic mice at days 0, 8, and 16 of differentiation. Mainly the 42-kDa (p42) and 30-kDa (p30) C/EBPα isoforms and the 32- to 35-kDa (LAP) and 20-kDa (LIP) C/EBPβ isoforms were detected. Actin was used as an internal control.
The adipogenic capacity of bone marrow mesenchymal progenitor cells was also studied. Long-term culture of wild-type primary bone marrow stromal cells with ascorbic acid and β-glycerophosphate to induce osteoblast differentiation typically produces both osteoblasts and clusters of mature lipid-filled adipocytes. We had previously found, however, that marrow cells from the NSE-ΔFosB mice formed markedly fewer adipocytes in culture than did those from control animals (47). When bone marrow stromal cells from control, OG2-ΔFosB, and NSE-ΔFosB mice were cultured with ascorbic acid and β-glycerophosphate, the adipogenic capacity of bone marrow cultures from OG2-ΔFosB transgenic mice, shown by staining with Oil Red O, was comparable to that of cultures established from control mice, while adipocyte formation was dramatically reduced in cultures from NSE-ΔFosB transgenic mice, as reported previously (25, 47) (Fig. 3C).
To examine adipogenesis at the molecular level, total cell lysates were isolated from control, OG2-ΔFosB, and NSE-ΔFosB bone marrow cultures at days 0, 8, and 16 of differentiation, and the adipocytic transcription factors C/EBPα and C/EBPβ were quantified by Western blotting (Fig. 3D). The major isoforms of C/EBPα are p42 (42-kDa) and p30 (30-kDa) proteins, whereas C/EBPβ mRNA produces mainly a 32- to 35-kDa isoform, termed LAP, and a 20-kDa isoform, termed LIP. The expression of both the 42-kDa and 30-kDa isoforms of C/EBPα was clearly increased with progressive adipocyte differentiation in extracts from control and OG2-ΔFosB mouse cell cultures but not in NSE-ΔFosB mouse cell cultures, further confirming the lack of an adipocytic effect in the osteoblast-specific transgenic mice. In contrast, there was little change in the expression of C/EBPβ isoforms in any of the cultures.
ΔFosB inhibits the expression of adipogenic transcription factors at an early stage of stem cell commitment.
The unchanged adipogenesis in the OG2-ΔFosB transgenic mice suggested that the inhibitory effects on adipocyte differentiation in NSE-ΔFosB transgenic mice were the result of direct effects of the transgene product in adipocytes or their precursors. In order to test this hypothesis and to elucidate at which stage of adipocyte differentiation the inhibition occurs, we examined the effect of ΔFosB expression on differentiation of the preadipocytic cell line 3T3-L1, which undergoes a well-characterized adipocytic differentiation program (27, 42), as well as in the multipotential mesenchymal cell line ST2, which has the potential to differentiate along both the osteoblastic and adipocytic lineages (35, 39, 63) and thus represents an earlier stage of mesenchymal cell differentiation.
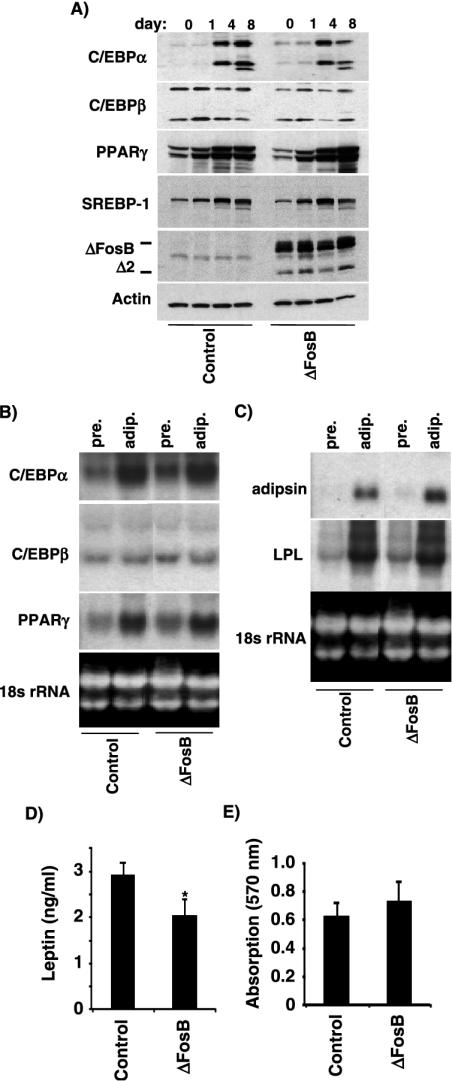
3T3-L1 cells stably transfected with ΔFosB or an empty vector control were grown to confluence and then induced to undergo differentiation by treatment with adipogenic agents. Total cell lysates were isolated from preadipocytes (day 0), differentiating adipocytes (days 1 and 4), and fully mature adipocytes (day 8), and the expression levels of several adipocyte transcription factors were examined by Western blotting. As shown in Fig. 4A, the presence of ΔFosB had little or no effect on the clear increase in the protein level of C/EBPα isoforms, PPARγ, and sterol regulatory element binding protein 1 (SREBP-1) transcription factors that occurred in 3T3-L1 cells cultured in adipogenic medium. The early adipogenic transcription factor C/EBPβ remained essentially unchanged in both control-transfected and ΔFosB-expressing cells throughout the experiment. We also examined steady-state mRNA levels in undifferentiated and differentiated 3T3-L1 cells by Northern blot analysis (Fig. 4B). In these already committed preadipocytic cells, ΔFosB had no effect on the levels of C/EBPα and C/EBPβ RNA transcripts in either the preadipocytes or the mature adipocytes, consistent with the results obtained by Western blotting.
FIG. 4.
ΔFosB has little effect on 3T3-L1 adipocyte differentiation. (A) Western blot analysis of the adipogenic transcription factors C/EBPα, C/EBPβ, PPARγ, and SREBP-1 in cell extracts from 3T3-L1 adipocytes stably transfected with ΔFosB or the empty vector control at days 0, 1, 4, and 8 of adipocyte differentiation. Mainly the 42-kDa (p42) and 30-kDa (p30) C/EBPα isoforms and the 32- to 35-kDa (LAP) and 20-kDa (LIP) C/EBPβ isoforms were detected. Actin served as an internal control. (B) Northern blot analysis of C/EBPα, C/EBPβ, and PPARγ in preadipocytes (pre) and mature adipocytes (adip). (C) Northern blot analysis of two markers of mature adipocytes, lipoprotein lipase (LPL) and adipsin. 18S rRNA served as an internal control. (D) Secreted leptin in conditioned medium from control- and ΔFosB-transfected cells at day 8 of adipocyte differentiation was quantified as described in Materials and Methods (*, P < 0.05). (E) 3T3-L1 cells stably transfected with ΔFosB or empty vector were stained with Oil Red O at day 8 of differentiation, and the degree of staining was quantified as described in Materials and Methods. Data are presented as means ± standard error of the mean. *, P < 0.05, Student's t test.
We also examined adipocyte markers expressed at later stages of adipocyte differentiation. Both adipsin and lipoprotein lipase mRNA levels were strongly increased in mature adipocytes compared to preadipocytes in both control and ΔFosB-expressing 3T3-L1 cells (Fig. 4C). Finally, at day 8 of differentiation, at which point more than 80% of the cells had acquired the phenotype of mature adipocytes, secreted leptin (Fig. 4D) and lipid accumulation (Fig. 4E) were measured as described in Materials and Methods. Although overexpression of ΔFosB reduced leptin secretion by about 30% (Fig. 4D), there were no statistically significant differences in lipid accumulation by the ΔFosB and control cultures and the morphologies of the ΔFosB and control cultures were similar, supporting the finding that ΔFosB overexpression has little effect on adipocyte differentiation in 3T3-L1 preadipocytes.
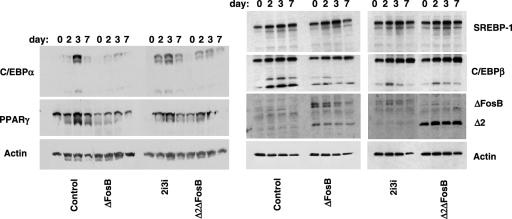
The findings that ΔFosB had little effect on the development of fully mature adipocytes from the cell-line 3T3-L1, which is considered already committed to the adipocytic lineage, but repressed adipogenesis from the primary bone marrow progenitor cells from NSE-ΔFosB mice suggested that ΔFosB exerts its inhibitory effects at early stages of stem cell commitment. This possibility was tested by overexpressing ΔFosB in ST2 cells, which have the potential to differentiate along both the osteoblastic and adipocytic lineages and thus represent an earlier stage of mesenchymal cell differentiation. Western blot analysis of adipocyte transcription factors at days 0, 2, 3, and 7 of adipocyte differentiation (Fig. 5) demonstrated that stable overexpression of ΔFosB repressed the increase in C/EBPα and PPARγ protein levels that occurred in control cells. No clear change in expression levels of the high-molecular-weight isoform of C/EBPβ (LAP) was observed, but expression of the short C/EBPβ isoform (LIP) as well as a band of slightly higher molecular weight that most likely represents a posttranslationally modified form of LIP was reduced in the ΔFosB-transfected cells. In contrast, the expression pattern of the adipocyte transcription factor SREBP-1 was unchanged.
FIG. 5.
ΔFosB isoforms inhibit the expression of adipogenic transcription factors in the ST2 bone marrow stromal cell line. Western blot analysis of C/EBPα, C/EBPβ, PPARγ, and SREBP-1 in cell extracts from ST2 cells stably transfected with ΔFosB, the full-length 2i3i isoform, the truncated Δ2ΔFosB isoform, or empty vector at days 0, 2, 3, and 7 of adipocyte differentiation. Mainly two C/EBPα isoforms at 42 to 45 kDa (probably p42 and a posttranslationally modified form) were detected, whereas less of the 30-kDa (p30) isoform was seen. For C/EBPβ, mostly the 32- to 35-kDa (LAP) isoform and less of the 20-kDa (LIP) isoform were detected. At higher expression levels, a third band, most likely representing a posttranslationally modified LIP form, was observed. Actin served as an internal control.
The ΔFosB mRNA transcript gives rise to both the ΔFosB protein and a further truncated isoform, termed Δ2ΔFosB, that is lacking the N-terminal Fos homology domain of ΔFosB. In order to elucidate the relative importance of these two isoforms in regulating adipocyte differentiation, ST2 cells were transfected with a Δ2ΔFosB cDNA construct or with a mutated ΔFosB construct, termed 2i3i, that cannot generate the Δ2ΔFosB protein. Transfection with either 2i3i or Δ2ΔFosB caused a decrease in PPARγ, C/EBPα, and the LIP isoform of C/EBPβ but had no effect on SREBP-1, similar to the pattern seen when the cells were transfected with the native ΔFosB cDNA (Fig. 5). Thus, the Fos homology domain, which is present in ΔFosB and 2i3i but not in the further truncated Δ2ΔFosB isoform, is not required for the ΔFosB-induced changes in the expression of the adipogenic transcription factors.
ΔFosB interacts with transcription factor C/EBPβ.
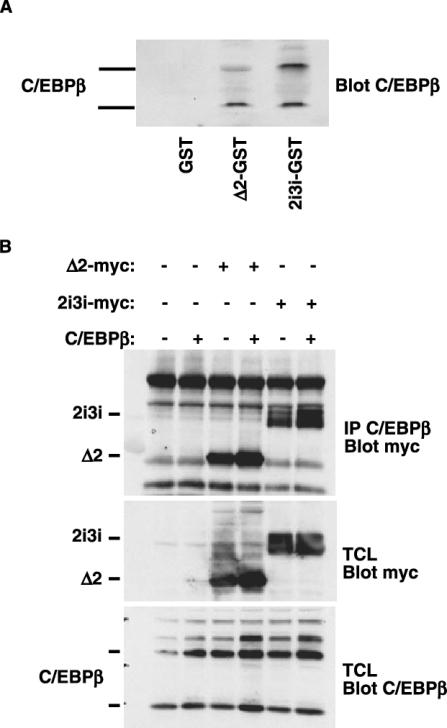
Since the inhibitory effect of ΔFosB on adipogenesis appeared to occur at an early stage of adipocyte differentiation, ΔFosB could interact with one or more transcription factors known to be involved in the early commitment to adipogenesis. C/EBPβ is one of the earliest inducers of adipogenesis and, like ΔFosB, is a basic leucine zipper transcription factor. We therefore investigated the possibility that the two proteins interact. For this purpose, we generated chimeric proteins of GST fused to the full-length 2i3i isoform or to Δ2ΔFosB and used them in pull-down experiments with total cell lysates from ST2 cells that overexpressed a C/EBPβ construct (Fig. 6A). Both GST-Δ2ΔFosB and GST-2i3i fusion proteins bound C/EBPβ in the cell extracts, whereas GST by itself did not, demonstrating in vitro an interaction between ΔFosB and C/EBPβ.
FIG. 6.
ΔFosB interacts with the early adipogenic transcription factor C/EBPβ. (A) Pull-down experiments were performed with GST-ΔFosB and GST-Δ2ΔFosB and cellular extracts from ST2 cells transfected with a C/EBPβ construct. Bound proteins were analyzed by Western blotting with an anti-C/EBPβ antibody. (B) C/EBPβ was immunoprecipitated (IP) from ST2 cells transfected with C/EBPβ and either the Myc-Δ2ΔFosB or Myc-2i3i isoform, and the immune complexes were blotted for Myc to detect the Myc-tagged ΔFosB isoforms. Western blot analysis of c-Myc and C/EBPβ in total cell lysates (TCL) from transfected cells verified equal expression levels of the proteins.
To confirm that this interaction also occurs in a cellular environment, ST2 cells were transfected with C/EBPβ and either Myc-tagged Δ2ΔFosB or Myc-tagged 2i3i. Immunoprecipitation of C/EBPβ followed by immunoblotting of the immune complexes with an anti-Myc antibody identified both ΔFosB isoforms (Fig. 6B), confirming that the C/EBPβ and ΔFosB transcription factors were present in the same complex. The endogenous level of C/EBPβ was sufficient to precipitate some of the Myc-tagged ΔFosB proteins, although not as much as when C/EBPβ was overexpressed. No interactions between ΔFosB and C/EBPα or C/EBPδ were detected by either GST pull-down or coimmunoprecipitation (data not shown).
Δ2ΔFosB enhances binding of C/EBPβ to its consensus DNA-binding site.
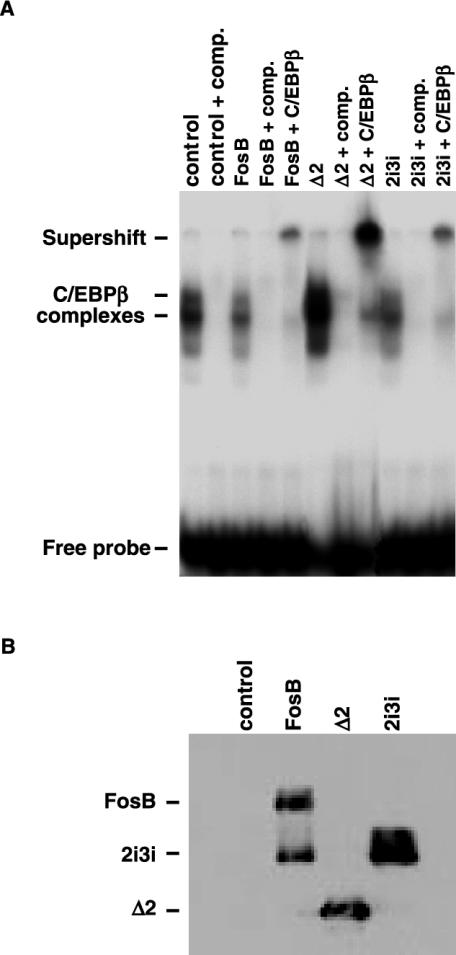
The interaction of ΔFosB isoforms with C/EBPβ suggests that the interaction might affect the ability of C/EBPβ to heterodimerize with other transcription factors and to bind DNA response elements, thereby affecting the regulation of the transcription of downstream target genes (11, 27). We therefore examined the effect of ΔFosB isoforms on C/EBPβ binding to its consensus response element (11, 41) in an electrophoretic mobility shift assay. As shown in Fig. 7A, incubation of the C/EBPβ response element with nuclear extracts from control-transfected ST2 cells produced several complexes. The complexes observed were shown to be specific by competition with unlabeled probe. When nuclear extracts from cells overexpressing FosB or the full-length ΔFosB isoform were used, the levels of the complexes were slightly reduced from the levels seen with the control nuclear extracts. In contrast, overexpression of the truncated Δ2ΔFosB isoform dramatically enhanced complex formation. Supershift analysis with an antibody against C/EBPβ indicated that C/EBPβ was part of all the complexes formed on the response element. The expression of FosB isoforms in the nuclear extracts used for the electrophoretic mobility shift assay was verified by Western blotting (Fig. 7B).
FIG. 7.
Truncated Δ2ΔFosB isoform enhances binding of C/EBPβ to its consensus DNA response element. (A) Electrophoretic mobility shift assay of nuclear extracts from ST2 cells transfected with empty vector (control) or cells overexpressing FosB, the truncated Δ2ΔFosB isoform, or the full-length 2i3i isoform. Complex formation was inhibited with excess unlabeled oligonucleotide (comp), or protein complexes were supershifted with an anti-C/EBPβ antibody. (B) Western blot analysis of nuclear extracts to verify overexpression of ΔFosB isoforms.
DISCUSSION
It is widely believed that osteoprogenitors and adipocyte progenitors originate from common mesenchymal stem cells located in the bone marrow (40, 43). In addition, several lines of evidence have suggested that differentiation of osteoblasts and adipocytes is regulated reciprocally (4, 11, 32). Park et al. reported that differentiated adipocytes from human bone marrow could dedifferentiate and then redifferentiate into osteoblasts (38), indicating a high degree of plasticity in the osteoblast and adipocyte lineages. In vivo, an inverse relationship between the number of osteoblasts and bone marrow adipocytes has been demonstrated in several forms of osteopenia, where decreased bone mass is often associated with increased adipogenesis (6, 29, 57, 60). Conversely, we have reported an osteosclerotic phenotype accompanied by decreased adipogenesis in transgenic mice that overexpress the AP-1 transcription factor ΔFosB under the control of the NSE promoter (25, 47, 53).
In our initial study of NSE-ΔFosB mice, we found that the NSE promoter directs transgene expression to both bone and fat as well as to several other tissues (47). Thus, the concurrence of increased osteoblast formation and function and decreased adipocyte formation could result from the independent action of ΔFosB in the two cell types. On the other hand, given the postulated reciprocity in the development of the two cell lineages, the change in one cell type could be a consequence of the action of ΔFosB in the other. We previously examined the possibility that the decreased circulating leptin levels in these mice contributed to the bone phenotype, since it has been suggested that the adipocyte-secreted hormone leptin acts as a mediator coupling adipocyte differentiation to osteoblast function (14). While we found that correcting the level of circulating leptin had little effect on the excessive bone formation (25), this did not completely exclude the possibility of interdependence between the increased osteoblast differentiation and decreased adipocyte differentiation observed in the transgenic animals.
To further understand how ΔFosB induces the changes in osteoblast and adipocyte differentiation, and especially to explore the possible role of indirect effects, we generated transgenic mice that express ΔFosB in a bone-specific manner, with the mouse OG2 (osteocalcin) promoter used to drive the expression of the tetracycline transactivator. Osteocalcin is considered a late marker of differentiating osteoblasts (54), and while the OG2 promoter produces a lower level of expression than the collagen 1a1 promoter (22), it has been used successfully to direct transgene expression specifically to mature osteoblastic cells (10, 22, 28), without any expression in osteoclasts (65, 66). The phenotype of the bitransgenic OG2-tTA × TetOp-ΔFosB mice clearly showed that the effects of ΔFosB on osteoblast and adipocyte differentiation are independent of each other.
Complete histomorphometric analysis showed that the restricted expression of ΔFosB in osteoblasts was sufficient to cause a significant increase in all bone formation parameters without affecting bone resorption, as had been seen in the NSE-ΔFosB transgenic mice. Thus, these results confirm our previous conclusion that ΔFosB-induced osteosclerosis is a direct effect of ΔFosB overexpression in osteoblasts (25, 47). However, in sharp contrast to the clear inhibition of adipogenesis in NSE-ΔFosB mice, no differences were observed in either the abdominal fat or the adipogenic capacity of the bone marrow cells of OG2-ΔFosB transgenic mice. This study therefore also indicates that the decreased adipogenesis in the NSE-ΔFosB mice is not simply a consequence of the increased osteoblast differentiation, but rather is probably the direct result of ΔFosB overexpression in preadipocytes or adipocytes. While we cannot rule out the possibility that the absence of the adipocyte phenotype in the OG2-ΔFosB mice is due in part to a lower level of expression of ΔFosB in the osteoblasts of these mice than in the osteoblasts of the NSE-ΔFosB mice, we think that this is unlikely, given our earlier findings that the abdominal fat weights were essentially identical in the high-expressing and low-expressing lines of NSE-ΔFosB mice, while the additional bone formation differed by more than 10-fold between those lines (47), and also that when the NSE-ΔFosB mice are given doxycycline to prevent transgene expression, the low weight of the fat pad is essentially unchanged at a time (2 weeks of treatment) when bone formation and osteoblast number have been reduced to levels well below those of the control littermates (53).
These results suggest that, if anything, the fat phenotype is more likely to be induced by low levels of ΔFosB expression than the bone phenotype. Thus, ΔFosB most likely affects both osteoblast and adipocyte differentiation directly and independently. Whether ΔFosB regulates the two events by similar mechanisms is unknown. Interestingly, a similar osteosclerotic phenotype has been described in mice that overexpress another Fos family member, Fra-1 (19). In contrast to ΔFosB, however, Fra-1 overexpression had no effect on adipogenesis in vivo, much like the phenotype seen in the OG2-ΔFosB mice, but it enhanced osteoclastogenesis in vitro.
Differentiation of mesenchymal cells into adipocytes involves a cascade of transcriptional events, including the induction of the early adipocyte transcription factors C/EBPβ and C/EBPδ. These transcription factors in turn activate the expression of C/EBPα and PPARγ, which are essential for the stimulation of several adipocyte-specific genes (7, 61, 62). In fact, knocking out either the C/EBPα gene or the PPARγ gene results in severely diminished adipogenesis (44, 55, 58).
We found that C/EBPα expression was downregulated in primary bone marrow stromal cell cultures from NSE-ΔFosB mice but not in those from OG2-ΔFosB mice (Fig. 3), suggesting that ΔFosB might be altering this key adipogenic regulatory mechanism when expressed in adipocytes or their precursors. We therefore explored the possible mechanism of a direct effect of ΔFosB on adipocyte differentiation by overexpressing the ΔFosB isoforms in the preadipocytic 3T3-L1 cell line and the less committed ST2 bone marrow stromal cell line. Overexpression of either of the ΔFosB isoforms in ST2 cells inhibited the induction of C/EBPα and PPARγ, further supporting the possibility that downregulation of the expression of these transcription factors could account, at least in part, for the low-fat phenotype of the NSE-ΔFosB mice. However, in contrast to the results obtained in ST2 cells, overexpression of ΔFosB in the more committed 3T3-L1 cells had little effect on the induction of C/EBPα or PPARγ expression. Consistent with this failure of ΔFosB to inhibit expression of the adipocyte master regulators in 3T3-L1 cells, its presence had no effect on the development of adipocyte morphology, lipid accumulation, or the expression of the late adipocyte markers adipsin and lipoprotein lipase, although the amount of secreted leptin decreased by about 30%. Collectively, these data suggest that ΔFosB exerts its antiadipogenic effect at an early stage of adipogenesis yet beyond the presumed branching point of the osteoblast and adipocyte lineages. The partial downregulation of leptin secretion by the 3T3-L1 cells suggests that it may also act in a more limited way during later stages of differentiation.
Since ΔFosB is a splicing variant of the AP-1 transcription factor FosB, it is likely to exert its effects by altering transcriptional regulation. No changes in bone or fat formation have been observed in mice that lack the fosB gene (5, 17; our unpublished observations). This indicates that none of the FosB isoforms (FosB, ΔFosB, and Δ2ΔFosB) are strictly required for osteoblast and adipocyte differentiation to occur and suggests that the effects of overexpressed ΔFosB are not due to the displacement of full-length FosB from some critically important complex or to an increased amount of a required ΔFosB-containing complex.
ΔFosB and the further N-terminally truncated Δ2ΔFosB lack one and both of FosB's transactivation domains, respectively, but contain both the DNA-binding and heterodimerization domains (31). Based on these structural considerations, it is likely that the truncated isoforms bind transcription factors and DNA response elements that are similar or identical to those bound by full-length FosB but that they alter the transcriptional activity of the complexes. Overexpressing the ΔFosB and Δ2ΔFosB proteins could decrease transcriptional activity by reducing the DNA-binding affinity or altering the DNA-binding specificity of the protein complex or by preventing the interaction with transcriptional coactivators. Alternatively, transcription could be increased due to the displacement of transcriptional repressors. (Since the Δ2ΔFosB isoform, which lacks ΔFosB's transcriptionally active N-terminal Fos homology domain [13, 21], appears to be sufficient to induce the observed effects in the ST2 cells, the phenotype is unlikely to be a consequence of direct transcriptional activity of ΔFosB.) Thus, the identification of transcription factors that interact with overexpressed ΔFosB during osteoblast or adipocyte differentiation will contribute to understanding the mechanisms that are critically important for the differentiation of these cells.
The ability of ΔFosB to prevent the increased expression of C/EBPα that normally occurred in primary bone marrow stromal cells and ST2 cells in response to adipogenic conditions suggested that ΔFosB interacts with a factor that promotes the expression of C/EBPα. We therefore examined whether ΔFosB interacts with or affects the function of C/EBPβ, another basic leucine zipper transcription factor that promotes adipocyte differentiation by upregulating the expression of several adipogenic genes, including C/EBPα (27, 42, 45), and which has also recently been implicated in osteoblast differentiation (18). We found that ΔFosB indeed bound to C/EBPβ but did not bind to other C/EBP proteins, indicating the specificity of the interaction. Furthermore, the presence of the ΔFosB isoforms altered the binding of C/EBPβ-containing protein complexes to a consensus C/EBPβ response element.
Interestingly, the full-length ΔFosB isoform, like FosB itself, caused a small reduction in C/EBPβ DNA binding, while the Δ2ΔFosB isoform strongly increased the binding of C/EBPβ to the DNA. The strong potentiation of the C/EBPβ-DNA interaction by the N-terminally truncated Δ2ΔFosB isoform, in contrast to the markedly weak effects of FosB and ΔFosB, suggests that the Δ2ΔFosB isoform might be the actual mediator of changes in osteoblast and adipocyte differentiation observed in the NSE-ΔFosB transgenic mice. Indeed, recent data show that overexpressing the Δ2ΔFosB isoform by itself under control of the NSE promoter is sufficient to cause both the osteoblast and the adipocyte phenotypes in mice (unpublished data).
Together, the binding of ΔFosB isoforms to C/EBPβ, the alteration of C/EBPβ-DNA interactions by ΔFosB isoforms, and the ΔFosB-induced changes in the amount of the small C/EBPβ isoform in the ST2 cells provide a strong indication of the involvement of C/EBPβ in the mechanism by which ΔFosB inhibits adipocyte formation. C/EBPβ biology is complex, however, presenting challenges to elucidating its possible role in the ΔFosB-dependent mechanisms. Differential usage of initiation methionines in a single C/EBPβ mRNA generates isoforms that activate (LAP) and inhibit (LIP) gene expression (12, 34). The levels of these isoforms are differentially regulated (1), possibly in a species- and cell type-specific manner, and the specific response of target genes depends on the relative amounts of the different isoforms (12). This may explain the apparently conflicting reports that C/EBPβ both positively (12) and negatively (56) regulates the expression of the albumin gene, where it interacts with the same C/EBPβ-binding site that we used in these experiments.
The challenge of elucidating the ΔFosB mechanism is further increased by the existence of the full-length and Δ2ΔFosB isoforms of ΔFosB and the different effects of those isoforms on the C/EBPβ-DNA interaction and probably on the transcriptional activity of the resulting complexes. While elucidation of the combined effect of ΔFosB and the C/EBPβ isoforms on the C/EBPα promoter is thus beyond the scope of this study, such an analysis might reveal that interactions between C/EBPβ and ΔFosB contribute to both the increase in osteoblastogenesis and the inhibition of adipocyte differentiation seen in the NSE-ΔFosB mice.
In conclusion, this study establishes that ΔFosB-induced osteosclerosis is a direct effect of the overexpression of ΔFosB in osteoblasts. In addition, under these conditions, osteosclerosis is induced independently of decreased adipogenesis, suggesting that ΔFosB isoforms exert direct effects on both osteoblast and adipocyte differentiation processes. Finally, the inhibitory effect of ΔFosB on adipogenesis is also cell autonomous and appears to occur at an early stage of stem cell commitment, possibly via an interaction with C/EBPβ.
Acknowledgments
We thank Karen Ford and Wayne Grant for excellent technical assistance. We are grateful to J. Gimble and D. P. Ramji for supplying various cDNA constructs.
This work was supported by a postdoctoral fellowship from the Danish Research Council to M.K. and for the most part by a grant from the National Institutes of Health (AR48218) to R.B. Other support included grants from the NIH to W.M.P. (DE12616) and to the Yale Core Center for Musculoskeletal Diseases (AR46032) and from Aventis Pharma and Proskelia to R.B.
REFERENCES
- 1.An, M. R., C.-C. Hsieh, P. D. Reisner, J. P. Rabek, S. G. Scott, D. T. Kuninger, and J. Papaconstantinou. 1996. Evidence for posttranscriptional regulation of C/EBPαand C/EBPβisoform expression during the lipopolysaccharide-mediated acute-phase response. Mol. Cell. Biol. 16:2295-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baron, R., A. Vignery, L. Neff, A. Silverglate, and A. Santa Maria. 1983. Processing of undecalcified bone specimens for bone histomorphometry, p. 13-35. In R. R. Recker (ed.), Bone histomorphometry: techniques and interpretation, vol. 1. CRC Press, Boca Raton, Fla.
- 3.Bellows, C. G., J. N. M. Heersche, and J. E. Aubin. 1990. Determination of the capacity for proliferation and differentiation of osteoprogenitor cells in the presence and absence of dexamethasone. Dev. Biol. 140:132-138. [DOI] [PubMed] [Google Scholar]
- 4.Beresford, J. N., J. H. Bennett, C. Devlin, P. S. Leboy, and M. E. Owen. 1992. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J. Cell Sci. 102:341-351. [DOI] [PubMed] [Google Scholar]
- 5.Brown, J. R., H. Ye, R. T. Bronson, P. Dikkes, and M. E. Greenberg. 1996. A defect in nurturing in mice lacking the immediate early gene fosB. Cell 86:297-309. [DOI] [PubMed] [Google Scholar]
- 6.Burkhardt, R., G. Kettner, W. Bohm, M. Schmidmeier, R. Schlag, B. Frisch, B. Mallmann, W. Eisenmenger, and T. Gilg. 1987. Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone 8:157-164. [DOI] [PubMed] [Google Scholar]
- 7.Cao, Z., R. M. Umek, and S. L. McKnight. 1991. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 5:1538-1552. [DOI] [PubMed] [Google Scholar]
- 8.Chen, J., M. B. Kelz, G. Zeng, N. Sakai, C. Steffen, P. E. Shockett, M. R. Picciotto, R. S. Duman, and E. J. Nestler. 1998. Transgenic animals with inducible, targeted gene expression in brain. Mol. Pharmacol. 54:495-503. [DOI] [PubMed] [Google Scholar]
- 9.Chomczynski, P. 1993. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. BioTechniques 15:532-534, 536-537. [PubMed] [Google Scholar]
- 10.Corral, D. A., M. Amling, M. Priemel, E. Loyer, S. Fuchs, P. Ducy, R. Baron, and G. Karsenty. 1998. Dissociation between bone resorption and bone formation in osteopenic transgenic mice. Proc. Natl. Acad. Sci. USA 95:13835-13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darlington, G. J., S. E. Ross, and O. A. MacDougald. 1998. The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem. 273:30057-30060. [DOI] [PubMed] [Google Scholar]
- 12.Descombes, P., and U. Schibler. 1991. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell 67:569-579. [DOI] [PubMed] [Google Scholar]
- 13.Dobrzanski, P., T. Noguchi, K. Kovary, C. A. Rizzo, P. S. Lazo, and R. Bravo. 1991. Both products of the fosB gene, FosB and its short form, FosB/SF, are transcriptional activators in fibroblasts. Mol. Cell. Biol. 11:5470-5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ducy, P., M. Amling, S. Takeda, M. Priemel, A. F. Schilling, F. T. Beil, J. Shen, C. Vinson, J. M. Rueger, and G. Karsenty. 2000. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197-207. [DOI] [PubMed] [Google Scholar]
- 15.Ducy, P., and G. Karsenty. 1995. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol. Cell. Biol. 15:1858-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grigoriadis, A. E., K. Schellander, Z. Q. Wang, and E. F. Wagner. 1993. Osteoblasts are target cells for transformation in c-fos transgenic mice. J. Cell Biol. 122:685-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruda, M. C., J. van Amsterdam, C. A. Rizzo, S. K. Durham, S. Lira, and R. Bravo. 1996. Expression of FosB during mouse development: normal development of FosB knockout mice. Oncogene 12:2177-2185. [PubMed] [Google Scholar]
- 18.Gutierrez, S., A. Javed, D. K. Tennant, M. van Rees, M. Montecino, G. S. Stein, J. L. Stein, and J. B. Lian. 2002. CCAAT/enhancer-binding proteins (C/EBP) β and δ activate osteocalcin gene transcription and synergize with Runx2 at the C/EBP element to regulate bone-specific expression. J. Biol. Chem. 277:1316-1323. [DOI] [PubMed] [Google Scholar]
- 19.Jochum, W., J.-P. David, C. Elliott, A. Wutz, H. Plenk, Jr., K. Matsuo, and E. F. Wagner. 2000. Increased bone formation and osteosclerosis in mice overexpressing the transcription factor Fra-1. Nat. Med. 6:980-984. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, R. S., B. M. Spiegelman, and V. Papaioannou. 1992. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell 71:577-586. [DOI] [PubMed] [Google Scholar]
- 21.Jooss, K. U., M. Funk, and R. Muller. 1994. An autonomous N-terminal transactivation domain in Fos protein plays a crucial role in transformation. EMBO J. 13:1467-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalajzic, Z., P. Liu, I. Kalajzic, Z. Du, A. Braut, M. Mina, E. Canalis, and D. W. Rowe. 2002. Directing the expression of a green fluorescent protein transgene in differentiated osteoblasts: comparison between rat type I collagen and rat osteocalcin promoters. Bone 31:654-660. [DOI] [PubMed] [Google Scholar]
- 23.Karin, M., Z. Liu, and E. Zandi. 1997. AP-1 function and regulation. Curr. Opin. Cell Biol. 9:240-246. [DOI] [PubMed] [Google Scholar]
- 24.Kelz, M. B., J. Chen, W. A. Carlezon, Jr., K. Whisler, L. Gilden, A. M. Beckmann, C. Steffen, Y.-J. Zhang, L. Marotti, D. W. Self, T. Tkatch, G. Baranauskas, D. J. Surmeier, R. L. Neve, R. S. Duman, M. R. Picciotto, and E. J. Nestler. 1999. Expression of the transcription factor ΔFosB in the brain controls sensitivity to cocaine. Nature 401:272-276. [DOI] [PubMed] [Google Scholar]
- 25.Kveiborg, M., R. Chiusaroli, N. A. Sims, M. Wu, G. Sabatakos, W. C. Horne, and R. Baron. 2002. The increased bone mass in ΔFosB transgenic mice is independent of circulating leptin levels. Endocrinology 143:4304-4309. [DOI] [PubMed] [Google Scholar]
- 26.Lai, C.-F., and S.-L. Cheng. 2002. Signal transductions induced by bone morphogenetic protein-2 and transforming growth factor-β in normal human osteoblastic cells. J. Biol. Chem. 277:15514-15522. [DOI] [PubMed] [Google Scholar]
- 27.Lane, M. D., Q.-Q. Tang, and M.-S. Jiang. 1999. Role of the CCAAT enhancer binding proteins (C/EBPs) in adipocyte differentiation. Biochem. Biophys. Res. Commun. 266:677-683. [DOI] [PubMed] [Google Scholar]
- 28.Liu, S., R. Guo, Q. Tu, and L. D. Quarles. 2002. Overexpression of Phex in osteoblasts fails to rescue the Hyp mouse phenotype. J. Biol. Chem. 277:3686-3697. [DOI] [PubMed] [Google Scholar]
- 29.Martin, R. B., B. D. Chow, and P. A. Lucas. 1990. Bone marrow fat content in relation to bone remodeling and serum chemistry in intact and ovariectomized dogs. Calcif. Tissue Int. 46:189-194. [DOI] [PubMed] [Google Scholar]
- 30.Mumberg, D., F. C. Lucibello, M. Schuermann, and R. Muller. 1991. Alternative splicing of fosB transcripts results in differentially expressed mRNAs encoding functionally antagonistic proteins. Genes Dev. 5:1212-1223. [DOI] [PubMed] [Google Scholar]
- 31.Nakabeppu, Y., and D. Nathans. 1991. A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell 64:751-759. [DOI] [PubMed] [Google Scholar]
- 32.Nuttall, M. E., and J. M. Gimble. 2000. Is there a therapeutic opportunity to either prevent or treat osteopenic disorders by inhibiting marrow adipogenesis? Bone 27:177-184. [DOI] [PubMed] [Google Scholar]
- 33.Nuttall, M. E., A. J. Patton, D. L. Olivera, D. P. Nadeau, and M. Gowen. 1998. Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: implications for osteopenic disorders. J. Bone Miner. Res. 13:371-382. [DOI] [PubMed] [Google Scholar]
- 34.Ossipow, V., P. Descombes, and U. Schibler. 1993. CCAAT/enhancer-binding protein mRNA is translated into multiple proteins with different transcription activation potentials. Proc. Natl. Acad. Sci. USA 90:8219-8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otsuka, E., A. Yamaguchi, S. Hirose, and H. Hagiwara. 1999. Characterization of osteoblastic differentiation of stromal cell line ST2 that is induced by ascorbic acid. Am. J. Physiol. 277:C132-C138. [DOI] [PubMed] [Google Scholar]
- 36.Owen, M. 1988. Marrow stromal stem cells. J. Cell Sci. Suppl. 10:63-76. [DOI] [PubMed] [Google Scholar]
- 37.Parfitt, A. M., M. K. Drezner, F. H. Glorieux, J. A. Kanis, H. Malluche, P. J. Meunier, S. M. Ott, and R. R. Recker. 1987. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2:595-610. [DOI] [PubMed] [Google Scholar]
- 38.Park, S. R., R. O. Oreffo, and J. T. Triffitt. 1999. Interconversion potential of cloned human marrow adipocytes in vitro. Bone 24:549-554. [DOI] [PubMed] [Google Scholar]
- 39.Pereira, R. C., A. M. Delany, and E. Canalis. 2002. Effects of cortisol and bone morphogenetic protein-2 on stromal cell differentiation: correlation with CCAAT-enhancer binding protein expression. Bone 30:685-691. [DOI] [PubMed] [Google Scholar]
- 40.Pittenger, M. F., A. M. Mackay, S. C. Beck, R. K. Jaiswal, R. Douglas, J. D. Mosca, M. A. Moorman, D. W. Simonetti, S. Craig, and D. R. Marshak. 1999. Multilineage potential of adult human mesenchymal stem cells. Science 284:143-147. [DOI] [PubMed] [Google Scholar]
- 41.Poli, V., F. P. Mancini, and R. Cortese. 1990. IL-6DBP, a nuclear protein involved in interleukin-6 signal transduction, defines a new family of leucine zipper proteins related to C/EBP. Cell 63:643-653. [DOI] [PubMed] [Google Scholar]
- 42.Rangwala, S. M., and M. A. Lazar. 2000. Transcriptional control of adipogenesis. Annu. Rev. Nutr. 20:535-559. [DOI] [PubMed] [Google Scholar]
- 43.Rickard, D. J., M. Kassem, T. E. Hefferan, G. Sarkar, T. C. Spelsberg, and B. L. Riggs. 1996. Isolation and characterization of osteoblast precursor cells from human bone marrow. J. Bone Miner. Res. 11:312-324. [DOI] [PubMed] [Google Scholar]
- 44.Rosen, E. D., P. Sarraf, A. E. Troy, G. Bradwin, K. Moore, D. S. Milstone, B. M. Spiegelman, and R. M. Mortensen. 1999. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 4:611-617. [DOI] [PubMed] [Google Scholar]
- 45.Rosen, E. D., and B. M. Spiegelman. 2000. Molecular regulation of adipogenesis. Annu. Rev. Cell Dev. Biol. 16:145-171. [DOI] [PubMed] [Google Scholar]
- 46.Sabatakos, G., G. E. Davies, M. Grosse, A. Cryer, and D. P. Ramji. 1998. Expression of the genes encoding CCAAT-enhancer binding protein isoforms in the mouse mammary gland during lactation and involution. Biochem. J. 334:205-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sabatakos, G., N. A. Sims, J. Chen, K. Aoki, M. B. Kelz, M. Amling, Y. Bouali, K. Mukhopadhyay, K. Ford, E. J. Nestler, and R. Baron. 2000. Overexpression of ΔFosB transcription factor(s) increases bone formation and inhibits adipogenesis. Nat. Med. 6:985-990. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 49.Schreiber, E., P. Matthias, M. M. Muller, and W. Schaffner. 1989. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sen, A., Y. R. Lea-Currie, D. Sujkowska, D. M. Franklin, W. O. Wilkison, Y.-D. C. Halvorsen, and J. M. Gimble. 2001. Adipogenic potential of human adipose derived stromal cells from multiple donors is heterogeneous. J. Cell Biochem. 81:312-319. [DOI] [PubMed] [Google Scholar]
- 51.Shockett, P., M. Difilippantonio, N. Hellman, and D. G. Schatz. 1995. A modified tetracycline-regulated system provides autoregulatory, inducible gene expression in cultured cells and transgenic mice. Proc. Natl. Acad. Sci. USA 92:6522-6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sims, N. A., P. Clement-Lacroix, F. Da Ponte, Y. Bouali, N. Binart, R. Moriggl, V. Goffin, K. Coschigano, M. Gaillard-Kelly, J. Kopchick, R. Baron, and P. A. Kelly. 2000. Bone homeostasis in growth hormone receptor-null mice is restored by IGF-I but independent of Stat5. J. Clin. Investig. 106:1095-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sims, N. A., G. Sabatakos, J.-S. Chen, M. B. Kelz, E. J. Nestler, and R. Baron. 2002. Regulating ΔFosB expression in adult Tet-Off-ΔFosB transgenic mice alters bone formation and bone mass. Bone 30:32-39. [DOI] [PubMed] [Google Scholar]
- 54.Stein, G. S., J. B. Lian, and T. A. Owen. 1990. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 4:3111-3123. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka, T., N. Yoshida, T. Kishimoto, and S. Akira. 1997. Defective adipocyte differentiation in mice lacking the C/EBPβ and/or C/EBPδ gene. EMBO J. 16:7432-7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trautwein, C., T. Rakemann, A. Pietrangelo, J. Plumpe, G. Montosi, and M. P. Manns. 1996. C/EBP-β/LAP controls down-regulation of albumin gene transcription during liver regeneration. J. Biol. Chem. 271:22262-22270. [DOI] [PubMed] [Google Scholar]
- 57.Wang, G.-J., D. E. Sweet, S. I. Reger, and R. C. Thompson. 1977. Fat-cell changes as a mechanism of avascular necrosis of the femoral head in cortisone-treated rabbits. J. Bone Joint Surg. Am. 59:729-735. [PubMed] [Google Scholar]
- 58.Wang, N., M. J. Finegold, A. Bradley, C. N. Ou, S. V. Abdelsayed, M. D. Wilde, L. R. Taylor, D. R. Wilson, and G. J. Darlington. 1995. Impaired energy homeostasis in C/EBPα knockout mice. Science 269:1108-1112. [DOI] [PubMed] [Google Scholar]
- 59.Wang, Z.-Q., C. Ovitt, A. E. Grigoriadis, U. Mohle-Steinlein, U. Ruther, and E. F. Wagner. 1992. Bone and haematopoietic defects in mice lacking c-fos. Nature 360:741-745. [DOI] [PubMed] [Google Scholar]
- 60.Wronski, T. J., C. C. Walsh, and L. A. Ignaszewski. 1986. Histologic evidence for osteopenia and increased bone turnover in ovariectomized rats. Bone 7:119-123. [DOI] [PubMed] [Google Scholar]
- 61.Wu, Z., N. L. Bucher, and S. R. Farmer. 1996. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPβ, C/EBPδ, and glucocorticoids. Mol. Cell. Biol. 16:4128-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu, Z., Y. Xie, N. L. R. Bucher, and S. R. Farmer. 1995. Conditional ectopic expression of C/EBPβin NIH-3T3 cells induces PPARγ and stimulates adipogenesis. Genes Dev. 9:2350-2363. [DOI] [PubMed] [Google Scholar]
- 63.Yamaguchi, A., T. Ishizuya, N. Kintou, Y. Wada, T. Katagiri, J. M. Wozney, V. Rosen, and S. Yoshiki. 1996. Effects of BMP-2, BMP-4, and BMP-6 on osteoblastic differentiation of bone marrow-derived stromal cell lines, ST2 and MC3T3-G2/PA6. Biochem. Biophys. Res. Commun. 220:366-371. [DOI] [PubMed] [Google Scholar]
- 64.Yen, J., R. M. Wisdom, I. Tratner, and I. M. Verma. 1991. An alternative spliced form of FosB is a negative regulator of transcriptional activation and transformation by Fos proteins. Proc. Natl. Acad. Sci. USA 88:5077-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao, G., M. C. Monier-Faugere, M. C. Langub, Z. Geng, T. Nakayama, J. W. Pike, S. D. Chernausek, C. J. Rosen, L. R. Donahue, H. H. Malluche, J. A. Fagin, and T. L. Clemens. 2000. Targeted overexpression of insulin-like growth factor I to osteoblasts of transgenic mice: increased trabecular bone volume without increased osteoblast proliferation. Endocrinology 141:2674-2682. [DOI] [PubMed] [Google Scholar]
- 66.Zimmerman, D., F. Jin, P. Leboy, S. Hardy, and C. Damsky. 2000. Impaired bone formation in transgenic mice resulting from altered integrin function in osteoblasts. Dev. Biol. 220:2-15. [DOI] [PubMed] [Google Scholar]