Abstract
Purpose
We investigated the clinical outcome of bone marrow (BM) involvement in patients with diffuse large B-cell lymphoma (DLBCL) who received rituximab-based therapy.
Materials and Methods
A total of 567 consecutive patients with newly diagnosed DLBCL treated with rituximab-CHOP (RCHOP) between November 2001 and March 2010 were included in the current study. All of the patients underwent a BM study at the initial staging and the clinical characteristics and prognosis of these patients with or without BM involvement were analyzed retrospectively.
Results
The total cohort included 567 patients. The overall incidence of BM involvement was 8.5%. With a median follow-up duration of 33.2 months (range, 0.1 to 80.7 months) for patients who were alive at the last follow-up, the five-year overall survival (OS) and event-free survival (EFS) rate in patients without BM involvement (76.3% and 67.5%, p<0.001) was statistically higher than that in patients with BM involvement (44.3% and 40.1%, p<0.001). In multivariate analysis, among total patients, BM involvement showed a significant association with OS and EFS. In univariate and multivariate analyses, even among stage IV patients, a significant association with worse EFS was observed in the BM involvement group.
Conclusion
BM involvement at diagnosis affected the survival of patients with DLBCL who received RCHOP. Although use of RCHOP can result in significant improvement of the therapeutic effect of DLBCL, BM involvement is still a negative prognostic factor of DLBCL patients in the era of rituximab.
Keywords: Diffuse large B-cell lymphoma, Bone marrow, Rituximab
Introduction
Involvement of bone marrow (BM) has been reported in 10-30% of cases of diffuse large B-cell lymphoma (DLBCL) [1,2]. Due to its prognostic and therapeutic implications, BM involvement in patients with DLBCL is of critical importance. In general, patients with BM involvement are known to have a more aggressive clinical course and advanced disease than patients without BM involvement [3]. Thus, BM involvement showed an association with significantly shorter survivals in patients with DLBCL [4].
Rituximab is a chimeric monoclonal antibody against the protein CD20, which is found primarily on the surface of malignant B-cells [5]. The addition of rituximab to cyclophosphamide, vincristine, adriamycin, and prednisolone (CHOP) has led to a notable improvement in the response rate and survival outcomes for patients with DLBCL [6]. Several recent studies have demonstrated that inclusion of rituximab in combination chemotherapy can result in a significantly improved therapeutic effect on DLBCL with BM involvement. Some studies have also reported that rituximab could relieve the negative impact of BM involvement [7,8]. Nevertheless, it is still uncertain whether rituximab plus CHOP (RCHOP) can alter the clinical outcome of patients with BM involvement in DLBCL. Accordingly, we evaluated the prognosis of DLBCL patients with BM involvement who received RCHOP.
Materials and Methods
1. Patients and treatment
We conducted a retrospective review of the medical records of 567 patients with newly diagnosed DLBCL who received RCHOP at six centers between November 2001 and March 2010. All of the patients were evaluated using standard laboratory tests, computed tomography (CT) scans, and a unilateral BM aspirate and biopsy at the time of diagnosis. All of the BM biopsies were analyzed by a pathologist and a hematologist using standard immunohistochemistry, along with a visual assessment. Additional information was also abstracted, including age, sex, performance status, presence of B symptoms (fever, night sweats, and weight loss), presence of bulky disease (defined as tumor size>10 cm), presence of extranodal disease, presence of BM involvement, International Prognostic Index (IPI) scoring system [9], serum lactate dehydrogenase (LDH), hemoglobin (Hb), white blood cells (WBC), and platelets (Plt). All patients were staged according to the Ann Arbor Staging classification using CT scans [10]. An elevated LDH was defined as greater than 480 U/L according to the upper normal limit. All of the patients were treated with six cycles of RCHOP, while patients with a bulky disease received six cycles of RCHOP with radiotherapy. This study was approved by the institutional review board at each center.
2. Statistical analysis
The descriptive statistics are reported as the proportion and median. Overall survival (OS) was defined as the time from diagnosis to death from any cause. Event-free survival (EFS) was defined as the time from diagnosis to failure or death from any cause [11]. OS and EFS were analyzed using the Kaplan-Meier test, and each group was compared using a log-rank test. Cox regression model was used to determine the clinical predictors for OS and EFS. An effect was considered statistically significant when p<0.05. All analyses were performed using the SPSS ver. 14 (SPSS Inc., Chicago, IL).
Results
1. Patient characteristics
A summary of patient characteristics is shown in Table 1. The total cohort included 567 patients. The median age of patients was 61.0 years (range, 16 to 89 years) and 56.3% were male; 230 (40.6%) patients had stage III and IV at diagnosis, and 107 patients (40.6%) were categorized as high or high-intermediate risk according to their IPI. The overall incidence of BM involvement was 8.5%. In patients with normal LDH and a normal complete blood count (CBC), 1.8% had BM involvement. Among these 48 patients with BM involvement, the median age was 60.0 years (range, 17 to 78 years) and 64.6% were male. Thirty one patients had an elevated LDH. The median CBC values were Hb 11.1 g/dL, WBC 6.5×109/L, and Plt 152×109/L. Sixteen patients had B symptoms, and eight patients had a bulky disease. Twenty six patients remain alive, while 20 patients relapsed and 26 patients later died.
Table 1.
Baseline characteristics according to BM involvement
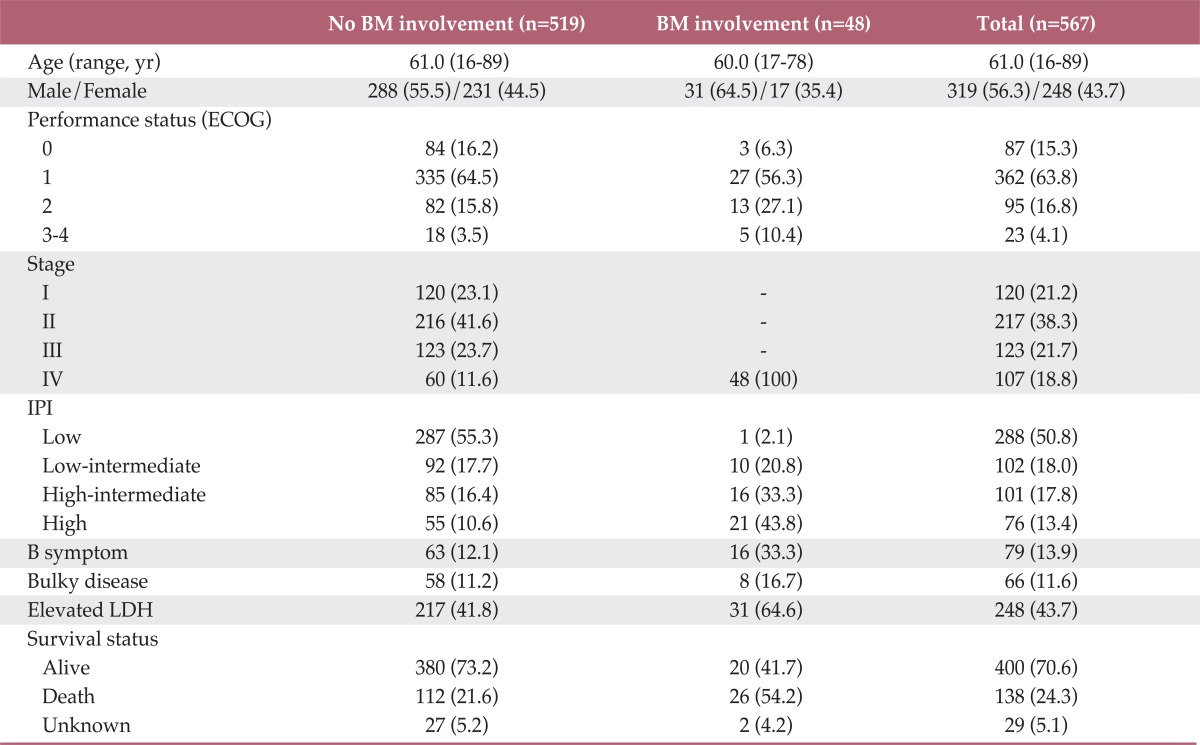
Values are presented as number (%). BM, bone marrow; ECOG, Eastern Cooperation Oncology Group; IPI, International Prognostic Index; LDH, lactate dehydrogenase.
2. Survival
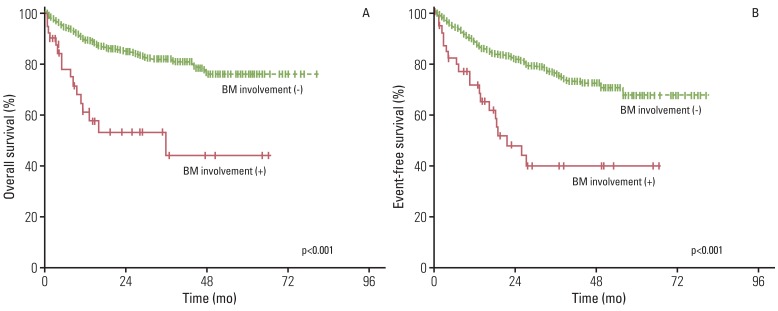
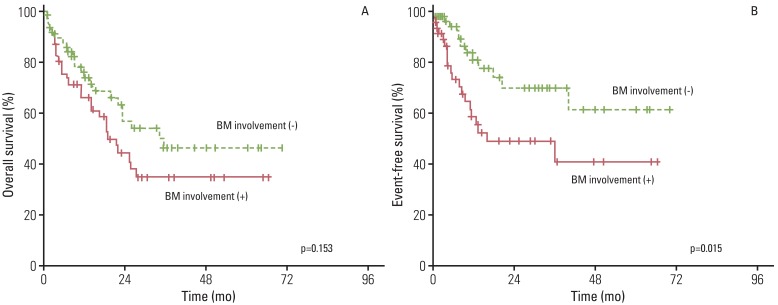
With a median follow-up duration of 33.2 months (range, 0.1 to 80.7 months), among patients who were alive at the last follow-up, the five-year OS and EFS rate was 74.6% and 65.4%, respectively. As shown in Fig. 1, the five-year OS and EFS rate in patients without BM involvement (76.3% and 67.5%, p<0.001) was statistically higher than that in patients with BM involvement (44.3% and 40.1%, p<0.001). In multivariate analysis, among total patients, BM involvement showed a significant association with OS and EFS (Table 2). In univariate and multivariate analyses, even among stage IV patients, BM involvement group showed a significant association with worse EFS (Table 3, Fig. 2).
Fig. 1.
Kaplan-Meier analysis of overall survival (A) and event-free survival (B) for patients with and without bone marrow (BM) involvement.
Table 2.
Multivariate analysis for factors affecting EFS and OS
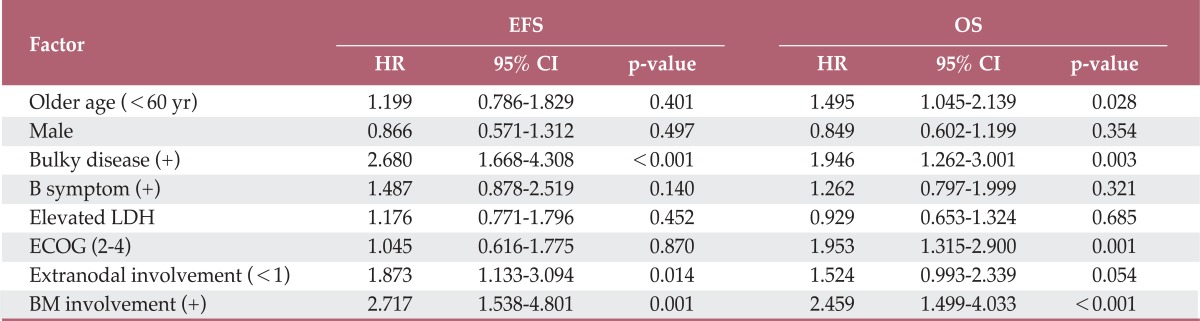
EFS, event-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; LDH, lactate dehydrogenase; ECOG, Eastern Cooperation Oncology Group; BM, bone marrow.
Table 3.
Multivariate analysis for factors affecting EFS among stage IV patients
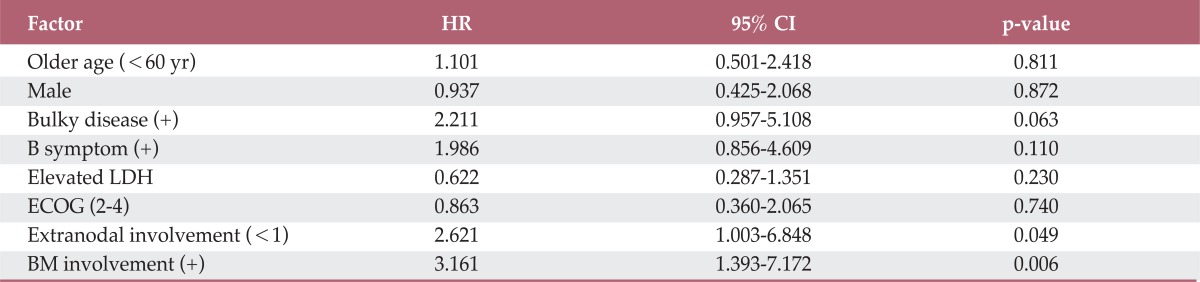
EFS, event-free survival; HR, hazard ratio; CI, confidence interval; LDH, lactate dehydrogenase; ECOG, Eastern Cooperation Oncology Group; BM, bone marrow.
Fig. 2.
Kaplan-Meier analysis of overall survival (A) and event-free survival (B) for stage IV patients with and without bone marrow (BM) involvement.
Discussion
The result of the current study demonstrates that patients with BM involvement have a worse prognosis than patients without BM involvement, even after receiving rituximab-containing therapy. The poor prognosis of patients with BM involvement has already been described in a number of studies. Conlan et al. [4] reported that BM involvement predicted a short survival. In a separate study, the results consistently confirmed that patients with marrow involvement had the worst prognosis [12]. However, the significant improvement in treatment outcomes observed following the introduction of rituximab has altered risk assessment in DLBCL. In fact, the IPI is a powerful tool for predicting the clinical outcome in patients with aggressive non-hodgkin lymphoma [9]. However, only a few studies have used the IPI score for prediction and examination of the prognosis in DLBCL with BM involvement. Chung et al. [12] recently reported an association of concordant BM involvement with poor prognosis, which was independent of the IPI. Likewise, in the current study, the distribution of IPI scores for the patients also varied from 2 to 5.
This study pursued an in-depth report on the 48 patients with BM involvement. Although BM involvement itself is a poor risk, the common features associated with primary BM DLBCL include an advanced lymphoma stage with relative old age and poor performance [13]. Most of our patients were also found to have poor clinical factors, including advanced age, poor performance status, elevated LDH level, and B symptoms at diagnosis. These findings are consistent with those of a previous report on the features of BM involvement [3].
Addition of rituximab to CHOP has led to a notable improvement in the response rate and survival outcomes for patients with DLBCL [6]. Recent studies have also demonstrated that the inclusion of rituximab in combination chemotherapy has a significantly improved therapeutic effect on DLBCL with BM involvement [7,8,14]. Although the precise relationship between rituximab and BM involvement is not well understood, rituximab appears to efficiently eradicate neoplastic lymphoid cells in BM. In general, rituximab is a therapeutic antibody that binds to a particular protein, the CD20 antigen, on the surface of normal and malignant B-cells. It then recruits the body's natural defences to attack and kill the marked B-cells. Stem cells in BM lack the CD20 antigen, allowing for regeneration of healthy B-cells after treatment and return to normal levels within several months [5]. According to Abe et al. [15], rituximab treatment for relapsed CD20 positive acute lymphoblastic leukemia resulted in complete prevention of regrowth of bone tumors. Some studies have also reported that lymphoma patients who presented with only BM disease at diagnosis were treated successfully with rituximab and combination chemotherapy [16,17]. More specifically, rituximab therapy in the case of autoimmune disease shows selective depletion of pathogenic autoreactive B-cell clones in BM, as demonstrated by molecular analysis [18]. Therefore, these observations indicated that BM involvement may not have a significant impact on the prognosis of patients who receive RCHOP. However, in the current study, a significant difference in five-year OS and EFS was observed between patients with and without BM involvement who received RCHOP. In addition, after adjusting for other factors, multivariate analysis according to OS and EFS also showed that BM involvement had a negative influence on survival. In univariate and multivariate analyses, even among stage IV patients, BM involvement group showed a significant association with worse EFS. This finding suggests that treatment approach to DLBCL with BM involvement should be considered separately from those for DLBCL with normal BM. Some data support the use of intensive multiagent chemotherapy in eradication of neoplastic cells in BM [19]. In addition, more intensive regimens have been used in lymphoblastic lymphoma, which typically presents as a widely disseminated disease with frequent BM involvement [20].
Conclusion
Our data showed that BM involvement at diagnosis had a negative impact on survival in patients with DLBCL who received RCHOP. These results suggest that BM involvement is still a negative prognostic factor of DLBCL patients in the era of rituximab. Prognostic subgroup analysis should be evaluated and validated for patients with BM involvement, and a more suitable treatment compatible with BM involvement will be further investigated in patients with BM involvement.
Acknowledgments
This study was supported by a grant from the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (no. A111345).
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Zhang QY, Foucar K. Bone marrow involvement by hodgkin and non-hodgkin lymphomas. Hematol Oncol Clin North Am. 2009;23:873–902. doi: 10.1016/j.hoc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Arber DA, George TI. Bone marrow biopsy involvement by non-Hodgkin's lymphoma: frequency of lymphoma types, patterns, blood involvement, and discordance with other sites in 450 specimens. Am J Surg Pathol. 2005;29:1549–1557. doi: 10.1097/01.pas.0000182405.65041.8b. [DOI] [PubMed] [Google Scholar]
- 3.Kajiura D, Yamashita Y, Mori N. Diffuse large B-cell lymphoma initially manifesting in the bone marrow. Am J Clin Pathol. 2007;127:762–769. doi: 10.1309/2GW5W7KQBXF6LFAW. [DOI] [PubMed] [Google Scholar]
- 4.Conlan MG, Bast M, Armitage JO, Weisenburger DD. Bone marrow involvement by non-Hodgkin's lymphoma: the clinical significance of morphologic discordance between the lymph node and bone marrow. Nebraska Lymphoma Study Group. J Clin Oncol. 1990;8:1163–1172. doi: 10.1200/JCO.1990.8.7.1163. [DOI] [PubMed] [Google Scholar]
- 5.Zwick C, Murawski N, Pfreundschuh M German High-Grade Non-Hodgkin Lymphoma Study Group. Rituximab in high-grade lymphoma. Semin Hematol. 2010;47:148–155. doi: 10.1053/j.seminhematol.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 7.Yi SH, Xu Y, Zou DH, An G, Zhao YZ, Qi JY, et al. Prognostic impact of bone marrow involvement (BMI) and therapies in diffuse large B cell lymphoma. Zhonghua Xue Ye Xue Za Zhi. 2009;30:307–312. [PubMed] [Google Scholar]
- 8.Li QC, Yuan XL, Wang YF, Zou DH, Zhao YZ, Qiu LG. Clinical outcomes of different regimens for non-Hodgkin's lymphoma with bone marrow involvement: analysis of 148 cases. Zhonghua Yi Xue Za Zhi. 2008;88:254–257. [PubMed] [Google Scholar]
- 9.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkins Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 10.Armitage JO. Staging non-Hodgkin lymphoma. CA Cancer J Clin. 2005;55:368–376. doi: 10.3322/canjclin.55.6.368. [DOI] [PubMed] [Google Scholar]
- 11.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 12.Chung R, Lai R, Wei P, Lee J, Hanson J, Belch AR, et al. Concordant but not discordant bone marrow involvement in diffuse large B-cell lymphoma predicts a poor clinical outcome independent of the International Prognostic Index. Blood. 2007;110:1278–1282. doi: 10.1182/blood-2007-01-070300. [DOI] [PubMed] [Google Scholar]
- 13.Chang H, Hung YS, Lin TL, Wang PN, Kuo MC, Tang TC, et al. Primary bone marrow diffuse large B cell lymphoma: a case series and review. Ann Hematol. 2011;90:791–796. doi: 10.1007/s00277-010-1129-4. [DOI] [PubMed] [Google Scholar]
- 14.Gaudio F, Giordano A, Perrone T, Pastore D, Curci P, Delia M, et al. High Ki67 index and bulky disease remain significant adverse prognostic factors in patients with diffuse large B cell lymphoma before and after the introduction of rituximab. Acta Haematol. 2011;126:44–51. doi: 10.1159/000324206. [DOI] [PubMed] [Google Scholar]
- 15.Abe T, Kitajima T, Honma K, Kurasaki T, Okazuka K, Shibasaki Y, et al. Effective combination chemotherapy with rituximab for acute lymphoblastic leukemia with bone relapse after bone marrow transplantation. Rinsho Ketsueki. 2008;49:1556–1561. [PubMed] [Google Scholar]
- 16.Jardin F, Callonnec F, Contentin N, Picquenot JM, Gueit I, Heron F, et al. Intravascular large B-Cell lymphoma with bone marrow involvement and superior sagittal sinus thrombosis: report of a case successfully treated with a CHOP/rituximab combination regimen. Clin Lymphoma. 2005;6:46–49. doi: 10.3816/clm.2005.n.027. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi T, Minato M, Tsukuda H, Yoshimoto M, Tsujisaki M. Successful treatment of intravascular large B-cell lymphoma diagnosed by bone marrow biopsy and FDG-PET scan. Intern Med. 2008;47:975–979. doi: 10.2169/internalmedicine.47.0808. [DOI] [PubMed] [Google Scholar]
- 18.Quartuccio L, Salvin S, Fabris M, Sacco S, De Vita S. Disappearance of bone marrow B cell clonal expansion in patients with type II hepatitis C virus-related cryoglobulinemic glomerulonephritis after clinical efficient rituximab therapy. Ann Rheum Dis. 2008;67:1494–1495. doi: 10.1136/ard.2007.084939. [DOI] [PubMed] [Google Scholar]
- 19.Sieniawski M, Bhartia S, Wilkinson J, Proctor SJ. Incidence and outcome of patients with diffuse large B cell lymphoma with marrow involvement and preliminary experience of an adult acute lympho-blastic leukemia protocol (NEALL VI) in cyclophosphamide, doxorubicin, vincristine, and prednisolone: rituximab refractory patients. Leuk Lymphoma. 2009;50:1726–1730. doi: 10.1080/10428190903144667. [DOI] [PubMed] [Google Scholar]
- 20.Sweetenham JW. Treatment of lymphoblastic lymphoma in adults. Oncology (Williston Park) 2009;23:1015–1020. [PubMed] [Google Scholar]