Abstract
Purpose
A number of factors related to overall survival (OS) have been addressed in advanced non-small cell lung cancer (NSCLC). This study was conducted to determine the impact of whole-body metastatic regions on survival outcome in advanced non-squamous NSCLC.
Materials and Methods
Between March 2005 and February 2011, 112 eligible patients with newly confirmed stage IV non-squamous NSCLC, available for epidermal growth factor receptor (EGFR) mutation status 18-21 analysis, and accessible for the determination of pretreatment whole-body metastatic regions were enrolled in this retrospective study. The total number of synchronous metastatic regions was scored according to the following disease sites: abdomen/pelvis, lung to lung/pulmonary lymphangitic spread, bone, pleura/pleural effusion/pericardial effusion, neck/axillary lymph nodes, other soft tissue, brain.
Results
The median age of the cohort was 65 years (range, 31 to 88 years). The median whole-body metastatic score was 2 (range, 1 to 6), and bone and lung to lung were the most common metastatic sites. EGFR mutations were observed in 40 (35.7%) patients with a deletion in exon 19 and Leu858Arg mutation in exon 21 being detected in 16 (40.0%) and 19 (47.5%) patients, respectively. Multivariate analysis for OS revealed that treatment factors (p=0.005), performance status (p=0.006), whole-body metastatic score (p<0.001), and EGFR mutation status (p=0.095) were significantly or marginally associated with OS.
Conclusion
The results of the present study demonstrated that whole-body metastatic extent strongly affects survival outcome, even after adjustment for other significant variables in advanced non-squamous NSCLC. The clinical validity of more curative multimodal approaches in cohorts with limited metastases remains to be explored.
Keywords: Epidermal growth factor receptor, Mutation, Adenocarcinoma of lung, Neoplasm metastasis, Non-small cell lung carcinoma, Prognosis
Introduction
Approximately 80% of primary lung cancers are classified as non-small cell lung cancer (NSCLC), and approximately two thirds of NSCLC patients present with locally advanced or advanced disease that is not amenable to curative surgery [1]. In Korea, although lung cancer incidence rates are gradually declining in men and increasing in women, lung cancer constitutes the leading cause of cancer deaths in both groups [2].
With the change of lung cancer epidemiology, the frequency of non-squamous histology, especially adenocarcinoma of the lung, is steadily increasing [3-5]. Non-squamous NSCLC has a differentiated disease entity than squamous cell lung cancer in terms of a common etiology of carcinogenesis and the frequent association with activation of genetic alterations that can become specific therapeutic targets for recently developed innovative agents [4,6,7]. The discovery of epidermal growth factor receptor (EGFR) somatic mutations in the tyrosine kinase domain contributed to the paradigm shift of therapeutic strategies in non-squamous NSCLC owing to the unique biological responsiveness and characteristics of these tumors [6,8].
Although there are diverse known prognostic factors affecting overall survival (OS) in advanced NSCLC, there are conflicting results among studies, and it remains uncertain which parameters definitively influence the overall outcomes [7,9-14]. In the present study, we hypothesized that the examination of clinical features of long-term survivors in advanced non-squamous NSCLC would provide a more curative approach to enhance patient prognosis, despite the dismal oncologic outcomes in the majority of patients with metastatic diseases. The prognostic importance of whole-body metastatic extent in advanced NSCLC remains uncertain, and we wished to clarify the impact of pretreatment whole-body metastatic extent on survival outcome relative to other known significant elements in advanced non-squamous NSCLC.
Materials and Methods
1. Patient population
Between March 2005 and February 2011, 112 eligible patients were enrolled for inclusion in this retrospective study. The inclusion criteria were as follows: patients with newly confirmed stage IV non-squamous NSCLC as determined by the 7th American Joint Committee on Cancer classification system; patients who were evaluated for EGFR mutation status 18-21 from tumor specimens prior to treatment; and patients with available pretreatment whole-body imaging studies for the assessment of metastatic regions. We obtained approval from the Institutional Review Board of Catholic Medical Center ethics committee for this retrospective study. Performance status (PS) based on the Eastern Cooperative Oncology Group (ECOG) PS scale and smoking history were routinely examined and recorded in electronic medical charts.
2. Review of imaging study and whole-body metastatic score
Imaging studies were performed prior to the start of treatment for the detection of synchronous metastatic regions, including whole body 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT), CT of the whole chest and abdomen/pelvis, and magnetic resonance imaging (MRI) or CT of the brain. 18F-FDG-PET/CT was performed in 106 of the 112 patients, while six patients underwent CT of the whole chest and abdomen/pelvis instead of 18F-FDG-PET/CT. Whole body bone scans and MRI of the spine were selectively performed when skeletal metastasis was suspected or clinically indicated. Nuclear medicine specialists and radiologists reviewed the findings of the distant metastases. When an accurate discrimination of metastatic disease and reactive changes were difficult, serial image findings or standard uptake value changes in [18F]-FDG-PET scans were used. Except for cases of primary tumors with metastatic lymph nodes in the thorax with only pleural or pericardial effusions, routine cytologic examination for effusions was not performed for diagnostic purposes.
Based on the imaging studies, we conducted the application of a whole-body metastatic scoring system in the same manner reported in our previous study [15] to estimate the whole-body extent of metastatic spread. Advantages and shortcomings of our whole-body scoring system to measure tumor extent are described in our previous publication. In brief, the total amount of tumor burden in metastatic disease was difficult to determine accurately. Therefore, we scored the total number of synchronous metastatic sites according to disease sites, and the whole-body metastatic score was recorded as 1 to 7 based on summation of each score. Categorization of the seven sites was as follows: abdomen/pelvis (including adrenal gland, lymph nodes, and other abdominal or pelvic organs), lung to lung or pulmonary lymphangitic spread, bone (skeletal system), pleura and/or pleural effusion and/or pericardial effusion, neck and/or axillary lymph nodes, other soft tissue, and brain.
3. Mutational analysis of EGFR
Formalin fixed paraffin-embedded lung tumor tissues from surgical resections or bronchoscopic biopsies were investigated. Representative sections were stained with hematoxilin and eosin, and the presence of tumor was verified by an experienced pathologist. To identify tumor areas containing at least 80% tumor cells, 10 µm thick unstained sections were analyzed under the microscope. DNA was extracted from microdissected tumor cells with xylene and ethanol to remove the paraffin and then placed in 1% sodium dodecyl sulfate/proteinase K (10 mg/mL) at 56℃ overnight. DNA was extracted using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. After polymerase chain reaction (PCR) amplification of the exons (18-21) of EGFR, PCR fragments were separated via agarose gel electrophoresis and purified using a QIAquick PCR Purification Kit (Qiagen) according to the manufacturer's recommendations. Direct sequencing of the PCR amplicons was performed for both strands using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) on an ABI3730XL DNA analyzer (Applied Biosystems).
4. Survival assessment
Information on OS status and date of death for all patients was retrieved from the survival data registered at the National Cancer Center (Goyang, Korea) and Seoul St. Mary's Hospital. Among the 112 eligible patients, eight were lost to follow-up from our institution after initial screening for staging and EGFR mutational analysis without further treatment. However, the final status of the entire patient population was available from the national registration records.
5. Statistical analysis
Pearson chi-square or Fisher's exact tests were used for comparison of the proportion of variables. OS duration was defined as the time from the date of pathological diagnosis to the date of death from any cause. The Kaplan-Meier method was performed for survival estimation, and survival difference was analyzed using the log-rank test. The Cox proportional hazards model was used for multivariate analysis of OS. All statistical analyses were two sided, and a p-value of <0.05 was considered to be statistically significant.
Results
1. Patient and tumor characteristics
Table 1 summarizes the characteristics of the patients and tumors. The median age of the patients was 65 years (range, 31 to 88 years), while 61 (54.5%) patients were male. The majority of the patients (92.8%) had a histology that was consistent with adenocarcinoma. The composition of the other histologies was two poorly differentiated (PD) carcinomas and two NSCLCs without further detailed information. Ninety-one (81.1%) patients had a PS of ECOG score of 0-1. Patients' smoking history was categorized as never-smoker or ever-smoker (current+former smoker). The frequency of ever-smokers was 50.9%. EGFR mutation was noted in 40 (35.7%) patients.
Table 1.
Patient and tumor characteristics
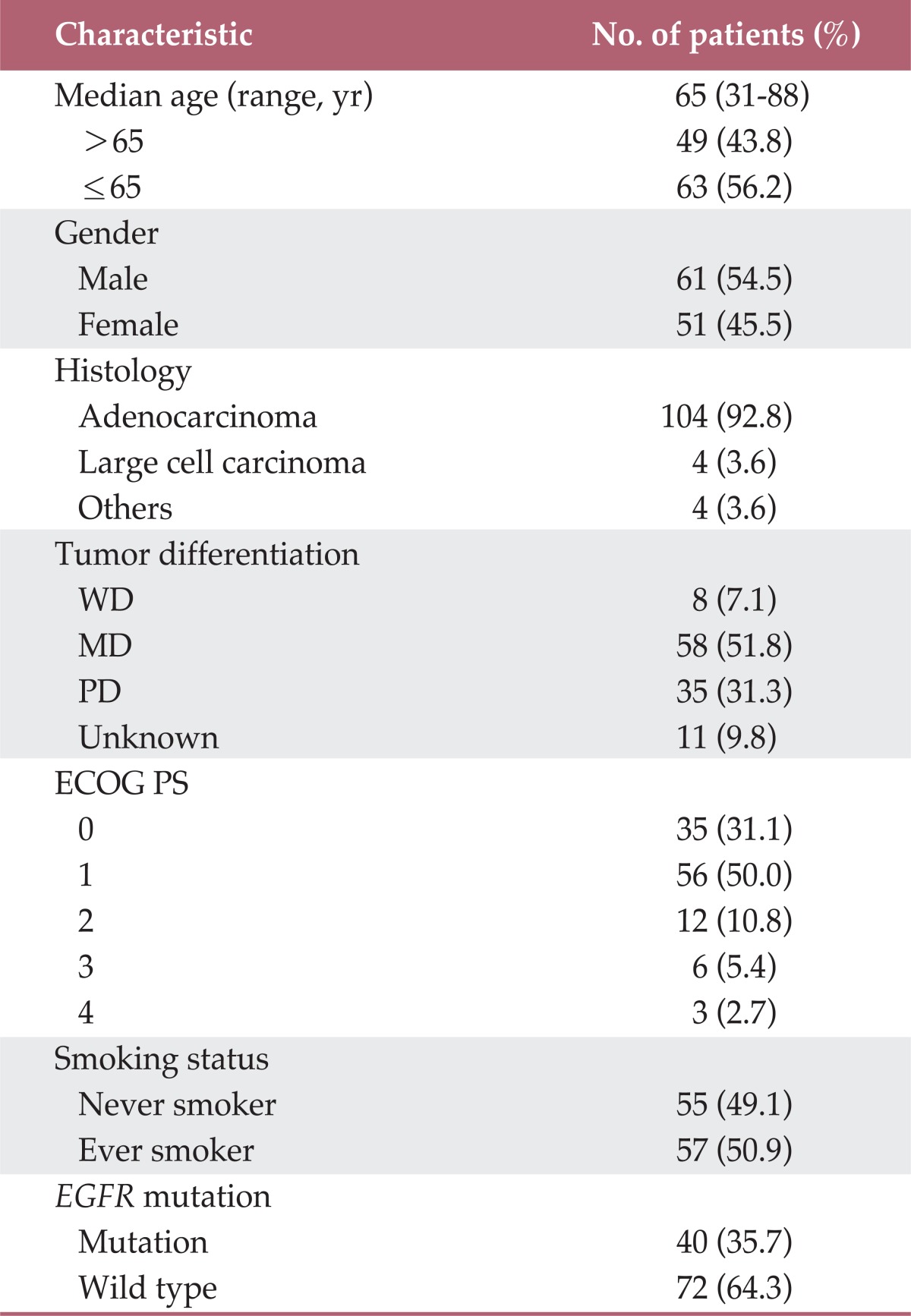
WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor.
2. Distribution of metastatic sites and characteristics of metastasis confined to a single system
The median whole-body metastatic score in the entire cohort was 2 (range, 1 to 6). Ninety-eight (87.5%) patients had whole-body metastatic regions limited within three systems, and 32 (28.6%) had a single whole-body metastatic score. The most common sites of metastatic regions were pulmonary and skeletal systems. Sixty-nine (61.6%) patients had bone metastasis, while lung-to-lung metastasis or lymphangitic lung spread was observed in 67 (59.8%) patients. The detailed characteristics of the distribution of whole-body metastatic sites are summarized in Table 2.
Table 2.
Distribution of total metastatic score and metastatic regions
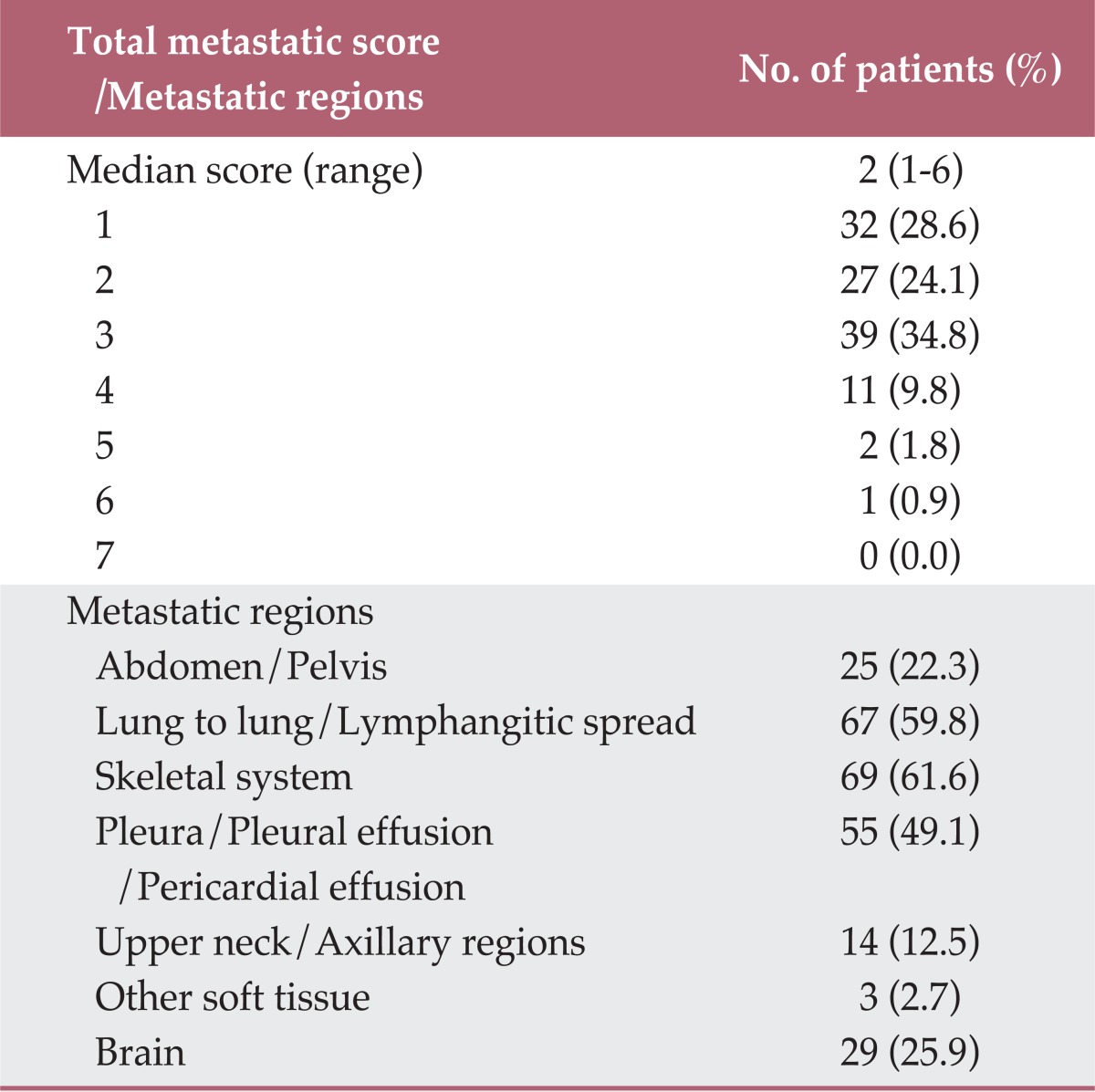
The metastatic distribution of cohorts with a single whole-body metastatic score was as follows: lung-to-lung/lymphangitic lung spread in 12 patients, bone metastasis in nine, pleural metastasis/pleural effusion/pericardial effusion in six, abdomen/pelvis in two, brain in two, and neck/axillary in one.
3. Correlation between EGFR mutation status and other characteristics
EGFR mutation was more frequently detected in female patients (p=0.002), never-smokers (p<0.001), and patients with well to moderately differentiated tumors (p=0.017). Among 61 male and 51 female patients, 14 (23%) and 26 (51%) had activating EGFR mutations, respectively. Similarly, among 55 never and 57 ever smokers, 29 (52.7%) and 11 (19.3%) showed activating EGFR mutations, respectively. Among 35 tumors with PD histology, 7 (20%) exhibited activating EGFR mutations. In contrast, 29 (43.9%) out of 66 well-to-moderate differentiated tumors revealed activating EGFR mutations.
4. Detailed characteristics of EGFR mutation
A deletion in exon 19 and Leu858Arg mutation in exon 21 were detected in 16 (40.0%) and 19 (47.5%) patients, respectively. A double mutation in exon 19 (lle744Met) and 21 (Leu858Arg) was noted in one patient and a Leu861Gln mutation in exon 21 was found in one patient. The remaining four patients showed an activating EGFR mutation in exon 20. Of the entire cohort of 112 patients, a Gln787Gln silent mutation in exon 20 was noted in 19 patients. The median OS time was 28, 6.9, and 11 months in EGFR 19, 20, and 21 mutant carriers, respectively, but this difference was not statistically significant (p=0.120).
5. Treatment characteristics
All patients were regarded as having metastatic stage IV disease and were scheduled to undergo systemic treatment. However, 95 (84.8%) patients received systemic chemotherapy or targeted therapy as the first-line treatment at our institution. Before April 2011, the cost of treatment with targeted agents as the first line of treatment in patients with EGFR mutations was not covered by our national insurance. Thus, prior to that period, the majority of patients were treated with first-line chemotherapy as the first treatment in NSCLC. First-line targeted agents were only selectively prescribed in patients who were enrolled in clinical trials or those who wished to pay for therapy themselves.
The most common regimens for doublet chemotherapy were gemcitabine plus cisplatin (42.9%) followed by docetaxel plus cisplatin (11.6%). Other chemotherapeutic regimens were composed of paclitaxel/carboplatin in ten patients, pemetrexed/cisplatin in seven, docetaxel monotherapy in six, gemcitabine/carboplatin in two, vinorelbine in one, and gemcitabine/vinorelbine in one. Bevacizumab was used in combination with standard doublet chemotherapy in two patients. Six of the seven patients treated with first-line targeted agents received geftinib, while one received erlotinib. In 33 patients, targeted agents were subsequently administered after first-line chemotherapy due to disease progression. Among 40 patients who exhibited EGFR mutations, 21 had received at least one course of targeted agents. Among these 21 patients, the median OS was 23 months (95% confidence interval [CI], 17.3 to 28.7 months). In nine patients, the first-line systemic treatment could not be delivered due to poor general condition or patients' decisions. Instead, these patients received palliative radiotherapy to the metastatic regions and the best available supportive care. Treatment information for eight patients was not available due to loss to follow-up after pathological and staging work-up.
6. Univariate and multivariate analysis for OS
At the time of analysis, 84 (75%) patients had died. The median OS period was 12.8 months (range, 1 to 55.8 months). The proportion of patients living more than two years after pathological diagnosis was 16.1% (n=18). Although both EGFR mutation and smoking status were significant prognosticators for OS in the univariate analysis, only EGFR mutation status was included for survival analysis due to strong correlation between the two variables. In the univariate analysis for OS, EGFR mutation status (p=0.005), ECOG PS (p=0.026), whole-body metastatic score (p=0.001), and treatment characteristics (p=0.012) were significantly associated with OS. The median OS in patients with EGFR mutation and EGFR wild type was 20 months (95% CI, 6.6 to 33.4 months) and 11 months (95% CI, 7.7 to 14.3 months), respectively, and those of patients with a whole-body metastatic score of 1 and more than 1 were 36 months (95% CI, 16.9 to 55.1 months) and 10.6 months (95% CI, 9.0 to 12.2 months), respectively. The median OS for patients treated with no systemic agent/unavailable information, systemic chemotherapy only, and at least one course of targeted agents were 6 months (95% CI, 3.1 to 8.9 months), 12.6 months (95% CI, 3.7 to 21.5 months), and 14 months (95% CI, 6.0 to 22.0 months), respectively. Age (p=0.064) and gender (p=0.086) were marginally associated with OS. Upon multivariate analysis, ECOG PS (p=0.006), treatment characteristics (p=0.005), and whole-body metastatic score (p<0.001) remained significantly associated, but EGFR mutation status (p=0.095) did not. The results of the univariate and multivariate analyses are shown in Table 3. When we separately performed log-rank survival analyses according to the whole-body metastatic score, a separation of the survival curves in the subgroups between whole-body metastatic score≤2 and whole-body metastatic score>2 (p=0.030) was observed, but no such separation was observed between whole-body metastatic score ≤3 and whole-body metastatic score >3 (p=0.781).
Table 3.
Univariate and multivariate analysis for overall survival
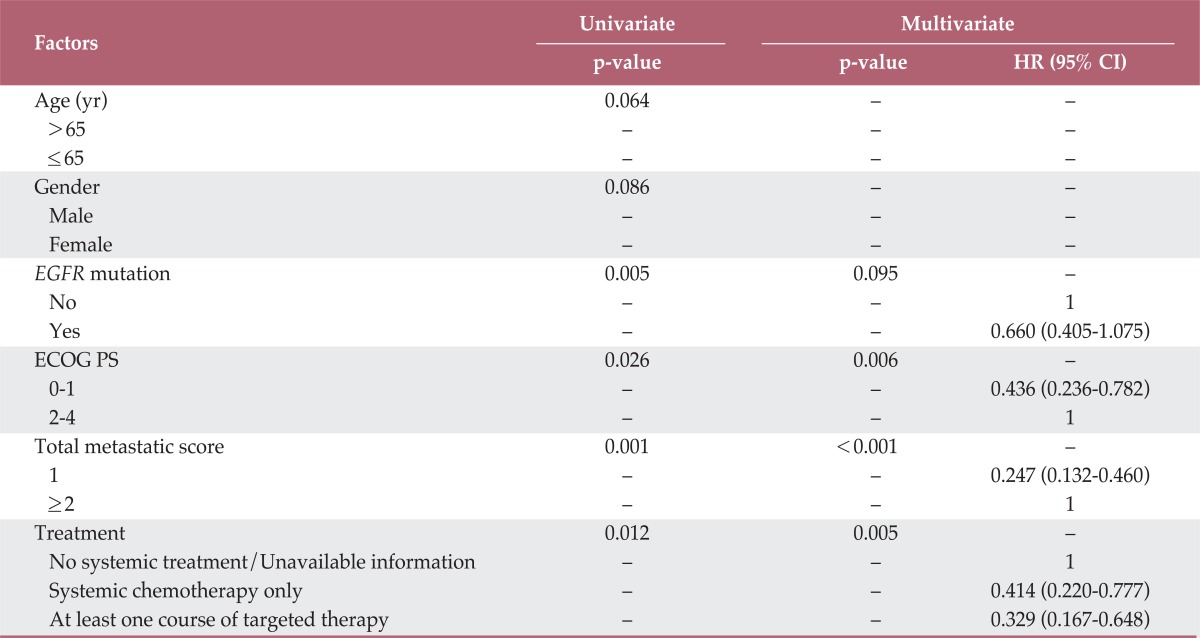
HR, hazard ratio; CI, confidence interval; EGFR, epidermal growth factor receptor; ECOG PS, Eastern Cooperative Oncology Group performance status.
Among seven patients treated with first-line targeted agents, two had a deletion in exon 19, one had a Leu858Arg mutation in exon 21 and the remaining four patients had no EGFR mutations. The OS of three EGFR-mutated patients were 5.8 (21 mut), 22.4 (19 mut) and 25.5 (19 mut) months, while those of patients with the four wild type EGFR status were 1, 1, 6, 11, 8, and 13.1 months.
Discussion
We aimed to clarify the main prognostic factors related to OS in metastatic non-squamous NSCLC and identify a subset of patients who could be approached with curative intent at the time of their initial diagnosis from the radiation oncologists' perspectives.
Previous publications have suggested various prognostic factors involved in advanced NSCLC using heterogeneous patient populations [7,9-14]. In the present study, ECOG PS, treatment factors and whole-body metastatic score were the major determinants for OS in advanced non-squamous NSCLC.
Similar to previous studies, we found that EGFR mutations were more frequently detected in female patients, never-smokers and patients with well to moderately differentiated tumors [7,8,11,16,17]. Although the EGFR mutation status significantly influenced OS, the importance was marginal upon multivariate analysis. Additionally, the types of administered treatment were important factors for survival outcome. Although the median OS was 14 months when all patients treated with at least one course of targeted agents were considered, it was 23 months in those exhibiting EGFR mutations. Even though this difference was not statistically significant in previous studies [7,18], an OS difference among EGFR mutation subtypes was also observed in our study.
The whole-body extent of tumors was estimated by scoring the involved metastatic regions. In a previous study [15], we observed a significant difference in tumor marker values between the low and high whole-body metastatic score groups. We wanted to reveal the impact of whole-body metastatic score on OS, and thus included that variable in survival analysis. Although there are some limitations to our scoring system, there has been no other method to accurately determine the total burden of metastatic disease. Similar findings were observed in a more recent study [14]; notably, although a specific method was not described, the number of involved metastatic organs was a significant variable in the multivariate and univariate survival analysis.
In the present study, a whole-body metastatic score for patients was a significant factor for OS, and the separation of OS curves tended to disappear when an increase in whole-body metastatic score was included as a variable. There was a significant OS difference in subgroups between the whole-body metastatic score ≤ 1 vs. whole-body metastatic score>1, and between the whole-body metastatic score≤2 vs. whole-body metastatic score>2. However, similar findings were not observed between the whole-body metastatic score≤3 vs. the whole-body metastatic score>3. Therefore, the number of whole-body metastatic regions can be considered a comprehensive and robust prognostic factor in advanced non-squamous NSCLC. We also indirectly observed the patterns of metastatic spread in patients with a single whole-body metastatic score. Pulmonary and skeletal systems were the most common sites of initial metastatic regions observed in the single whole-body metastatic score group, as well as among the overall metastatic patterns. Our findings suggest that, even in disseminated diseases, there is an initial spectrum of subgroups that can be cured by more aggressive treatment.
A limitation of our scoring system is that patients with a single whole-body metastatic score included those with malignant pleural effusion, pericardial effusion or pulmonary lymphangitic spread. These patients often have a more dismal prognosis than other single whole-body metastatic score patients. However, despite this limitation, the whole-body metastatic score showed a consistent prognostic role, and the results of the study suggest that patients with multiple gross lesions confined to limited regions may be good candidates for more curative synchronous multi-target ablative treatment using high precision radiotherapy techniques. Previous reports attempted to clarify the benefit of these approaches [19-24]. Milano et al. [21] reported the results of a prospective pilot study of curative-intent stereotactic body radiation therapy in patients with five or fewer oligometastatic lesions. In their study, a greater sum of gross tumor volume predicted worse OS, local control (LC) and distant control, and patients with breast cancer conferred better significant outcomes. However, neither the number of metastatic lesions nor the number of involved organs was a significant predictor of outcome. The LC rate was not deteriorated (2-year 87% vs. 6-year 87%) in their long-term follow-up study [22]; however, the freedom from distant metastasis rate decreased significantly from 52% at 2-years to 36% at 6-years. In a study by Arrieta et al. [19], a promising OS outcome of 31.8 months was reported for patients with NSCLC with brain only metastasis. In their study, patients were treated with whole brain radiation therapy and two cycles of paclitaxel/cisplatin induction chemotherapy. If disease did not progress, concurrent chemoradiation of paclitaxel/carboplatin chemotherapy with 60 Gy of irradiation followed by selective adjuvant chemotherapy was employed. It is still unclear whether patients with limited metastatic regions will ultimately achieve clear clinical benefits in terms of survival outcomes and inhibition of new metastatic lesions following the eradication of all metastatic sites using these approaches; accordingly, future studies using well-designed future randomized trials are needed. Even though the cost effectiveness of more curative approaches has not yet been demonstrated, recent advances in novel radiation delivery methods enabled synchronous multi-target treatments, even in remote meta-static diseases within a large treatment field [25].
It should be noted that the present study has the following limitations. Our eligible criteria might result in several biases. Although the whole-body metastatic scoring system reflects the tumor extents, the development of more accurate and efficacious methods to determine the total tumor burden in the disseminated state should be addressed. Despite the composition of heterogeneous treatment characteristics, our data demonstrated the consistent prognostic importance of whole-body metastatic score and ECOG PS, which is the traditionally established prognostic factor in NSCLC. Our study was carried out under tumor histology of non-squamous NSCLC; however, further studies are needed to determine if the same results can be drawn for squamous NSCLC or other types of cancer.
Conclusion
A good PS, administration of appropriate systemic treatment and metastases confined to limited regions were strong prognostic indicators of long-term survival in advanced non-squamous NSCLC. Future investigations should be conducted to determine whether the patient subsets with those characteristics could be suitable candidates for achieving improved treatment outcomes with more aggressive multi-modal and multi-target approaches. Moreover, our results warrant the introduction of an optimal scoring system to accurately predict the patient survival outcomes in advanced non-squamous NSCLC based on large cohort studies.
Acknowledgments
This research was supported by Seoul St. Mary's Clinical Medicine Research Program year of 2009 through the Catholic University of Korea.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Martin J, Ginsberg RJ, Venkatraman ES, Bains MS, Downey RJ, Korst RJ, et al. Long-term results of combined-modality therapy in resectable non-small-cell lung cancer. J Clin Oncol. 2002;20:1989–1995. doi: 10.1200/JCO.2002.08.092. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011;43:1–11. doi: 10.4143/crt.2011.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssen-Heijnen ML, Coebergh JW. The changing epide-miology of lung cancer in Europe. Lung Cancer. 2003;41:245–258. doi: 10.1016/s0169-5002(03)00230-7. [DOI] [PubMed] [Google Scholar]
- 4.Torok S, Hegedus B, Laszlo V, Hoda MA, Ghanim B, Berger W, et al. Lung cancer in never smokers. Future Oncol. 2011;7:1195–1211. doi: 10.2217/fon.11.100. [DOI] [PubMed] [Google Scholar]
- 5.Travis WD, Lubin J, Ries L, Devesa S. United States lung carcinoma incidence trends: declining for most histologic types among males, increasing among females. Cancer. 1996;77:2464–2470. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2464::AID-CNCR8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 6.Dy GK, Adjei AA. Emerging therapeutic targets in non-small cell lung cancer. Proc Am Thorac Soc. 2009;6:218–223. doi: 10.1513/pats.200808-099LC. [DOI] [PubMed] [Google Scholar]
- 7.Wu M, Zhao J, Song SW, Zhuo M, Wang X, Bai H, et al. EGFR mutations are associated with prognosis but not with the response to front-line chemotherapy in the Chinese patients with advanced non-small cell lung cancer. Lung Cancer. 2010;67:343–347. doi: 10.1016/j.lungcan.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto H, Toyooka S, Mitsudomi T. Impact of EGFR mutation analysis in non-small cell lung cancer. Lung Cancer. 2009;63:315–321. doi: 10.1016/j.lungcan.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal C, Langer CJ. Older age, poor performance status and major comorbidities: how to treat high-risk patients with advanced nonsmall cell lung cancer. Curr Opin Oncol. 2012;24:130–136. doi: 10.1097/CCO.0b013e32834ea6ea. [DOI] [PubMed] [Google Scholar]
- 10.Giroux Leprieur E, Lavole A, Ruppert AM, Gounant V, Wislez M, Cadranel J, et al. Factors associated with long-term survival of patients with advanced non-small cell lung cancer. Respirology. 2012;17:134–142. doi: 10.1111/j.1440-1843.2011.02070.x. [DOI] [PubMed] [Google Scholar]
- 11.Kalikaki A, Koutsopoulos A, Hatzidaki D, Trypaki M, Kontopodis E, Stathopoulos E, et al. Clinical outcome of patients with non-small cell lung cancer receiving front-line chemotherapy according to EGFR and K-RAS mutation status. Lung Cancer. 2010;69:110–115. doi: 10.1016/j.lungcan.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Leung EY, Scott HR, McMillan DC. Clinical utility of the pretreatment glasgow prognostic score in patients with advanced inoperable non-small cell lung cancer. J Thorac Oncol. 2012;7:655–662. doi: 10.1097/JTO.0b013e318244ffe1. [DOI] [PubMed] [Google Scholar]
- 13.O'Byrne KJ, Gatzemeier U, Bondarenko I, Barrios C, Eschbach C, Martens UM, et al. Molecular biomarkers in non-small-cell lung cancer: a retrospective analysis of data from the phase 3 FLEX study. Lancet Oncol. 2011;12:795–805. doi: 10.1016/S1470-2045(11)70189-9. [DOI] [PubMed] [Google Scholar]
- 14.Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. Prognostic factors in patients with advanced non-small cell lung cancer: data from the phase III FLEX study. Lung Cancer. 2012;77:376–382. doi: 10.1016/j.lungcan.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Lee DS, Kim YS, Jung SL, Lee KY, Kang JH, Park S, et al. The relevance of serum carcinoembryonic antigen as an indicator of brain metastasis detection in advanced non-small cell lung cancer. Tumour Biol. 2012;33:1065–1073. doi: 10.1007/s13277-012-0344-0. [DOI] [PubMed] [Google Scholar]
- 16.Kosaka T, Yatabe Y, Onozato R, Kuwano H, Mitsudomi T. Prognostic implication of EGFR, KRAS, and TP53 gene mutations in a large cohort of Japanese patients with surgically treated lung adenocarcinoma. J Thorac Oncol. 2009;4:22–29. doi: 10.1097/JTO.0b013e3181914111. [DOI] [PubMed] [Google Scholar]
- 17.Mak RH, Doran E, Muzikansky A, Kang J, Neal JW, Baldini EH, et al. Outcomes after combined modality therapy for EGFR-mutant and wild-type locally advan-ced NSCLC. Oncologist. 2011;16:886–895. doi: 10.1634/theoncologist.2011-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riely GJ, Pao W, Pham D, Li AR, Rizvi N, Venkatraman ES, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12(3 Pt 1):839–844. doi: 10.1158/1078-0432.CCR-05-1846. [DOI] [PubMed] [Google Scholar]
- 19.Arrieta O, Villarreal-Garza C, Zamora J, Blake-Cerda M, de la Mata MD, Zavala DG, et al. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastasis treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol. 2011;6:166. doi: 10.1186/1748-717X-6-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harita S, Mizuta A, Kuyama S, Kikuchi T. Long-term survival following concurrent chemoradiotherapy in patients with non-small-cell lung cancer with concomitant brain metastases only. Int J Clin Oncol. 2005;10:63–68. doi: 10.1007/s10147-004-0436-y. [DOI] [PubMed] [Google Scholar]
- 21.Milano MT, Katz AW, Muhs AG, Philip A, Buchholz DJ, Schell MC, et al. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer. 2008;112:650–658. doi: 10.1002/cncr.23209. [DOI] [PubMed] [Google Scholar]
- 22.Milano MT, Katz AW, Zhang H, Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. 2012;83:878–886. doi: 10.1016/j.ijrobp.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 23.Norihisa Y, Nagata Y, Takayama K, Matsuo Y, Sakamoto T, Sakamoto M, et al. Stereotactic body radiotherapy for oligometastatic lung tumors. Int J Radiat Oncol Biol Phys. 2008;72:398–403. doi: 10.1016/j.ijrobp.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Takeda A, Kunieda E, Ohashi T, Aoki Y, Koike N, Takeda T. Stereotactic body radiotherapy (SBRT) for oligome-tastatic lung tumors from colorectal cancer and other primary cancers in comparison with primary lung cancer. Radiother Oncol. 2011;101:255–259. doi: 10.1016/j.radonc.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 25.Welsh JS, Patel RR, Ritter MA, Harari PM, Mackie TR, Mehta MP. Helical tomotherapy: an innovative technology and approach to radiation therapy. Technol Cancer Res Treat. 2002;1:311–316. doi: 10.1177/153303460200100413. [DOI] [PubMed] [Google Scholar]


