Abstract
Objectives
Current evidence to support non-medical prescribing is predominantly qualitative, with little evaluation of accuracy, safety and appropriateness. Our aim was to evaluate a new model of service for the Australia healthcare system, of inpatient medication prescribing by a pharmacist in an elective surgery preadmission clinic (PAC) against usual care, using an endorsed performance framework.
Design
Single centre, randomised controlled, two-arm trial.
Setting
Elective surgery PAC in a Brisbane-based tertiary hospital.
Participants
400 adults scheduled for elective surgery were randomised to intervention or control.
Intervention
A pharmacist generated the inpatient medication chart to reflect the patient's regular medication, made a plan for medication perioperatively and prescribed venous thromboembolism (VTE) prophylaxis. In the control arm, the medication chart was generated by the Resident Medical Officers.
Outcome measures
Primary outcome was frequency of omissions and prescribing errors when compared against the medication history. The clinical significance of omissions was also analysed. Secondary outcome was appropriateness of VTE prophylaxis prescribing.
Results
There were significantly less unintended omissions of medications: 11 of 887 (1.2%) intervention orders compared with 383 of 1217 (31.5%) control (p<0.001). There were significantly less prescribing errors involving selection of drug, dose or frequency: 2 in 857 (0.2%) intervention orders compared with 51 in 807 (6.3%) control (p<0.001). Orders with at least one component of the prescription missing, incorrect or unclear occurred in 208 of 904 (23%) intervention orders and 445 of 1034 (43%) controls (p<0.001). VTE prophylaxis on admission to the ward was appropriate in 93% of intervention patients and 90% controls (p=0.29).
Conclusions
Medication charts in the intervention arm contained fewer clinically significant omissions, and prescribing errors, when compared with controls. There was no difference in appropriateness of VTE prophylaxis on admission between the two groups.
Trial Registration
Registered with ANZCTR—ACTR Number ACTRN12609000426280
Article summary.
Article focus
A doctor–pharmacist collaborative prescribing model provides as least as high a quality of care as usual care, with regard to safety, access, appropriateness, effectiveness, efficiency and consumer participation.
Workforce shortages are prompting a review of the way the current workforce is utilised, and whether different roles could be taken on by healthcare professionals to alleviate some of the pressures within the system.
Research on non-medical prescribing so far is predominantly qualitative in nature. Our study has analysed quantitative data on the safety, accuracy and appropriateness of prescribing to try and assess whether this model is at least as good as usual care.
Key messages
Pharmacists’ skills in medication management are currently underutilised, and with appropriate training and education they could be contributing to medication management much more effectively by taking on a prescribing role.
The prescribing is collaborative and driven by guidelines and under the supervision of a medical team. Diagnosis is not within the scope of practice of the prescribing pharmacist.
This model of care has been proved to be highly effective in this study, with an increased accuracy, safety and appropriateness of prescribing within the intervention arm.
Strengths and limitations of this study
The results with regard to the accuracy and safety of medication charts produced in the study are emphatic and statistically significant.
The intervention is reproducible in other settings with a pharmacist of appropriate experience, training and education.
The study assessed one pharmacist prescriber versus a cohort of medical prescribers. While this has been accounted for in the analysis, it also reflects what usual practice would be in a model care such as this. The authors recognise and acknowledge it as a limitation.
Introduction
Prescribing involves four stages: information gathering, clinical decision-making, communication of decision and monitoring.1 Taking a medication history, continuing, ceasing and withholding of medications and initiating new medications are critical components of prescribing associated with an admission for surgery. Medication errors are common, occur most often at the time of prescribing, and frequently on the day of hospital admission, resulting in discrepancies between regular medications and admission orders.2–4 A small, but significant, proportion of errors result in adverse drug events (ADEs).5 Errors have been defined as when there is “a failure to communicate essential information; the use of drugs or doses is inappropriate for the individual patient; and transcription error.”6 To be able to communicate a clinical decision safely and effectively in the form of a written prescription, it is necessary to select the correct drug, together with the route, form, dose, frequency and duration.7 Multiple interventions have been suggested in an attempt to improve prescribing, with suggestions that increased training of the individual, a controlled environment and a change in organisational culture are necessary.8
Within hospital, the medication chart provides instructions for safe medication supply and administration, and ensures the patient access to medications as an inpatient. It is an integral part of communication between doctors, pharmacists and nurses about prescribing decisions and is used as the primary source of information regarding medications on discharge. The pharmacy service in the Princess Alexandra Hospital (PAH) preadmission clinic (PAC) began in 1998 to provide timely, accurate and comprehensive information about medication as patients crossed between healthcare settings. It ensured accurate transfer of information at admission, during the inpatient stay and at discharge, the benefits of which were a reduction in both readmissions and contact with community healthcare providers postdischarge.9 The importance of accurate transfer of information across the whole surgical care pathway from preadmission to discharge, including information about medications, has been highlighted in a recent study that reported how communication failures led to patient morbidity and mortality. Standardisation and systemisation of communication processes, along with other interventions targeted at the entire surgical pathway, were recommended with a view to improving information transfer and quality of care.10
Pharmacists in PACs have been shown to improve the accuracy of medication histories and medication orders, when compared with standard care, and the efficacy of prescribing perioperatively in line with recognised guidelines.11 12 Only with an accurate history of medication usage can decisions be made safely regarding the perioperative management of medications. Medication histories are elicited from a variety of sources of information: patient's own medications, the patient or carer, general practitioner summaries, community pharmacies, previous hospital admissions and nursing home records. A number of sources may be consulted to build an accurate record of medication that the patient is taking, both regularly and occasionally.
The range of prescribers has been expanded in a number of countries, with changes in legislation to allow for extension of prescribing privileges to non-medical professionals, including pharmacists. The objective of this was to make greater use of the skills and specialisation of pharmacists so that a more flexible system for the prescribing, supply and administration of medicines could be developed, while maintaining safe and appropriate access to medicines.13 14
In response to the documented workforce shortages in Australia, Brooks et al described possible solutions, including ‘task substitution’, and a focus has been placed recently on non-medical prescribers within the healthcare system.15–19 Pharmacists, with training in pharmacology and therapeutics, are potentially well placed to undertake prescribing roles. An Australian study identified the main driver behind pharmacist prescribing as the desire to work collaboratively with medical and nursing staff to:
Provide consumers with improved, responsible and safe access to prescription medicines;
Optimise use of pharmacists’ and doctors’ skills and time;
Reduce inefficient use of health resources.20
Evidence to support non-medical prescribing so far has been mainly qualitative, with minimal evaluation of access, safety and appropriateness. One recent review concluded that acceptability of non-medical prescribing services is based on the perceived value to the health service.21 This lack of evidence has led to calls to prove the safety and effectiveness of non-medical prescribing services in Australia.22 The aim of the data analysis discussed in this paper was to compare a doctor—pharmacist collaborative prescribing model with usual care, with regard to safety, access, appropriateness and effectiveness; the null hypothesis being that no difference exists between the two models of care.23
Methods
The study was conducted between June and September 2009 in the surgical PAC at PAH, a 750-bed tertiary teaching hospital in Queensland.
The definition of error used in the study was: “a failure to communicate essential information; the use of drugs or doses is inappropriate for the individual patient; and transcription error.”3
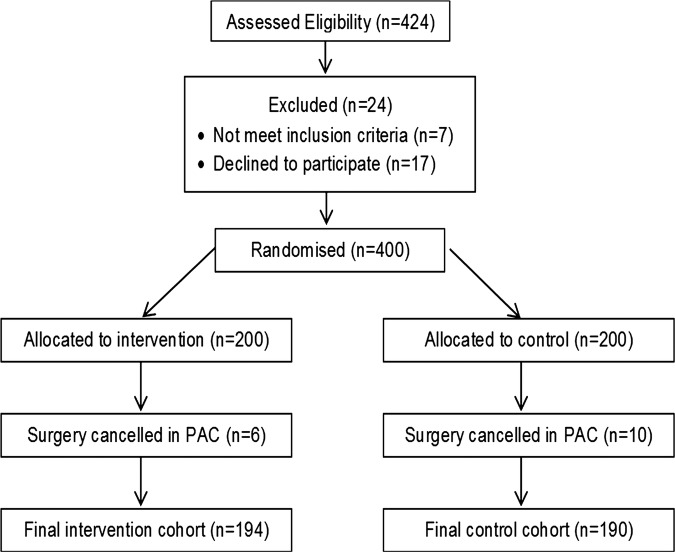
All patients who attended PAC and could provide written informed consent were considered for participation. Patients were excluded if they were under 18 years of age, unable to communicate due to language difficulties or undergoing day surgery (see figure 1).
Figure 1.
Randomisation flow chart.
Patients were approached on arrival at the clinic and written consent was obtained. After consent, patients were randomised using a computer generated randomisation list, in blocks of 10 (Microsoft Excel). Sealed envelopes (not prepared by the recruiting researcher) contained a zero or one as per the computer list; the next envelope was opened after consent to determine whether a patient entered the control or intervention arm, respectively. If a patient had been randomised and their surgery cancelled during PAC, the patient was removed from the study and not replaced.
A previous pilot study in the PAC showed an error rate of 12% of orders.24 Using an expected error rate of 8% in the intervention arm, a sample size of 932 orders per group was calculated to be required for a power of 80%. Assuming an average of five orders per patient, approximately 200 patients per arm would be required.
Only one pharmacist in the PAC, with 3 years’ experience as a hospital pharmacist and having a postgraduate diploma in clinical pharmacy, was trained to be a prescriber. The pharmacist attended a prescribing course which was accredited by the General Pharmaceutical Council, UK as an Independent Pharmacist Prescribing Course.25
Training included a minimum of 12 days of ‘period of learning in practice’ under a ‘designated medical practitioner’ (DMP), who was the consultant anaesthetist for PAC. The training included case studies and sessions on venous thromboembolism (VTE) prophylaxis with a consultant vascular physician and the clinical nurse consultant (CNC) for VTE prophylaxis at PAH. The DMP endorsed the pharmacist's competency to prescribe before the study could start.
For the pilot, an amendment was facilitated to the Queensland Health (Drugs and Poisons) Regulation 1996 to allow ‘Pharmacists registered in Queensland who are employed or contracted to Queensland Health and working in the Pharmacist Prescribing Pilot’ to prescribe controlled drugs, restricted drugs and schedule 2 and 3 poisons.
Intervention cohort
Patients were seen by a nurse, prescribing pharmacist, Resident Medical Officer (RMO) and anaesthetist. Patients had to be seen by the pharmacist before they were seen by the RMO to allow usual RMO duties and a countersignature of the pharmacist prescriptions, a site requirement.
The pharmacist undertook all pharmacist duties as per usual care, as well as prescribing medications on the medication chart. The scope of prescribing was continuing or withholding regular medications and prescribing VTE prophylaxis according to local and national guidelines, following a risk and contraindication assessment.26
Directors of surgery were consulted prior to the start of the trial for permission to include patients in prescribing of VTE prophylaxis, according to their specific unit guidelines, which had been defined in advance in collaboration with the CNC for VTE prophylaxis at PAH. Urology and renal transplant patients were excluded (N=43 control, N=34 intervention) from VTE prophylaxis prescribing as the director of urology was unavailable to confirm the scope of the project, and the director for transplant requested exclusion on the grounds that VTE prophylaxis in these patients was driven more by consultant discretion as opposed to being driven by guidelines.
Control cohort
Patients were seen by all four healthcare professionals in clinic, in no particular order, as per usual care. Either pharmacist in the clinic saw control patients for documentation of medication history. The prescribing of the medication chart was the responsibility of the RMO. In both arms, review and monitoring were undertaken, both by RMOs in clinic at countersignature and by RMOs and clinical pharmacists at the ward level, once the patient was admitted. Changes made by RMOs to intervention patient medication charts in clinic were recorded.
Outcome measures
The primary endpoint for the study was the accuracy of medication charts, with regard to concordance of the medication chart with the medication history, the plan for medications perioperatively and the quality of the individual orders related to legality and safety for administration purposes. The secondary endpoint was the appropriateness of prescribing for both chemical and mechanical VTE prophylaxis according to local and national guidelines.26
Analysis of scanned copies of medication charts, for the primary outcomes of omissions and errors, was conducted in tandem by two assessors, one a member of the research team and the other an external assessor, both trained in the use of validated audit tools1 and blinded to randomisation. Any ambiguities were clarified by consensus.
Appropriateness of VTE prophylaxis prescribed in both arms in clinic was analysed using scanned copies of medication charts, in tandem by two assessors, one a member of the research team and the other a CNC for VTE prophylaxis at PAH. Prescribing was also assessed on admission to the ward to ensure that VTE prophylaxis was appropriate.
An expert panel, comprising a surgeon, a clinical pharmacologist, an anaesthetist, a RMO, a pharmacist and a nurse, was convened to assess the clinical significance of omissions in a randomly selected 5% sample of the total cohort of patients from both arms (N=10 control, N=9 intervention). Panel members were blinded to randomisation.
Tables 1 and 2 describe the collection methods and definitions of these endpoints.
Table 1.
Analysis to assess the accuracy and safety of medication charts generated in the study
| Measure | Definition | Method | Assessing |
|---|---|---|---|
| Omissions | Medication in patient's medication history not prescribed on medication chart, with no reason documented in patient chart | Every medication in patient's medication history checked against medication chart—omissions from medication chart noted | Whether or not medication is prescribed |
| Prescribing errors | Anomaly in drug name, strength, dose, frequency or route, with no documentation in patient chart | Every medication in patient's medication history checked against medication chart—anomalies noted | Whether or not prescription is accurate in terms of drug name, strength, dose, frequency and route |
| Communication errors | Unclear prescription in terms of name, route, dose, frequency, slow release medication notification or intermittent order prescribing | Every prescription written checked using a validated tool—unclear prescribing noted, as agreed by both researchers | Whether or not prescription is safe for administration purposes |
Table 2.
Analysis to assess accuracy of VTE risk and contraindication assessments and appropriateness of VTE prescribing
| Measure | Definition | Method | Assessing |
|---|---|---|---|
| VTE-risk assessment | Patient categorised into low or high risk for VTE, as per guidelines | Every patient medical record checked for a documented VTE risk assessment | Risk assessment documented Y/N Risk assessment correct Y/N |
| VTE contraindication assessment | Patient highlighted as inappropriate for mechanical or chemical prophylaxis, as per guidelines | Every patient medical record checked for a documented contraindication assessment | Contraindication assessment documented Y/N Contraindication assessment correct Y/N |
| VTE prescribing | Whether patient prescribed mechanical and/or chemical VTE prophylaxis, as per guidelines | Prescribing of mechanical and chemical VTE prophylaxis checked against agreed local and national guidelines | VTE prescribing appropriate according to guidelines and individual patient factors Y/N |
VTE,venous thromboembolism.
Categorical data were compared using χ² tests for independence. When any one cell had a count of less than 10, Fisher's exact test was substituted. Logistic regression was used to analyse the overall omissions between the two groups. The number of regular and ‘PRN’ medications that the patient was currently taking was included as an explanatory variable in the model as it was deemed more likely that an individual medication would be omitted in a patient taking a large number of medications. Logistic regression was also used to analyse the overall communications prescribing errors between the two groups. The assumption of independence between observations is clearly violated as multiple observations exist for most patients. As such, robust SEs clustered by patient were calculated. No other covariates were adjusted for. All reported p values are two-sided using a level of significance of 0.05. All statistical analysis and sample size calculations were conducted using Stata V.11.2 (StataCorp, College Station, Texas, USA).
Results
The demographics of the patients randomised into the trial were similar, except for the higher number of medications taken by patients in the control arm (see table 3).
Table 3.
Characteristics of study population
| Control | Intervention | |
|---|---|---|
| Total patients | 190 | 194 |
| Age* | 57.6 (18–89) | 55.8 (18–86) |
| Male (%) | 58 | 59 |
| Regular medications†‡ | 4 (0–16) | 3 (0–18) |
| When required ‘PRN’ medications†§ | 2 (0–7) | 1 (0–4) |
| Complementary and alternative medicines (CAM)† | (0) (0–9) | (0) (0–6) |
| Over the counter (OTC) medications† | (0) (0–2) | (0) (0–2) |
| Total medications | 1364 | 983 |
| Total medications (regular and PRN only) | 1217 | 887 |
| Medication charts prescribed | 161 (85%) | 194 (100%) |
*Mean (range).
†Regular medications are defined as medications prescribed with the intent to be taken on a regular basis.
‡Median (range).
§Pro Re Nata (PRN) medications are defined as medications prescribed with the intent to be taken only when required.
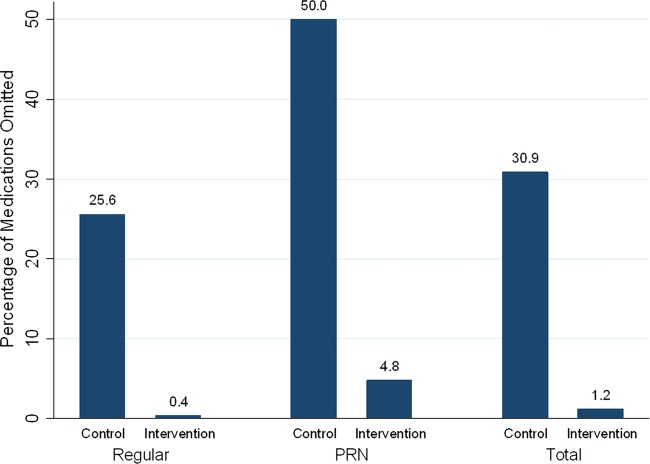
Omissions
Total unintentional medication omissions from medication charts were higher for control patients (31.5%) compared with interventions (1.2%) The OR for an order in the control group to be omitted, compared with that for the intervention group, was 41.0 (95% CI 20.6 to 81.8; p<0.001 logistic regression) after adjusting for the number of medications the patient was currently taking (see table 4 and figure 2). There were 59 prescribers in the control arm, 54 of whom reviewed patients who were currently taking regular or PRN medications at home, and as such had the opportunity to omit a patient's medication. Of these 54 prescribers, the median percentage of medications that were omitted per prescriber in the control arm was 21 (range 0–100).
Table 4.
Medication omissions from medication chart
| Type of medication and perioperative plan | Control (N) [%] | Intervention (N) [%] |
|---|---|---|
| Regular | ||
| Continue | 179 (805) [22.2] | 3 (620) [0.5] |
| Withhold prior to surgery | 46 (75) [7.4] | 0 (48) |
| Withhold on morning of surgery | 21 (54) [38.9] | 0 (39) |
| Adjust dose | 1 (5) [20.0] | 0 (5) |
| Review | 1 (7) [14.2] | 0 (6) |
| Cease | 0 (1) | 0 (2) |
| PRN | ||
| Continue | 128 (248) [51.6] | 6 (142) [4.2] |
| Withhold prior to surgery | 7 (12) [58.3] | 2 (13) [15.4] |
| Adjust dose | 0 (2) [20.0] | 0 (1) |
| Review | 0 (8) [14.3] | 0 (11) |
| Total omissions | 383 (1217) [31.5] | 11 (887) [1.2] |
| Complementary and alternative medicines (CAMs)* | 126 | 87 |
| Over-the-counter medications (OTC)* | 21 | 9 |
*CAM and OTC medications were not classed as omissions in either arm if they were not prescribed on the inpatient medication chart.
Figure 2.
Percentage of medications omitted.
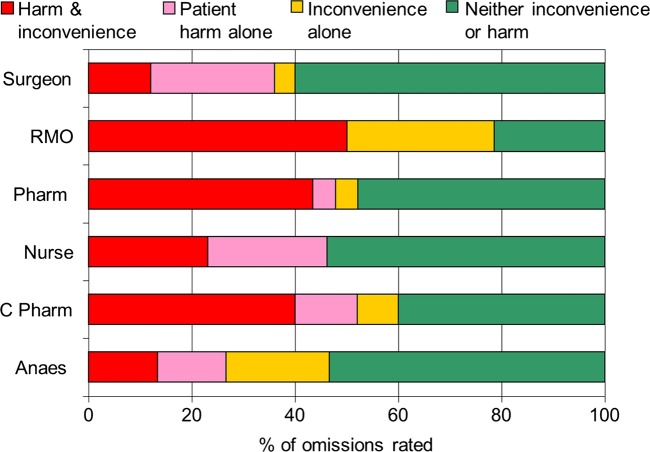
Clinical significance of omissions
Omissions from a randomly selected 5% of the total cohort were evaluated for clinical significance. Of the 89 regular medications in the patients’ medication histories in the control arm, 25 (28%) were omitted from the medication charts, compared with 1 of 55 (2%) in the control arm. When asked to assess the severity of omission, the average across the panel showed that 52% of omissions in the control arm had the potential for patient harm or ward inconvenience (see figure 3). Only one reviewer thought the omission in the intervention arm was significant.
Figure 3.
Assessment of clinical significance of omissions.
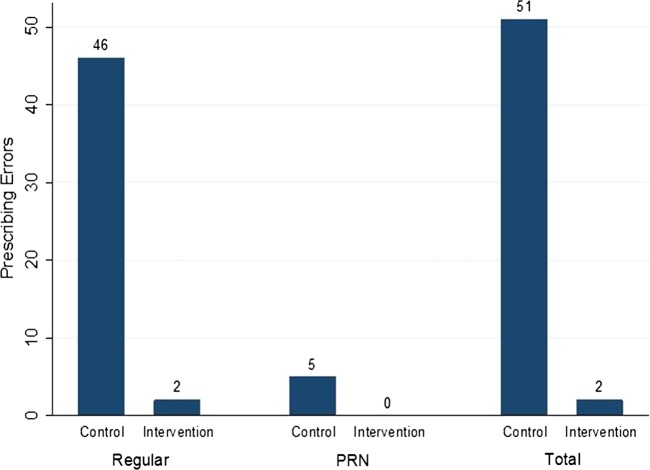
Prescribing errors related to drug, dose and frequency selection
Overall, 53 errors were identified where the drug strength, dose or frequency prescribed did not match the medication history or perioperative plan (see figure 4). This equates to 6.3% of control orders compared with 0.2% of intervention orders (p<0.001, Fisher's exact test).
Figure 4.
Number of prescribing errors.
Communication errors
Communication errors, where prescriptions were rated as ambiguous or unclear, were significantly higher in the control arm compared with the intervention arm. The OR for an order in the control arm to have a communication error compared with an order in the intervention arm was 2.52 (95% CI 1.96 to 3.27; logistic regression p<0.001). As there were multiple orders per patient, robust SEs, clustered by patient, were utilised (see table 5). Individually, communication errors were significantly higher in the control arm for all types of error except the route of administration (p=0.57 χ2 test).
Table 5.
Prescribing errors with an ambiguity in at least one component of the prescription
| Control number of errors (% of total orders) | Intervention number of errors (% of total orders) | p Value | |
|---|---|---|---|
| Total orders | 1034 | 904 | |
| Orders with at least one communication error | 445 (43) | 208 (23) | <0.001*† |
| Prescribing communication errors | 667 | 229 | |
| Prescribing communication errors | |||
| Drug name | 23 (2.1) | 0 | <0.001‡ |
| Route | 79 (7.6) | 76 (8.4) | 0.57† |
| Dose | 48 (4.6) | 5 (0.6) | <0.001‡ |
| Frequency | 190 (18.4) | 96 (10.6) | <0.001‡ |
| Administration times incorrect or missing | 117 (14.9) (781 orders) | 4 (0.5%) (762 orders) | <0.001‡ |
| PRN maximum dose missing | 178 (74.5) (241 orders) | 47 (32.6) (142 orders) | <0.001‡ |
| Slow release not specified | 15 (30.0) (50 orders) | 1 (1.5) (66 orders) | <0.001‡ |
| Intermittent order not specified | 17 (57.5) (30 orders) | 0 (38 orders) | <0.001‡ |
*Logistic regression.
†χ2 test.
‡Fisher's exact test.
From the control arm prescribers, 44 of them prescribed medication on the medication charts, with a median number of orders of 21 (range 1–85). The median percentage of orders in the control arm that contained at least one communication error per prescriber was 38 (range 0–100).
VTE prophylaxis
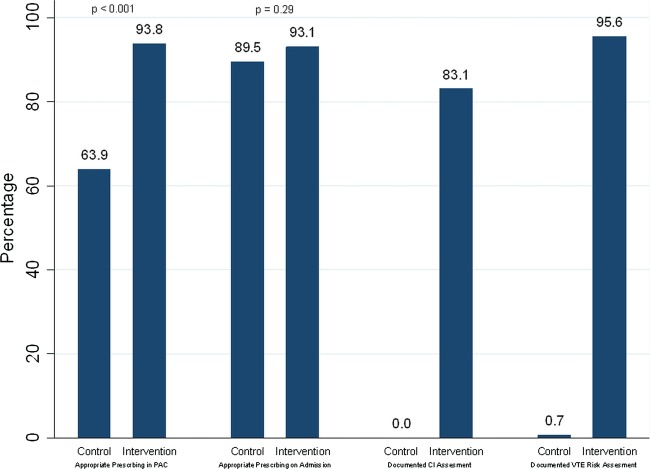
Patients in the intervention arm were significantly more likely than controls to have appropriate VTE prophylaxis prescribed on the medication chart in PAC and to have documented VTE assessment (see figure 5). On admission to the ward, approximately 90% of both intervention and control patients were prescribed appropriate VTE prophylaxis.
Figure 5.
Venous thromboembolism prophylaxis assessments and prescribing.
Discussion
This study has built on the findings from previous research of pharmacists prescribing in PAC settings, which have found improved accuracy of information gathered, and improved prescribing according to guidelines.9 27 Similar studies of pharmacist interventions in different settings have shown improvements in clinical endpoints such as blood pressure control, increased appropriateness of prescribing and reductions in ADEs, such as warfarin-associated bleeds.28 29
The traditional scope of practice for the PAC pharmacist consists of taking a medication history, using guidelines, clinical judgement and referral to the surgical team to suggest a plan for medications perioperatively, and providing this information to the RMOs to generate the medication charts. This scope has been extended in our study by providing an appropriately trained pharmacist to generate the medication chart and prescribe VTE prophylaxis, which has led to a significant reduction in omissions and prescribing errors, ensuring that patients get the correct medication while in hospital. The evaluation of VTE prophylaxis prescribing was essential to assess the safety and appropriateness of initiation of a new medication, within guidelines, by the prescribing pharmacist. The results from this study have shown the prescribing to be as appropriate as usual care at the time the patient is admitted to the ward. Issues still remain with the prescribing, especially with the use of inappropriate abbreviations.30 For example, a large proportion of communication errors in the intervention arm were due to the use of s/c to indicate subcutaneous, which has informed the researchers on future educational requirements of prescribers, especially with regard to safe prescribing.
Electronic prescribing may be one solution to such errors involving legibility and inappropriate abbreviations, but studies have shown that the systems introduce errors of their own.31 These errors need to be fully assessed and appreciated if the quality of prescribing is to be improved by the introduction of computerised prescribing into the healthcare system.
The results presented in this paper are part of a larger study. Further work is required to assess the appropriateness of prescribing of medication charts and consumer participation of this new model of care.23 There are a number of limitations. Even though the trial was randomised, the total number of medications that the patients were taking was higher in the control arm (1364) compared with the intervention arm (983). The explanation for this is unknown but may in part be due to large randomisation block sizes, possibly meaning that a number of consecutive patients were randomised to the control arm during clinic sessions, where patients were more likely to have a higher burden of medication, for example, during a vascular surgery clinic. There was more opportunity for omissions from the control arm as a result of more medications needing to be continued, and this was allowed for in the analysis.
RMOs in clinic during the study were aware of the intervention pharmacist's role, which may have led to an increased number and quality of medication charts prescribed in the control arm. Even with this potential effect, the study still showed a significant improvement in the safety and accuracy of medication charts.
Review of medication orders is not a role that an RMO routinely undertakes. All RMOs were educated with regard to the requirement of a countersignature of pharmacist orders, and to amend anything as required prior to sign off. In the trial, 10 charts were amended—5 changes were minor, 3 were addition of analgesics out of the pharmacist's prescribing scope and 2 changes actually resulted in inappropriate VTE prophylaxis. Despite the legislative changes, countersignature of pharmacist orders was a local requirement owing to the concern that junior doctors may become deskilled as a result of being removed from the prescribing process. However, the authors suggest that having an appropriately trained prescribing pharmacist in clinic, for the RMOs to use as guidance and to provide feedback on any prescribing errors, may increase the effectiveness of the learning environment.
Having only one pharmacist prescribing in the intervention arm and multiple RMOs prescribing in the control arm is a potential source of bias that is unavoidable where individual knowledge, skills and capabilities determine the quality of prescribing. It has been suggested that medical undergraduate training may not prepare graduates to prescribe, which if addressed may reduce this individual variance.32 The model of care tested in our study was successful as we were able to reduce the variance within a group by training one individual pharmacist to manage medications perioperatively, within a set scope of practice, and to include prescribing. It could be argued that the same results may have been obtained by providing the RMOs with extra prescribing training, and that the improved performance may not necessarily be solely due to the introduction of a new professional discipline. The authors acknowledge that the improved results may well be multifactorial, but would also suggest that the underlying competencies of an experienced, ‘advanced level’ pharmacist, plus the prescribing training provided, have ensured appropriate competencies to prescribe in the model of care in which the prescribing took place.33
The order of consultation in the intervention arm was set by trial design. The order in the control arm was not set, which is a true reflection of usual care, where the patient could see the RMO prior to the pharmacist. This may have impacted on the quality of control medication charts prescribed by the RMO, without information available from the pharmacist history. While this could be classed as a limitation, this does reflect usual care in PAC and highlights the collaborative nature of the existing model of care.
The prescribing pharmacist was able to see control patients for usual care duties of a medication history, which may be perceived as introducing bias. However, as both pharmacists have received the same undergraduate and general-level pharmacist training, the quality of medication history gathered for the RMO to use to prescribe the medication chart would be the same.
Another limitation is the potential sustainability of the model of care, and capacity to train pharmacists as prescribers. This was only one pharmacist in one hospital who had received special training to be able to prescribe. Evaluation of the requirements of non-medical prescribing courses is underway, but substantial further thought needs to be applied to ensure reproducibility of these results in a larger sample and consistent production of safe and effective prescribers.34
Further work is required to address the actual and perceived medicolegal implications for both doctors and pharmacists in such collaborations.
Conclusion
Medication charts in the intervention arm were significantly safer and more accurate with regard to the patients’ regular medications than medication charts in the control arm.
There was no difference in appropriateness of VTE prophylaxis prescribing between arms on admission to the ward.
Our study has shown that the pharmacist in a PAC was able to effectively gather all the information required to collaboratively formulate a clinical decision in clinic within an agreed scope of practice, and communicate the decisions safely and accurately onto the medication chart.
A collaborative doctor–pharmacist prescribing model in a PAC was as safe and accurate as usual care in ensuring that patients were prescribed the medication required on admission for elective surgery.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the following people without whom the study would not have been possible. Professor Stephen Lynch, Chair of Surgery, Princess Alexandra Hospital, Associate Professor Lynette Loy, Director of Pharmacy, Princess Alexandra Hospital, Ms Ching-Ting Hung, Senior Pharmacist, Princess Alexandra Hospital, Ms Renea Collins, Clinical Nurse Consultant, VTE Prophylaxis, Princess Alexandra Hospital, all of the staff in Pre Admission Clinic.
Footnotes
Contributors: ARH, IDC, JS, KW, EM and LN contributed to the project concept and study design. ARH collected all data and was responsible for the running of the study. ARH, IDC, JS and LN were involved in the evaluation of data. ARH and DMD were responsible for database design, data evaluation and statistical reporting. All authors contributed significantly to the write-up of the project. All authors have read and approved the final manuscript.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None.
Ethics approval: Princess Alexandra Hospital Human Ethics Research Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Extra data can be accessed via the Dryad data repository at http://datadryad.org/ with the doi:10.5061/dryad.81tr1.
References
- 1.Coombes I, Reid C, McDougall D, et al. Pilot of a National Inpatient Medication Chart in Australia: improving prescribing safety and enabling prescribing training. Br J Clin Pharmacol 2011;72:338–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bates D, Cullen D, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA 1995;274:29–34 [PubMed] [Google Scholar]
- 3.Bobb A, Gleason K, Husch M, et al. The epidemiology of prescribing errors: the potential impact of computerized prescriber order entry. Arch Intern Med 2004;164:785–92 [DOI] [PubMed] [Google Scholar]
- 4.Cornish P, Knowles S, Marchesano R, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med 2005;165:424–9 [DOI] [PubMed] [Google Scholar]
- 5.Bates D, Boyle D, Vander Villet M, et al. Relationship between medication errors and adverse drug events. J Gen Intern Med 1995;10:199–205 [DOI] [PubMed] [Google Scholar]
- 6.Dean B, Barber N, Schacter M. What is a prescribing error? Qual Health Care 2000;9:232–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aronson JK. Balanced prescribing. Br J Clin Pharmacol 2006;62:629–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barber N, Rawlins M, Dean Franklin B. Reducing prescribing error: competence, control, and culture. Qual Saf Health Care 2003;12:i29–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stowasser D. Medication information and health outcomes—development and evaluation of a medication liaison service, in School of Pharmacy 2000. Brisbane: University of Queensland, 2000 [Google Scholar]
- 10.Nagpal K, Arora S, Vats A, et al. Failures in communication and information transfer across the surgical care pathway: interview study. BMJ Qual Saf 2012;21:843–9 [DOI] [PubMed] [Google Scholar]
- 11.Bhanji A, Fallon K, LeBlanc S. Pharmacy involvement in a surgery preadmission program. Am J Health Syst Pharm 1993;50:483–6 [PubMed] [Google Scholar]
- 12.Wright P, Khachi H, Vercaeren S, et al. Medicines reconciliation in preadmission clinics can improve medicines prescribing. Pharm Pract 2008;18:44–6 [Google Scholar]
- 13.Crown J. Review of prescribing, supply and administration of medicines. Final report London, 1999 [Google Scholar]
- 14.Courtenay M, Carey N. Nurse independent prescribing and nurse supplementary prescribing practice: national survey. J Adv Nurs 2008;61:291–9 [DOI] [PubMed] [Google Scholar]
- 15.Australian Institute of Health and Welfare Medical labour force 2005. Canberra: AIHW, 2008. (AIHW Cat. no. HWL 41, Series number 40). [Google Scholar]
- 16.National Health Workforce Taskforce. 2009. Health Workforce in Australia and Factors for Current Shortages. http://www.nhwt.gov.au/documents/NHWT/The%20health%20workforce%20in%20Australia%20and%20factors%20influencing%20current%20shortages.pdf (accessed Jan 2012)
- 17.Brooks P, Lapsley H, Butt D. Medical workforce issues in Australia: ‘tomorrow's doctors—too few, too far’. Med J Aust 2003:206–8 [PubMed] [Google Scholar]
- 18.Health Workforce Australia, HWA Work Plan 2011/12. 2011. Approved by Australian Health Ministers’ Conference, 26 September.
- 19.National Health Workforce Research and Planning Collaboration, Non-Medical Prescribing. An exploration of likely nature of, and contingencies for, developing a nationally consistent approach to prescribing by non-medical health professionals. 2010. Final Report.
- 20.Bessell T, Marriott J, Emmerton L, et al. Improving Australians’ Access to Prescription Medicines: Development of Pharmacy Practice Models. 2005. Final Report.
- 21.Bhanbhro S, Drennan V, Grant R, et al. Assessing the contribution of prescribing in primary care by nurses and professionals allied to medicine: a systematic review of the literature. BMC Health Serv Res 2011;11:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Der Weyden M. Doctor displacement: a political agenda or a health care imperative? Med J Aust 2008;189:608–9 [DOI] [PubMed] [Google Scholar]
- 23.National Health Information Standards and Statistics Committee (NHISSC) 2009. The National Health Performance Framework (2nd Edition).
- 24.Hale A, Nissen L.2010. Does the Consultation Order in a Multidisciplinary Elective Surgical Pre-Admission Clinic Influence the Frequency and Quality of Prescribing for Inpatient Medication Charts? Australasian Pharmaceutical Science Association, Brisbane 6–9 December.
- 25.General Pharmaceutical Council, Accreditation of Independent Prescribing Programmes. 2010, General Pharmaceutical Council.
- 26.Australia and New Zealand Working Party on the Management and Prevention of Venous Thromboembolism Prevention of venous thromboembolism best practice guidelines for Australia and New Zealand. 4th edn Sydney: Health Education and Management Innovations, 2007 [Google Scholar]
- 27.Marotti S, Kerridge R, Grimer M. A randomised controlled trial of pharmacist medication histories and supplementary prescribing on medication errors on postoperative medications. J Anaesth Intensive Care 2011;29:1064–70 [DOI] [PubMed] [Google Scholar]
- 28.Reid F, Murray P, Storrie M. Implementation of a pharmacist led clinic for hypertensive patients in primary care—a pilot study. Pharm World Sci 2005;27:202–7 [DOI] [PubMed] [Google Scholar]
- 29.Burns N. Evaluation of warfarin dosing by pharmacists for elderly medical in-patients. Pharm World Sci 2004;26:232–7 [DOI] [PubMed] [Google Scholar]
- 30.Australian Commission on Safety and Quality in Healthcare. 2006. National terminology, abbreviations and symbols to be used in the prescribing and administering of medicines in Australian hospitals.
- 31.Koppel R, Metlay J, Cohen A. Role of computerized physician order entry systems in facilitating medication errors. JAMA 2005;292:1197–203 [DOI] [PubMed] [Google Scholar]
- 32.Pillans P. How prepared are medical graduates to begin prescribing? Intern Med J 2009;39:425–7 [DOI] [PubMed] [Google Scholar]
- 33.Morris S, Coombes I. The right to prescribe: towards core prescribing competencies for all prescribers. Aust Prescriber 2011;34:126 [Google Scholar]
- 34.Coombes I, Wheeler A, Hale A. Evaluation of effectiveness and relevance of Safe Medication Practice Tutorials as a course for pharmacist prescribers in New Zealand. Pharm Educ 2011;11:95–8 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.