Abstract
Lipids and lipid metabolites have long been known to play biological roles that go beyond energy storage and membrane structure. In age-related macular degeneration and diabetes, for example, dysregulation of lipid metabolism is closely associated with disease onset and progression. At the same time, some lipids and their metabolites can exert beneficial effects in the same disorders. This review summarises our current knowledge of the contributions of lipids to both the pathogenesis and treatment of neovascular eye disease. The clinical entities covered are exudative age-related macular degeneration, diabetic retinopathy and retinopathy of prematurity, with a special emphasis on the potential therapeutic effects of ω3-(also known as n-3) polyunsaturated fatty acids.
INTRODUCTION
Angiogenesis research began to evolve into an academic specialty in its own right as early as the 1970s.1,2 An impressive body of literature has since accumulated, comprising close to 50 000 articles in PubMed. However, only about 2000 (4%) of these publications relate to lipids (PubMed search September 2010). This is in stark contrast to the increasing evidence of a potent role for lipid mediators particularly in the development of neovascular eye disease. Progression of diabetic retinopathy, for example, can be modulated by altering a patient’s lipid profile.3–5 Similarly, progression of age-related macular degeneration (AMD) and retinopathy of prematurity (ROP) have been linked to differences in lipid intake and metabolism.6–14
One class of lipid-based mediators found to modulate neovascular eye disease particularly are ω3-(also known as n-3) polyunsaturated fatty acids (PUFAs).7,8,11,15,16 ω3-PUFAs are essential fatty acids that cannot be synthesised in sufficient amounts by humans and must therefore be obtained from the diet. The retina has the highest ω3-PUFA concentration of all tissues (20%) and ω3-PUFAs serve vital functions in normal retinal architecture and function.17 Conversely, excess dietary intake of the structurally similar ω6-PUFAs appears to convey very different, even opposing effects in the retina: while ω3-PUFAs appear to be associated with a reduced risk of AMD,18 ω6-PUFAs seem to be associated with an increased risk.8
These observations illustrate that it is crucial to understand the role of lipid pathways and lipid-derived mediators in retinal health and disease in order to develop targeted, lipid-based therapies. It may well be that among the multitude of ω3-PUFA-derived lipid metabolites, only few are relevant for conveying beneficial effects during proliferative retinopathy or AMD. A direct analogy may be drawn from proteins, where vascular endothelial growth factor (VEGF) stands out as a major angiogenic mediator from a plethora of other, less relevant proteins. This review will provide an overview of our current knowledge of lipid-based mediators and their relative contribution to both the pathogenesis and potential treatment of neovascular eye diseases. The clinical entities covered will comprise wet AMD, diabetic retinopathy and retinopathy of prematurity (ROP), three of the most prevalent blinding eye diseases.19–21
NON-ENZYMATIC LIPID PATHWAYS: LIPID PEROXIDATION
Late-stage neovascular AMD (exudative or wet AMD) is characterised by progressive dysfunction of macular retinal pigment epithelium (RPE) and formation of choroidal neovascularisation (CNV).22,23 While the complex pathogenesis of AMD is not yet fully elucidated, clinical and experimental evidence support a key role for reactive lipid mediators generated by oxidative damage in the outer retina.24,25 The retina exceeds all other human tissues in its concentration of PUFAs. Due to their polyunsaturated structure, these PUFAs are particularly susceptible to oxidative degradation by non-enzymatic lipid peroxidation. Within the retina, PUFAs are especially enriched in the photoreceptor outer segment (POS) membranes, putting the outer retina at high risk for damage by lipid peroxidation products.
During non-enzymatic lipid peroxidation, oxygen-derived free radicals interact with unsaturated PUFA double bonds26 to generate a variety of highly reactive aldehyde intermediates, the most widely studied being malondialdehyde (MDA), 4-hydroxynonenal (HNE) and carboxyethylpyrrole (CEP).25,27 These molecules rapidly attach to cellular proteins forming covalent adducts (advanced lipid peroxidation end products, ALE), thereby impairing protein stability and function. Accumulation of lipid peroxidation products and ALEs occurs in vivo as a result of both photo-oxidative damage and ageing.28–30 ALEs are also found in RPE-derived lipofuscin isolated from human donor eyes31 as well as in drusen and Bruch’s membrane of AMD patients.32
Photoreceptor cells cope with damage by continuous shedding and renewal of their PUFA-rich outer segments (POS). The shed POS are phagocytosed by RPE cells that metabolise and clear the toxic lipid peroxidation products, mainly through lysosomal enzymes. Thus RPE lysosomal enzymes are important modulators in this process as well as likely targets for lipid peroxidation-mediated damage. This notion is supported by in vitro observations that phagocytosis of lipid peroxidation-modified POS by RPE can induce profound lysosomal dysfunction,33,34 resulting in increased lipofuscin generation, impaired cellular self-renewal by autophagy, and release of undegraded POS proteins into the sub-RPE space.9,35 Furthermore, lipid peroxidation-related lysosomal dysfunction induces VEGF secretion by RPE cells in vitro (Bergmann et al, unpublished results) and subretinal injection of ALEs exacerbates laser-induced CNV in vivo.36 Interestingly, ALEs also serve as haptens that induce autoantibody formation against lipid peroxidation-modified retinal proteins inducing a subsequent inflammatory response in the sub-RPE space that may contribute to complement activation and RPE damage in AMD.27,37
Combined, these results from in vitro and in vivo studies suggest that lipid peroxidation products contribute to the pathogenesis of exudative AMD. In accordance with this, the Age-Related Eye Disease Study (AREDS)1 demonstrates a protective effect of antioxidative treatment on AMD progression,38 indicating that under specific circumstances oxidative processes in AMD are susceptible to therapeutic intervention. However, other clinical studies targeting lipid peroxidation, for example by carbonyl scavenging, have not yielded conclusive results.39 Further research is needed to evaluate if interference with lipid peroxidation is useful for prophylactic and therapeutic intervention in AMD.
Similar processes as illustrated above for AMD may also play a role in proliferative retinopathies. While less research has been done in these areas, non-enzymatic lipid peroxidation initiated by reactive oxygen species has been implicated in the pathogenesis of both diabetic retinopathy and ROP.40–42 Similarly, the conversion of arachidonic acid (AA) into trans-AA under nitrative stress was found to mediate apoptosis of microvascular cells via upregulation of thrombospondin-1 (TSP-1) and activation of the CD36 receptor on endothelial cells.43 Approaches to reduce oxidative and nitrative stress using antioxidative therapy would attenuate these detrimental non-enzymatic lipid peroxidation processes. However, antioxidative therapies have produced inconsistent results thus far44–46 and more studies are needed to evaluate if antioxidants can play a significant role in attenuating proliferative retinopathies.
ENZYMATIC LIPID PATHWAYS: ATX, PLA2, COX, LOX AND CYP450
Beyond non-enzymatic lipid peroxidation, many specific lipid-metabolising enzymes play a role in angioproliferative diseases.47 One example is autotaxin (ATX), an enzyme that converts lysophosphatidylcholine (LPC) into lysophosphatidic acid (LPA), a bioactive signalling molecule. Once generated, LPA can upregulate VEGF-C expression and induce tube formation in endothelial cells.48 One other important example is phospholipase A2 (PLA2), an enzyme that becomes activated following ischaemia/ reperfusion injuries.49–51 PLA2 catalyses the hydrolysis of lipids from cell membranes, liberating them for further metabolism and signalling. The amount of each fatty acid released from the cell membrane depends on the relative amount of that particular fatty acid present in the membrane. For essential fatty acids (which cannot be adequately synthesised by humans) this available amount of lipid substrate, in turn, is dependent on dietary intake.18
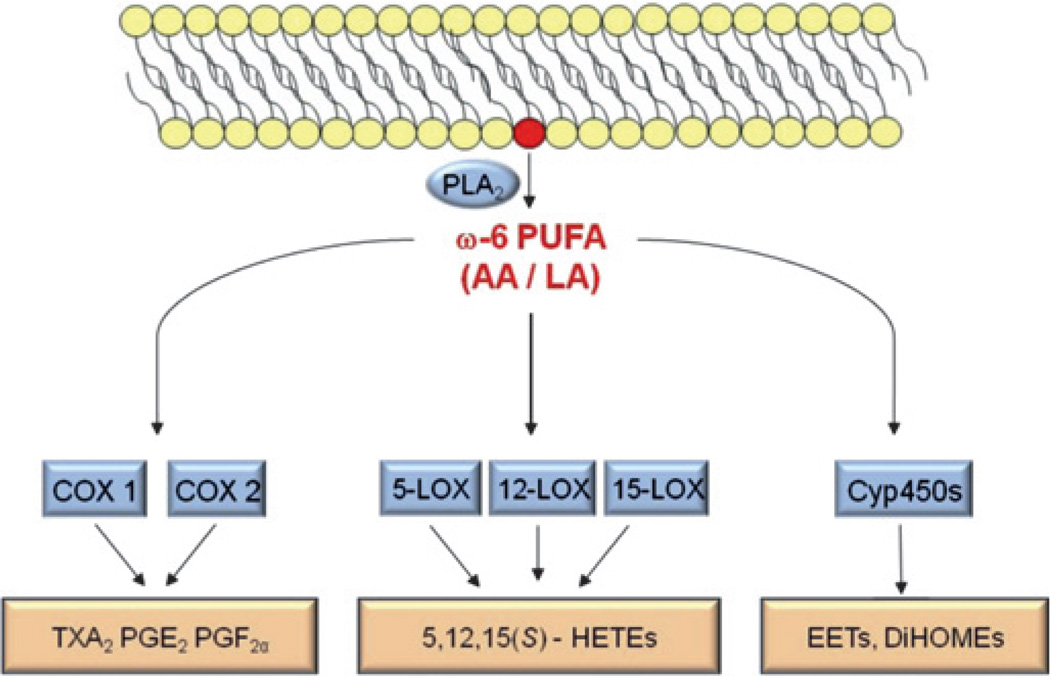
An important lipid that is liberated by PLA2 from the cell membrane is AA, a member of the family of ω6-PUFAs. Once liberated, AA is rapidly further metabolised by three major pathways: the cyclooxygenase (COX), lipoxygenase (LOX) and cytochrome P450 (Cyp450) pathways (figure 1). COX-2 in particular is among the immediate-early gene products that is upregulated in response to retinal ischemia, for example in diabetic retinopathy.52,53 The same enzymes (PLA2, COX, LOX, Cyp450) that metabolise ω6-PUFAs also process the structurally similar ω3-PUFAs, which can be exploited for potential therapeutic approaches (see below).
Figure 1.
Schematic of the major enzymatic pathways metabolising ω6-polyunsaturated fatty acids (PUFAs). Phospholipase A2 (PLA2) liberates ω6-PUFAs (eg, arachidonic acid (AA) or linoleic acid (LA)) from the cell membrane. These lipids are rapidly further metabolised by cyclooxygenase (COX), lipoxygenase (LOX) and cytochrome P450 (Cyp450) enzymes, generating the pathway-specific lipid metabolites thromboxane A2 (TXA2), prostaglandin (PG) E2, PGF2α, hydroxyeicosatetraenoic acids (HETEs), epoxyeicosatrienoic acids (EETs), dihydroxyeicosatrienoic acids (DiHOMEs) and others.
ω6-PUFA metabolites TXA2, PGE2 and 5-HETE
Increased PLA2 and COX-2 activities can have important implications for proliferative retinopathies. For example, increased production of ω6-PUFA-derived thromboxane A2 (TXA2) via PLA2 and COX-2 can lead to a time- and concentration-dependent death of retinal endothelial cells.54–57 Interestingly, TXA2 is generated more abundantly in the stressed newborn retina compared with adults, potentially giving this lipid-derived metabolite an important role in the pathogenesis of ROP.47 In contrast to the endotheliotoxic effects of TXA2, other ω6-PUFA-derived mediators from the COX-2 pathway have potent pro-angiogenic properties during retinopathy. Prostaglandin (PG) E2, for example, can stimulate the formation of pathological retinal neovessels through binding to its PGE receptor 3 (EP3).53,58 Importantly, ω6-PUFA-derived PGE2 and ω3-PUFA-derived PGE3 exert opposing effects on endothelial cell proliferation. While PGE2 increases the production of angio-poietin-2 (ANG2) and matrix metalloproteinase-9 to stimulate endothelial tube formation, PGE3 inhibits the same processes.59
It is not only cyclooxygenase-dependent metabolites that are differentially regulated during neovascular eye diseases. The lipoxygenase-dependent ω6-PUFA product 5-hydroxyeicosatetraenoic acid (5-HETE), for example, is increased in vitreous of patients with diabetic retinopathy.60 These findings, along with reports on ω6-PUFA-derived 5-HETE and epoxyeicosatrienoic acids (EETs) being involved in mediating both inflammatory and angiogenic processes61–63 illustrate that not only COX-dependent metabolites of arachidonic acid but also lipoxygenase- and/or Cyp450-dependent lipid metabolites of ω6-PUFAs may be potent modulators of proliferative retinopathies. This contribution of enzymatically created lipid mediators to the pathogenesis of retinopathy appears especially relevant in patients with diabetes where strong evidence points towards a significant contribution of dyslipidaemia to retinopathy progression.3,64–67 In AMD, the AREDS1 indicates that higher intake of ω6-PUFAs may be associated with a higher prevalence of exudative AMD.8
LIPID MEDIATORS AS THERAPEUTICS FOR NEOVASCULAR EYE DISEASE
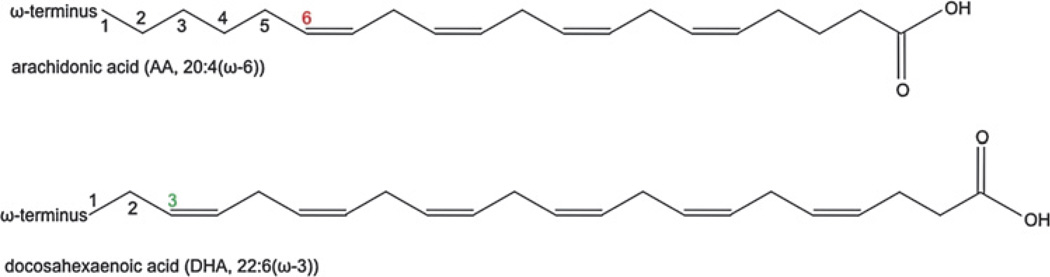
As illustrated above, many lipid-derived mediators can potently induce or amplify neovascular eye disease. Increasing evidence, however, indicates that other lipid-derived mediators can improve the same pathologies. This is especially the case with ω3-PUFAs, which convey beneficial effects in several models of neovascular eye disease.11,15,16 Structurally, ω3-PUFAs are very similar to ω6-PUFAs and differ solely in the location of their double bonds: ω3-PUFAs have the first double bond after the third carbon atom when counting from the ω end; ω6-PUFAs have the first double bond after the sixth carbon atom (figure 2).
Figure 2.
Chemical structure of arachidonic acid (AA), an ω6-polyunsaturated fatty acid (PUFA), and docosahexaenoic acid (DHA), an ω3-PUFA. Coloured numbers indicate the third (green) and sixth (red) carbon atom from the omega end after which the first double bond is located.
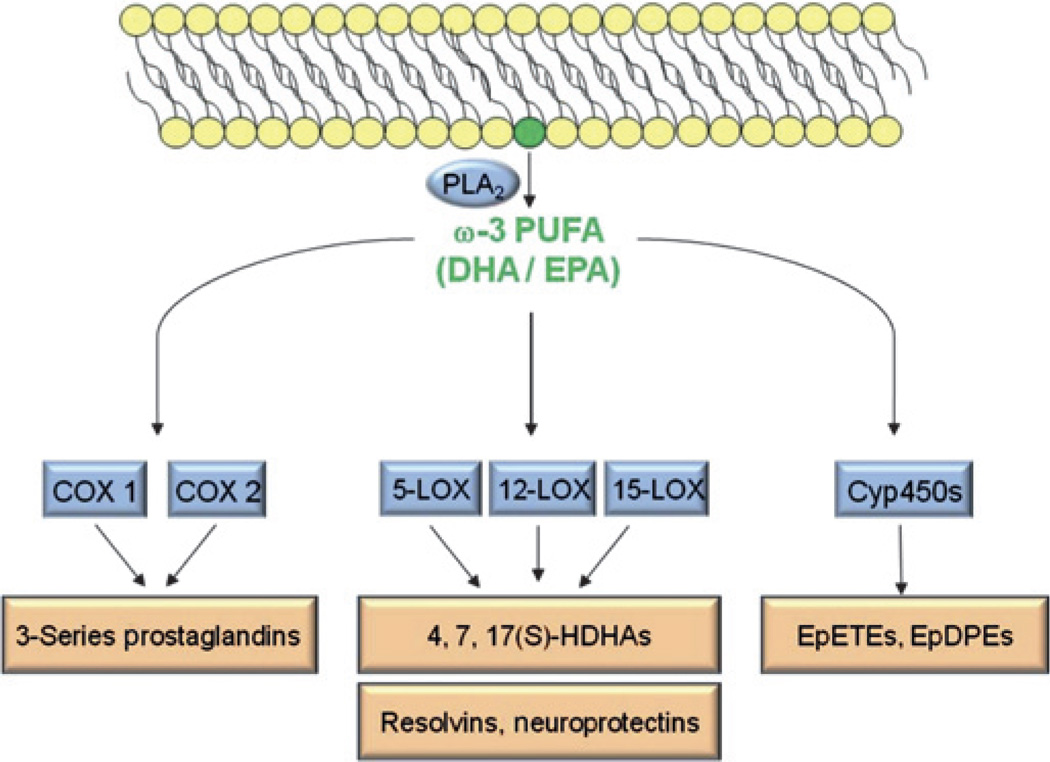
The minor differences in structure between ω3- and ω6-PUFAs translate into significant differences in biological function. Although ω3-PUFAs are metabolised by the same enzymes as ω6-PUFAs, unique metabolites are generated from ω3-PUFAs (figure 3). Pioneering work by Serhan and colleagues identified resolvins and neuroprotectins as potent anti-inflammatory and anti-angiogenic ω3-PUFA metabolites.68–70 Studies in animal models of proliferative retinopathy11 and choroidal neovascularisation15 found that these ω3-PUFA metabolites significantly attenuated neovascular eye disease. In addition, the ω3-PUFA metabolite neuroprotectin promotes RPE survival during oxidative stress,71,72 a process thought to be involved in the pathogenesis of AMD.
Figure 3.
Schematic of the major enzymatic pathways metabolising ω3-polyunsaturated fatty acids (PUFAs). Phospholipase A2 (PLA2) liberates ω3-PUFAs (eg, docosahexaenoic acid (DHA) or eicosapentaenoic acid (EPA)) from the cell membrane. These lipids are rapidly further metabolised by cyclooxygenase (COX), lipoxygenase (LOX) and cytochrome P450 (Cyp450) enzymes, generating the pathway-specific lipid metabolites 3-series prostaglandins, hydroxydocosahexaenoic acids (HDHAs), resolvins, neuroprotectins, epoxyeicosatetraenoic acids (EpETEs) and epoxydocosapentaenoic acids (EpDPEs).
Lipid mediators as therapeutics for exudative AMD
Both ω3- and ω6-PUFAs are essential fatty acids obtained from diet.73 This has led to studies investigating the correlation between dietary ω3-PUFA intake (mainly in the form of fish) and the risk for neovascular eye disease. The largest set of data on ω3-PUFAs and AMD risk comes from the AREDS1 study. While AREDS1 was not initially designed to evaluate the effect of ω3-PUFAs on progression of AMD, retrospective data analysis found that ω3-PUFA intake is inversely associated with neovascular AMD.8 Conversely, higher intake of AA (an ω6-PUFA mainly obtained from ingestion of meat) is associated with greater prevalence of neovascular AMD.8 As retrospective data and reliance on patient-reported data may introduce confounding variables,74 the ongoing AREDS2 study was specifically designed to prospectively evaluate the impact of ω3-PUFA supplementation on AMD prevalence and progression. With definitive results from the placebo-controlled, double-blind AREDS2 study not available before 2013, the currently available data from both pre-clinical15,71,72 and retrospective clinical studies7,8 must be interpreted carefully, but on the whole, they point towards beneficial effects of ω3-PUFAs in AMD. In RPE cell cultures ω3-PUFAs reduced oxidative stress-induced apoptosis by over 70%72 and in a mouse model of laser-induced CNV, neuroprotectin D1 (an ω3-PUFA metabolite) reduced CNV areas by 68%.15 Clinically, the retrospective AREDS1 data analysis suggests higher intake of ω3-PUFAs to be associated with a decreased likelihood for AMD progression8 and participants who reported the highest ω3-PUFA intake were 30% less likely to develop late stage AMD over a 12-year period.7 No apparent risks were associated with increased ω3-PUFA intake in patients at risk of AMD.
Lipid mediators as therapeutics for diabetic retinopathy and ROP
While exudative AMD, diabetic retinopathy and ROP all share the final common pathway of retinal (or subretinal) neovascularisation, it has to be stated clearly that very disease-specific variables influence disease onset and progression in these three entities. While in AMD degenerative changes in RPE and Bruch’s membrane play an important pathogenic role,75,76 diabetic retinopathy is characterised by severe metabolic dysregulation4,5 and ROP by an overall immaturity not only of the retina but the whole organism.77,78 All these disease-specific factors can significantly alter lipidomic profiles and the expression of lipid-processing enzymes and thus may differentially affect the success of lipid-based therapies. Therefore, it will be important to evaluate independently whether the positive findings from the AREDS1 cohort7,8 can be extended to diabetic retinopathy and ROP.
With regard to diabetic retinopathy, it is intriguing that recent studies have identified peroxisome proliferator-activated receptor (PPAR)γ as one of the target receptors of ω3-PUFAs.16 Activation of the nuclear receptor PPARγ by ω3-PUFAs was found to be crucial in mediating the beneficial effect of ω3-PUFAs in experimental retinopathy.16 Interestingly, activation of the same receptor by rosiglitazone, a drug that is used to treat insulin resistance in type 2 diabetes, was found to have similar beneficial effects on progression of diabetic retinopathy. 79 An independent study with over 10 000 patients also reported beneficial effects on progression of diabetic retinopathy when patients were treated with fenofibrate, a pharmacological activator of PPARγ.3 PPARγ shares high structural homology with PPARγ and can similarly be activated by ω3-PUFAs.80 The prospect that ω3-PUFAs exert their beneficial effects on retinopathy, at least in part, through activation of PPARs 16 may offer the prospect of using ω3-PUFAs as alternative or supplement to established pharmacological PPAR activators in the treatment of diabetic complications, including retinopathy.3,16,79
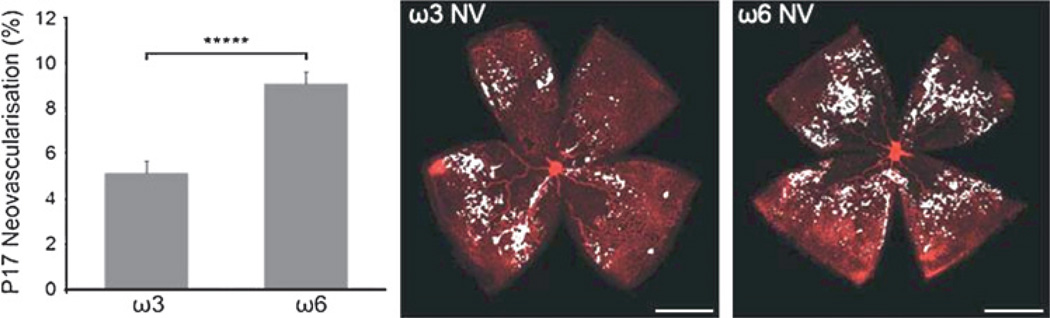
With regard to ROP, data from the mouse model of oxygen-induced retinopathy (OIR)81–84 strongly indicates a beneficial effect of ω3-PUFAs on both functional vascularisation of avascular retina11 as well as reduction of pre-retinal neovascularisation. 16 The effect of ω3-PUFAs in this model is profound and comparable in its extent to the effects that are achievable with anti-VEGF therapy during OIR (figure 4).85
Figure 4.
ω3-PUFAs attenuate proliferative retinopathy in the mouse model of oxygen-induced retinopathy (OIR) by more than 40%, an effect comparable to anti-vascular endothelial growth factor (VEGF) therapy in the same model. NV, neovascularization; P17, postnatal day 17; *****p<0.00001. Figure reproduced with permission from Stahl et al.17
Importantly, in premature infants ω3-PUFA supplementation may have implications that reach far beyond ROP. During normal third trimester development, ω3-PUFAs are provided in utero from the mother to her fetus. This physiological supply is interrupted by premature birth. Recent studies found that newborn infants may exhibit daily deficits of up to 44% in docosahexaenoic acid (DHA; an ω3-PUFA).86 However, total parenteral nutrition (TPN) for premature infants is in many cases completely devoid of ω3-PUFAs, thus not replenishing this deficit. Clinical studies that evaluate the effects of increased ω3-PUFAs in TPN protocols have suggested a beneficial effect of ω3-PUFAs on the progression of TPN-associated liver disease.87,88 Combined with the results from OIR animal studies11,16 these data suggest that supplementing ω3-PUFAs in preterm infants to levels that are seen in utero during a healthy third trimester pregnancy might improve not only ROP but be beneficial for overall postnatal development.
Lipid mediators and over-the-counter COX inhibitors
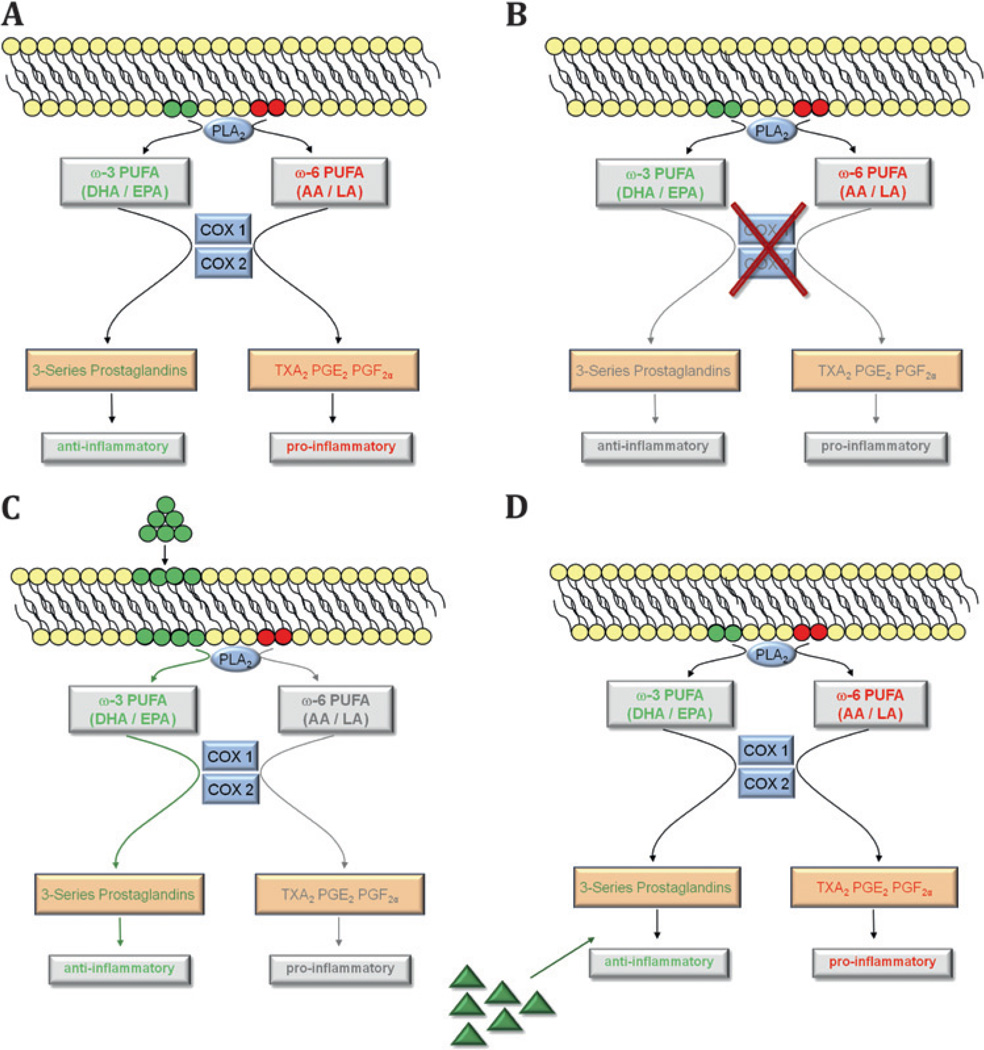
Inhibitors of the COX pathways are among the most frequently used over-the-counter drugs. These COX inhibitors significantly alter lipid metabolism by blocking parts of the major enzymatic lipid pathways (figures 1 and 3). However, no concordant results have been reported from studies investigating the effect of COX inhibitors on neovascular eye disease.89–91 One explanation might be that inhibiting COX activity decreases production of both potentially beneficial as well as potentially negative metabolites (figure 5A,B) or that beneficial metabolites are produced through another enzyme system. Altering the substrate pool of available lipids (figure 5C) or administering beneficial lipid metabolites (figure 5D) might be a more efficient approach to shift the retina towards a more advantageous composition in lipid-derived mediators.
Figure 5.
The cyclooxygenase pathways used as examples for different lipid-based treatment approaches. (A) With both ω3- and ω6-polyunsaturated fatty acids (PUFAs) available, the enzymatic cascade of phospholipase A2 (PLA2) and cyclooxygenase (COX)-1 and -2 create anti-inflammatory 3-series prostaglandins and inflammatory 2-series prostaglandins (thromboxane A2 (TXA2), prostaglandin (PG) E2 and PGF2α). (B) Pharmacological inhibition of COX enzymes with COX-inhibitors limits both the generation of ω3- and ω6-derived metabolites thus reducing the pool of both inflammatory and anti-inflammatory mediators in the retina. (C) In contrast, increasing the amount of available ω3-PUFA substrates for enzymatic processing yields more anti-inflammatory ω3-derived mediators and less inflammatory ω6-metabolites. (D) A different treatment approach would be to directly administer lipid metabolites that have been identified as having beneficial effects during retinopathy. Similar rationales apply to other lipid-metabolising pathways (lipoxygenase (LOX) or cytochrome P450 (Cyp450)) where the pool of lipid metabolites can be shifted towards a more beneficial profile. AA, arachidonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid.
CONCLUSION
Results from animal studies and clinical trials suggest that lipid-based mediators are likely to emerge as a novel group of modifiable variables in neovascular eye disease. As lipid mediators can convey both positive and negative effects on retinopathy, inhibition of lipid-metabolising enzymes (eg, by COX inhibitors) may reduce both positive and negative metabolites. Whether the optimal approach for effective lipid-based treatments will lie in supplementation of beneficial lipid substrates such as ω3-PUFAs or rather in the selective use of beneficial lipid metabolites or enzyme inhibitors will largely depend on ongoing research aimed at identifying the specific lipid metabolites that convey beneficial effects in retinopathy. Given our current knowledge, an important conclusion at this stage is that lipid mediators can play important roles in both pathogenesis and treatment of neovascular eye disease and that a thorough understanding of retinal lipid metabolism must form the foundation of any lipid-based intervention. The clinical potential of this relatively new field in ophthalmology research and the promising findings obtained so far render lipid-based therapies a very interesting target to investigate further in order to harness lipids and their metabolites as future therapies of neovascular eye disease.
Acknowledgments
Funding Deutsche Forschungsgemeinschaft (AS); Canadian Institutes of Health Research, Canadian National Institute for the Blind (PS); Juvenile Diabetes Research Foundation International (JC); NIH (EY017017, EY017017-S1), V. Kann Rasmussen Foundation, Roche Foundation for Anemia Research, Children’s Hospital Boston Mental Retardation and Developmental Disabilities Research Center, Research to Prevent Blindness Senior Investigator Award, Alcon Research Institute Award, MacTel Foundation (LEHS).
Footnotes
Competing interests None to declare.
Provenance and peer review Commissioned; externally peer reviewed.
REFERENCES
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Brem SS, Gullino PM, Medina D. Angiogenesis: a marker for neoplastic transformation of mammary papillary hyperplasia. Science. 1977;195:880–882. doi: 10.1126/science.402692. [DOI] [PubMed] [Google Scholar]
- 3.Chew EY, Ambrosius WT, Davis MD, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyons TJ, Jenkins AJ, Zheng D, et al. Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest Ophthalmol Vis Sci. 2004;45:910–918. doi: 10.1167/iovs.02-0648. [DOI] [PubMed] [Google Scholar]
- 5.Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 6.Bartoli M, Al-Shabrawey M, Labazi M, et al. HMG-CoA reductase inhibitors (statin) prevents retinal neovascularization in a model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2009;50:4934–4940. doi: 10.1167/iovs.08-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sangiovanni JP, Agron E, Meleth AD, et al. u3 Long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: a prospective cohort study from the Age-Related Eye Disease Study. Am J Clin Nutr. 2009;90:1601–1607. doi: 10.3945/ajcn.2009.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SanGiovanni JP, Chew EY, Clemons TE, et al. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch Ophthalmol. 2007;125:671–679. doi: 10.1001/archopht.125.5.671. [DOI] [PubMed] [Google Scholar]
- 9.Krohne TU, Stratmann NK, Kopitz J, et al. Effects of lipid peroxidation products on lipofuscinogenesis and autophagy in human retinal pigment epithelial cells. Exp Eye Res. 2010;90:465–471. doi: 10.1016/j.exer.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Tuo J, Ross RJ, Herzlich AA, et al. A high omega-3 fatty acid diet reduces retinal lesions in a murine model of macular degeneration. Am J Pathol. 2009;175:799–807. doi: 10.2353/ajpath.2009.090089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Y, Scheef EA, Wang S, et al. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood. 2009;113:744–754. doi: 10.1182/blood-2008-03-145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinberger B, Nisar S, Anwar M, et al. Lipid peroxidation in cord blood and neonatal outcome. Pediatr Int. 2006;48:479–483. doi: 10.1111/j.1442-200X.2006.02257.x. [DOI] [PubMed] [Google Scholar]
- 14.Leduc M, Kermorvant-Duchemin E, Checchin D, et al. Hypercapnia- and transarachidonic acid-induced retinal microvascular degeneration: implications in the genesis of retinopathy of prematurity. Semin Perinatol. 2006;30:129–138. doi: 10.1053/j.semperi.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Sheets KG, Zhou Y, Ertel MK, et al. Neuroprotectin D1 attenuates laser-induced choroidal neovascularization in mouse. Mol Vis. 2010;16:320–329. [PMC free article] [PubMed] [Google Scholar]
- 16.Stahl A, Sapieha P, Connor KM, et al. PPARg mediates a direct antiangiogenic effect of u3-PUFAs in proliferative retinopathy. Circ Res. 2010;107:476–484. doi: 10.1161/CIRCRESAHA.110.221317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Tan JS, Wang JJ, Flood V, et al. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol. 2009;127:656–665. doi: 10.1001/archophthalmol.2009.76. [DOI] [PubMed] [Google Scholar]
- 19.Good WV, Hardy RJ. The multicenter study of early treatment for retinopathy of prematurity (ETROP) Ophthalmology. 2001;108:1013–1014. doi: 10.1016/s0161-6420(01)00540-1. [DOI] [PubMed] [Google Scholar]
- 20.Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007;14:179–183. doi: 10.1080/09286580701396720. [DOI] [PubMed] [Google Scholar]
- 21.Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 22.Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122:598–614. doi: 10.1001/archopht.122.4.598. [DOI] [PubMed] [Google Scholar]
- 23.Roth F, Bindewald A, Holz FG. Key pathophysiologic pathways in age-related macular disease. Graefes Arch Clin Exp Ophthalmol. 2004;242:710–716. doi: 10.1007/s00417-004-0976-x. [DOI] [PubMed] [Google Scholar]
- 24.Beatty S, Koh H, Phil M, et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 25.Kopitz J, Holz FG, Kaemmerer E, et al. Lipids and lipid peroxidation products in the pathogenesis of age-related macular degeneration. Biochimie. 2004;86:825–831. doi: 10.1016/j.biochi.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 26.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 27.Gu X, Meer SG, Miyagi M, et al. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem. 2003;278:42027–42035. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- 28.Kapphahn RJ, Giwa BM, Berg KM, et al. Retinal proteins modified by 4-hydroxynonenal: identification of molecular targets. Exp Eye Res. 2006;83:165–175. doi: 10.1016/j.exer.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Tanito M, Elliott MH, Kotake Y, et al. Protein modifications by 4-hydroxynonenal and 4-hydroxyhexenal in light-exposed rat retina. Invest Ophthalmol Vis Sci. 2005;46:3859–3868. doi: 10.1167/iovs.05-0672. [DOI] [PubMed] [Google Scholar]
- 30.Spaide RF, Ho-Spaide WC, Browne RW, et al. Characterization of peroxidized lipids in Bruch’s membrane. Retina. 1999;19:141–147. doi: 10.1097/00006982-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Schutt F, Bergmann M, Holz FG, et al. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44:3663–3668. doi: 10.1167/iovs.03-0172. [DOI] [PubMed] [Google Scholar]
- 32.Crabb JW, Miyagi M, Gu X, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaemmerer E, Schutt F, Krohne TU, et al. Effects of lipid peroxidation-related protein modifications on RPE lysosomal functions and POS phagocytosis. Invest Ophthalmol Vis Sci. 2007;48:1342–1347. doi: 10.1167/iovs.06-0549. [DOI] [PubMed] [Google Scholar]
- 34.Krohne TU, Kaemmerer E, Holz FG, et al. Lipid peroxidation products reduce lysosomal protease activities in human retinal pigment epithelial cells via two different mechanisms of action. Exp Eye Res. 2010;90:261–266. doi: 10.1016/j.exer.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Krohne TU, Holz FG, Kopitz J. Apical-to-basolateral transcytosis of photoreceptor outer segments induced by lipid peroxidation products in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:553–560. doi: 10.1167/iovs.09-3755. [DOI] [PubMed] [Google Scholar]
- 36.Ebrahem Q, Renganathan K, Sears J, et al. Carboxyethylpyrrole oxidative protein modifications stimulate neovascularization: implications for age-related macular degeneration. Proc Natl Acad Sci U S A. 2006;103:13480–13484. doi: 10.1073/pnas.0601552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hollyfield JG, Bonilha VL, Rayborn ME, et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Negre-Salvayre A, Coatrieux C, Ingueneau C, et al. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol. 2008;153:6–20. doi: 10.1038/sj.bjp.0707395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kesavulu MM, Giri R, Kameswara Rao B, et al. Lipid peroxidation and antioxidant enzyme levels in type 2 diabetics with microvascular complications. Diabetes Metab. 2000;26:387–392. [PubMed] [Google Scholar]
- 41.Winterbourn CC, Chan T, Buss IH, et al. Protein carbonyls and lipid peroxidation products as oxidation markers in preterm infant plasma: associations with chronic lung disease and retinopathy and effects of selenium supplementation. Pediatr Res. 2000;48:84–90. doi: 10.1203/00006450-200007000-00015. [DOI] [PubMed] [Google Scholar]
- 42.Verdejo C, Marco P, Renau-Piqueras J, et al. Lipid peroxidation in proliferative vitreoretinopathies. Eye (Lond) 1999;13:183–188. doi: 10.1038/eye.1999.48. [DOI] [PubMed] [Google Scholar]
- 43.Kermorvant-Duchemin E, Sennlaub F, Sirinyan M, et al. Trans-arachidonic acids generated during nitrative stress induce a thrombospondin-1-dependent microvascular degeneration. Nat Med. 2005;11:1339–1345. doi: 10.1038/nm1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pazdro R, Burgess JR. The role of vitamin E and oxidative stress in diabetes complications. Mech Ageing Dev. 2010;131:276–286. doi: 10.1016/j.mad.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Lee CT, Gayton EL, Beulens JW, et al. Micronutrients and diabetic retinopathy a systematic review. Ophthalmology. 2010;117:71–78. doi: 10.1016/j.ophtha.2009.06.040. [DOI] [PubMed] [Google Scholar]
- 46.Johnson EJ. Age-related macular degeneration and antioxidant vitamins: recent findings. Curr Opin Clin Nutr Metab Care. 2010;13:28–33. doi: 10.1097/MCO.0b013e32833308ff. [DOI] [PubMed] [Google Scholar]
- 47.Hardy P, Beauchamp M, Sennlaub F, et al. Inflammatory lipid mediators in ischemic retinopathy. Pharmacol Rep. 2005;(Suppl 57):169–190. [PubMed] [Google Scholar]
- 48.Lin CI, Chen CN, Huang MT, et al. Lysophosphatidic acid upregulates vascular endothelial growth factor-C and tube formation in human endothelial cells through LPA(1/3), COX-2, and NF-kappaB activation- and EGFR transactivation-dependent mechanisms. Cell Signal. 2008;20:1804–1814. doi: 10.1016/j.cellsig.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Bazan NG. Free arachidonic acid and other lipids in the nervous system during early ischemia and after electroshock. Adv Exp Med Biol. 1976;72:317–335. doi: 10.1007/978-1-4684-0955-0_26. [DOI] [PubMed] [Google Scholar]
- 50.Phillis JW, O’Regan MH. A potentially critical role of phospholipases in central nervous system ischemic, traumatic, and neurodegenerative disorders. Brain Res Brain Res Rev. 2004;44:13–47. doi: 10.1016/j.brainresrev.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Bonventre JV. Roles of phospholipases A2 in brain cell and tissue injury associated with ischemia and excitotoxicity. J Lipid Mediat Cell Signal. 1996;14:15–23. doi: 10.1016/0929-7855(96)00503-2. [DOI] [PubMed] [Google Scholar]
- 52.Matsuo Y, Kihara T, Ikeda M, et al. Role of platelet-activating factor and thromboxane A2 in radical production during ischemia and reperfusion of the rat brain. Brain Res. 1996;709:296–302. doi: 10.1016/0006-8993(95)01324-5. [DOI] [PubMed] [Google Scholar]
- 53.Sennlaub F, Valamanesh F, Vazquez-Tello A, et al. Cyclooxygenase-2 in human and experimental ischemic proliferative retinopathy. Circulation. 2003;108:198–204. doi: 10.1161/01.CIR.0000080735.93327.00. [DOI] [PubMed] [Google Scholar]
- 54.Beauchamp MH, Martinez-Bermudez AK, Gobeil F, Jr, et al. Role of thromboxane in retinal microvascular degeneration in oxygen-induced retinopathy. J Appl Physiol. 2001;90:2279–2288. doi: 10.1152/jappl.2001.90.6.2279. [DOI] [PubMed] [Google Scholar]
- 55.Stevens MK, Yaksh TL. Time course of release in vivo of PGE2, PGF2 alpha, 6-keto-PGF1 alpha, and TxB2 into the brain extracellular space after 15 min of complete global ischemia in the presence and absence of cyclooxygenase inhibition. J Cereb Blood Flow Metab. 1988;8:790–798. doi: 10.1038/jcbfm.1988.134. [DOI] [PubMed] [Google Scholar]
- 56.Flower RW, McLeod DS, Wajer SD, et al. Prostaglandins as mediators of vasotonia in the immature retina. Pediatrics. 1984;73:440–444. [PubMed] [Google Scholar]
- 57.Zhu Y, Park TS, Gidday JM. Mechanisms of hyperoxia-induced reductions in retinal blood flow in newborn pig. Exp Eye Res. 1998;67:357–369. doi: 10.1006/exer.1998.0535. [DOI] [PubMed] [Google Scholar]
- 58.Barnett JM, McCollum GW, Penn JS. Role of cytosolic phospholipase A(2) in retinal neovascularization. Invest Ophthalmol Vis Sci. 2010;51:1136–1142. doi: 10.1167/iovs.09-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Szymczak M, Murray M, Petrovic N. Modulation of angiogenesis by omega-3 polyunsaturated fatty acids is mediated by cyclooxygenases. Blood. 2008;111:3514–3521. doi: 10.1182/blood-2007-08-109934. [DOI] [PubMed] [Google Scholar]
- 60.Schwartzman ML, Iserovich P, Gotlinger K, et al. Profile of lipid and protein autacoids in diabetic vitreous correlates with the progression of diabetic retinopathy. Diabetes. 2010;59:1780–1788. doi: 10.2337/db10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeng ZZ, Yellaturu CR, Neeli I, et al. 5(S)-Hydroxyeicosatetraenoic acid stimulates DNA synthesis in human microvascular endothelial cells via activation of Jak/STAT and phosphatidylinositol 3-kinase/Akt signaling, leading to induction of expression of basic fibroblast growth factor 2. J Biol Chem. 2002;277:41213–41219. doi: 10.1074/jbc.M204508200. [DOI] [PubMed] [Google Scholar]
- 62.Michaelis UR, Fisslthaler B, Medhora M, et al. Cytochrome P450 2C9-derived epoxyeicosatrienoic acids induce angiogenesis via cross-talk with the epidermal growth factor receptor (EGFR) FASEB J. 2003;17:770–772. doi: 10.1096/fj.02-0640fje. [DOI] [PubMed] [Google Scholar]
- 63.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch. 2010;459:881–895. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jenkins AJ, Rowley KG, Lyons TJ, et al. Lipoproteins and diabetic microvascular complications. Curr Pharm Des. 2004;10:3395–3418. doi: 10.2174/1381612043383188. [DOI] [PubMed] [Google Scholar]
- 65.Ferris FL, 3rd, Chew EY, Hoogwerf BJ. Serum lipids and diabetic retinopathy. Early Treatment Diabetic Retinopathy Study Research Group. Diabetes Care. 1996;19:1291–1293. doi: 10.2337/diacare.19.11.1291. [DOI] [PubMed] [Google Scholar]
- 66.Chew EY, Klein ML, Ferris FL, 3rd, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol. 1996;114:1079–1084. doi: 10.1001/archopht.1996.01100140281004. [DOI] [PubMed] [Google Scholar]
- 67.Ucgun NI, Yildirim Z, Kilic N, et al. The importance of serum lipids in exudative diabetic macular edema in type 2 diabetic patients. Ann N Y Acad Sci. 2007;1100:213–217. doi: 10.1196/annals.1395.021. [DOI] [PubMed] [Google Scholar]
- 68.Jin Y, Arita M, Zhang Q, et al. Anti-angiogenesis effect of the novel anti-inflammatory and pro-resolving lipid mediators. Invest Ophthalmol Vis Sci. 2009;50:4743–4752. doi: 10.1167/iovs.08-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calandria JM, Bazan NG. Neuroprotectin D1 modulates the induction of pro-inflammatory signaling and promotes retinal pigment epithelial cell survival during oxidative stress. Adv Exp Med Biol. 2010;664:663–670. doi: 10.1007/978-1-4419-1399-9_76. [DOI] [PubMed] [Google Scholar]
- 72.Calandria JM, Marcheselli VL, Mukherjee PK, et al. Selective survival rescue in 15-lipoxygenase-1-deficient retinal pigment epithelial cells by the novel docosahexaenoic acid-derived mediator, neuroprotectin D1. J Biol Chem. 2009;284:17877–17882. doi: 10.1074/jbc.M109.003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Le HD, Meisel JA, de Meijer VE, et al. The essentiality of arachidonic acid and docosahexaenoic acid. Prostaglandins Leukot Essent Fatty Acids. 2009;81:165–170. doi: 10.1016/j.plefa.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.The Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case—control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology. 2000;107:2224–2232. doi: 10.1016/s0161-6420(00)00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai J, Nelson KC, Wu M, et al. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 76.Stahl A, Paschek L, Martin G, et al. Combinatory inhibition of VEGF and FGF2 is superior to solitary VEGF inhibition in an in vitro model of RPE-induced angiogenesis. Graefes Arch Clin Exp Ophthalmol. 2009;247:767–773. doi: 10.1007/s00417-009-1058-x. [DOI] [PubMed] [Google Scholar]
- 77.Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 78.Jones M. Effect of preterm birth on airway function and lung growth. Paediatr Respir Rev. 2009;10(Suppl 1):9–11. doi: 10.1016/S1526-0542(09)70005-3. [DOI] [PubMed] [Google Scholar]
- 79.Shen LQ, Child A, Weber GM, et al. Rosiglitazone and delayed onset of proliferative diabetic retinopathy. Arch Ophthalmol. 2008;126:793–799. doi: 10.1001/archopht.126.6.793. [DOI] [PubMed] [Google Scholar]
- 80.Krey G, Braissant O, L’Horset F, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997;11:779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 81.Connor KM, Krah NM, Dennison RJ, et al. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stahl A, Connor KM, Sapieha P, et al. The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci. 2010;51:2813–2826. doi: 10.1167/iovs.10-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 84.Stahl A, Connor KM, Sapieha P, et al. Computer-aided quantification of retinal neovascularization. Angiogenesis. 2009;12:297–301. doi: 10.1007/s10456-009-9155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92:10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lapillonne A, Jensen CL. Reevaluation of the DHA requirement for the premature infant. Prostaglandins Leukot Essent Fatty Acids. 2009;81:143–150. doi: 10.1016/j.plefa.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 87.Cheung HM, Lam HS, Tam YH, et al. Rescue treatment of infants with intestinal failure and parenteral nutrition-associated cholestasis (PNAC) using a parenteral fish-oil-based lipid. Clin Nutr. 2009;28:209–212. doi: 10.1016/j.clnu.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 88.de Meijer VE, Gura KM, Le HD, et al. Fish oil-based lipid emulsions prevent and reverse parenteral nutrition-associated liver disease: the Boston experience. JPEN J Parenter Enteral Nutr. 2009;33:541–547. doi: 10.1177/0148607109332773. [DOI] [PubMed] [Google Scholar]
- 89.Chew EY, Kim J, Coleman HR, et al. Preliminary assessment of celecoxib and microdiode pulse laser treatment of diabetic macular edema. Retina. 2010;30:459–467. doi: 10.1097/IAE.0b013e3181bcf1a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Christen WG, Glynn RJ, Chew EY, et al. Low-dose aspirin and medical record-confirmed age-related macular degeneration in a randomized trial of women. Ophthalmology. 2009;116:2386–2392. doi: 10.1016/j.ophtha.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Jong PT. Aspirin and age-related macular degeneration. Ophthalmology. 2010;117:1279–1280. doi: 10.1016/j.ophtha.2010.03.011. author reply 1280. [DOI] [PubMed] [Google Scholar]