Abstract
OBJECTIVES
The objectives of this study were prospective evaluation of MR enterographic accuracy for detecting Crohn's disease imaging features in pediatric patients compared with a CT reference standard, as well as determination of MR enterographic accuracy for detecting active bowel inflammation and fibrosis using a histologic reference standard.
MATERIALS AND METHODS
The study group for this blinded prospective study included 21 pediatric subjects with known Crohn's disease scheduled for clinical CT imaging and histological bowel sampling for symptomatic exacerbation. All subjects and their parents gave informed consent to also undergo MR enterography. CT and MR enterography examinations were independently reviewed by 2 radiologists and scored for Crohn's disease features. All bowel histology specimens were reviewed by a single pathologist for presence of active mucosal inflammation and mural fibrosis, followed by imaging-histological correlation.
RESULTS
All 21 subjects underwent MR enterography and histological sampling, 18 of whom also underwent CT. MR enterography demonstrated high sensitivity for detecting Crohn's imaging features (bowel wall thickening, mesenteric inflammation, lymphadenopathy, fistula, abscess) compared with CT, with individual sensitivity values ranging from 85.1-100%. Out of a total of 53 abnormal bowel segments with MRI-histology correlation, MR enterography demonstrated 86.7% accuracy (90.0% sensitivity, 82.6% specificity) for detecting active inflammation (P < 0.001). Accuracy of MR enterography for detecting mural fibrosis overall was 64.9% compared with histology, but increased to 88.2% (P < 0.05) for detecting fibrosis without superimposed active inflammation.
CONCLUSIONS
MR enterography can substitute for CT as the first-line imaging modality in pediatric Crohn's patients, based on its ability to detect intestinal pathology in both small and large bowel as well as extraintestinal disease manifestations. Additionally, MR enterography provides an accurate noninvasive assessment of Crohn's disease activity and mural fibrosis and can aid in formulating treatment strategies for symptomatic patients and assessing therapy response.
Introduction
Crohn's disease is an idiopathic bowel disorder affecting approximately 600,000 people in North America, with an associated incidence of 10,000-47,000 new cases per year ([1-3]). Crohn's disease demonstrates a bimodal demographic distribution with an initial peak incidence occurring in the 15-30 year age range([2]), leading to a significant incidence and prevalence within the pediatric population. Recent data estimate an age-specific incidence of Crohn's disease in the pediatric population to be 16 per 100,000 persons[4], with pediatric patients comprising 25-30% of the total Crohn's population[5]. Crohn's disease is characterized by multiple, discontinuous areas of bowel inflammation distributed throughout the gastrointestinal tract, often complicated by strictures, abscesses, and fistula formation. Crohn's disease patients typically follow a chronic, relapsing disease course, with patients often experiencing episodic symptom recurrence over the course of their lifetime.
Imaging plays an important role in the evaluation of symptomatic Crohn's patients. The aims of imaging include evaluation of initial primary diagnosis including presence, severity, and extent of disease; disease activity; treatment response; and extraintestinal complications ([6-7]). Although historically the standard imaging modality for evaluating Crohn's disease has been barium fluoroscopy, over the last decade CT has become the primary imaging modality for evaluating Crohn's disease due to its widespread availability, fast scanning time, and ability to detect extraintestinal disease manifestations ([8-9]). Currently, routine CT evaluation of Crohn's disease includes assessment of bowel wall thickening, perienteric and pericolonic mesenteric inflammation; lymph node size and number; extraluminal collections (fistulae, abscesses, sinuses); and extraintestinal complications ([10]).
Although CT has proved to be an effective imaging modality for Crohn's disease, one significant limitation is its associated patient exposure to ionizing radiation. The issue of ionizing radiation risk to patients associated with diagnostic radiology examinations has received much attention in recent years; especially for children, in whom the relative cancer mortality risk per unit radiation dose is significantly higher compared with adults ([11-12]). Diagnostic imaging radiation exposure is particularly relevant for the pediatric Crohn's population, in whom the chronic relapsing nature of the disease often results in patients undergoing multiple CT examinations over time due to symptomatic recurrence. Recent epidemiological studies suggest a nonzero radiation-induced cancer risk at exposure levels as low as 50-100 mSv ([13]), which are likely to be exceeded in patients diagnosed with Crohn's disease at an early age ([14]). A recent European study following Crohn's patients over a 15 year period found that more than 15% of patients exceeded 75 mSv in cumulative radiation exposure due to imaging, and that the risk of high radiation exposure more than doubled if the patient were diagnosed with Crohn's under the age of 17 ([8]).
Magnetic resonance imaging (MRI) provides superior soft tissue contrast relative to CT, without associated ionizing radiation exposure to patients, and is a very promising alternative imaging modality for patients with Crohn's disease. The recent development of MRI pulse sequences that provide motion-free, high resolution images with T1 or T2 contrast has made MR imaging of intestinal pathology possible ([15]). MRI is already in routine clinical use for detecting perianal and perirectal fistulae in Crohn's patients due to its superior depiction of the anal sphincter complex and small fistulous tracks relative to CT ([16-17]). Additionally, certain bowel wall MRI features, such as T2 hyperintensity and layered wall enhancement, have been shown to correlate with active inflammation in Crohn's disease ([18, 19, Del Vescovo Abd Imag 2008, 20]). MR enterography (MR-E) is a specific MRI technique that combines large volume oral contrast distention of the bowel with intravenous gadolinium administration, analogous to CT-E, to increase sensitivity for detecting bowel wall abnormalities ([21]).
This study compared MR enterography with contrast enhanced CT in a prospective fashion to determine whether MR-E can substitute for CT as the first-line imaging examination for symptomatic pediatric Crohn's patients. The potential benefit would be a reduction in CT utilization in this patient population and a decrease in lifetime patient radiation exposure. Another aim of this study was to determine whether MR-E can accurately discriminate between active and inactive inflammation in areas of bowel abnormality, using a histological gold standard. A reliable imaging index of disease activity would be a helpful triaging tool for predicting which Crohn's disease patients with bowel wall abnormalities are likely to respond to medications such as TNF-α directed therapies (active inflammation) and which may require surgical bowel resection (inactive disease/fibrosis) ([20, 22]).
Materials and Methods
Patient enrollment
This single institutional prospective study was conducted with approval from the institutional review board and in accordance with the Health Insurance Portability and Accountability Act. All enrolled subjects were patients with histologically-proven Crohn's disease under 18 years of age who were scheduled to undergo CT imaging for symptom exacerbation. Patients over the age of 18 were eligible to participate if they were under the care of a pediatric gastroenterologist. Informed consent was obtained for performance of the MR enterography examination, which was the research study. For subjects under 18 years of age, informed consent was obtained from a parent. Study exclusion criteria included inability to undergo MRI without conscious sedation, presence of MRI-incompatible metallic hardware, and renal impairment (estimated glomerular filtration rate < 60 mL/min/1.73 m2).
CT and MR enterography technique
All CT exams were performed on a 16-detector multislice CT scanner (GE Lightspeed; Milwaukee, WI) using standard departmental oral (Redi-CAT; E-Z EM, Lake Success, NY) and intravenous (Isovue 370; Bracco Diagnostics) contrast administration, with images acquired during portal venous phase (70 seconds post contrast injection). Standard departmental pediatric CT dose reduction techniques were employed using weight-based automated exposure control (Auto-mA, GE; mA range 110-220, kVp 120, Noise Index 12). Images were primarily interpreted at 5mm slice thickness, followed by reconstruction to 1.25mm thin sections at 1mm intervals for secondary review and generation of coronal reformations.
All MRI exams were performed on a 1.5T clinical scanner (Siemens Magnetom; Malvern, PA) equipped with Total Imaging Matrix surface coil design in the abdominopelvic configuration (12 RF channels). For the MR enterography protocol, subjects consumed a total of 1200 cc of oral contrast over the course of 60 minutes just prior to MR scanning. The oral preparation consisted of a mixture of 900 cc dilute barium and sorbitol (VoLumen; E-Z EM, Lake Success, NY) combined with 300 cc superparamagnetic iron oxide solution (GastroMARK; Mallinckrodt, St. Louis, MO); the former is given to distend the bowel and the latter to increase contrast between lumen and bowel wall on post-contrast and T2-weighted images. MR enterography sequences included 3-plane localizers, coronal and axial T2 single-shot images through entire abdomen/pelvis (HASTE, 5mm/0sp, TR N/A, TE=100msec, 320 × 256 matrix), coronal balanced steady state images through the abdomen/pelvis (TRUE-FISP, 4mm/0sp, TR=5.4msec, TE=2.7msec, 320 × 256, breath-hold), axial T2 forced recovery fast spin echo fat-suppressed images through the abdomen and pelvis (RESTORE, TR=2100, TE=100, ETL=15, 320 × 290, free-breathing with navigator triggering), coronal dynamic T1 3D volumetric gradient echo fat-suppressed images through the abdomen and pelvis post-contrast (VIBE, 4mm/0sp, TR=5, TE=2.5, 320 × 290, breath hold) with acquisitions at 1, 3, and 5 minutes post-injection, and axial T1 2D spoiled gradient fat-suppressed high-resolution post-gadolinium images through the abdomen and pelvis (5mm/5sp, TR=200, TE=2.5, FOV=40cm, 512×512, multiple breath holds). Administered intravenous contrast was gadopentetate dimeglumine (Magnevist; Bayer, Pittsburgh) at a dose of 0.1 mmol/kg injected at 2 ml/sec using a power injector followed by a 20 ml saline flush at the same rate. Total MR-E scan times ranged from 45-60 minutes.
Image interpretation
Assessment of Crohn's disease imaging features
Two radiologists with expertise in Crohn's disease imaging independently reviewed all CT and MR enterography exams. The readers were blinded to all clinical and demographic information related to the subjects, the clinical interpretation of the studies, as well as each other's reads. Separate review sessions were scheduled for the CT and MR enterography exams, spaced by at least one month apart. The readers scored each exam for the presence and location of the following Crohn's disease imaging features: 1) bowel wall thickening (>3mm), 2) mesenteric inflammatory changes, 3) lymphadenopathy (≥1cm short axis diameter), 4) abscesses (defined as peripherally enhancing extraluminal collections), and 5) fistulae (defined as abnormal communications between bowel and another organ or body space). All results were individually recorded on a standard spreadsheet template and later compared. If the two readers disagreed on whether a finding was present, a third reader (also a board-certified radiologist with expertise in abdominal and pediatric imaging) served as arbiter. MR-E exams were then scored using the CT results as the imaging gold standard.
Imaging assessment of Crohn's disease activity
For each segment of bowel wall abnormality identified on MR enterography (presence of bowel wall thickening and/or adjacent mesenteric inflammation), an imaging determination of Crohn's activity was made based on whether the following features of active disease were present: 1) prominent mucosal enhancement followed by progressive transmural enhancement on dynamic post-gadolinium images, 2) prominent mesenteric vasa recta, 3) bowel wall T2 hyperintensity relative to muscle. In addition, an imaging assessment for transmural fibrosis was made based on the presence or absence of the following fibrosis features: 1) absent or minimal transmural enhancement on post-gadolinium images, 2) low bowel wall T2 signal intensity (typically isointense or hypointense relative to muscle).
Histological assessment of Crohn's disease activity
All subjects had histological bowel sampling, either via endoscopic biopsy or full-thickness surgical bowel resection. Resection and biopsy specimens were routinely processed as part of clinical care, including formalin fixation, paraffin embedding, sectioning and hemotoxylin/eosin staining. Two pathologists reviewed representative histological specimens from the enrolled patients under multi-head microscope to define pathologic criteria for this study. Subsequently, a single pathologist, who was blinded to the original clinical specimen interpretation, the clinical history, and the imaging findings, assessed all histology specimens from the enrolled patients in a single review session. All biopsy and surgical resection specimens were evaluated for presence or absence of active inflammation, while only surgical excisions were additionally evaluated for presence or absence of mural fibrosis. Active inflammation was defined by neutrophilic infiltration of intestinal crypts (cryptitis and/or crypt abscess), while mural fibrosis was defined as collagen fiber replacement within the bowel wall involving at least submucosal and mucosal layers. Histological specimens were localized anatomically within the bowel based on either the operative (surgical excisions) or endoscopic (biopsies) report, for the purposes of imaging correlation. For surgical excision specimens, the location of each sectioned area was also oriented with respect to fixed anatomic landmarks (e.g. ileocecal valve or appendix) present in the gross specimen. Surgical gross excision specimens were sectioned and marked every 10 cm per usual clinical protocol, which enable multiple segments to be evaluated per each gross excision specimen.
Statistical analysis
Degree of agreement between CT and MR-E for detection of Crohn's disease imaging features was determined using Cohen's kappa test. Significance of association between MR-enterographic and histologic assessment of Crohn's disease activity was determined using Fisher's exact test. Both statistical tests were performed using Graphpad software (La Jolla, CA).
Results
Study population
A total of 21 subjects were included in the study (Table 1). This includes 18 subjects who underwent CT, MR-E and histological sampling, as well as 3 additional subjects who underwent MR-E and histological sampling without recent CT. Mean subject age was 17.7 years (range 12-22). There were 10 males and 11 females in the study.
Table 1.
MR enterographic detection of Crohn's imaging features compared with CT reference standard.
| Number of subjects | 18 | |||
| Mean subject age | 17.7 years (range 12-22) | |||
| Mean days between exams | 3.1 days (range 0-20) | |||
| Crohn's imaging feature | Total number | # detected (MR-E) | MR-E sensitivity (%) | Kappa |
| LAD | 47 | 40 | 85.1 | 0.85 (0.75-0.96) |
| Thickened bowel wall | 24 | 21 | 87.5 | 0.88 (0.74-1.00) |
| Fluid collections | 7 | 6 | 85.7 | 0.86 (0.59-1.00) |
| Mesenteric inflammation | 9 | 8 | 88.9 | 0.89 (0.68-1.00) |
| Fistulae | 8 | 8 | 100.0 | 1.00 |
MR enterographic detection of Crohn's imaging features
In the 18 subjects who underwent both CT and MR-E, MR-E detection of Crohn's disease imaging features was assessed using CT as the reference standard. The mean number of days between the two studies was 3.1 days. The Crohn's disease imaging features assessed were lymphadenopathy (defined as lymph nodes ≥ 1 cm in short axis diameter), bowel wall thickening (defined as wall thickness ≥ 3mm diameter), focal fluid collections, mesenteric inflammation, and fistulae (Figure 1). The presence and number of each of these imaging features were recorded for the CT and MR-E exams independently, followed by a joint review session in which the findings on both exams were compared to determine the level of concordance. Compared with CT, MR-E proved highly sensitive for detection of these Crohn's imaging features, with sensitivity values ranging from 85.1% for lymphadenopathy to 100% for fistulae (Table 1). MR-E demonstrated almost perfect agreement with CT for all five of the imaging features based on inter-rater reliability measurement, with kappa values ranging from 0.85-1.00.
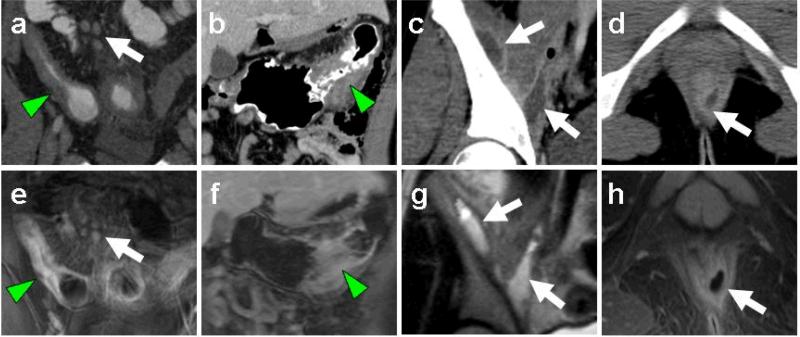
Fig. 1.
MR enterography and CT depiction of Crohn's imaging features. Representative image pairs from CT (a-d) and MR-E (e-h) studies demonstrate wall thickening of small bowel (a, e arrowheads) and colon (b, f arrowheads), lymphadenopathy (a, d arrows), intersphincteric fistula formation (d, h arrows), and iliopsoas abscesses (c, g arrows).
MR enterographic detection of active bowel inflammation compared with histological reference
All subjects underwent histological bowel sampling (either colonoscopic biopsy or surgical bowel excision) as part of their standard medical care. The location of each histology specimen obtained was determined from the surgical or endoscopy procedure note. For each segment of bowel that was sampled, an independent MR-E determination of active bowel inflammation was made and compared with the histologic evaluation. MR-E criteria for active inflammation included prominent mucosal enhancement followed by progressive transmural enhancement on dynamic post-contrast images, prominent mesenteric vasa recta, and bowel wall T2 hyperintensity (Figure 2). Histological detection of active inflammation relied upon detection of neutrophilic infiltration of the mucosal crypts, which could be detected on either mucosal biopsy or full-thickness excision specimens. A total of 53 bowel segments were available for imaging-pathology correlation, including 16 endoscopic biopsies and 37 surgical excision segments (Table 2). By anatomic location, 30 of the bowel segments were in the small bowel and 23 were in the colon. The most common anatomic bowel region sampled was the terminal ileum (18/53 or 34%). Using histology as a reference, MR-E had an accuracy of 86.7% (46/53) for detection of active inflammation, which was statistically significant (P < 0.001, Fisher's exact test). This was associated with a sensitivity of 90.0% and a specificity of 82.6%.
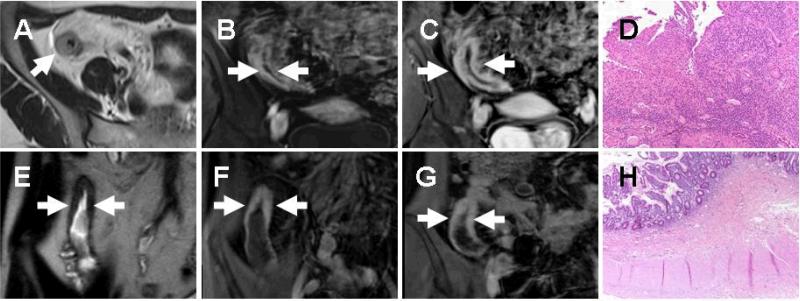
Fig. 2.
MR enterographic detection of active inflammation and fibrosis. Imaging is depicted from abnormal small bowel segments (arrows) in 2 subjects, the first demonstrates bowel wall T2 hyperintensity (a) early mucosal enhancement (b) and later progressive transmural enhancement (c) suggestive of active inflammation, while the second demonstrates bowel wall T2 hypointensity (e) and minimal wall enhancement (f) that does not increase with time (g) suggestive of fibrosis. Histological assessment of corresponding surgical excision specimens confirms the MR findings, with active inflammation characterized by neutrophilic invasion of the mucosal glands (d) and submucosal collagen deposition indicative of mural fibrosis (h).
Table 2.
MR enterographic assessment of Crohn's disease activity compared with histologic reference standard.
| Number of subjects | 21 |
| Total number of bowel segments | 53 |
| -Endoscopic biopsy | 16 |
| -Surgical resection | 37 |
| Bowel segment locations: | |
| -Small bowel upstream of TI | 12 |
| -Terminal ileum | 18 |
| -Cecum/ascending colon | 12 |
| -Transverse/descending colon | 8 |
| -Rectosigmoid | 2 |
| Detecting active inflammation (compared with biopsy or surgical excision histology): | |
| Accuracy | 86.7%* (46/53; P < 0.001) |
| Sensitivity | 90.0% |
| Specificity | 82.6% |
| Detecting mural fibrosis (overall, compared with surgical excision histology): | |
| Accuracy | 64.9% (24/37; P = 0.082) |
| Sensitivity | 58.3% |
| Specificity | 76.9% |
| Detecting mural fibrosis (excluding cases with co-existing active inflammation): | |
| Accuracy | 88.2%* (15/18; P = 0.013) |
| Sensitivity | 83.3% |
| Specificity | 83.3% |
MR enterographic detection of bowel wall fibrosis compared with surgical bowel resection histological reference
Histological determination of bowel wall fibrosis is based on detection of collagen fiber replacement within the bowel wall involving at least submucosal and mucosal layers. Therefore, in order to assess the ability of MR-E to detect bowel fibrosis, we only evaluated those bowel segments where surgical excision specimens were available. Imaging features suggestive of fibrosis included bowel wall T2 hypointensity and absent or minimal transmural enhancement without mucosal prominence (Figure 2). 37 total bowel segments were identified. The accuracy of MR-E for detecting mural fibrosis overall was 64.9% (24/37), with a sensitivity of 58.3% and a specificity of 76.9%. The accuracy and sensitivity of MR-E for detecting fibrosis were considerably lower compared with MR-E detection of active inflammation. The fact that specificity remained high suggested that the low accuracy for MR-E detection of fibrosis was principally due to a high false negative rate. During a secondary review of the results, we identified 7 false negative cases of fibrosis in which there was histological evidence of active inflammation superimposed upon fibrosis (Figure 3). In all seven of these cases, histological examination revealed active inflammatory changes extending beyond the mucosa into the deeper bowel wall layers where there was ulceration and mural abscess formation (Figure 3F). When these cases of concomitant active inflammation and mural fibrosis were excluded, the accuracy of MR-E for detecting chronic fibrosis alone increased to 88.2% (15/18), which was statistically significant (P = 0.013, Fisher's exact test). This was also associated with increased sensitivity (88.3%) and specificity (88.3%) values.
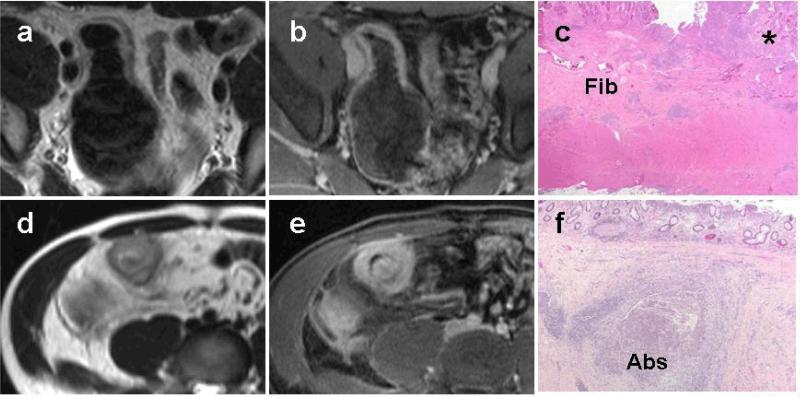
Fig. 3.
Superimposed active inflammation can obscure MR enterographic detection of bowel wall fibrosis. Representative T2-weighted (a, d) and T1 fat-suppressed post-contrast (b, e) images from 2 subjects demonstrate focal small bowel wall thickening, T2 hyperintensity, and marked mural enhancement suggestive of active inflammation. Corresponding histology images (c and f) demonstrate mucosal inflammation (c, asterisk) superimposed upon submucosal fibrosis (c, “Fib”) as well as a large intramural abscess (f, “Abs”).
Discussion
This study evaluated MR enterography as a potential first-line imaging modality for assessing pediatric Crohn's disease. Traditionally, endoscopy and barium fluoroscopy have been the standard techniques for detecting bowel pathology in Crohn's patients. However, neither modality is sensitive for detecting extraluminal and extraintestinal disease complications, which often need to be excluded in patients presenting with acute symptomatic exacerbation. In recent years, CT has become the primary imaging modality for Crohn's disease evaluation because of its ability to detect both gastrointestinal tract pathology and extraintestinal complications ([15]). Additional advantages of CT include widespread availability, multiplanar imaging capability, and rapid image acquisition ([6]). Recent attention has focused on the potential ionizing radiation risk associated with CT scanning, particularly when utilized in diseases such as Crohn's that frequently are diagnosed during childhood and necessitate repetitive imaging during disease remission and relapse ([11]). The pediatric population is at increased risk for ionizing radiation-induced malignancy relative to adults because of its relatively high rate of cell division and longer latency period to develop cancer ([23]). MR enterography potentially offers the diagnostic benefits of CT without the ionizing radiation exposure risk. Our results indicate that MR enterography has high sensitivity for detecting Crohn's disease imaging features compared with CT reference standard, with sensitivity values ranging from 85.1% for lymphadenopathy up to 100% for fistulae. MR-E demonstrated almost perfect agreement with CT for all five of the imaging features (lymphadenopathy, bowel wall thickening, mesenteric inflammation, abscess, fistula) based on inter-rater reliability analysis. The imaging features selected are widely considered to be imaging hallmarks of Crohn's disease ([10]) and include extraluminal complications disease requiring urgent intervention.
This study also evaluated the ability of MR enterography to discriminate active from inactive bowel inflammation, an important distinction during followup imaging of Crohn's patients undergoing medical therapy ([24]). This distinction is particularly important given the recent advent of biological therapies targeting specific inflammatory molecules such as TNF-α, which are more likely to be effective in patients with active inflammation ([22]). MR-E had an accuracy of 86.7% compared with histological reference standard, with a sensitivity of 90.0% and a specificity of 82.6%. The association is statistically significant (P < 0.001, Fisher's exact test) and indicates that MR-E is a reliable noninvasive method for detecting active inflammation in pediatric Crohn's patients.
We also examined the ability of MR-E to detect mural fibrosis, which is a histological hallmark of obstructing fibrostenotic strictures that often require surgical excision ([25]). MR-E sensitivity (58.3%) and accuracy (64.9%) for detecting fibrosis overall were decreased compared with detection of active inflammation. However, after our initial analysis we identified seven cases in which MR enterography missed mural fibrosis due to superimposed active inflammation. In all of these cases, the active inflammatory changes had extended beyond the mucosa into the bowel wall and were associated with mural ulceration and abscess formation. These mural ulcers and abscesses likely account for the T2 hyperintensity and strong mural enhancement seen on MRI in these cases, which obscured the underlying fibrosis. Excluding these cases of concomitant active inflammation and fibrosis, the sensitivity of MR-E for detecting chronic fibrosis alone was 83.3% and the accuracy was statistically significant (P < 0.05, Fisher's exact test). The ability of MR-E to discriminate chronic fibrosis from fibrosis with superimposed active inflammation is clinically important, and suggests that MR-E can help triage pediatric Crohn's patients with obstructing bowel strictures into medical (with concomitant active disease) and surgical (chronic fibrosis alone) intervention groups.
To our knowledge, this study is the first to evaluate MR enterography as the primary imaging modality in pediatric Crohn's disease assessment, using CT imaging and histology as reference standards. A 2004 study demonstrated high sensitivity and specificity of gadolinium-enhanced MRI to discriminate Crohn's disease from ulcerative colitis in pediatric IBD patients[26], while a 2009 study of MR enterography in pediatric IBD patients showed an association between abnormal small bowel enhancement and serum c-reactive proteins levels[27]. Both studies demonstrate MR enterography to be feasible in a pediatric IBD population but are limited by lack of independent imaging or histological reference for the abnormal bowel segments detected by MRI. The MR enterography technique has been validated by multiple investigators for IBD evaluation in adults, including a recent study in adult Crohn's patients demonstrating MR-E to be comparable to barium small bowel series for detecting bowel pathology ([28]). Few studies have performed direct comparison of MR enterography with CT. One study from 2000 compared contrast-enhanced MRI and CT for detecting bowel wall abnormalities in adult Crohn's disease patients ([29]); however, the CT (nonhelical) and MRI (oral and rectal contrast) protocols used in that study do not reflect current imaging technique. Two recent studies have demonstrated MR-E to be comparable to CT enterography for detection of active small bowel inflammation in adult Crohn's disease patients ([30-31]). Our study extends upon this prior work by demonstrating MR-E to be sensitive for detecting active inflammation in both small and large bowel, as well as detecting extraintestinal manifestations of Crohn's disease. These results suggest that MR-E can serve as a first-line Crohn's imaging modality. Also, our study included histological specimens from all subjects, including a significant proportion of full-thickness surgical excisions, which provided reference standards for evaluating ability of MR-E to detect not only active inflammation, but also mural fibrosis. In this regard, our work extends beyond previous studies of MR-E in adult Crohn's disease ([19-20]) that relied predominantly on endoscopic biopsies and only discriminated active from inactive inflammation.
The MR-E protocol utilized in this study was well-tolerated by the study population, with scan times ranging from 45-60 minutes and no subjects being excluded due to inability to complete the exam. Importantly, none of the pediatric patients involved in the study required conscious sedation. The ability to perform MR enterography without sedation is an important feature that will facilitate more widespread application of the technique in the pediatric population. We do note that some subjects did require persistent encouragement to consume the required volume of oral contrast. In this regard, addition of sugar-free fruit flavored crystals to the contrast agent significantly increased subject compliance. Also, in order to minimize patient discomfort during the exam, we kept the number of breath-hold sequences to a minimum by utilizing fast motion freezing sequences (e.g. HASTE, True FISP) that could be acquired with quiet respiration, as well as navigator respiratory triggering for the longer T2 fat-suppressed sequences. Parallel imaging acceleration was also utilized to decrease imaging time for the dynamic post-gadolinium T1 fat-suppressed sequences.
Limitations of this study include the fact that CT and MR-E exams were not performed on the same day in all subjects due to scheduling limitations. However, the close temporal spacing of the two imaging studies (mean time between studies = 3.1 days) did facilitate direct comparison of CT and MR-E features. Also, the CT protocol used in our study was the standard abdominopelvic technique with positive oral and intravenous contrast, rather than CT enterography protocol with large volume neutral enteric contrast and thin slice technique, which has become the preferred CT technique for evaluating inflammatory bowel disease ([32]). Theoretically, CT enterography may have revealed additional areas of bowel pathology that may have been missed by conventional CT, which could have affected the MR-E vs CT comparison. CT enterography is not routinely used at our institution to evaluate pediatric Crohn's disease patients, due to increased radiation exposure relative to conventional CT and reduced ability to discriminate abscess collections from neutral contrast-filled bowel loops. We are planning a future study comparing MR-E with CT-E in pediatric Crohn's patients. An additional limitation of this study is that our study population was limited to symptomatic subjects because of the requirement for medical indication for CT performance. As such, we did not evaluate the ability of MR-E to assess disease severity and activity in asymptomatic patients. Similarly, surgical excision specimens were only obtained from symptomatic patients with resection margins determined by gross surgical appearance, potentially leading to some selection bias toward abnormal bowel features.
Our results indicate that MR enterography can substitute for CT as the primary imaging modality for pediatric Crohn's disease evaluation. It is acknowledged that large-scale replacement of CT by MR-E as the primary Crohn's disease imaging modality would be associated with significant cost and availability issues ([33]). Additionally, the large volume oral preparation and relatively long scan times associated with MR-E may not be well-tolerated by some acutely ill patients, meaning that CT is likely to remain the imaging study of choice for evaluation of Crohn's patients in the acute/emergent setting. At the least,, substituting CT with MR-E in the pediatric Crohn's population makes sense, given the higher radiation sensitivity and increased imaging-associated radiation exposure of this group compared with the adult Crohn's population ([8]). Such a change in practice would be consistent with recent calls in the radiology literature for increased utilization of MR enterography in the adult Crohn's population[34]. It is likely that a combination of CT dose reduction techniques and increased MR enterography utilization will become a standard part of Crohn's disease imaging evaluation in the coming years.
Acknowledgments
Grant support: This work is supported by a Catalyst award from NIH-National Center for Research Resources and Harvard Medical School (UL1 RR025758-02 to M.S.G.)
References
- 1.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 2.Friedman S, Blumberg RS. Inflammatory Bowel Disease. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Harrison's Principles of Internal Medicine. McGraw-Hill; New York: 2001. pp. 1679–1692. [Google Scholar]
- 3.Loftus EV., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 4.Hyams JS. Crohn's disease in children. Pediatr Clin North Am. 1996;43:255–277. doi: 10.1016/s0031-3955(05)70405-3. [DOI] [PubMed] [Google Scholar]
- 5.Diefenbach KA, Breuer CK. Pediatric inflammatory bowel disease. World J Gastroenterol. 2006;12:3204–3212. doi: 10.3748/wjg.v12.i20.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patak MA, Mortele KJ, Ros PR. Multidetector row CT of the small bowel. Radiol Clin North Am. 2005;43:1063–1077. viii. doi: 10.1016/j.rcl.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Mackalski BA, Bernstein CN. New diagnostic imaging tools for inflammatory bowel disease. Gut. 2006;55:733–741. doi: 10.1136/gut.2005.076612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desmond AN, O'Regan K, Curran C, et al. Crohn's disease: factors associated with exposure to high levels of diagnostic radiation. Gut. 2008;57:1524–1529. doi: 10.1136/gut.2008.151415. [DOI] [PubMed] [Google Scholar]
- 9.Horton KM, Fishman EK. The current status of multidetector row CT and three-dimensional imaging of the small bowel. Radiol Clin North Am. 2003;41:199–212. doi: 10.1016/s0033-8389(02)00121-5. [DOI] [PubMed] [Google Scholar]
- 10.Gore RM, Balthazar EJ, Ghahremani GG, Miller FH. CT features of ulcerative colitis and Crohn's disease. AJR Am J Roentgenol. 1996;167:3–15. doi: 10.2214/ajr.167.1.8659415. [DOI] [PubMed] [Google Scholar]
- 11.Brenner DJ, Elliston CD, Hall EJ, Berdon WE. Estimates of the cancer risks from pediatric CT radiation are not merely theoretical: comment on “point/counterpoint: in x-ray computed tomography, technique factors should be selected appropriate to patient size. against the proposition”. Med Phys. 2001;28:2387–2388. doi: 10.1118/1.1415074. [DOI] [PubMed] [Google Scholar]
- 12.Rice HE, Frush DP, Farmer D, Waldhausen JH. Review of radiation risks from computed tomography: essentials for the pediatric surgeon. J Pediatr Surg. 2007;42:603–607. doi: 10.1016/j.jpedsurg.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Pierce DA, Preston DL. Radiation-related cancer risks at low doses among atomic bomb survivors. Radiat Res. 2000;154:178–186. doi: 10.1667/0033-7587(2000)154[0178:rrcral]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Brenner DJ. Should computed tomography be the modality of choice for imaging Crohn's disease in children? The radiation risk perspective. Gut. 2008;57:1489–1490. doi: 10.1136/gut.2008.156265. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa A, Saotome T, Yamasaki M, et al. Cross-sectional imaging in Crohn disease. Radiographics. 2004;24:689–702. doi: 10.1148/rg.243035120. [DOI] [PubMed] [Google Scholar]
- 16.Koelbel G, Schmiedl U, Majer MC, et al. Diagnosis of fistulae and sinus tracts in patients with Crohn disease: value of MR imaging. AJR Am J Roentgenol. 1989;152:999–1003. doi: 10.2214/ajr.152.5.999. [DOI] [PubMed] [Google Scholar]
- 17.Szurowska E, Wypych J, Izycka-Swieszewska E. Perianal fistulas in Crohn's disease: MRI diagnosis and surgical planning : MRI in fistulazing perianal Crohn's disease. Abdom Imaging. 2007 doi: 10.1007/s00261-007-9188-2. [DOI] [PubMed] [Google Scholar]
- 18.Maccioni F, Viscido A, Broglia L, et al. Evaluation of Crohn disease activity with magnetic resonance imaging. Abdom Imaging. 2000;25:219–228. doi: 10.1007/s002610000004. [DOI] [PubMed] [Google Scholar]
- 19.Koh DM, Miao Y, Chinn RJ, et al. MR imaging evaluation of the activity of Crohn's disease. AJR Am J Roentgenol. 2001;177:1325–1332. doi: 10.2214/ajr.177.6.1771325. [DOI] [PubMed] [Google Scholar]
- 20.Del Vescovo R, Sansoni I, Caviglia R, et al. Dynamic contrast enhanced magnetic resonance imaging of the terminal ileum: differentiation of activity of Crohn's disease. Abdom Imaging. 2007 doi: 10.1007/s00261-007-9267-4. [DOI] [PubMed] [Google Scholar]
- 21.Fidler J. MR imaging of the small bowel. Radiol Clin North Am. 2007;45:317–331. doi: 10.1016/j.rcl.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Clark M, Colombel JF, Feagan BC, et al. American gastroenterological association consensus development conference on the use of biologics in the treatment of inflammatory bowel disease, June 21-23, 2006. Gastroenterology. 2007;133:312–339. doi: 10.1053/j.gastro.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Brenner DJ. Estimating cancer risks from pediatric CT: going from the qualitative to the quantitative. Pediatr Radiol. 2002;32:228–223. doi: 10.1007/s00247-002-0671-1. discussion 242-224. [DOI] [PubMed] [Google Scholar]
- 24.Del Campo L, Arribas I, Valbuena M, Mate J, Moreno-Otero R. Spiral CT findings in active and remission phases in patients with Crohn disease. J Comput Assist Tomogr. 2001;25:792–797. doi: 10.1097/00004728-200109000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Maglinte DD, Gourtsoyiannis N, Rex D, Howard TJ, Kelvin FM. Classification of small bowel Crohn's subtypes based on multimodality imaging. Radiol Clin North Am. 2003;41:285–303. doi: 10.1016/s0033-8389(02)00117-3. [DOI] [PubMed] [Google Scholar]
- 26.Darbari A, Sena L, Argani P, Oliva-Hemker JM, Thompson R, Cuffari C. Gadolinium-enhanced magnetic resonance imaging: a useful radiological tool in diagnosing pediatric IBD. Inflamm Bowel Dis. 2004;10:67–72. doi: 10.1097/00054725-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Alexopoulou E, Roma E, Loggitsi D, et al. Magnetic resonance imaging of the small bowel in children with idiopathic inflammatory bowel disease: evaluation of disease activity. Pediatr Radiol. 2009;39:791–797. doi: 10.1007/s00247-009-1272-z. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein CN, Greenberg H, Boult I, Chubey S, Leblanc C, Ryner L. A prospective comparison study of MRI versus small bowel follow-through in recurrent Crohn's disease. Am J Gastroenterol. 2005;100:2493–2502. doi: 10.1111/j.1572-0241.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 29.Low RN, Francis IR, Politoske D, Bennett M. Crohn's disease evaluation: comparison of contrast-enhanced MR imaging and single-phase helical CT scanning. J Magn Reson Imaging. 2000;11:127–135. doi: 10.1002/(sici)1522-2586(200002)11:2<127::aid-jmri8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 30.Lee SS, Kim AY, Yang SK, et al. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009;251:751–761. doi: 10.1148/radiol.2513081184. [DOI] [PubMed] [Google Scholar]
- 31.Siddiki HA, Fidler JL, Fletcher JG, et al. Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn's disease. AJR Am J Roentgenol. 2009;193:113–121. doi: 10.2214/AJR.08.2027. [DOI] [PubMed] [Google Scholar]
- 32.Paulsen SR, Huprich JE, Fletcher JG, et al. CT enterography as a diagnostic tool in evaluating small bowel disorders: review of clinical experience with over 700 cases. Radiographics. 2006;26:641–657. doi: 10.1148/rg.263055162. discussion 657-662. [DOI] [PubMed] [Google Scholar]
- 33.Lin MF, Narra V. Developing role of magnetic resonance imaging in Crohn's disease. Curr Opin Gastroenterol. 2008;24:135–140. doi: 10.1097/MOG.0b013e3282f49b14. [DOI] [PubMed] [Google Scholar]
- 34.Oommen J, Oto A. Contrast-enhanced MRI of the small bowel in Crohn's disease. Abdom Imaging. 2010 doi: 10.1007/s00261-010-9617-5. [DOI] [PubMed] [Google Scholar]